Abstract
Use of a novel reagent has been established for the synthesis of a series of 4,5-diaryl-2H-1,2,3-triazoles (6a-i and 9a-e) from cyanostilbene analogs of benzo[b]thiophene, benzo[b]furan and indole, catalyzed by L-proline via Lewis base-catalyzed one-step [3+2]cycloaddition of azide. This method provides an efficient, simple and environmentally benign procedure that affords good yields and relatively short reaction times.
Keywords: 4,5-Diaryl-2H-1,2,3-triazoles; L-Proline; Heteroaryl cyanostilbenes; Sodium azide; [3+2] Cycloaddition of azide
Recent studies on cyanostilbene anticancer agents (Fig 1. 1-3a) have focused on the construction of bridge units between the two aromatic ring systems to overcome the instability of the molecules due to cis, trans-isomerization in solution.1-5 Diverse biological activities have been documented for drug molecules that incorporate the triazole ring system, including anti-cancer,6-8 anti-bacterial,9 anti-fungal10 and anti-convulsant activities.11 Recently, we reported the synthesis of a variety of heterocyclic cyanstilbene analogs as anti-cancer agents.12 In the view of the promising biological activity of the triazole analogs and in continuation of our research work on cyanostilbene analogs (Fig 1; 2 and 3a)12 as novel anticancer agents, we focused on the incorporation of a triazole bridge unit between the two aromatic ring systems of such molecules by chemical modification of cyanostilbene moiety (Scheme 1). Unlike compounds 1-3a, the resulting 4,5-disubstituted-2H-1,2,3-triazole analogs cannot undergo cis-trans isomerization, providing molecules with greater stability (e.g. Scheme 1; 6a).
Figure 1.
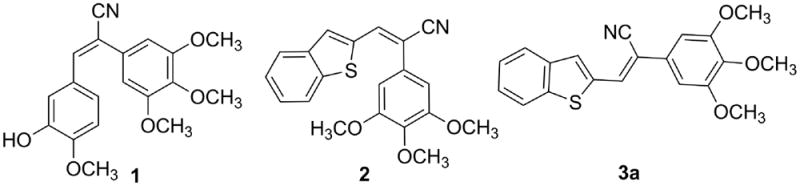
Cyanostilbene analogs
Scheme 1.
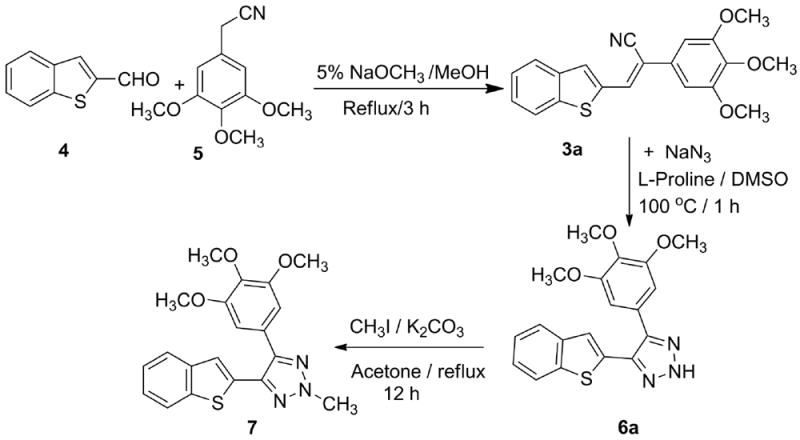
Synthesis of 4-(benzo[b]thiophen-2-yl)-5-(3,4,5-tri methoxyphenyl)-2H-1,2,3-triazole (6a) and 4-(benzo[b] thiophen-2-yl)-2-methyl-5-(3,4,5-trimethoxyphenyl)-1,2,3-(2H)-triazole (7).
The formation of 4,5-disubstitued-1H- and 2H-1,2,3-triazoles ring system is well known in the literature as “click chemistry” reaction products from Cu-catalyzed azide-alkyne 1,3-dipolar cycloaddition (CuAAC) reactions.13-14 Although the thermal reaction between alkyne and NaN3 “click chemistry” synthesis of 4,5-disubstitued-2H-1,2,3-triazoles is well known, the low yields, difficulty in preparation of substituted alkynes and cost effectiveness are limitations of this reaction.15 Zard et al.16 reported the synthesis of 1,2,3-(2H)-triazoles by the reaction of α-substituted nitroalkenes with excess of NaN3 at 80-90 °C in 54-96% yield. In this reaction the yield of the products depends on the substrate but the preparation of the olefin precursor became challenge. Synthesis of 4,5-disubstituted-2H-1,2,3-triazoles was also reported by cycloaddition of internal alkynes with alkyl azides or metal azides, this procedure has limitations such as low yields, if the alkyne reactant is conjugated with an aryl group containing electron donating substituents.17 Another approach for the synthesis of 4,5-disubstitued-2H-1,2,3-triazoles from nitroalkenes was reported utilizing NaN3 and L-proline to afford product yields in the range 40-89%.18 However, for this reaction the vinyl group is essential, and the reaction has to be carried out at room temperature, necessitating elongated reaction times, since facile polymerization of the nitroalkene reactant occurs at elevated temperatures. Very recently, we reported the synthesis of 4,5-substituted 2H-1,2,3-triazoles from (Z)-2,3-diphenyl substituted acrylonitriles using excess NaN3 (3 equivs) and NH4Cl in DMF at reflux temperatures for 12 h in 59-87% yields.19
In the present communication we describe the synthesis of 4,5-diaryl-2H-1,2,3-triazoles from heteroaryl cyanostilbenes utilizing L-proline as a Lewis base and NaN3 in dimethyl sulfoxide at 100 °C. Under these conditions, the reaction time is 1-6 h, and yields are in the range 75-96%.
In an earlier communication we reported the one-step synthesis of heteroaryl cyanostilbene analogs by the reaction of benzthiophene-2-aldehyde with phenyl acetonitriles in 5% sodium methoxide/methanol solution for 3 h (Scheme 1).12 This reaction predominantly forms the trans-isomer (3a) which slowly converts to the respective cis-isomer (2) in organic solvents; more rapid trans to cis conversion takes place in the presence of protic solvents and on exposure to light. To avoid this geometrical isomerism we sought to lock the geometry of cyanostilbenes (3a-i) by converting them to their corresponding 1,2,3-(2H)-triazole analogs (6a-i) by reaction with NaN3/DMSO in the presence of a Lewis base such as L-proline (Scheme 2). The initial reaction conditions utilized for the synthesis of the representative compound 6a from cyanostilbene 3a, are summarized in Table 1. The formation of the 4,5-disubstitued-2H-1,2,3-triazole 6a was confirmed by single crystal X-ray diffraction analysis (Figure 2), and from subsequent regiospecific N-2 methylation with methyl iodide/K2CO3 in acetone to form 7 (Scheme 1; Figure 2).
Scheme 2.
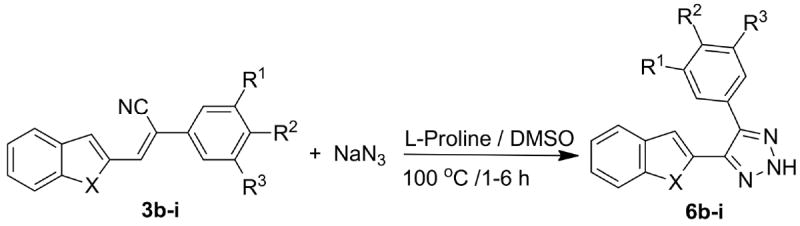
Synthesis of substituted 4-heteroaryl-5-phenyl-2H-1,2,3-triazoles (6b-i)
Table 1.
Different reaction conditions for the formation of 6a from 3a via 3+2 cycloaddition of azide.
| Catalyst | Solvent | NaN3 | Temp | Time | Yieldc |
|---|---|---|---|---|---|
|
| |||||
| Equiv. | (°C) | (h) | (%) | ||
| NH4Cl (2.0) | DMSO | 2 | 120 | 18 | 45 |
| NH4Cl (2.0) | DMSO | 3 | 120 | 18 | 51 |
| NH4Cl (2.0) | DMF | 2 | 120 | 20 | 40 |
| - | DMSO | 2 | 120 | 36 | 15 |
| TMSAa(1.1) | DMSO | 2 | 120 | 20 | 65 |
| TMSA (1.1) | DMF | 2 | 120 | 24 | 62 |
| L-pro | DMSO | 1 | rt | 20 | 75 |
| L-pro | DMSO | 1 | 100 | 1 | 96 |
| L-pro | DMSO | 1 | 130 | 1 | 89 |
| L-pro | Acetonitrile | 2 | 82 | 5 | 0 |
| TEA (1.2)b | DMSO | 1 | 100 | 10 | 18 |
| Pyrrolidine (1.2) | DMSO | 1 | 100 | 6 | 33 |
| KOt-Bu (1.2) | DMSO | 1 | 100 | 12 | 15 |
TMSA: trimethyl silyl azide,
TEA: Triethyl amine,
isolated yields.
Figure 2.
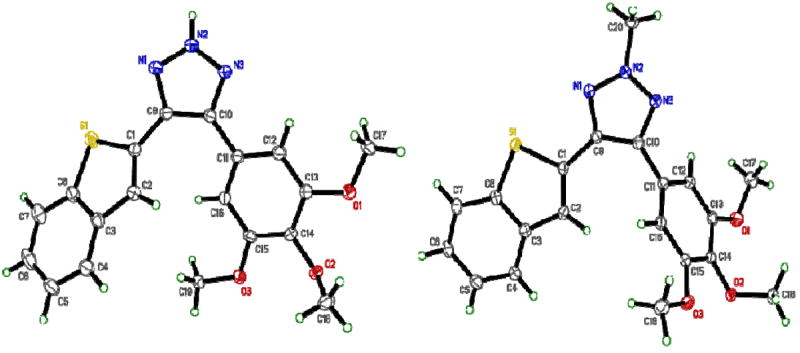
Single crystal X-ray structures of 4-(benzo[b]thiophen-2-yl)-5-(3,4,5-trimethoxyphenyl)-2H-1,2,3-triazole (6a) and 4-(benzo [b] thiophen-2-yl)-2-methyl-5-(3,4,5-trimethoxyphenyl)-2H-1,2,3-(2H)-triazole (7).
Heating (Z)-3-(benzo[b]thiophen-2-yl)-2-(3,4,5-trimethoxy phenyl)acrylonitrile (3a) with NaN3 (2 equivs) in DMSO under reflux for 36 h in the absence of Lewis base afforded poor yields (15%) of 6a. The yield of 6a was significantly improved when the reaction was carried out using excess NaN3 (3 equivs) in the presence of NH4Cl in DMSO at 120 °C for 18 h (51%). In attempts to further improve yield and reduce reaction time, different base catalysts were utilized (i.e. L-proline, TMSA, TEA, pyrrolidine, and KOt-But). L-proline (96%) was found to be the most suitable catalyst for this reaction, with the additional advantages of being efficient, and environmentally benign.
A variety of different heteroaryl trans-cyanostilbene analogs (3a-i and 8a-e) containing variously substituted phenyl ring systems were used to study the scope of the reaction (Scheme 2 and 3), and the effect of structure on reaction rate and yield; the results obtained are provided in Tables 2 and 3. This reaction was also carried out using a cis-cyanostilbene analog as starting material, i.e. (E)-3-(benzo[b]thiophen-2-yl)-2-(3,4,5-trimethoxy phenyl)acrylonitrile (2) as a model reactant, to compare the rate of reaction and yield of 6a in comparison with its corresponding Z-isomer (3a). The rate of reaction using E-isomer 2 was about half the rate of the reaction using the Z-isomer 3a, and the yield of 6a from 2 was only 78%. Overall yields for the 2- and 3-benzo[b]thiophenyl-, 2- and 3-indolyl- and 2-benzo[b]furanyl-2H-1,2,3-triazole products were in the range 96-75%.
Scheme 3.
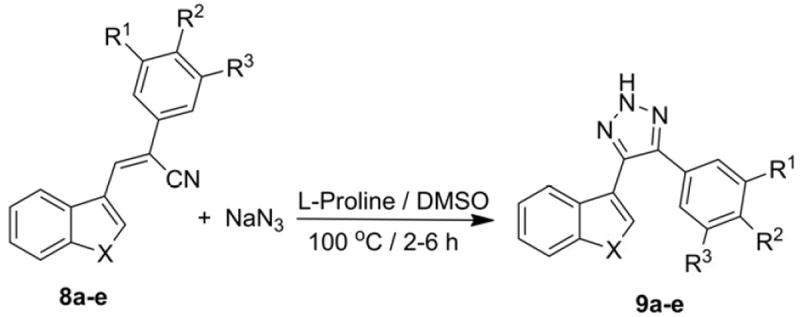
Synthesis of substituted 4-(benzo[b]thiophen-3-yl)-5-phenyl-2H-1,2,3-triazoles (9a-e).
Table 2.
Effect of different heteroaryl-2-yl cyanostilbenes in the synthesis of a variety of 2H-1,2,3-triazoles (6a-i).
| Entry | X | R1 | R2 | R3 | Time | Yielda |
|---|---|---|---|---|---|---|
|
| ||||||
| (h) | (%) | |||||
| 6a | S | OCH3 | OCH3 | OCH3 | 1 | 96 |
| 6b | S | OCH3 | H | OCH3 | 3 | 94 |
| 6c | S | H | OCH3 | H | 4 | 90 |
| 6d | S | OH | OCH3 | H | 6 | 75 |
| 6e | S | 3,4-methylene dioxy | H | 5 | 86 | |
| 6f | S | H | SCH3 | H | 5 | 84 |
| 6g | O | OCH3 | OCH3 | OCH3 | 3 | 93 |
| 6h | O | OCH3 | H | OCH3 | 4 | 91 |
| 6i | NH | OCH3 | OCH3 | OCH3 | 4 | 91 |
isolated yields.
Table 3.
Effect of different heteroaryl-3-yl cyanostilbenes in the synthesis of 2H-1,2,3-triazoles (9a-e).
| Entry | X | R1 | R2 | R3 | Time | Yielda |
|---|---|---|---|---|---|---|
|
| ||||||
| (h) | (%) | |||||
| 9a | S | OCH3 | OCH3 | OCH3 | 2 | 92 |
| 9b | S | OCH3 | OCH3 | H | 6 | 88 |
| 9c | S | H | OCH3 | H | 5 | 84 |
| 9d | NH | OCH3 | OCH3 | OCH3 | 4 | 91 |
| 9e | NH | OCH3 | H | OCH3 | 5 | 89 |
isolated yields.
A plausible mechanism for the above reaction is provided in Scheme 4. Initial Michael addition of azide (N3-) ion generates carbanion 10 followed by internal cyclization to form the triazole species 11. Loss of the cyano group then yields 12, followed by Lewis base-catalyzed prototropy to afford the 2H-1,2,3-triazole product 6a.
Scheme 4.
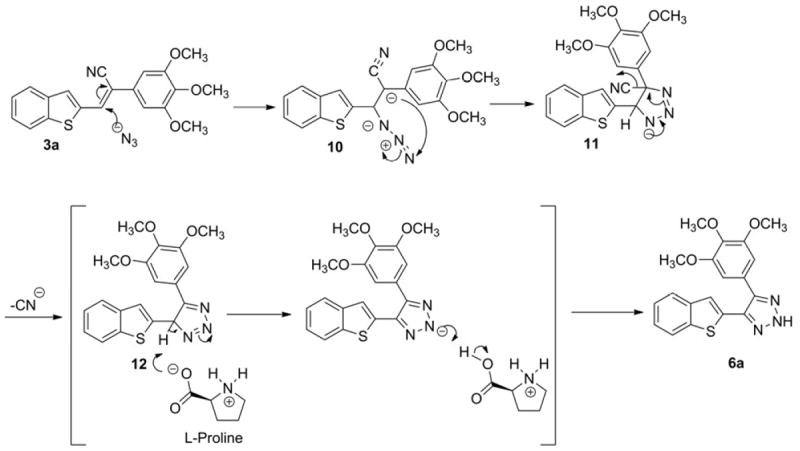
Plausible mechanism for the formation of 4-(benzo [b]thiophen-2-yl)-5-(3,4,5-trimethoxyphenyl)-2H-1,2,3-triazole (6a) from 3a via 3+2 cycloaddition of azide.
In conclusion, a novel one-step synthesis of a series of benzothiophenyl, benzofuranyl, and indolyl, 2H-1,2,3-triazoles from corresponding trans-cyanostilbene precursors has been developed. This method involves use of a novel reagent, L-proline, a Lewis base-catalyzed [3+2]cycloaddition of azide across the cyanostilbene double bond. The procedure affords good yields and short reaction times, and provides an efficient, simple and eco-friendly procedure for the synthesis of a variety of 4,5-diaryl-2H-1,2,3-triazoles.
Supplementary Material
Acknowledgments
The authors thank the NIH/National Cancer Institute (CA140409), and the Arkansas Research Alliance for financial support.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/XXXx/j.tetlet.2014.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jalily PH, Hadfield JA, Hirst N, Rossington SB. Bioorg Med Chem Lett. 2012;22:6731–6734. doi: 10.1016/j.bmcl.2012.08.089. [DOI] [PubMed] [Google Scholar]
- 2.Tarleton M, Gilbert J, Sakoff JA, McCluskey A. Eur J Med Chem. 2012;57:65–73. doi: 10.1016/j.ejmech.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 3.Ohsumi K, Hatanaka T, Fujita K, Nakagawa R, Fukuda Y, Nihei Y, Suga Y, Morinaga Y, Akiyama Y, Tsuji T. Bioorg Med Chem Lett. 1998;8(22):3153–3158. doi: 10.1016/s0960-894x(98)00579-4. [DOI] [PubMed] [Google Scholar]
- 4.Ohsumi K, Nakagawa R, Fukuda Y, Hatanaka T, Morinaga Y, Nihei Y, Ohishi K, Suga Y, Akiyama Y, Tsuji T. J Med Chem. 1998;41:3022–3032. doi: 10.1021/jm980101w. [DOI] [PubMed] [Google Scholar]
- 5.Penthala NR, Janganti VM, Bommagani S, Madadi NR, Crooks PA. Medchemcomm. 2014;5:886–890. [Google Scholar]
- 6.LeBlanc R, Dickson J, Brown T, Stewart M, Pati HN, VanDerveer D, Arman H, Harris J, Pennington W, Holt HL, Jr, Lee M. Bioorg Med Chem. 2005;13:6025–6034. doi: 10.1016/j.bmc.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 7.Odlo K, Hentzen J, dit Chabert JF, Ducki S, Gani OA, Sylte I, Skrede M, Florenes VA, Hansen TV. Bioorg Med Chem. 2008;16:4829–4838. doi: 10.1016/j.bmc.2008.03.049. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Q, Peng Y, Wang XI, Keenan SM, Arora S, Welsh WJ. J Med Chem. 2007;50:749–754. doi: 10.1021/jm061142s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eswaran S, Adhikari AV, Shetty NS. Eur J Med Chem. 2009;44:4637–4647. doi: 10.1016/j.ejmech.2009.06.031. [DOI] [PubMed] [Google Scholar]
- 10.(a) Plech T, Wujec M, Siwek A, Kosikowska U, Malm A. Eur J Med Chem. 2011;46:241–248. doi: 10.1016/j.ejmech.2010.11.010. [DOI] [PubMed] [Google Scholar]; (b) Meerpoel L, Backx LJ, Van der Veken LJ, Heeres J, Corens D, De Groot A, Odds FC, Van Gerven F, Woestenborghs FA, Van Breda A, Oris M, van Dorsselaer P, Willemsens GH, Vermuyten KJ, Marichal PJ, Vanden Bossche HF, Ausma J, Borgers M. J Med Chem. 2005;48:2184–2193. doi: 10.1021/jm0494772. [DOI] [PubMed] [Google Scholar]
- 11.(a) Guan LP, Jin QH, Tian GR, Chai KY, Quan ZS. J Pharm Pharm Sci. 2007;10:254–262. [PubMed] [Google Scholar]; (b) Wagle S, Adhikari AV, Kumari NS. Eur J Med Chem. 2009;44:1135–1143. doi: 10.1016/j.ejmech.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 12.Penthala NR, Sonar VN, Horn J, Leggas M, Yadlapalli JS, Crooks PA. Medchemcomm. 2013;4:1073–1078. doi: 10.1039/C3MD00130J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.(a) Rostovtsev VV, Green LG, Fokin VV, Sharpless KB. Angew Chem Int Ed Engl. 2002;41:2596–2599. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]; (b) Kolb HC, Finn MG, Sharpless KB. Angew Chem Int Ed Engl. 2001;40:2004–2021. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]; (c) Moses JE, Moorhouse AD. Chem Soc Rev. 2007;36:1249–1262. doi: 10.1039/b613014n. [DOI] [PubMed] [Google Scholar]; (d) Bock VD, Perciaccante R, Jansen TP, Hiemstra H, van Maarseveen JH. Org Lett. 2006;8:919–922. doi: 10.1021/ol053095o. [DOI] [PubMed] [Google Scholar]
- 14.Kamijo S, Jin T, Huo Z, Yamamoto Y. J Am Chem Soc. 2003;125:7786–7787. doi: 10.1021/ja034191s. [DOI] [PubMed] [Google Scholar]
- 15.(a) Loren JC, Krasiński A, Fokin VV, Sharpless KB. Synlett. 2005;18:2847–2850. [Google Scholar]; (b) Shchetnikov G, Peregudov A, Osipov S. Synlett. 2007;2007:0136–0140. [Google Scholar]; (c) Skarpos H, Osipov SN, Vorob’eva DV, Odinets IL, Lork E, Roschenthaler GV. Org Biomol Chem. 2007;5:2361–2367. doi: 10.1039/b705510b. [DOI] [PubMed] [Google Scholar]; (d) Yap AH, Weinreb SM. Tetrahedron Letters. 2006;47:3035–3038. [Google Scholar]
- 16.Quiclet-Sire B, Zard SZ. Synthesis. 2005;19:3319–3326. [Google Scholar]
- 17.(a) Majireck MM, Weinreb SM. J Org Chem. 2006;71:8680–8683. doi: 10.1021/jo061688m. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Tsai CW, Yang SC, Liu YM, Wu MJ. Tetrahedron. 2009;65(40):8367–8372. [Google Scholar]
- 18.Sengupta S, Duan H, Lu W, Petersen JL, Shi X. Org Lett. 2008;10:1493–1496. doi: 10.1021/ol8002783. [DOI] [PubMed] [Google Scholar]
- 19.Madadi NR, Penthala NR, Crooks PA. Tetrahedron Lett. 2014;55:4207–4211. doi: 10.1016/j.tetlet.2014.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


