Abstract
We present a long term followup (13 years) of spinal hydatid disease with multiple recurrences and intradural dissemination of the disease at the last followup. Intradural extension of the disease in our case was supposedly through the dural rent which has not been reported in English literature. An early followup of the same case has been reported previously by the authors. A 53 year-old female came with progressive left leg pain and difficulty in walking since 2 months. On examination, she had grade four power of ankle and digit dorsiflexors (L4 and L5 myotomes) on the left side (Medical Research Council grade). There was no sensory loss, no myelopathy and sphincters were intact. Plain radiographs showed consolidation at D10-D11 (old operated levels) with stable anterior column and there were no implant related problems. Magnetic resonance imaging showed a cystic lesion at L3-L4, signal intensity same as of cerebrospinal fluid in T2 and T1, displacing the cauda equina roots. The proximal extent of the lesion could not be identified because of artifacts from previous stainless steel instrumentation. Computed tomography myelogram showed complete block at L3-L4 junction with “meniscus sign”. This is the longest followup of hydatid disease of the spine that has ever been reported. Hydatid disease should always be included in the differential diagnosis of destructive or infectious lesions of the spine. Aggressive radical resection whenever possible and chemotherapy is the key to good results. Recurrence is known to occur even after that. Disease can have long remission periods. Possibility of intradural dissemination through dural injury is highly likely. Hence, it should always be repaired whenever possible.
Keywords: Hydatid cyst, intradural dissemination, recurrence
MeSH terms: Hydatid, cyst, spinal diseases
INTRODUCTION
Hydatid disease is caused by the larval form of parasitic tapeworm - Echinococcus granulosus. It is common in countries with poor socio-sanitary conditions.1 Humans act as intermediate hosts and get infected either through contact with definitive host (dogs, wolves) or its feces or by ingesting the infected food.2 Due to its subtle presentation and absence of characteristic findings in plain radiographs, diagnosis is commonly missed. Differential diagnoses include potts disease, mycosis, abscesses and neoplasms.1,3 Multiple recurrences are common. Mortality from this disease ranges from 3% to 50%1 and mean life expectancy has been reported to be 5 years.1,3,4 We present 13 year followup of spinal hydatid disease with multiple recurrences and intradural dissemination of the disease at the last followup. Intradural extension of the disease in our case was supposedly through the dural rent which has not been reported in English literature to the best of our knowledge. An early followup of the same case has been reported previously by the senior author.5
CASE REPORT
A 40 year old female presented with history of progressive left leg pain and difficulty in walking since 2 months. Her walking capacity was restricted to 10 m. On examination, she had grade four power of ankle and digit dorsiflexors (L4 and L5 myotomes) on left side (Medical Research Council grade). There was no sensory loss and left knee jerk was absent. There were no signs of myelopathy and sphincters were intact. Plain radiographs showed consolidation at D10-D11 (old operated levels) with stable anterior column and there were no implant related problems [Figure 1a]. Magnetic resonance imaging showed a cystic lesion at L3-L4, signal intensity same as of cerebrospinal fluid (CSF) in T2 and T1, displacing the cauda equina roots. The proximal extent of the lesion could not be identified to certainty because of the artifacts from previous stainless steel instrumentation which was extending up to L3 level. Computed tomography (CT) myelogram showed complete block at L3-L4 intervertebral disc level [Figure 1b] which pointed toward possibility of intradural lesion. Again the proximal extent could not be determined on CT myelogram. Ultrasonography abdomen-pelvis and CT scan chest and brain ruled out any other primary focus. In the year 1997, patient had progressive onset of weakness in lower limbs, for which she underwent posterior decompression at D10-D11 level in peripheral hospital. She was started with anti tuberculosis treatment. At 1½ year postsurgery (1998) when symptoms didn’t improve, patient presented to us. We then did excision biopsy of lesion through transpedicular approach along with posterior stabilization with Hartshill rectangle and sublaminar wires. Intraoperatively we found pearly white daughter cysts and histopathology proved it to be hydatid cyst. Medical management in the form of tablet albendazole was then started in consultation with infectious disease physician. Postsurgery patient became ambulatory but again had a recurrence at the same level (D10-D11) after 2 years and had to be reoperated for paraplegia. This time, a left sided transthoracic decompression was done. Intraoperatively, cord got exposed due to a dural rent. It could not be repaired because dura was adherent to cyst wall and could not be separated. No reconstruction was done as vertebral bodies were found to be intact. Patient went on to complete neurological improvement postsurgery. She had further two recurrences at the same level (D10-D11) which presented with paraparesis and right transthoracic resection of lesion (2002) and anterior corpectomy of D10-D11 with screw fixation with cement augmentation polymethyl methacrylate (PMMA) (2004) was done. Each time patient recovered and became fully ambulatory. She had a long asymptomatic period of 7 years till latest presentation.
Figure 1.
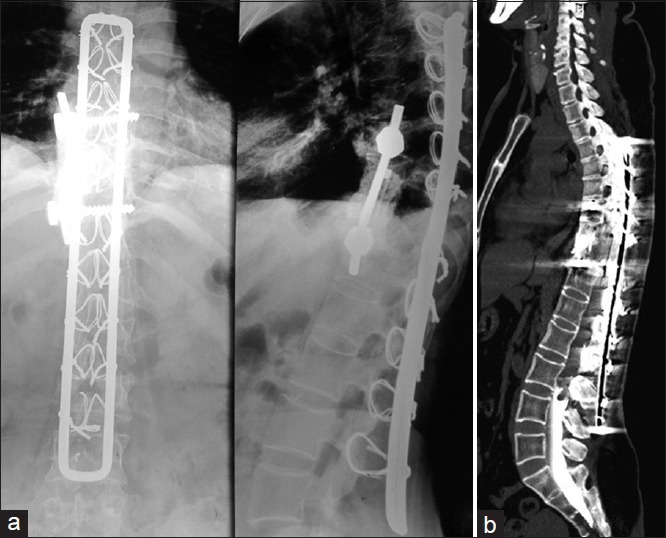
(a) Plain radiographs anteroposterior and lateral views showing instrumentation with Hartshill and sublaminar wires showing fusion at D10-D11 and no implant breakage or cut-out (b) Computed tomography myelogram showing complete cut off of dye flow at L3-L4 level
A working diagnosis of recurrent hydatid cyst was established. Surgical decompression was contemplated in view of deficit and claudication.
Plan of surgery was to expose previous operated thoracic (D10-D11) and lumbar level (L3-L4). Through a posterior approach, Hartshill rectangle was removed. Site of previous surgery (D10-D11) revealed a large cyst with multiple daughter cysts [Figure 2]. Cyst wall was continuous with the dural sac wall along with a dural rent. There was no extra dural extension of the disease from the primary thoracic area (D10-D11) to the lumbar region (L3-L4). Laminectomy was done at L2-L3-L4 but no extra dural mass was found. Dura appeared to be bulging which indicated intradural mass. Durotomy was done from L4 to D12. Multiple small cysts were found from L4 to D12 largest being 1 × 1 × 0.5 cm. Morphology of cysts was typical of a hydatid cyst with translucent wall and clear fluid content. Few cysts were adherent to the cauda equina roots and were not excised completely to prevent root injury. Histopathological examination reconfirmed the diagnosis of recurrent hydatid cyst. Combination therapy of albendazole (10 mg/kg) and praziquantel (50 mg/kg) daily was started.
Figure 2.
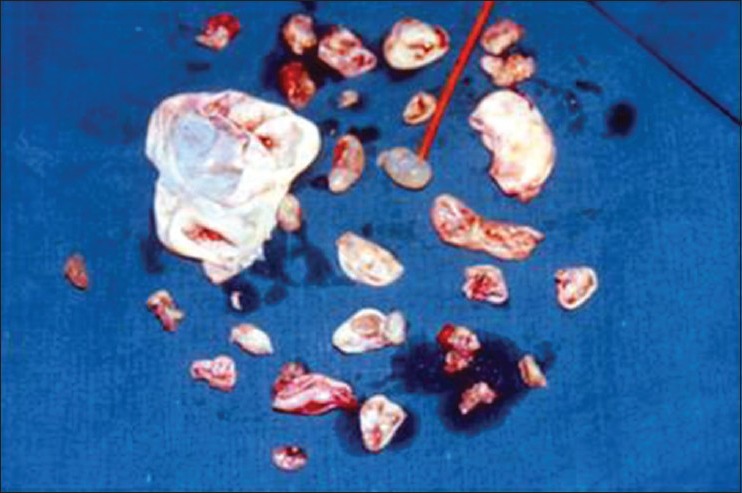
Multiple daughter cysts taken out from primary as well as the site of dissemination
Postoperative course was uneventful and at the latest followup of 1 year, patient was independently ambulatory with no complaints and showing complete neurological recovery.
DISCUSSION
Hydatid involvement of the liver and lungs has been most commonly described (90%).2,3 Bone affection is rare (0.5-4%) of which spine involvement is seen in half of the cases.2 Thoracic spine is most commonly affected (52%), followed by lumbar (37%) sacral (5.5%) and cervical (5.5%) levels.6,7
Involvement of the spine is through portovenous anastomoses or direct extension from pulmonary focus. Daughter cysts infiltrate the cancellous trabeculae of vertebral body, breach the bone and extend to the paravertebral soft tissue or extra dural space causing neurologic compression.2,7,8 Intervertebral disc involvement is unusual.
Braithwaite9 has described five pathologies for vertebral hydatidosis: (1) Primary intramedullary cyst (2) intradural extra medullary cyst (3) extra dural intraspinal cyst (4) vertebral body disease (5) paravertebral cyst. The first three groups are very rare.1,4,7,10 Our case had an initial primary thoracic vertebral involvement (T7-T8) (Braithwaite type 4) with spread to paravertebral tissue and extra dural space (Braithwaite type 3 and 5). The patient had multiple recurrences with neurological involvement which required surgeries to decompress the cord. On each occasion, complete neural recovery was noted and was maintained at long term followup (minimum 2 years to maximum 7 years). At last followup, there was intradural involvement of the disease with a block at the lumbar level (Braithwaite type 2). We believe that the possible mechanism of spread to the intradural space was through the rent in the dura mater that incurred during the second surgery. At surgery there was no continuity of cyst at thoracolumbar region with the parent mass at the thoracic level; either intradural or extra dural, thus indicating free fall of the daughter cyst in the CSF column and settling under gravity at the lumbar level. This is similar to drop metastasis described in literature.6
Magnetic resonance imaging is the gold standard for the diagnosis of hydatid disease.1,2,11 Cyst has thin single wall, no septation and same intensity as of CSF.2,11 In our case, the role of magnetic resonance imaging (MRI) was limited because of stainless steel implant. MRI suggested recurrence of the disease; however the presence of steel implant hindered with the determination of the actual extent and location of the disease. CT myelogram helped in determining the distal extent of the lesion. Proximal extent could be found out only after exposing the lesion. Diagnostic aspiration of the cyst has been described for primary hydatid disease though it should be avoided in view of possible risk of anaphylaxis and further spillage of the daughter cyst.2
Dural injury, though unfortunate, are highly likely due to the peridural fibrosis both due to repeated surgery and the pathology itself. Also the dural rent is unlikely to heal spontaneously due to fibrosis. Furthermore the hydatid cyst wall may become continuous with the dural sac as was seen in our case at the thoracic level making complete excision of the sac impossible. This type of spread has not been reported previously for a parasitic infestation.4 Kaen et al.4 have reported a similar case of primary hydatid disease of lungs and liver with thoracic spine involvement. Patient had multiple recurrences with intradural dissemination. Primary hematic dissemination was regarded as most plausible mechanism, as there was no dural injury. Güneçs et al.12 have described a case of primary pulmonary hydatid disease with multiple intradural dissemination. The mode of spread in their case was postulated to be through local transforaminal invasion. In our case, dural tear at the time of previous surgery can be suggested as the most plausible mechanism of spread.
Overall recurrence in spinal hydatid disease has been reported to be as high as 30-100%.1 Incomplete primary removal of cyst due to extensive bony involvement, spillage during excision, improper chemotherapy and undetected local spread are few of the factors toward high recurrence rate. Adhesions between the cyst wall and the roots in the dural sac further complicate the situation for the fear of root injury if aggressive debridement is contemplated. In most of the cases reported previously there was symptomatic recurrence every 6 months-1 year. Our case had symptom free interval of average 2 years between each surgery. The last symptom free interval was 7 years before the current intradural spread. Possibly, extensive radical resection at each surgery helped us with long remission periods. However this time, intradural involvement prohibited radical removal of the cyst for the fear of neurological injury.4 We agree that the patient may have further intradural recurrences; therefore regular imaging during asymptomatic period may be justified.
PMMA was used for reconstruction of defect at the time of fourth surgery. PMMA has been reported to have anthelmintic effect.3 Medical management of the disease has been a matter of concern and debate. Prabhakar et al.13 had reported frequent recurrences even after continuation of albendazole therapy. Albendazole is definitely preferred over mebendazole considering its better oral absorption and higher intracystic penetration.1 Combination of albendazole with praziquantel has better scolicidal effect.10
This is one of the longest followup of hydatid disease of spine that has ever been reported. This case highlights upon mode of progression, diagnosis, recurrence and modalities of management of hydatid disease. Hydatid disease of spine should be kept in mind in the differential diagnosis of destructive or infectious lesions of spine. Aggressive radical resection whenever possible and chemotherapy is the key to good results. Recurrence is known to occur even after successful evacuation of lesion and prolonged medical management. Disease can have long remission periods. Therefore, high index of suspicion is the key to early diagnosis and long followup with critical clinical evaluation is necessary. Possibility of intradural dissemination through dural injury is highly likely. Hence, it should always be repaired whenever possible.
Footnotes
Source of Support: Nil
Conflict of Interest: None
REFERENCES
- 1.Schnepper GD, Johnson WD. Recurrent spinal hydatidosis in North America. Case report and review of the literature. Neurosurg Focus. 2004;17:E8. doi: 10.3171/foc.2004.17.6.8. [DOI] [PubMed] [Google Scholar]
- 2.Moharamzad Y, Kharazi HH, Shobeiri E, Farzanegan G, Hashemi F, Namavari A. Disseminated intraspinal hydatid disease. J Neurosurg Spine. 2008;8:490–3. doi: 10.3171/SPI/2008/8/5/490. [DOI] [PubMed] [Google Scholar]
- 3.Ozdemir HM, Ogün TC, Tasbas B. A lasting solution is hard to achieve in primary hydatid disease of the spine: Long term results and an overview. Spine (Phila Pa 1976) 2004;29:932–7. doi: 10.1097/00007632-200404150-00022. [DOI] [PubMed] [Google Scholar]
- 4.Kaen A, Lagares A, Perez-Nuñez A, Rivas JJ, Ramos A, Lobato RD. Intradural extra medullary spinal hydatidosis: Case report. Neurocirugia (Astur) 2009;20:282–7. doi: 10.1016/s1130-1473(09)70169-1. [DOI] [PubMed] [Google Scholar]
- 5.Bhojraj SY, Shetty NR. Primary hydatid disease of the spine: An unusual cause of progressive paraplegia. Case report and review of the literature. J Neurosurg. 1999;91:216–8. doi: 10.3171/spi.1999.91.2.0216. [DOI] [PubMed] [Google Scholar]
- 6.Rao AJ, Gultekin SH, Neuwelt EA, Cintrón-Colón HR, Ragel BT. Late occurrence of drop metastasis to the spine in a case of esthesioneuroblastoma. J Neurosurg Spine. 2011;15:571–5. doi: 10.3171/2011.6.SPINE11157. [DOI] [PubMed] [Google Scholar]
- 7.Akhan O, Dinçer A, Saatçi I, Gülekon N, Besim A. Spinal intradural hydatid cyst in a child. Br J Radiol. 1991;64:465–6. doi: 10.1259/0007-1285-64-761-465. [DOI] [PubMed] [Google Scholar]
- 8.Kalinova K, Proichev V, Stefanova P, Tokmakova K, Poriazova E. Hydatid bone disease: A case report and review of the literature. J Orthop Surg (Hong Kong) 2005;13:323–5. doi: 10.1177/230949900501300321. [DOI] [PubMed] [Google Scholar]
- 9.Braithwaite PA, Lees RF. Vertebral hydatid disease: Radiological assessment. Radiology. 1981;140:763–6. doi: 10.1148/radiology.140.3.7280247. [DOI] [PubMed] [Google Scholar]
- 10.Lam KS, Faraj A, Mulholland RC, Finch RG. Medical decompression of vertebral hydatidosis. Spine (Phila Pa 1976) 1997;22:2050–5. doi: 10.1097/00007632-199709010-00023. [DOI] [PubMed] [Google Scholar]
- 11.Kahilogullari G, Tuna H, Aydin Z, Colpan E, Egemen N. Primary intradural extra medullary hydatid cyst. Am J Med Sci. 2005;329:202–4. doi: 10.1097/00000441-200504000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Güneçs M, Akdemir H, Tuğcu B, Günaldi O, Gümüçs E, Akpinar A. Multiple intradural spinal hydatid disease: A case report and review of literature. Spine (Phila Pa 1976) 2009;34:E346–50. doi: 10.1097/BRS.0b013e3181a01b0f. [DOI] [PubMed] [Google Scholar]
- 13.Prabhakar MM, Acharya AJ, Modi DR, Jadav B. Spinal hydatid disease: A case series. J Spinal Cord Med. 2005;28:426–31. doi: 10.1080/10790268.2005.11753843. [DOI] [PMC free article] [PubMed] [Google Scholar]


