Abstract
Acromegaly often involves the presence of different pathologies of the thyroid gland. Long-lasting stimulation of the follicular epithelium by growth hormone (GH) and insulin-like growth factor 1 (IGF-1) can cause disorders in thyroid function, an increase in its mass and the development of goitre. Acromegalic patients present most frequently with non-toxic multinodular goitre. Nodules are more prevalent in patients with active acromegaly. It has been suggested that then thyroid size increases and it can be reduced through treatment with somatostatin analogues. The relationship between thyroid volume and the level of IGF-1 and the duration of the disease is unclear. Each acromegalic patient requires a hormonal and imaging evaluation of the thyroid when the diagnosis is made, and an accurate evaluation during further observation and treatment. Although the data concerning the co-occurrence of acromegaly and thyroid cancer still remain controversial, it is particularly important to diagnose the patient early and to rule out thyroid cancer.
Keywords: acromegaly, thyroid gland, goitre, insulin-like growth factor 1
Introduction
Acromegaly, a chronic disease caused by overproduction of growth hormone, co-occurs with different thyroid diseases, the most frequent pathology being goitre. Research carried out in the years 1960–2008 showed that goitre developed in 20% to 90% of patients with acromegaly (in 55% on average), usually in an active form [1–6]. Non-toxic nodular goitre (39.9%) and non-toxic diffuse goitre (17.8%) are the most frequent, while toxic nodular goitre is less prevalent (14.3%) [3, 7]. Unnikrishann et al. [7] estimate that goitre occurs in approximately 30% of acromegalic patients living in areas with iodine deficiency, while only 5% have thyroid hyperactivity.
It has also been reported that 13% to 17.5% of acromegalic patients have undergone thyroidectomies and most of them were performed before pituitary adenomas were diagnosed [8, 9].
The co-occurrence of autoimmune thyroid diseases with acromegaly is not common. So far only a handful of cases of Graves-Basedow disease in acromegalic patients have been reported [9, 10], while Hashimoto's disease occurs more frequently (4.6%). Other variations of autoimmune thyroiditis, including painless (silent) thyroiditis, rarely cause thyrotoxicosis and its clinical symptoms [11].
Goitre and acromegaly
Mechanisms underlying the intracellular activity of IGF-1
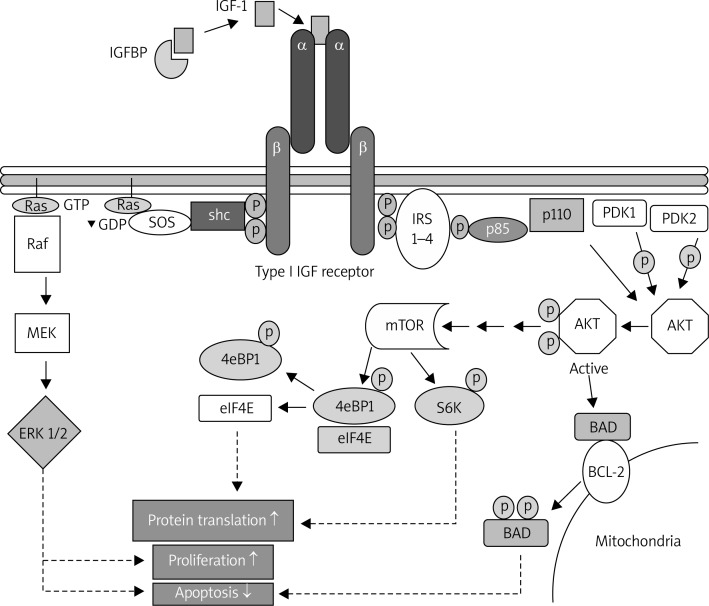
Already in the 1950s Salmon and Daughaday postulated that there was a specific factor, later labelled insulin-like growth factor 1 (IGF-1) or somatomedin, which growth hormone (GH) acts through [12]. The system of transmitting IGF-1 consists of IGF-1 growth factor and IGF-1R receptor, which is composed of two α and two β chains linked by two disulfide bonds and belongs to the class of tyrosine kinase receptors that are internally and enzymatically active [13]. This system also includes at least six types of IGF-binding proteins (IGFBPs), which transport IGF-1 in the blood and other bodily fluids, as well as proteases [14–16]. Insulin-like growth factor 1 is transported primarily in the form of 150-kDa ternary complexes, which additionally consist of IGFBP3 and an acid-labile subunit. These complexes modulate the activity of IGF: they protect it from proteolytic degradation and extend its half-life. The release of IGF from the complex is related to the activity of IGFBP proteases. The ratio between free and bound IGF in the blood is of great importance for cell proliferation [17]. The mechanism of IGF-1 action consists in binding somatomedin with the external domain of the IGF-1R receptor, which causes the autophosphorylation of the catalytic cytosolic domain of the tyrosine kinase. Next, several intracellular signalling pathways, such as kinase cascades Ras-Raf-MEK-ERK and PI3K-AKT-mTOR, are activated, as shown in Figure 1 [18]. The Ras-Raf-MEK-ERK signalling pathway consists of a small monomeric membrane G protein, which binds GTP, called GTP-ase or Ras protein, and a serine/threonine protein kinase Raf, which phosphorylates the kinase of MAP kinase called MEK. After the activation of Ras protein, with the use of signalling protein, shc adaptor protein and the exchange of guanine nucleotides SOS, there is another stage of the cascade: Ras-GTP binds with the domain of the Raf kinase and activates it, which causes the binding of Raf and the phosphorylation of MAP kinases. MEK phosphorylates and activates serine/threonine MAP kinase, which phosphorylates other kinases, such as ERK1/2 (kinases regulated by extracellular signals). Activated kinases translocate to the nucleus where they phosphorylate a number of transcription factors which induce and control the expression programs of several genes. Phosphatidylinositol 3-kinase (PI 3-kinase), on the other hand, plays the main role in the PI3K-AKT-mTOR signalling pathway. It is a heterodimer consisting of two subunits, the regulatory (p85) and catalytic (p110) subunits, which has the activity of serine/ threonine and phosphatidylinositol kinase. PI-3 is responsible, among others, for the conversion of phosphatidylinositol-4.5-bisphosphate (PIP2) into phosphatidylinositol-3,4,5-trisphosphate (PIP3), which activates the serine/threonine kinase AKT, also termed B protein kinase. In order for the pathway to function properly, including proper AKT phosphorylation, insulin receptor substrates IRS 1-4 and phosphatidylinositol-dependent kinases PDK1 and PDK2 are necessary. The stimulation of AKT kinase causes the phosphorylation of serine residues of the protein homologue of the BAD protein which is a member of the Bcl-2- family. Phosphorylated BAD protein binds with adaptor proteins and undergoes cytoplasmic sequestration, which creates an antiapoptotic effect [13, 19]. The signalling pathway that participates in the transduction of the signal initiated by IGF-1, and was discovered the most recently, is the serine/threonine kinase mTOR pathway, which is also activated by AKT kinase. The family of TOR proteins participates in the regulation of many cellular processes, including the initiation of mRNA transcription and the translation of proteins. Activated mTOR kinase phosphorylates the effector protein S6 kinase 1 (S6K1) and protein binding the early eukaryotic initiation factor 4E (eIF4E), i.e. 4EBPI. S6K1 and eIF4E initiate the process of translating proteins which are necessary for the transition of the cell from phase G1 to phase S of the mitotic cycle [19]. To sum up, the activation of the signalling pathways mentioned above causes an increase in cell proliferation and a significant reduction in apoptosis [18].
Figure 1.
Signal transmission pathways in the cell which are activated after IGF-1 binds with the IGF-1R receptor, according to Bruchim [18] (see description above)
The relationship between acromegaly and the prevalence of goitre was noticed already in the 1930s. In 1936 Rolleston [20] found – based on palpation – that acromegalic patients had enlarged thyroid glands. Several years later Herrmann et al. [4] examined 73 patients with acromegaly and they noticed that goitre was more prevalent in this group of patients; it was diagnosed in 82.2% of patients who were being treated and in 90.4% of patients with active acromegaly, while in the control group it was found in 18.1% of the population. Nodular goitre occurred in 63% of patients, including as many as 71.2% of persons with active acromegaly.
The impact of the duration of the disease on the development of pathologies in the thyroid has also been analysed [5, 21]. Cheung and Boyages [5] emphasise that in the initial stage of acromegaly a diffuse goitre appears, thyroid autonomy gradually develops, and patients with a longer disease duration develop nodular goitre, which is explained by the prolonged exposure of thyroid cells to an increased level of IGF-1.
Research confirms that the enlargement of the thyroid in acromegaly is related to an increased level of IGF-1. This explains why patients with active acromegaly, characterised by a higher level of IGF-1, have higher thyroid volume [5, 22–24].
In a study conducted by Brzozowska et al. [25] which involved 480 children, a positive correlation was found between IGF-1 concentration and thyroid size; mean IGF-1 levels in children with enlarged thyroids were statistically significantly higher than in the control group. Similar observations were made by Völzke et al. [26] in a study involving 3,662 healthy persons (no diagnosed thyroid pathologies, no acromegaly) living in the northern part of Germany, in whom an increased IGF-1 level was associated with goitre. Wüster et al. [9] noted that in their group of acromegalic patients the average preoperative level of GH was higher in patients with an enlarged thyroid than in those without goitre. Moreover, goitre additionally correlated with the duration of an increased level of GH in the serum. Cheung and Boyages [5] postulate that the longer the duration of acromegaly and the higher the IGF-1 level, the greater the volume of the thyroid is: it may be even five times higher compared to the control group. In the study by Miyakawa et al. [24] IGF-1 and GH levels had an analogous correlation with thyroid volume. Tramontano et al. [23] investigated the impact of IGF-1 in rats on the proliferation of follicular cells in the thyroid incubated with IGF-1 and observed the increase in DNA synthesis and cellular proliferation in this model system. It has been confirmed that long-lasting stimulation of follicular epithelium of the thyroid by GH and IGF-1 can cause enlargement of the thyroid and disorders in its function in acromegalic patients [1].
However, the correlation of thyroid volume with the level of IGF-1 and the duration of the disease is unclear. Gasperi et al. [3] observed that acromegalic patients had almost twice as large thyroids as the persons in the control group, which had a positive correlation with the duration of the disease. No correlation was found between the levels of IGF-1 and GH. Similarly, Cannavo et al. [2] did not find such a correlation in their patients, but although they did not find a positive correlation, they suggest that a decrease in IGF-1 and GH levels in the serum has a positive impact on the thyroid.
The role of thyroid-stimulating hormone (TSH)
The impact of thyroid-stimulating hormone (TSH) on the development of goitre in acromegalic patients is complex. Both Cheung and Boyages [5] and Miyakawa et al. [24] suggest that there is an inverse correlation between thyroid volume and the level of TSH. On the other hand, it is presumed that goitre develops independently of TSH, although TSH is an important factor which supports the influence of IGF-1 on the thyroid. In studies conducted using FRTL-5 (Fischer rat thyroid follicle-5 cell) cell lines IGF-1 had a minimal effect when there was no TSH, but pre-incubation of those cells in TSH caused a significant increase in the effect of IGF-1 on the incorporation of thymidine, which was 7 times greater than in cells which had no contact with TSH [27]. Moreover, studies have shown that thyroid volume does not increase in persons with a deficiency of GH who are undergoing GH substitution therapy, if there is co-occurring TSH deficiency [28]. For that reason TSH is thought to play an important role in the development of goitre in an early stage of acromegaly, but at a later stage, when thyroid autonomy has already developed, the presence of TSH is not necessary for thyroid volume to increase further [5, 24, 28].
The level of hormones in patients with acromegaly
Most acromegalic patients have euthyroidism (67%) and about 25% have hypothyroidism [2]. Hyperthyroidism has been found in 3.5–26% of patients [29]. Thyrotoxicosis is not frequent [7, 29], but it increases cardiovascular risk, particularly when is coupled with high GH and IGF-1 levels, and for that reason it is important to gain euthyroidism as fast as possible [7]. Hyperthyroidism should be taken into consideration in diagnosing a patient with acromegaly and weight loss, after excluding cancer [10]. Moreover, the response of TSH to TRH in acromegalic patients is lower compared to the control group, and sometimes it does not occur at all [9, 11, 30]. Gemsenjäger et al. emphasise that this could suggest subclinical hyperthyroidism [31, 32].
Several factors affect the secretion of TSH in the pituitary. The synthesis and secretion of TSH are controlled mostly by the stimulating influence of TRH and the negative feedback of thyroid hormones (T3 and T4). Other regulators including leptin, dopamine, GH, IGF-1 and somatostatin are of lesser importance in the process [33–35]. The autonomous nervous system additionally modulates thyroid sensitivity to TSH [36]. It has been proved that increased sympathetic activity can inhibit the response of the thyroid gland to TSH, which explains the lack of a significant increase in the levels of T3 and T4 in the blood, in response to a dramatic increase in TSH levels in the study conducted on rats [37]. TSH secretion is important for maintaining energy homeostasis and basic heat production [38]. Hypothalamic centres involved in controlling this homeostasis affect the paraventricular nucleus, which contains TSH-secreting neurons [34].
Studies also show that GH and IGF-1 directly or indirectly modulate the secretion of TSH by thyrotroph cells [39].
Dopamine inhibits the synthesis and secretion of TSH through D2 receptors stimulated in thyreotropic cells, which causes a decrease in the amplitude of TSH pulses, but does not affect their frequency. Dopamine also stimulates the secretion of TRH through neurons in the paraventricular nucleus [40]. However, experimental studies which would prove the thesis suggesting that an excess of GH stimulates the central dopaminergic system have not been conducted on humans or animals so far.
Somatostatin inhibits the secretion of TSH by activating two receptor subtypes, SST2 and SST5, in thyreotropic cells [41]. Under physiological conditions GH exerts a feedback mechanism on the secretion of hypothalamic somatostatin, increasing its release and reducing the secretion of growth -hormone-releasing hormone (GHRH) [42]. Thus, an increase in the amount of somatostatin and its impact on thyreotropic cells can inhibit the secretion of TSH. The GHRH, on the other hand, has a synergistic effect on the secretion of TSH after TRH both in healthy persons and in patients with acromegaly [43].
One important metabolic signal which modulates the activity of the hypothalamic-pituitary-thyroid axis in animals and humans is leptin; it has a stimulating impact on the synthesis and release of TRH both directly and indirectly via neurons which express pro-opio-melanocortin, and cocaine- and amphetamine-related transcript in the arcuate nucleus [35, 44–47].
Roelfsema et al. [39] have put forward a hypothesis that the production and secretion of TSH in active acromegaly can be reduced using a number of mechanisms.
One of them is related to the inhibited transmission of leptin to the paraventricular nucleus, as leptin concentration is reduced in patients with active acromegaly. It increases after the pituitary adenoma has been surgically treated or during treatment with somatostatin analogues or a GH receptor blocker, pegvisomant [48–50]. Analysing TSH concentration in the serum while leptin is administered to acromegalic patients could help determine the precise role of this hormone for the hypothalamic-pituitary-thyroid axis in the future.
The second mechanism has to do with an increase in intracellular (intrapituitary) conversion of T3 to T4 in thyreotropic cells as a result of an excess of GH, which can reduce TSH synthesis and secretion [51]. Moreover, due to the feedback mechanism, the excess of GH with somatostatin participation has a suppressive effect on TSH secretion [33].
Roelfsema et al. [39] also highlight that untreated acromegaly is associated with reduced 24-hour TSH secretion both in terms of basic (non-pulse) values and pulses. They also found a strong reverse correlation between the daily level of TSH and the secretion of GH. They postulate that the frequency of TSH pulses remains unchanged, which has been confirmed with statistical methods, using the Weibull γ parameter. Although a daily rhythm of TSH levels was present in all patients, it had a reduced mesor (average daily value of the parameter) and amplitude (the maximum deviation from the mesor value or the difference between the maximum and minimum values) with an unchanging acrophase (time period during 24 h when the cycle peaks) [39, 52].
What is more, an important aspect is the decrease in sympathetic activity – triggered by an excess of GH and a low leptin level – which inhibits the sensitivity of the thyroid to TSH [39].
Since acromegalic patients have reduced TSH secretion, one could expect a low concentration of T4 in the blood, which, however, has not been confirmed by research [4, 53]. This suggests either increased biological activity of TSH through a change in the oligosaccharide chain of the TSH particle in the post-translational process or the impact of the autonomous nervous system [39]. Andersson et al. [54] demonstrated that GH-transgenic mice manifest a reduction in sympathetic reaction and in noradrenaline levels in the serum and in the tissues. Resmini et al. [55], on the other hand, observed a reduction in sympathetic cardiac activity in acromegalic patients. Increased sensitivity of the thyroid to TSH, modulated by an increase in sympathetic reaction, explains the normal concentration of T4 in the blood despite a reduced level of TSH in patients with active acromegaly [39].
The presence of a pituitary tumour can also per se cause a reduction in TSH secretion [39].
Vascularisation of the thyroid and active acromegaly
Insulin-like growth factor-1 has also been reported to regulate angiogenesis and the production of vascular endothelial growth factor [56, 57]. Bogazzi et al. [58] showed in colour Doppler ultrasonography that patients with active acromegaly have increased blood flow in the thyroid and vascularisation of the thyroid compared to patients treated with somatostatin analogues and patients with disease remission. The increased blood flow and vascularisation were not related to the duration of acromegaly.
Insulin-like growth factor-1 was reported to stimulate vascular endothelial growth factor (VEGF) mRNA expression and protein synthesis in a human colonic cancer cell line [56]. In addition, IGF-1 might play a role in angiogenesis because it has been observed that IGF-1 signalling is associated with the development of aberrant glioblastoma phenotypes related to aberrant angiogenesis [59]. Finally, it was shown that IGF-1 increases the stability of VEGF mRNA in an endometrial adenocarcinoma cell line, thereby increasing VEGF protein concentration [60]. Through these effects on VEGF, the increase in IGF-1 levels observed in acromegaly might lead to an increase in thyroid blood flow and the vascularity of the thyroid gland. Patients treated with somatostatin analogues have normal intrathyroidal blood flow and vascularisation of the thyroid gland due to proper control of acromegaly, as indicated by normal serum IGF-1 levels [58].
Thus an examination of the vascularisation of the thyroid is an additional parameter which can be used to assess the activity of acromegaly [11, 58].
Treating acromegaly depending on thyroid volume and thyroid hormone levels
Thyroid volume
It is known that not only genetic predisposition and environmental factors but also some drugs are involved in the regulation of thyroid volume [5, 24, 61]. Some studies suggest that in active acromegaly thyroid size increases and that it can be reduced as a result of treatment, along with a reduction in IGF-1 levels to reference levels [4, 5]. Herrman et al. [4] noted that patients with active acromegaly had increased thyroid volume of up to 20%. This volume decreased owing to treatment by approximately 25%, both after treatment using somatostatin analogues and after surgery. A similar reduction in the volume of the thyroid was achieved by Miyakawa [24] and Cheung and Boyages [5], in both cases during treatment with octreotide for over a year and 4 months after surgical transsphenoidal removal of the adenoma. Moreover, it should be emphasised that octreotide not only causes a reduction in the level of IGF-1, but also has an antiproliferative effect by blocking cells from going from phase G0 to G1, which was shown by Cheung and Boyages when they investigated the cell line which produced GH in the rat model [62], and somatostatin receptors are present in thyroid tissue [5, 63]. Studies into the presence of somatostatin receptors in the tissue have been conducted at several research centres. Boy et al. [64] did a PET/CT in 120 healthy persons using 68Ga-DOTATOC and RT-PCR with the aim of finding somatostatin receptors in tissue unaffected by the disease and proved its presence also in thyroid tissue (mainly subtype 2). The study showed statistically higher expression of SSTR-2 in men compared to women. Receptor subtypes 2 and 5 are present most frequently in the thyroid, subtypes 1 and 3 are more rare, while subtype 4 is never found.
This concerns healthy thyroid tissue as well as benign and malignant disease processes in the thyroid gland [65]. Similar results were obtained by Druckenthaner et al. [66]: they found subtype 2 somatostatin receptors in 94% of tissue samples examined using RT-PCR and in 87% of samples examined using specific anti-receptor antibodies.
Much the same discussion goes on about acromegaly and prostate. A chronic excess of GH and IGF-1 causes prostate overgrowth and other structural abnormalities such as calcifications, nodules, and cysts [67, 68]. Young patients (less than 40 years) with acromegaly have significant prostatic enlargement compared with age-matched controls. Although there is no evidence linking prostatic carcinoma and acromegaly, it has been suggested that a careful prostate screening should be included in the work-up and follow-up of acromegalic males. Similar to acromegaly, long-term treatment with octreotide can reverse prostate enlargement [67, 69].
Hormone levels
Octreotide and bromocriptine have a suppressive effect on the release of TSH, but long-term treatment with those substances does not change thyroid function in most patients [31, 70–72]. Roelfsema and Trölich [73] suggest that octreotide inhibits peripheral conversion of T4 to T3. This could be secondary to a decrease in GH levels caused by octreotide as GH stimulates peripheral deiodination of T4 [31, 74–76]. Other studies show that somatostatin can directly inhibit the release of T4 and T3 from autonomous thyroid tissue [31, 63].
Experimental studies on animals prove that GH modulates the activity of thyroxine deiodinase in the liver and other organs [77]. A change in the activity of the enzyme can cause an increase in T4 levels and a decrease in rT3 in the serum in patients with active acromegaly, although these levels are usually within reference ranges [53, 73, 78]. It has been confirmed that administering GH to rats increases the activity of deiodinase, resulting in an increase in intracellular T3 concentration. Some of the T3 goes into the circulatory system, but its concentration is usually normal [51, 79]. Moreover, the amount of intrapituitary T3 increases due to the administration of a large dose of GH in rats that had undergone thyroidectomy. It is thus possible that an increase in T3 synthesis through type 2 thyronine deiodinase present in thyreotrophic cells directly inhibits the synthesis and release of TSH in acromegaly [80]. It has also been suggested that folliculo-stellate cells of the pituitary, which secrete deiodinase, may participate in T3-dependent inhibition [81].
Thyroid cancer and acromegaly
Several studies have suggested that patients with acromegaly have an increased risk of benign and malignant neoplasms, particularly situated in intestines, brain, breast, thyroid, uterus, prostate, kidney and skin [69, 82, 83]. Thyroid cancer accounts for about 3% of malignant lesions. Fifteen to twenty-four percent of acromegaly patients die due to tumours. There has been indicated a 1.5–4-fold increase in risk of neoplasms compared to the general population, especially in patients with long-lasting uncontrolled acromegaly (longer than 5 years). Therefore, it is necessary to carry out effective treatment in order to decrease the prevalence of malignancies and mortality [83].
The data concerning the co-occurrence of acromegaly and thyroid cancer are ambiguous [3, 4].
Although simple goitre and multinodular goitre are very common in acromegalic patients, thyroid cancer is rare [84]. Studies indicate that IGF-1 can play a role in the development of both benign and malignant cancerous changes [85]. It is believed that a long-lasting excess of IGF-1 stimulates the proliferation of cells and induces an antiapoptotic effect in different types of cells and tissues, including thyroid cancer cells [84–87]. An increased level of IGF-1 can also stimulate the proliferation of cells and angiogenesis in the cancerous tumour and cause metastases [88, 89]. An increased prevalence of thyroid cancer in acromegalic patients still remains controversial [8, 22, 84, 90]. However, some reports show that the prevalence of thyroid cancer is only slightly increased compared to the general population and can occur in from 4.7% up to 5.6% of patients. In those studies the excess risk of carcinoma was confined to differentiated thyroid cancers; especially papillary cancer was more often observed [4, 8, 91, 92]. Among 86 patients examined by Ruchala et al., papillary cancer of the thyroid was found in 3 and follicular cancer in 2 of the patients. In 3 persons the lesions were multifocal, in one case the cancerous lesion infiltrated the capsule, and 1 patient manifested metastases to the lymph nodes [3]. Similar results were obtained by Tita et al., who found differentiated thyroid cancer in 5.6% of the 58 patients they observed [8]. Baris et al. [82] noted an increased prevalence of thyroid cancer (papillary or follicular) in women with acromegaly living in Sweden. Gasperi et al. [3], on the other hand, observed papillary thyroid cancer in 1.2% of 258 acromegalic patients, and this prevalence was not significantly higher than in the control group. Moreover, it should also be borne in mind that the increased number of the diagnoses of thyroid cancer in patients with acromegaly could be due to the fact that they are examined more accurately and more frequently than before [86]. It has been suggested that patients with a sustained increased level of IGF-1 should have their thyroid examined regularly, including undergoing an ultrasound-guided fine-needle aspiration biopsy (FNAB), particularly of nodules which have worrisome features in ultrasonography [84, 94–97].
The role of ultrasund-elastography in diagnosing thyroid cancer
Ultrasound-elastography (US-E), a procedure recently validated for the assessment of tissue elasticity, is currently considered a useful tool in the differential diagnosis of malignant lesions of the prostate, breast, pancreas, and lymph nodes. In an endeavour to find new diagnostic methods suitable for acromegalic patients, Scacchi et al. [22] assessed the role of US-E in the diagnosis of thyroid cancer. They examined 90 tumours in 25 patients with diagnosed acromegaly and they verified their results by performing an FNAB on suspected lesions. Initially, the results seemed promising, as the authors observed that the occurrence of nodules with increased hardness is significantly more frequent in the group examined (56.8%) than in the control group, which included patients without acromegaly but with multinodular goitre (16%). A further analysis of the lesions showed, however, that the increase in the hardness of thyroid tumours, found in US-E, did not prove that they were malignant. They were benign and their increased hardness was due to greater fibrosis [95] caused by an excess of GH and IGF-1, which increase the synthesis of collagen and its apposition in the tissues [96]. What is more, the study showed that a higher number of “hard” lesions was typical of patients with active acromegaly (68.9%), compared to patients who had been cured (44.4%) and to those with well-controlled disease (52.5%) [22]. Although elastosonographic evaluation of thyroid nodules in acromegaly suggests that they are harder in GH excess, it does not mean they are malignant. Thus US-E seems to be of limited significance for the diagnosis of thyroid cancer in patients with acromegaly.
Conclusions
The prevalence of thyroid pathologies in acromegalic patients is high. Since there is a wide variety of disorders which concern both the function and morphology of the thyroid, each acromegalic patient requires a hormonal and imaging evaluation of the thyroid when the diagnosis is made, and an accurate evaluation during further observation and treatment. It is particularly important to diagnose the patient early and to rule out thyroid cancer.
References
- 1.Kasagi K, Shimatsu A, Miyamoto S, Misaki T, Sakahara H, Konishi J. Goiter associated with acromegaly: sonographic and scintigraphic findings of the thyroid gland. Thyroid. 1999;9:791–6. doi: 10.1089/thy.1999.9.791. [DOI] [PubMed] [Google Scholar]
- 2.Cannavo S, Squadrito S, Finocchiaro MD, et al. Goiter and impairment of thyroid function in acromegalic patients: basal evaluation and follow-up. Horm Metab Res. 2000;32:190–5. doi: 10.1055/s-2007-978620. [DOI] [PubMed] [Google Scholar]
- 3.Gasperi M, Martino E, Manetti L, et al. Prevalence of thyroid diseases in patients with acromegaly: results of an Italian multicenter study. J Endocrinol Invest. 2002;25:240–5. doi: 10.1007/BF03343997. [DOI] [PubMed] [Google Scholar]
- 4.Herrmann BL, Baumann H, Janssen OE, Görges R, Schmid KW, Mann K. Impact of disease activity on thyroid disease in patients with acromegaly: basal evaluation and follow-up. Exp Clin Endocrinol Diabetes. 2004;112:225–30. doi: 10.1055/s-2004-817967. [DOI] [PubMed] [Google Scholar]
- 5.Cheung NW, Boyages SC. The thyroid gland in acromegaly: an ultrasonographic study. Clin Endocrinol (Oxf) 1997;46:545–9. doi: 10.1046/j.1365-2265.1997.1680985.x. [DOI] [PubMed] [Google Scholar]
- 6.Chanson P, Salenave S. Acromegaly. Orphanet J Rare Dis. 2008;25:3–17. doi: 10.1186/1750-1172-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Unnikrishnan AG, Agrawal NK, Kumar R, Thazhath SS, Reddy DV, Singh SK. Toxic thyroid adenoma and acromegaly: an unusual association. J Assoc Physicians India. 2003;51:412–3. [PubMed] [Google Scholar]
- 8.Tita P, Ambrosio MR, Scolio C, et al. High prevalence of differentiated thyroid carcinoma in acromegaly. Clin Endocrinol (Oxf) 2005;63:161–7. doi: 10.1111/j.1365-2265.2005.02316.x. [DOI] [PubMed] [Google Scholar]
- 9.Wüster C, Steger G, Schmelzle A, Gottswinter J, Minne HW, Ziegler R. Increased incidence of euthyroid and hyperthyroid goiters independently of thyrotropin in patients with acromegaly. Horm Metab Res. 1991;23:131–4. doi: 10.1055/s-2007-1003632. [DOI] [PubMed] [Google Scholar]
- 10.Burgos Peláez R, Simó Canonge R, Hernández-Pascual C, Mesa Manteca J. Acromegaly and Graves-Basedow disease. Report of 3 cases. Med Clin (Barc) 1994;103:179–80. [PubMed] [Google Scholar]
- 11.Saito T, Tojo K, Tajima N. Painless thyroiditis complicated by acromegaly. Intern Med. 2010;49:167–70. doi: 10.2169/internalmedicine.49.2782. [DOI] [PubMed] [Google Scholar]
- 12.Salmon WD, Jr, Daughaday WH. A hormonally controlled serum factor which stimulates sulfate incorporation by cartilage in vitro 1957. J Lab Clin Med. 1990;116:408–19. [PubMed] [Google Scholar]
- 13.Tarach JS. Badania in vitro megakariopoetycznych komórek progenitorowych szpiku kostnego, stymulowanych Il-3, GM-CSF oraz PEG-rHuMGDF, w wybranych przewlekłych zaburzeniach mieloproliferacyjnych; Polihymnia: Centrum Promocji Nauk Medycznych (Lublin); 2000. [Google Scholar]
- 14.Cohen P. Overview of the IGF-I system. Horm Res. 2006;65(Suppl 1):3–8. doi: 10.1159/000090640. [DOI] [PubMed] [Google Scholar]
- 15.Firth SM, Baxter RC. Cellular actions of the insulin-like growth factor binding proteins. Endocr Rev. 2002;23:824–54. doi: 10.1210/er.2001-0033. [DOI] [PubMed] [Google Scholar]
- 16.Holly J, Perks C. The role of insulin-like growth factor binding proteins. Neuroendocrinology. 2006;83:154–60. doi: 10.1159/000095523. [DOI] [PubMed] [Google Scholar]
- 17.Jogie-Brahim S, Min HK, Oh Y. Potential of proteomics towards the investigation of the IGF-independent actions of IGFBP3. Expert Rev Proteomics. 2005;2:71–86. doi: 10.1586/14789450.2.1.71. [DOI] [PubMed] [Google Scholar]
- 18.Bruchim I, Attias Z, Werner H. Targeting the IGF1 axis in cancer proliferation. Expert Opin Ther Targets. 2009;13:1179–81. doi: 10.1517/14728220903201702. [DOI] [PubMed] [Google Scholar]
- 19.Wysocki PJ. mTOR in renal cell cancer: modulator of tumor biology and therapeutic target. Expert Rev Mol Diagn. 2009;9:231–41. doi: 10.1586/erm.09.8. [DOI] [PubMed] [Google Scholar]
- 20.Rolleston HD. London: Oxford University Press; 1936. The endocrine organs in health and diseases. [Google Scholar]
- 21.Studer H, Peter HJ, Gerber H. Natural heterogeneity of thyroid cells: the basis for understanding thyroid function and nodular goitre growth. Endocr Rev. 1989;10:125–35. doi: 10.1210/edrv-10-2-125. [DOI] [PubMed] [Google Scholar]
- 22.Scacchi M, Andrioli M, Carzaniga C, et al. Elastosonographic evaluation of thyroid nodules in acromegaly. Eur J Endocrinol. 2009;161:607–13. doi: 10.1530/EJE-09-0558. [DOI] [PubMed] [Google Scholar]
- 23.Tramontano D, Cushing GW, Moses AC, Ingbar SH. Insuline-like growth factor-I stimulates the growth of rat thyroid cells in culture and synergizes the stimulation of DNA synthesis induced by TSH and Graves’-IgG. Endocrinology. 1986;119:940–2. doi: 10.1210/endo-119-2-940. [DOI] [PubMed] [Google Scholar]
- 24.Miyakawa M, Saji M, Tsushima T, Wakai K, Shizume K. Thyroid volume and serum thyroglobulin levels in patients with acromegaly: correlation with plasma insulin-like growth factor I levels. J Clin Endocrinol Metab. 1988;67:973–8. doi: 10.1210/jcem-67-5-973. [DOI] [PubMed] [Google Scholar]
- 25.Brzozowska M, Kinalska J, Krętowski A. The level of IGF1 and TGFbeta1 the blood serum and the thyroid size in children with normal ioduria. Endokrynol Diabetol Chor Przemiany Materii Wieku Rozw. 2005;11:215–20. [PubMed] [Google Scholar]
- 26.Völzke H, Friedrich N, Schipf S, et al. Association between serum insulin-like growth factor 1 levels and thyroid disorders in a population-based study. J Clin Endocrinol Metab. 2007;92:4039–45. doi: 10.1210/jc.2007-0816. [DOI] [PubMed] [Google Scholar]
- 27.Takahashi S, Conti M, Van Wyk JJ. Thyrotropin potentiation of insulin-like growth factor-I dependent deoxyribonucleic acid synthesis in FRTL-5 cells: mediatiom by an autocrine amplification factor(s) Endocrinology. 1990;126:736–45. doi: 10.1210/endo-126-2-736. [DOI] [PubMed] [Google Scholar]
- 28.Cheung NW, Lou JC, Boyages SC. Growth hormone does not increase thyroid size in the absence of TSH: a study in adults with hypopituitarism. J Clin Endocrinol Metab. 1996;81:1179–83. doi: 10.1210/jcem.81.3.8772597. [DOI] [PubMed] [Google Scholar]
- 29.Marzullo P, Cuocolo A, Ferone D, et al. Cardiac effect of thyrotoxicosis in acromegaly. J Clin Endocrinol Metab. 2000;85:1426–32. doi: 10.1210/jcem.85.4.6510. [DOI] [PubMed] [Google Scholar]
- 30.Tokuyama T, Yoshinari M, Okamura K, et al. The relationship between TSH response to TRH and GH response to dopaminergic agents in patients with acromegaly. Nihon Naibunpi Gakkai Zasshi. 1991;67:65–74. doi: 10.1507/endocrine1927.67.2_65. [DOI] [PubMed] [Google Scholar]
- 31.Sakane N, Yoshida T, Shimatsau A, Umekawa T, Kondo M. Octreotide and bromocriptine suppress thyroid hormone levels and thyroid nodule in an acromegalic patient with nontoxic autonomous goiter. Endocr J. 1997;44:305–10. doi: 10.1507/endocrj.44.305. [DOI] [PubMed] [Google Scholar]
- 32.Gemsenjäger E, Staub JJ, Girard J, Heitz P. Preclinical hyperthyroidism in multinodular goiter. J Clin Endocrinol Metab Res. 1976;43:810–6. doi: 10.1210/jcem-43-4-810. [DOI] [PubMed] [Google Scholar]
- 33.Mariotti S. Normal physiology of the hypothalamic-pituitary-thyroidal system and relation to the neural system and other endocrine gland. 2006. Chap 4. Thyroid disease manager. www.thyroidmanager.org.
- 34.Hollenberg AN. The role the thyrotropin-releasing hormone (TRH) neuronas metabolic sensor. Thyroid. 2008;18:131–9. doi: 10.1089/thy.2007.0251. [DOI] [PubMed] [Google Scholar]
- 35.Lechan RM, Fekete C. The TRH neuron: a hypothalamic integrator ofenergy metabolism. Prog Brain Res. 2006;153:209–35. doi: 10.1016/S0079-6123(06)53012-2. [DOI] [PubMed] [Google Scholar]
- 36.Kalsbeek A, Fliers E, Franke AN, Wortel J, Buijs RM. Functional connections between the suprachiasmatic nucleus and the thyroid gland as revealed by lesioning and viral tracing techniques in the rat. Endocrinology. 2000;141:3832–41. doi: 10.1210/endo.141.10.7709. [DOI] [PubMed] [Google Scholar]
- 37.Kalsbeek A, Fliers E, Franke AN, Wortel J, Buijs RM. Functional connections between the suprachiasmatic nucleus and the thyroid gland as revealed by lesioning and viral tracing techniques in the rat. Endocrinology. 2000;141:3832–41. doi: 10.1210/endo.141.10.7709. [DOI] [PubMed] [Google Scholar]
- 38.Morley JE. Neuroendocrine control of thyrotropin secretion. Endocr Rev. 1981;2:396–436. doi: 10.1210/edrv-2-4-396. [DOI] [PubMed] [Google Scholar]
- 39.Roelfsema F, Biermasz NR, Frolich M, Keenan DM, Veldhuis JD, Romijn JA. Diminished and irregular thyrotropin secretion with preserved diurnal rhytm in patients with active acromegaly. J Clin Endocrinol Metab. 2009;94:1945–50. doi: 10.1210/jc.2009-0174. [DOI] [PubMed] [Google Scholar]
- 40.Lewis BM, Dieguez C, Lewis MD, Scanlon MF. Dopamine stimulates release of thyrotrophin-releasing hormone from perfused intact rat hypothalamus via hypothalamic D2-receptors. J Endocrinol. 1987;115:419–24. doi: 10.1677/joe.0.1150419. [DOI] [PubMed] [Google Scholar]
- 41.Hofland LJ, Lamberts SW. Somatostatin receptor in pituitary function, diagnosis and therapy. Front Horm Res. 2004;32:235–52. doi: 10.1159/000079048. [DOI] [PubMed] [Google Scholar]
- 42.Giustina A, Veldhuis JD. Pathophysiology of the neuroregulation of growth hormone secretion in experimental animals and the human. Endocr Rev. 1998;19:717–97. doi: 10.1210/edrv.19.6.0353. [DOI] [PubMed] [Google Scholar]
- 43.Looij BJ, Roelfsema F, Frölich M, Nieuwenhuijzen Kruseman AC. The interaction of GHRH with TRH in acromegaly: a controlled study. Acta Endocrinol (Copenh) 1991;125:337–41. doi: 10.1530/acta.0.1250337. [DOI] [PubMed] [Google Scholar]
- 44.Seoane LM, Carro E, Tovar S, Casanueva FF, Dieguez C. Regulation of in vivo TSH secretion by leptin. Regul Pept. 2000;92:25–9. doi: 10.1016/s0167-0115(00)00145-2. [DOI] [PubMed] [Google Scholar]
- 45.Chan JL, Heist K, DePaoli AM, Veldhuis JD, Mentzoros CS. The role of falling leptin levels in the neuroendocrine and metabolic adaptation to shortterm starvation in healthy men. J Clin Invest. 2003;111:1409–21. doi: 10.1172/JCI17490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Légrádi G, Emerson CH, Ahima RS, Flier JS, Lechan RM. Leptin prevents fasting-induced suppression of prothyrotropin-releasing hormone messenger ribonucleic acid in neurons of the hypothalamic paraventricular nucleus. Endocrinology. 1997;138:2569–76. doi: 10.1210/endo.138.6.5209. [DOI] [PubMed] [Google Scholar]
- 47.Schurgin S, Canavan B, Koutkia P, Depadi AM, Grinspoon S. Endocrine and metabolic effects of physiologic r-metHuLeptin administration during acute caloric deprivation in normal-weight women. J Clin Endocrinol Metab. 2004;89:5402–9. doi: 10.1210/jc.2004-1102. [DOI] [PubMed] [Google Scholar]
- 48.Damjanović SS, Petakov MS, Raicević S, et al. Serum leptin levels in patients with acromegaly before and after correction of hypersomatotropism by transsphenoidal surgery. J Clin Endocrinol Metab. 2000;85:147–54. doi: 10.1210/jcem.85.1.6296. [DOI] [PubMed] [Google Scholar]
- 49.Tan KC, Tso AW, Lam KS. Effect of sandostatin LAR on serum leptin levels in patients with acromegaly. Clin Endocrinol (Oxf) 2001;54:31–5. doi: 10.1046/j.1365-2265.2001.01180.x. [DOI] [PubMed] [Google Scholar]
- 50.Parkinson C, Whatmore AJ, Yates AP, et al. The effect of pegvisomant-induced serum IGF-I normalization on serum leptin levels in patients with acromegaly. Clin Endocrinol (Oxf) 2003;59:168–74. doi: 10.1046/j.1365-2265.2003.01795.x. [DOI] [PubMed] [Google Scholar]
- 51.Geelhoed-Duijvestijn PH, Roelfsema F, Schröder-van der Elst JP, van Doorn J, van der Heide. Effect of administration of growth hormone on plasma and intracellular levels of thyroxine and tri-iodothyronine in thyroidectomized thyroxine-treated rats. J Endocrinol. 1992;133:45–9. doi: 10.1677/joe.0.1330045. [DOI] [PubMed] [Google Scholar]
- 52.Mantzoros CS, Ozata M, Negrao AB, et al. Synchronicity of frequently sampled thyrotropin (TSH) and leptin concentrations in healthy adults and leptin-deficient subjects: evidence for possible partial TSH regulation by leptin in humans. J Clin Endocrinol Metab. 2001;86:3284–91. doi: 10.1210/jcem.86.7.7644. [DOI] [PubMed] [Google Scholar]
- 53.Geelhoed-Duijvestijn PH, Bussemaker JK, Roelfsema F. Changes in basal and stimulated TSH and other parameters of thyroid function in acromegaly after transsphenoidal surgery. Acta Endocrinol (Copenh) 1989;121:207–15. doi: 10.1530/acta.0.1210207. [DOI] [PubMed] [Google Scholar]
- 54.Andersson IJ, Barlind A, Nyström HC, et al. Reduced sympathetic responsiveness as well as plasma and tissue noradrenaline concentration in growth hormone transgenic mice. Acta Physiol Scand. 2004;182:369–78. doi: 10.1111/j.1365-201X.2004.01368.x. [DOI] [PubMed] [Google Scholar]
- 55.Resmini E, Casu M, Patrone V, et al. Sympathovagal imbalance in acromegalic patients. J Clin Endocrinol Metab. 2006;91:115–20. doi: 10.1210/jc.2005-1506. [DOI] [PubMed] [Google Scholar]
- 56.Akagi Y, Liu W, Zebrowski B, et al. Regulation of vascular endothelial growth factor expression in human colon cancer by insulin-like growth factor-1. Cancer Res. 1998;58:4008–14. [PubMed] [Google Scholar]
- 57.Dunn SE. Insulin-like growth factor 1 stimulates angiogenesis and the production of vascular endothelial growth factor. Growth Horm IGF Res. 2000;10(Suppl A):S41–2. doi: 10.1016/s1096-6374(00)90020-0. [DOI] [PubMed] [Google Scholar]
- 58.Bogazzi F, Manetti L, Bartalena L, et al. Thyroid vascularity is increased in patients with active acromegaly. Clin Endocrinol (Oxf) 2002;57:65–70. doi: 10.1046/j.1365-2265.2002.01562.x. [DOI] [PubMed] [Google Scholar]
- 59.Hirano H, Lopes MB, Laws ER, Jr, et al. Insulin-like growth factor-1 content and pattern of expression correlates with histopathologic grade in diffusely infiltrating astrocytomas. Neurooncology. 1999;1:109–19. doi: 10.1093/neuonc/1.2.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bermont L, Lamielle F, Fauconnet S, et al. Regulation of vascular endothelial growth factor expression by insulin-like growth factor-1 in endometrial adenocarcinoma cells. Int J Cancer. 2000;85:117–23. doi: 10.1002/(sici)1097-0215(20000101)85:1<117::aid-ijc21>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 61.Abd El Nasar, Yamamah G, Kamel AF, Abd-El Dayem S, Hussein AS, Salama H. Thyroid volumes and iodine status in Egyptian South Sinai schoolchildren. Arch Med Sci. 2013;9:548–54. doi: 10.5114/aoms.2012.30952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cheung NW, Boyages SC. Somatostatin-14 and its analog octreotide exert a cytostatic effect on GH3 rat pituitary tumor cell proliferation via transient G0/G1 cycle block. Endocrinology. 1995;136:4174–81. doi: 10.1210/endo.136.10.7664634. [DOI] [PubMed] [Google Scholar]
- 63.Ain KB, Taylor KD. Somatostatin analogs affect proliferation of human thyroid carcinoma cell lines in vitro. J Clin Endocrinol Metab. 1994;78:1097–102. doi: 10.1210/jcem.78.5.7909817. [DOI] [PubMed] [Google Scholar]
- 64.Boy C, Heusner TA, Poeppel TD, et al. 68Ga-DOTATOC PET/CT and somatostatin receptor (sst1-sst5) expression in normal human tissue: correlation of sst2 mRNA and SUVmax. Eur J Nucl Med Mol Imaging. 2011;38:1224–36. doi: 10.1007/s00259-011-1760-x. [DOI] [PubMed] [Google Scholar]
- 65.Klagge A, Krause K, Schierle K, Steinert F, Dralle H, Fuhrer D. Somatostatin receptor subtype expression in human thyroid tumours. Horm Metab Res. 2010;42:237–40. doi: 10.1055/s-0029-1243636. [DOI] [PubMed] [Google Scholar]
- 66.Druckenthaner M, Schwarzer C, Ensinger C, et al. Evidence for somatostatin receptor 2 in thyroid tissue. Regul Pept. 2007;138:32–9. doi: 10.1016/j.regpep.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 67.Colao A, Marzullo P, Spiezia S, Lombardi G. Acromegaly and prostate cancer. Growth Horm IGF Res. 2000;10(Suppl A):S37–8. doi: 10.1016/s1096-6374(00)90018-2. [DOI] [PubMed] [Google Scholar]
- 68.Colao A, Marzullo P, Ferone D, et al. Prostatic hyperplasia: an unknown feature of acromegaly. J Clin Endocrinol Metab. 1998;83:775–9. doi: 10.1210/jcem.83.3.4645. [DOI] [PubMed] [Google Scholar]
- 69.Jenkins PJ. Cancers associated with acromegaly. Neuroendocrinology. 2006;83:218–23. doi: 10.1159/000095531. [DOI] [PubMed] [Google Scholar]
- 70.Kobberling J, Darragh A, Del Pozo E. Chronicdopamine receptor stimulation using bromocriptine: Failure to modify thyroid function. Clin Endocrinol (Oxf) 1979;11:367–70. doi: 10.1111/j.1365-2265.1979.tb03087.x. [DOI] [PubMed] [Google Scholar]
- 71.Barkan AL, Kelch RP, Hopwood NJ, Beitinis IZ. Treatment of acromegaly with the long acting somatostatin analogue SMS 201-995. J Clin Endocrinol Metab. 1988;66:16–23. doi: 10.1210/jcem-66-1-16. [DOI] [PubMed] [Google Scholar]
- 72.Ho KY, Weissberger AJ, Marbach P, Lanzarus L. Therapeutic efficacy of the somatostatin analog SMS 201-995 (octretotide) in acromegaly. Ann Int Med. 1990;112:173–81. doi: 10.7326/0003-4819-112-3-173. [DOI] [PubMed] [Google Scholar]
- 73.Roelfsema F, Frölich M. Pulsatile thyrotropin release and thyroid function in acromegalics before and during subcutaneous octreotide infusion. J Clin Endocrinol Metab. 1991;72:77–82. doi: 10.1210/jcem-72-1-77. [DOI] [PubMed] [Google Scholar]
- 74.Inada M, Sterling K. Thyroxine turnover and transport in active acromegaly. J Clin Endocrinol Metab. 1967;27:1019–27. doi: 10.1210/jcem-27-7-1019. [DOI] [PubMed] [Google Scholar]
- 75.Sato T, Suzukui Y, Taketani T, Ishiguro K, Masuyama T. Enhanced peripheral conversion of thyroxine to triiodothyronine during hGH therapy in GH deficient children. J Clin Endocrinol Metab. 1977;45:324–9. doi: 10.1210/jcem-45-2-324. [DOI] [PubMed] [Google Scholar]
- 76.Grunfeld C, Sherman BM, Cavalier RR. The acute effects of human growth hormone administration on thyroid function in normal men. J Clin Endocrinol Metab. 1988;67:1111–4. doi: 10.1210/jcem-67-5-1111. [DOI] [PubMed] [Google Scholar]
- 77.Gotzsche LS, Flyvbjerg A, Marshall S, Jorgensen KD, Weeke J. The influence of growth hormone and thyroxine on iodothyronine deiodinase activity in the liver, kidney and brown adipose tissue in hypophysectomized rats. Acta Endocrinol (Copenh) 1991;125:219–26. doi: 10.1530/acta.0.1250219. [DOI] [PubMed] [Google Scholar]
- 78.Eskildsen PC, Kruse A, Kirkegaard C. The pituitary-thyroid axis in acromegaly. Horm Metab Res. 1988;20:755–7. doi: 10.1055/s-2007-1010940. [DOI] [PubMed] [Google Scholar]
- 79.Kühn ER, Verheyen G, Chiasson RB, et al. Growth hormone stimulates the peripheral conversion of thyroxine into triiodothyronine by increasing the liver 5-momodeiodinase activity in the fasted and normal fed chicken. Horm Metal Res. 1987;19:304–8. doi: 10.1055/s-2007-1011806. [DOI] [PubMed] [Google Scholar]
- 80.Bianco AC, Salvatore D, Gereben B, Berry MJ, Lasrsen PR. Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr Rev. 2002;23:38–89. doi: 10.1210/edrv.23.1.0455. [DOI] [PubMed] [Google Scholar]
- 81.Fliers E, Unmehopa UA, Alkemade A. Functional neuroanatomy of thyroid hormone feedback in the human hypothalamus and pituitary gland. Mol Cell Endocrinol. 2006;251:1–8. doi: 10.1016/j.mce.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 82.Baris D, Gridley G, Ron E, et al. Acromegaly and cancer risk: a cohort study in Sweden and Denmark. Cancer Causes Control. 2002;13:395–400. doi: 10.1023/a:1015713732717. [DOI] [PubMed] [Google Scholar]
- 83.Ruchała M, Szczepanek-Parulska E, Komorska-Piotrowiak E. Diagnostyka i leczenie akromegalii. Oncoreview. 2011;4:240–7. [Google Scholar]
- 84.Siegel G, Tomer Y. Is there an association between acromegaly and thyroid carcinoma? A critical review of the literature. Endocr Res. 2005;31:51–8. doi: 10.1080/07435800500229177. [DOI] [PubMed] [Google Scholar]
- 85.Balkany C, Cushing GW. An association between acromegaly and thyroid carcinoma. Thyroid. 1995;5:47–50. doi: 10.1089/thy.1995.5.47. [DOI] [PubMed] [Google Scholar]
- 86.Onoda N, Ohmura E, Tsushima T, et al. Autocrine role of insulin-like growth factor (IGF)-I in a human thyroid cancer cell line. Eur J Cancer. 1992;28A:1904–9. doi: 10.1016/0959-8049(92)90033-x. [DOI] [PubMed] [Google Scholar]
- 87.Kurimoto M, Fukuda I, Hizuka K, Takano K. The prevalence of benign and malignant tumors in patients with acromegaly at single institute. Endocr J. 2008;55:67–71. doi: 10.1507/endocrj.k07e-010. [DOI] [PubMed] [Google Scholar]
- 88.Wong VW, Gurney H, Hazel JR. Multinodular goitre: an unusual case in a patient with acromegaly. Intern Med J. 2008;38:742–3. doi: 10.1111/j.1445-5994.2008.01774.x. [DOI] [PubMed] [Google Scholar]
- 89.Jenkins PJ, Mukherjee A, Shalet SM. Does growth hormone cause cancer? Clin Endocrinol (Oxf) 2006;64:115–21. doi: 10.1111/j.1365-2265.2005.02404.x. [DOI] [PubMed] [Google Scholar]
- 90.Colao A, Ferone D, Marzullo P, Lombardi G. Systemic complications of acromegaly: epidemiology, pathogenesis, and management. Endocr Rev. 2004;25:102–52. doi: 10.1210/er.2002-0022. [DOI] [PubMed] [Google Scholar]
- 91.Villar A, Zurro J, de Luis DA, Cuéllar L, Terroba C, Romero E. Acromegaly, multinodular toxic goiter and papillary carcinoma of the thyroid, potential role of G proteins. Ann Med Interna. 2002;19:79–80. [PubMed] [Google Scholar]
- 92.Gullu BE, Celik O, Nurperi G, Kadioglu P. Thyroid cancer is the most common cancer associated with acromegaly. Pituitary. 2010;13:242–8. doi: 10.1007/s11102-010-0224-9. [DOI] [PubMed] [Google Scholar]
- 93.Ruchała M, Skiba A, Gurgul E, Uruski P, Wasko R, Sowinski J. The occurence of thyroid focal lesions and need for fine needle aspiration biopsy in patients with acromegaly due to an increased risk of thyroid cancer. Neuro Endocrinol Lett. 2009;30:382–6. [PubMed] [Google Scholar]
- 94.Marchisotti FG, Umeda LM, Zach PL, Saldanha MD, First OS, Liberman B. Acromegaly and thyroid disease: prevalence of thyroid cancer. Arq Bras Endocrinol Metabol. 2005;49:843–9. doi: 10.1590/s0004-27302005000500027. [DOI] [PubMed] [Google Scholar]
- 95.Ertek S, Ersoy RU, Anil C, et al. Hypothyroidism, new nodule formation and increase in nodule size in patients who have undergone hemithyroidectomy. Arch Med Sci. 2012;8:263–9. doi: 10.5114/aoms.2012.28222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Słowińska-Klencka D, Popowicz B, Woźniak E, Sporny S, Klencki M. The influence of fine-needle aspiration biopsy of the thyroid gland on the size of the examined nodule and its ultrasound image. Arch Med Sci. 2012;8:1059–64. doi: 10.5114/aoms.2012.32415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cesur M, Akcil M, Ertek S, et al. Role of cytological characteristics of benign thyroid nodules on effectiveness of their treatment with levothyroxine. Arch Med Sci. 2013;9:1083–9. doi: 10.5114/aoms.2013.39796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dighe M, Bae U, Richardson ML, Dubinsky TJ, Minoshima S, Kim Y. Differential diagnosis of thyroid nodules with US elastography using carotid artery pulsation. Radiology. 2008;248:662–9. doi: 10.1148/radiol.2482071758. [DOI] [PubMed] [Google Scholar]
- 99.Fruchtman S, Simmons JG, Michaylira CZ, et al. Suppressor of cytokine signaling-2 modulates the fibrogenic actions of GH and IGF-1 in intestinal mesenchymal cells. Am J Physiol Gastrointest Liver Physiol. 2005;289:342–50. doi: 10.1152/ajpgi.00413.2004. [DOI] [PubMed] [Google Scholar]