Abstract
Introduction
Obstructive sleep apnoea (OSA) is a prevalent disorder characterised by repetitive upper-airway obstruction during sleep, and it is associated with type 2 diabetes. Continuous positive airway pressure (CPAP) is the primary treatment for OSA. Prior studies investigating whether CPAP can improve insulin resistance or glucose control in OSA patients have resulted in conflicting findings. This meta-analysis investigated whether CPAP treatment could improve glucose metabolism and insulin resistance in patients with OSA and type 2 diabetes.
Material and methods
We performed a systematic literature search using Medline, Cochrane, EMBASE, and Google Scholar databases for randomised controlled prospective studies that investigated the effect of CPAP on glycaemic control or insulin sensitivity in subjects with type 2 diabetes.
Results
The combined standard (STD) paired difference in mean change in the levels of glycated haemoglobin (HbA1c) was –0.073% (standard error (SE): 0.126), indicating that CPAP treatment did not alter HbA1c levels. The combined STD paired difference in mean change of insulin sensitivity was observed as 0.552 µmol/kg • min (SE = 0.196) and indicated insulin sensitivity significantly increased with CPAP treatment (p = 0.005).
Conclusions
We found that the CPAP treatment did not alter HbA1c levels but did significantly improve insulin resistance, indicating treating OSA can positively impact the symptoms of type 2 diabetes.
Keywords: obstructive airway apnoea, insulin resistance, glycaemic control, positive airway pressure
Introduction
Obstructive sleep apnoea (OSA) is a prevalent disorder characterised by repetitive upper-airway obstruction during sleep resulting in intermittent hypoxia and fragmentation of sleep. In the adult population, OSA is an independent risk factor for the development of diabetes mellitus [1–4]. Cross-sectional epidemiological studies found an association between OSA and deterioration in glycaemic control [5–10]. Obstructive sleep apnoea is present in about 73% of patients with type 2 diabetes [11]. However, it is currently unclear if this results from a connection between the two diseases or if it is just that both diseases are associated with obesity.
Continuous positive airway pressure (CPAP) is the primary treatment for OSA. Prior studies investigating whether CPAP can improve insulin resistance of glucose control in OSA patients have resulted in conflicting findings. Some prior work found CPAP treatment resulted in a significant reduction in glycated haemoglobin (HbA1c) [10, 12, 13], while other studies found no change [14–18]. Similarly, some but not all studies found CPAP therapy improved insulin sensitivity [12, 14, 15, 18–20]. Consequently, it is currently unclear if CPAP-therapy can have therapeutic benefit for type 2 diabetes in patients with OSA.
Three meta-analyses have investigated the effect of CPAP on measures of glycaemic control and insulin resistance [20–22]. Two of these studies evaluated patients with OSA but did not require patients to have type 2 diabetes [20–22], and the other included retrospective studies and did not evaluate changes in insulin resistance. In this study, we included prospective studies in patients with both OSA and diabetes, which measured HbA1c or insulin resistance to assess whether CPAP treatment in these patients was of benefit for diabetics.
Material and methods
Search strategy
We performed a systematic literature search using Medline, Cochrane, EMBASE, and Google Scholar (up to March 31, 2013) databases for randomised controlled prospective studies that investigated the effect of CPAP on glycaemic control or insulin sensitivity in subjects with type 2 diabetes. All relevant studies published prior to March 31, 2013 were included. All included studies had to be published in English and had to have investigated human subjects with stable, controlled type 2 diabetes, who received CPAP treatment for obstructive sleep apnoea. Retrospective or cohort studies and studies that did not report numerical results on HbA1c or insulin sensitivity (as evaluated by euglycaemic hyperinsulinaemic clamp) were excluded from the analysis. The overall search strategy combined search terms of diabetes mellitus, glycaemic control, insulin sensitivity, risk factors, sleep apnoea, obstructive sleep apnoea, and continuous positive airway pressure. Identified potential references were screened by two independent investigators. Disagreements were resolved by a third reviewer.
Data extraction
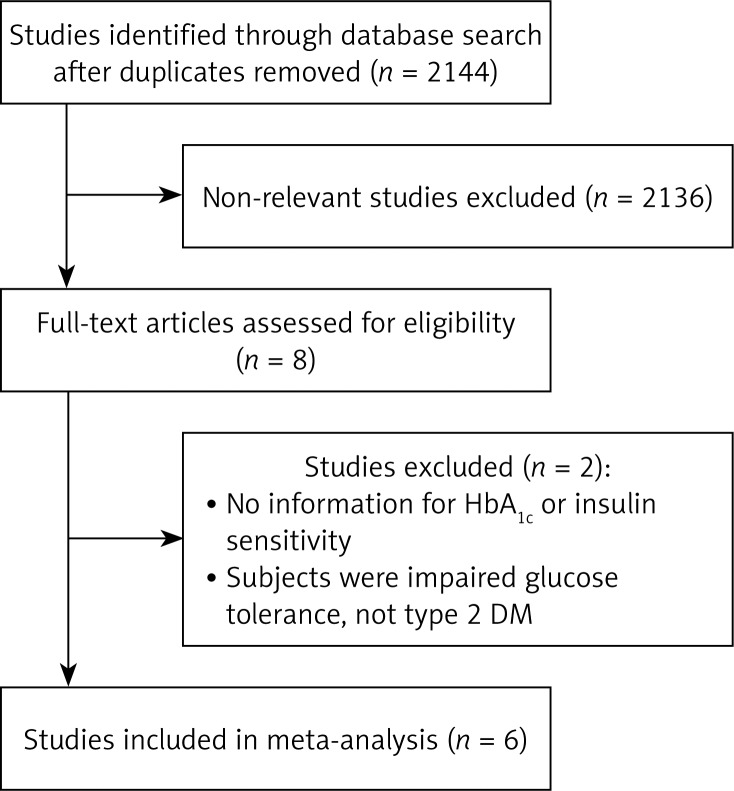
For studies that met the inclusion criteria (Figure 1), data was extracted into a standardised worksheet. Extracted data included the name of the first author, year of publication, study design, number of study subjects in each treatment group, the age and gender of subjects, the description of the CPAP protocol, and the results. We used a Delphi list to assess the included studies [23].
Figure 1.
Flow chart for study selection
Statistical analysis
The primary efficacy outcome was HbA1c levels following CPAP treatment. The secondary outcome was insulin sensitivity following CPAP therapy. The HbA1c and insulin sensitivity before and after CPAP treatment were used to evaluate treatment efficacy. Means with standard deviations (SD) were summarised for the major outcome, and the change before and after CPAP was evaluated as for the treatment effect. Combined summary statistics of the standardised (STD) paired difference in mean for the individual studies are shown. Combined STD paired differences in means were calculated and a 2-sided p-value < 0.05 was considered to indicate statistical significance. An χ2-based test of homogeneity was performed and the inconsistency index (I 2) statistic was determined. If I 2 was > 50% or > 75%, the studies were considered to be heterogeneous or highly heterogeneous, respectively. If I 2 was below 25%, the studies were considered to be homogeneous. If the I 2 statistic (> 50%) indicated that heterogeneity existed between studies, a random-effects model was calculated. Otherwise, fixed-effect models were calculated. Moreover, sensitivity analysis was performed based on the leave-one-out approach, and a funnel plot and the fail-safe N (which indicates whether the observed significance is spurious or not) were used to assess possible publication bias. All analyses were performed using Comprehensive Meta-Analysis statistical software, version 2.0 (Biostat, Englewood, NJ, USA).
Results
The database search identified eight studies potentially eligible for analysis (Figure 1). Two studies were excluded, because one of them had no information regarding HbA1c or insulin sensitivity and the subjects in the other did not have type 2 diabetes. Hence, a total of six studies met the inclusion criteria [12, 14, 15, 18, 19, 24]. The characteristics of the six studies included in the meta-analysis are summarised in Table I.
Table I.
Subject demographics and clinical outcomes for the 6 studies
| 1st AU (year) | Treatment | Randomisation | Comparison | Patient number | Age [years] | Male (%) | HbA1c [%] | Insulin sensitivity [µmol/kg · min] | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | |||||||
| Myhill (2012) | CAPA for 3 months | Randomised | CAPA initiated early (1 week) vs. CAPA initiated late (1–2 months) | 44 | 66.1 ±8.8 | 61.4 | Median = 6.9 (IQR = 6.1–7.3) | Median = 6.9 (IQR = 6.1–7.4) | N/A | N/A |
| Dawson (2008) | CAPA for 3 months | Non-randomised | Before CAPA vs. after CAPA | 20 | 59.8 ±10.2 | 60 | 7.1 ±1.3 | 7.2 ±1.3 | N/A | N/A |
| West (2007) | CAPA for 3 months | Randomised | CAPA for 3 months vs. placebo | 20 | 57.8 ±10.4 | 100 | 8.5 ±1.8 | N/A | 26.5 ±14.4 | N/A |
| Babu (2005) | CAPA for 1–3 months | Non-randomised | Before CAPA vs. after CAPA | 25 | 50.7 ±9 | 64 | 8.3 ±2.2 | 7.9 ±1.8 | N/A | N/A |
| Harsch (2004) | CAPA for about 3 months | Non-randomised | Before CAPA vs. after CAPA | 9 | 56.3 ±8.2 | 77.8 | 6.4 ±0.7 | 6.3 ±0.6 | 2.98 ±2.62 | 4.38 ±2.94 |
| Brooks (1994) | CAPA for 4 months | Non-randomised | Before CAPA vs. after CAPA | 10 | 50.8 ±9.6 | 70 | 8.9 ±1.5 | 8.9 ±1.2 | 11.4 ±6.2 | 15.1 ±4.6 |
N/A – not available
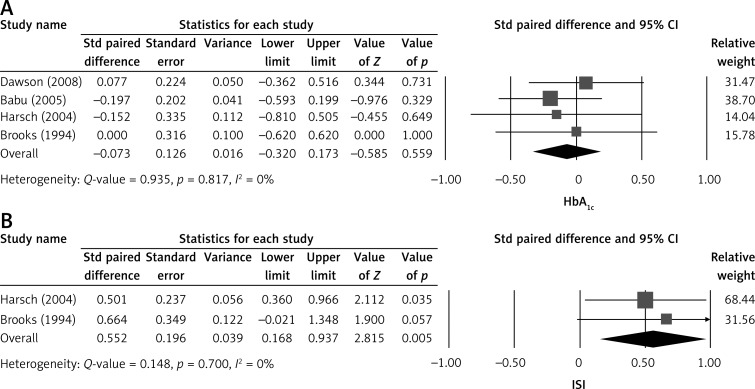
Four of the six included studies presented complete mean HbA1c levels before and after CPAP treatment. A fixed-effect model was used to evaluate this data according to the heterogonous test (Q = 0.148, I2 = 0%, p = 0.700) [12, 14, 15, 19]. The combined STD paired difference in mean change in the levels of HbA1c was –0.073% (standard error (SE): 0.126), indicating that CPAP treatment did not alter HbA1c levels (Figure 2A).
Figure 2.
Forest plot of major outcomes, (A) HbA1c level and (B) insulin sensitivity before and after CPAP treatment
Std – standardised, 95% CI – 95% confidence interval, ISI – insulin sensitivity
Two of the six studies reported changes in mean insulin sensitivity with CPAP therapy. A fixed-effect model was used according to the heterogeneous test (Q = 0.148, I 2 = 0%, p = 0.700). The combined STD paired difference in mean changes of insulin sensitivity was observed as 0.552 µmol/kg • min (SE = 0.196), and indicated insulin sensitivity significantly increased with CPAP treatment (p = 0.005) (Figure 2B).
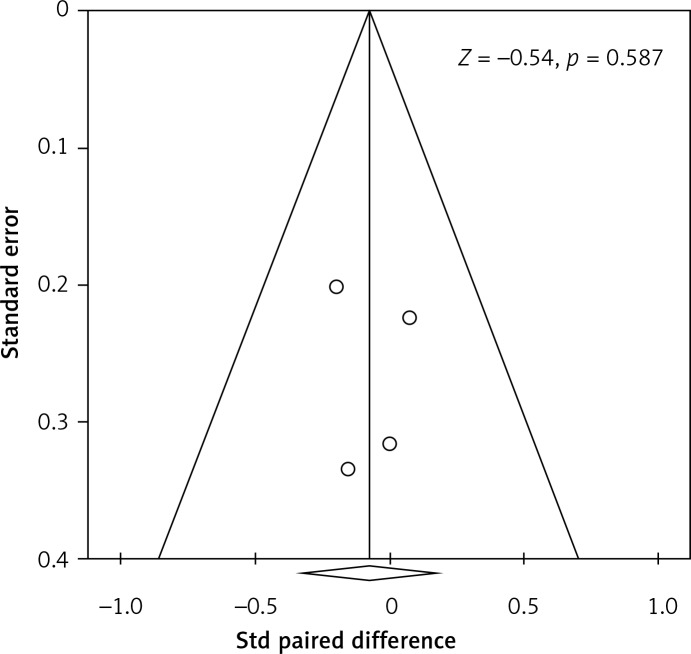
Sensitivity analysis of our HbA1c outcome indicated that removal of each study, one at a time, did not influence the direction or magnitude of the pooled estimate (Figure 3), indicating that no one study dominated the findings. In addition, funnel plot analysis demonstrated marked symmetry, indicating that there was no publication bias (Z value = –0.54; p = 0.587) (Figure 4). The quality assessment findings are summarised in Table II.
Figure 3.
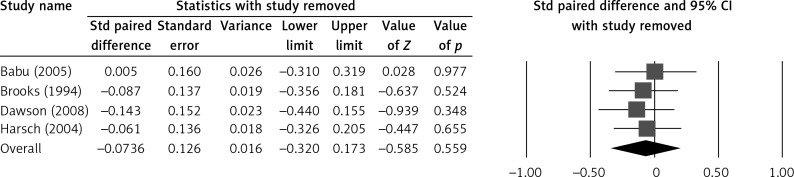
Sensitivity analysis for the influence of individual studies on pooled estimate by means of leave-one-out for HbA1c level before and after CPAP treatment
Std – standardised, 95% CI – 95% confidence interval, ISI – insulin sensitivity
Figure 4.
Funnel plot of HbA1c level
Table II.
Summary of data collected
| First author (year) | Was a method of randomisation used? | Were the groups similar at baseline regarding the most important prognostic indicators? | Were the eligibility criteria specified? | Was the outcome assessor blinded? | Was the care provider blinded? | Was the patient blinded? | Were point estimates and measures of variability presented for the primary outcome measures? | Did the analysis include an intention-to-treat analysis? |
|---|---|---|---|---|---|---|---|---|
| Myhill (2012) | Yes | Yes | Yes | NA | NA | NA | Yes | NA |
| Dawson (2008) | No | Yes | Yes | NA | NA | NA | Yes | NA |
| West (2007) | Yes | Yes | Yes | Yes | NA | NA | Yes | NA |
| Babu (2005) | No | Yes | Yes | NA | NA | NA | Yes | NA |
| Harsch (2004) | No | Yes | Yes | NA | NA | NA | Yes | NA |
| Brooks (1994) | No | Yes | Yes | NA | NA | NA | Yes | NA |
NA – information not available or not applicable
Discussion
This meta-analysis was designed to evaluate the effect of treatment of OSA with CPAP on glycaemic control in patients with type 2 diabetes. We found that CPAP treatment did not alter HbA1c levels but significantly improved insulin resistance, indicating treating OSA can positively impact the symptoms of type 2 diabetes in patients with OSA. Additional analysis supports our findings; we found no significant evidence of heterogeneity among the studies, funnel plot analysis showed there was no substantial publication bias, and the baseline characteristics were similar across the studies.
Our meta-analysis has some similarities and differences with prior meta-analyses that also evaluated CPAP effect on diabetes [20–22]. Two meta-analyses in patients with OSA, but who were not selected for having type 2 diabetes, also found that CPAP treatment had no significant impact on HbA1c [20, 22]. Subgroup analysis in one study also found no change in HbA1c levels in patients with type 2 diabetes following CPAP therapy [22]. A meta-analysis by Yang et al. evaluated glycaemic control by assessing fasting blood glucose data and found that CPAP treatment did not alter control [21]. In two meta-analyses that assessed insulin sensitivity, one found a significant decrease in insulin resistance as evaluated using homeostasis model assessment insulin resistance (HOMA-IR) ([fasting insulin] × [fasting glucose]/22.5]), which correlates well with glucose disposal rates derived from hyperinsulinaemic euglycaemic clamp [21, 25, 26]. In contrast to our findings, the other meta-analysis, which evaluated only randomised controlled trials in patients with OSA, found no change in insulin resistance following CPAP therapy [20]. The difference in findings between ours and this latter meta-analysis may reflect the types of studies included in the analyses. The study by Hecht et al. included trials that measured insulin resistance by HOMA-index, adiponectin, or Kitt-insulin-sensitivity index [20], and some studies excluded patients with type 2 diabetes [20]. In contrast, we only included studies that measured insulin resistance using the euglycaemic hyperinsulinaemic glucose clamp, which is a very sensitive measurement for this outcome. Moreover, our analysis was focused on trials with patients who had type 2 diabetes.
There are several limitations to our analysis that should be considered when interpreting the findings. Only a small number of studies qualified to be included in the meta-analysis. However, OSA is known to be an independent risk factor for diabetes mellitus, there are currently a limited number of studies that have evaluated the effect of CPAP treatment for OSA on symptoms of diabetes, in this case glucose metabolism. The fact that there are so few studies highlights the need for additional research into such a medically important question. In addition, heterogeneity existed among the included studies, and the length of CPAP therapy in these studies ranged from 41 days to 3 months, which may not be sufficient time to detect changes in HbA1c [20]. None of the included studies had a CPAP sham control group. A prior study by West et al. [18] that randomised male patients with type 2 diabetes and OSA to either CPAP (n = 20) or sham CPAP (placebo) (n = 22) found that CPAP did not significantly improve insulin resistance or glycaemic control. We did not include this study because the data presented in the manuscript could not be incorporated into our quantitative analysis. The study did not provide the numerical data for post-treatment insulin sensitivity and glycaemic control findings, but only the difference from pre-treatment. Based on statistical theory, it is incorrect to calculate the post-treatment values from pre-treatment and the difference between pre- and post-treatment values. The inconsistency of the results of West et al. and our own results further indicates the need for additional studies to investigate this question. Our study also had a number of strengths, including the fact that it was the first meta-analysis to evaluate the effect of CPAP on insulin sensitivity and HbA1c in patients with type 2 diabetes.
In conclusion, CPAP therapy in patients with type 2 diabetes and OSA improved insulin resistance without significant changes in glucose metabolism. The CPAP has also shown benefit for diseases associated with OSA, such as cardiovascular disease [27, 28]. It is possible that other biological markers, such as fetuin-A and leptin, in addition to HbA1c and insulin resistance, may be useful in monitoring the effect of CPAP on symptoms of diabetes in patients with OSA [29, 30]. Larger randomised controlled studies with greater treatment times from diverse geographical areas are needed to further investigate this question.
References
- 1.Ip MS, Lam B, Ng MM, Lam WK, Tsang KW, Lam KS. Obstructive sleep apnea is independently associated with insulin resistance. Am J Respir Crit Care Med. 2002;165:670–6. doi: 10.1164/ajrccm.165.5.2103001. [DOI] [PubMed] [Google Scholar]
- 2.Punjabi NM, Beamer BA. Alterations in glucose disposal in sleep-disordered breathing. Am J Respir Crit Care Med. 2009;179:235–40. doi: 10.1164/rccm.200809-1392OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Punjabi NM, Shahar E, Redline S, et al. Sleep-disordered breathing, glucose intolerance, and insulin resistance: the Sleep Heart Health Study. Am J Epidemiol. 2004;160:521–30. doi: 10.1093/aje/kwh261. [DOI] [PubMed] [Google Scholar]
- 4.Punjabi NM, Sorkin JD, Katzel LI, Goldberg AP, Schwartz AR, Smith PL. Sleep-disordered breathing and insulin resistance in middle-aged and overweight men. Am J Respir Crit Care Med. 2002;165:677–82. doi: 10.1164/ajrccm.165.5.2104087. [DOI] [PubMed] [Google Scholar]
- 5.Levy P, Bonsignore MR, Eckel J. Sleep, sleep-disordered breathing and metabolic consequences. Eur Respir J. 2009;34:243–60. doi: 10.1183/09031936.00166808. [DOI] [PubMed] [Google Scholar]
- 6.Spiegel K, Tasali E, Leproult R, Van Cauter E. Effects of poor and short sleep on glucose metabolism and obesity risk. Nat Rev Endocrinol. 2009;5:253–61. doi: 10.1038/nrendo.2009.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aronsohn RS, Whitmore H, Van Cauter E, Tasali E. Impact of untreated obstructive sleep apnea on glucose control in type 2 diabetes. Am J Respir Crit Care Med. 2010;181:507–13. doi: 10.1164/rccm.200909-1423OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okada M, Takamizawa A, Tsushima K, Urushihata K, Fujimoto K, Kubo K. Relationship between sleep-disordered breathing and lifestyle-related illnesses in subjects who have undergone health-screening. Intern Med. 2006;45:891–6. doi: 10.2169/internalmedicine.45.1592. [DOI] [PubMed] [Google Scholar]
- 9.Papanas N, Steiropoulos P, Nena E, et al. HbA1c is associated with severity of obstructive sleep apnea hypopnea syndrome in nondiabetic men. Vasc Health Risk Manag. 2009;5:751–6. doi: 10.2147/vhrm.s7057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steiropoulos P, Papanas N, Bouros D, Maltezos E. Obstructive sleep apnea aggravates glycemic control across the continuum of glucose homeostasis. Am J Respir Crit Care Med. 2010;182:286. doi: 10.1164/ajrccm.182.2.286. [DOI] [PubMed] [Google Scholar]
- 11.Pamidi S, Aronsohn RS, Tasali E. Obstructive sleep apnea: role in the risk and severity of diabetes. Best Pract Res Clin Endocrinol Metab. 2010;24:703–15. doi: 10.1016/j.beem.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Babu AR, Herdegen J, Fogelfeld L, Shott S, Mazzone T. Type 2 diabetes, glycemic control, and continuous positive airway pressure in obstructive sleep apnea. Arch Intern Med. 2005;165:447–52. doi: 10.1001/archinte.165.4.447. [DOI] [PubMed] [Google Scholar]
- 13.Shpirer I, Rapoport MJ, Stav D, Elizur A. Normal and elevated HbA1C levels correlate with severity of hypoxemia in patients with obstructive sleep apnea and decrease following CPAP treatment. Sleep Breath. 2012;16:461–6. doi: 10.1007/s11325-011-0525-x. [DOI] [PubMed] [Google Scholar]
- 14.Dawson A, Abel SL, Loving RT, et al. CPAP therapy of obstructive sleep apnea in type 2 diabetics improves glycemic control during sleep. J Clin Sleep Med. 2008;4:538–42. [PMC free article] [PubMed] [Google Scholar]
- 15.Harsch IA, Schahin SP, Bruckner K, et al. The effect of continuous positive airway pressure treatment on insulin sensitivity in patients with obstructive sleep apnoea syndrome and type 2 diabetes. Respiration. 2004;71:252–9. doi: 10.1159/000077423. [DOI] [PubMed] [Google Scholar]
- 16.Hassaballa HA, Tulaimat A, Herdegen JJ, Mokhlesi B. The effect of continuous positive airway pressure on glucose control in diabetic patients with severe obstructive sleep apnea. Sleep Breath. 2005;9:176–80. doi: 10.1007/s11325-005-0033-y. [DOI] [PubMed] [Google Scholar]
- 17.Smurra M, Philip P, Taillard J, Guilleminault C, Bioulac B, Gin H. CPAP treatment does not affect glucose-insulin metabolism in sleep apneic patients. Sleep Med. 2001;2:207–13. doi: 10.1016/s1389-9457(00)00079-4. [DOI] [PubMed] [Google Scholar]
- 18.West SD, Nicoll DJ, Wallace TM, Matthews DR, Stradling JR. Effect of CPAP on insulin resistance and HbA1c in men with obstructive sleep apnoea and type 2 diabetes. Thorax. 2007;62:969–74. doi: 10.1136/thx.2006.074351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brooks B, Cistulli PA, Borkman M, et al. Obstructive sleep apnea in obese noninsulin-dependent diabetic patients: effect of continuous positive airway pressure treatment on insulin responsiveness. J Clin Endocrinol Metab. 1994;79:1681–5. doi: 10.1210/jcem.79.6.7989475. [DOI] [PubMed] [Google Scholar]
- 20.Hecht L, Mohler R, Meyer G. Effects of CPAP-respiration on markers of glucose metabolism in patients with obstructive sleep apnoea syndrome: a systematic review and meta-analysis. Ger Med Sci. 2011;9 doi: 10.3205/000143. Doc20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang D, Liu Z, Yang H, Luo Q. Effects of continuous positive airway pressure on glycemic control and insulin resistance in patients with obstructive sleep apnea: a meta-analysis. Sleep Breath. 2013;17:33–8. doi: 10.1007/s11325-012-0680-8. [DOI] [PubMed] [Google Scholar]
- 22.Iftikhar IH, Blankfield RP. Effect of continuous positive airway pressure on hemoglobin A(1c) in patients with obstructive sleep apnea: a systematic review and meta-analysis. Lung. 2012;190:605–11. doi: 10.1007/s00408-012-9404-x. [DOI] [PubMed] [Google Scholar]
- 23.Verhagen AP, de Vet HC, de Bie RA, et al. The Delphi list: a criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by Delphi consensus. J Clin Epidemiol. 1998;51:1235–41. doi: 10.1016/s0895-4356(98)00131-0. [DOI] [PubMed] [Google Scholar]
- 24.Myhill PC, Davis WA, Peters KE, Chubb SA, Hillman D, Davis TM. Effect of continuous positive airway pressure therapy on cardiovascular risk factors in patients with type 2 diabetes and obstructive sleep apnea. J Clin Endocrinol Metab. 2012;97:4212–8. doi: 10.1210/jc.2012-2107. [DOI] [PubMed] [Google Scholar]
- 25.Bonora E, Targher G, Alberiche M, et al. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care. 2000;23:57–63. doi: 10.2337/diacare.23.1.57. [DOI] [PubMed] [Google Scholar]
- 26.Katsuki A, Sumida Y, Gabazza EC, et al. Homeostasis model assessment is a reliable indicator of insulin resistance during follow-up of patients with type 2 diabetes. Diabetes Care. 2001;24:362–5. doi: 10.2337/diacare.24.2.362. [DOI] [PubMed] [Google Scholar]
- 27.Zamarron C, Riveiro A, Gude F. Circulating levels of vascular endothelial markers in obstructive sleep apnoea syndrome. Effects of nasal continuous positive airway pressure. Arch Med Sci. 2011;6:1023–8. doi: 10.5114/aoms.2011.26615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kostapanos MS, Mikhailidis DP, Elisaf MS, Steiropoulos P, Papanas N. Obstructive sleep apnoea syndrome and cardiovascular risk. Arch Med Sci. 2010;6:1115–6. doi: 10.5114/aoms.2012.32425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ismail NA, Ragab S, El Dayem SM, et al. Fetuin-A levels in obesity: differences in relation to metabolic syndrome and correlation with clinical and laboratory variables. Arch Med Sci. 2012;8:826–33. doi: 10.5114/aoms.2012.31616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stępień M, Wlazeł RN, Paradowski M, et al. Serum concentrations of adiponectin, leptin, resistin, ghrelin and insulin and their association with obesity indices in obese normo- and hypertensive patients – pilot study. Arch Med Sci. 2012;8:431–6. doi: 10.5114/aoms.2012.29397. [DOI] [PMC free article] [PubMed] [Google Scholar]