Abstract
Objective:
Experimental pain models in human healthy volunteers are advantageous for early evaluation of analgesics. All efforts to develop nonsteroidal anti-inflammatory drugs (NSAIDs) which are devoid of gastrointestinal and cardiovascular system effects are still far from achieving a breakthrough. Hence we evaluated the analgesic activity of an ayurvedic drug, Boswellia serrata by using validated human pain models which has shown its analgesic activity both in-vitro and preclinical studies to evaluate the analgesic activity of single oral dose (125 mg, 2 capsules) of Boswellia serrata compared to placebo using mechanical pain model in healthy human subjects.
Materials and Methods:
After taking written informed consent, twelve healthy subjects were randomized (1:1) to receive single oral dose of Boswellia serrata (Shallaki®) 125 mg, 2 capsules or identical placebo in a crossover design. Mechanical pain was assessed using Ugo basile analgesymeter (by Randall Selitto test) at baseline and at 1 hr, 2 hrs and 3 hrs after test drug administration. Pain Threshold force and time and Pain Tolerance force and time were evaluated. Statistical analysis was done by paired t-test.
Results:
Twelve healthy volunteers have completed the study. Mean percentage change from baseline in Pain Threshold force and time with Boswellia serrata when compared to placebo had significantly increased [Force: 9.7 ± 11.0 vs 2.9 ± 3.4 (P = 0.05) and time: 9.7 ± 10.7 vs 2.8 ± 3.4 (P = 0.04)] at third hr. Mean Percentage change from baseline in Pain Tolerance force and time with Boswellia serrata when compared to placebo had significantly (P ≤ 0.01) increased at 1 hr, 2 hrs and 3 hrs.
Conclusion:
In the present study, Boswellia serrata significantly increased the Pain Threshold and Pain Tolerance force and time compared to placebo. Both study medications were well tolerated. Further multiple dose studies may be needed to establish the analgesic efficacy of the drug.
KEY WORDS: Boswellia serrata, human pain models, randall selitto test, ugo basile analgesymeter
Introduction
Pain is most prevalent health care problem. Early treatment is most important for the relief of pain in patients. Currently, non-opioid analgesics such as acetaminophen and other Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) including cyclo-oxygenase II (COX-2) inhibitors are available as medication regimens for chronic pain conditions like osteoarthritis. Although NSAIDs can effectively reduce pain and inflammation, prolonged use is reported to be associated with gastrointestinal bleeding, rise in blood pressure, heart failure and renal impairment. Due to high incidence of adverse events seen with non-selective and selective COX2 NSAID therapy, effective and safer alternative treatments for chronic pain conditions are urgently needed.[1] All efforts to develop NSAIDs that spare these adverse events are still far from achieving a breakthrough.
Experimental pain models in healthy volunteers are advantageous for evaluation of analgesic action, as this is often difficult to assess in the clinic because of confounding factors which may be present in a diseased condition. These pain models minimize the gap between knowledge gained in animal and human clinical studies. These tests measure subjective pain after inducing pain.[2]
In recent years, the gum resin extracted from the bark of an ancient herb, Boswellia serrata has gained considerable attention as a potent anti-inflammatory, anti-arthritic and analgesic agent.[3] Its main pharmacologically active ingredients are α and β boswellic acid and other pentacyclic triterpenic acids which have been shown to inhibit pro-inflammatory processes by their effects on 5-lipooxygenase, cyclo-oxygenase and complement system. Out of all boswellic acids, acetyl-11-keto-β-boswellic acid is the most potent inhibitor of 5-lipoxygenase, an enzyme responsible for inflammation.[4] The pharmacokinetic variables of Boswellia serrata are plasma half life is 5.97 ± 0.95 hrs and the plasma clearance is 296.10 ± 24.09 ml/min with maximum plasma concentration being 2.72 × 10-3 ± 0.18 μmoles/ml.[5]
A number of clinical studies support the anti-inflammatory and anti-arthritic properties of Boswellia serrata extract (BSE) and have showed a very good safety profile except mild adverse effects such as nausea, acid reflux and gastrointestinal upset.[6,7] There are no serious, long term or irreversible adverse effects and no evidence of serious interactions.[8] In one study, they stated that BSE is a promising alternative to NSAIDs, which warrants investigation in further pharmacological studies and clinical trials.[9] Being a herbal drug, it was not evaluated in a traditional path of modern drug development (by using validated human pain models). Osteoarthritis is a mechanical joint disease in which weight bearing causes pain, hence we used mechanical pain model to see its activity in acute pain. Due to paucity of data available on the evaluation of analgesic activity on human pain models, we studied in healthy subjects to distinguish whether Boswellia serrata is efficacious in reducing acute pain using one of the validated mechanical pain models[10] (Ugo basile analgesymeter) to provide the scientific validity for its analgesic activity as its anti-inflammatory activity is already proven on long term use.
The Primary endpoint of the study is mean percentage change from baseline in pain threshold force and time with Boswellia serrata when compared to placebo and mean percentage change from baseline in pain tolerance force and time with Boswellia serrata when compared to placebo. The Secondary endpoint is mean pain threshold force and time with Boswellia serrata when compared to placebo at baseline and post treatment and mean pain tolerance force and time with Boswellia serrata when compared to placebo at baseline and post treatment. Also, the safety and tolerability of Boswellia serrata in healthy participants was assessed.
Materials and Methods
The study was conducted according to a protocol approved by the Institutional ethics committee and in accordance with the Good Clinical Practice guidelines and the principles enunciated in the Declaration of Helsinki. All the subjects provided written informed consent before entering the study.
This study was conducted as a randomized, double blind, placebo controlled, crossover study in 12 healthy adult, male, human subjects to evaluate the analgesic activity of Boswellia serrata.
Study Subjects
The study included male human subjects aged 18-45yrs with a Body mass index (BMI) within normal range and healthy as evidenced by medical histories, complete physical examination, and routine laboratory tests performed [Complete blood picture, Electrocardiogram (ECG), Random Blood Sugar, blood urea, serum creatinine, Urine analysis, Liver function tests, HIV and HBsAg] within 21 days prior to commencement of the study. Participants with finger deformities and prior wounds or fractures on the tested extremity, pre-existing dyspepsia, gastritis, peptic ulcer, any acute or chronic drug or alcohol abuse, diabetes mellitus, inability to perform the test as per protocol procedure and participants using any analgesics, anti-inflammatory or any other drugs known to alter the pain sensation (topically and systemically) in the last 2 weeks were excluded. During the study period, subjects were not permitted to take any prescription and Over The Counter (OTC) medications. All the volunteers were housed in clinical facility and monitored for 48 hrs before dosing to ensure that the volunteers had abstained from any xanthine-containing food or beverages or alcoholic products and smoking for atleast 48 hrs prior to dosing and during each period.
Study Methodology
The healthy participants were trained on two separate occasions for the Randall Selitto test procedure using Ugo basile analgesymeter prior to participation in the study. The eligible subjects were randomized by computer generated random sequences in 1: 1 ratio to receive either two capsules (125 mg each) of Boswellia serrata (Shallaki®) or two identical placebo capsules as a single dose and efficacy of drugs was evaluated. Although the 99% of the drug is eliminated in 2 days, to be on safer zone and to avoid period effect of the analgesic model which induces pain (baseline value for pain would be reached with long washout period). After 2 weeks of washout period, they were crossed over in period 2 to evaluate the drug efficacy by the same procedures as in period 1. Randomization sequence was generated by a third person unrelated to study.
Mechanical Pain Model (Randall Selitto test using Ugo Basile Analgesimeter)
In the present study, we evaluated the analgesic activity of Boswellia serrata using a validated mechanical pain model by Randall Selitto test using Ugo Basile analgesymeter (Model No 03977, Ugo Basile, Milan, Italy) [Figure 1]. The pain fibres involved in the transmission of punctate pressure (noxious stimulus) through the TRPV1 receptors are myelinated A-delta (Aδ) and the unmyelinated (C fibres).[11,12] On the day of study, subjects were asked to report to the study site at 7:00 AM after a good overnight sleep, in fasting state. Then they were examined clinically and were asked to sit in a room at an ambient temperature of 22°C ± 2 °C for half an hour before the initiation of test procedure. Then, they were asked to perform the procedure as follows:
Figure 1.

Ugo Basile analgesymeter and Induction of Mechanical pain (Randall Selitto test)
Participants were asked to sit comfortably on a chair with eyes blindfolded. Participants were instructed to place the nail bed of index finger of non-dominant hand on a small plinth of Ugobasile analgesymeter, under a cone shaped pusher with a rounded tip [Figure 1]. Then, pedal switch was pressed by the investigator that exerts the force on the nail bed. The instrument is geometrically designed to increase the force on the finger at a constant rate of 80 grams per second (pressure of 54.42 g/cm.sec2). The participant was asked to lift his index finger of other hand when he first experienced pain and when he was unable to tolerate the pain. Then, pressure on the pedal switch was removed and the weight bearing arm of the instrument was lifted to release the pressure on the nail bed. Readings on the scale were recorded. Weight was lifted and moved left to place it in its original position.[13] During the test procedure, the readings on the scale of the instrument were recorded when the subject first experiences pain (noted as pain threshold) and when he is unable to tolerate the pain (described as pain tolerance). Three readings were taken at 5 minute intervals. Then average of the three readings was taken and the force (in grams) exerted on the nail bed and the threshold time and tolerance time were calculated.
Each unit movement on the scale of the instrument corresponds to 50 gram force exerted on the index finger. For instance, if the reading on the scale is 11, then the force exerted will be 50 times the reading that is, 550 g. The subjects were given light breakfast after baseline reading and within 15 min of breakfast, study drug was administered as a single dose. At 1hr, 2 hrs and 3 hrs of drug administration, pain threshold force and time and pain tolerance force and time were recorded using the same procedure.
After two weeks of wash out period, the second drug (Run 2) was administered according to the randomization sequence and the same procedures were repeated. All safety lab parameters were repeated after administration of both drugs.
Statistical Analysis
The data on pain threshold and tolerance time, were recorded in seconds and pain threshold and tolerance force were recorded in grams which are represented as mean ± SD. Paired student t-test was used to assess analgesic efficacy of Boswellia serrata with P < 0.05 considered to be statistically significant. All statistical analyses were performed using the Graph pad PRISM software 4 (Graph pad software Inc., USA).
Sample Size Calculation
A sample size of 12 volunteers was estimated to detect 20% difference in percentage increase in pain threshold from baseline with Boswellia serrata when compared to placebo at a standard deviation of 15 considering 90% power at 5% level of significance with a drop-out rate of 10%.
Results
In this study, 13 subjects were screened for participation into the study and there was one screen failure. Twelve eligible subjects have completed the study whose mean age was 36.6 ± 6.6yrs and average BMI was 21.55 ± 2.37 kg/m2. Analysis of variance (ANOVA) for each variable at different time points for both test drug and placebo was done, there is no statistical difference. But pair-wise comparisons (between drug and placebo at each time point showed statistical significance at certain time points for each specific variable (pain threshold force, pain threshold time, pain tolerance force and pain tolerance time).
Evaluation of Pain Threshold
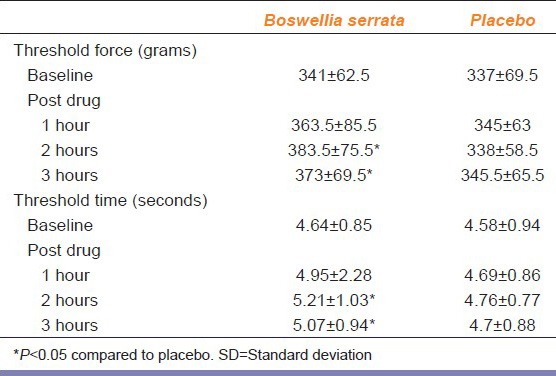
Mean pain threshold force and time with Boswellia serrata were significantly increased at 2 hrs and 3 hrs of drug administration compared to placebo with a P < 0.05. Mean pain threshold force and time with Boswellia serrata was also statistically significant at 3 hrs compared to baseline with a P < 0.05 [Table 1].
Table 1.
Pain threshold force (grams) and time (seconds) with Boswellia serrata and placebo at baseline and post drug values in mean±SD (n=12)
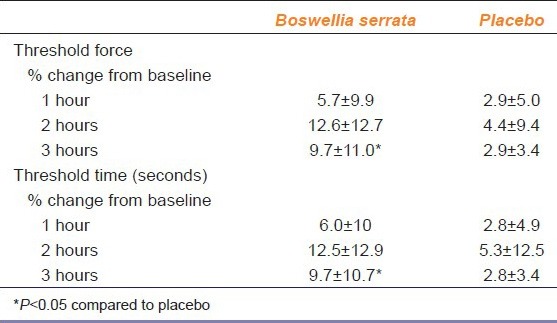
The Mean percentage change in pain threshold Force and Time with Boswellia serrata were increased significantly after 3 hrs of drug administration (pain threshold force, P = 0.05; pain threshold time, P ≤ 0.05) when compared to placebo [Table 2]
Table 2.
Percentage change in pain threshold force and time with Boswellia serrata and placebo after drug administration (n=12)
Evaluation of Pain Tolerance
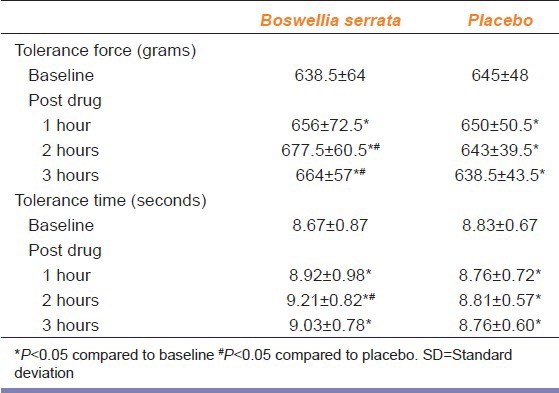
There was a significant increase in mean pain tolerance force and time with Boswellia serrata after 1 hr, 2 hrs and 3 hrs of drug administration when compared to baseline (P < 0.05). Mean pain tolerance force is significantly increased at 2 hrs and 3 hrs after drug administration (P ≤ 0.01, <0.05 respectively) where as mean pain tolerance time is significantly increased at 2 hrs when compared to placebo (P < 0.05) [Table 3].
Table 3.
Pain tolerance force (grams) and time (seconds) with Boswellia serrata and placebo at baseline and post drug values in mean±SD (n=12)
Mean percentage change from baseline in pain tolerance force and pain tolerance time has significantly increased with Boswellia serrata compared to placebo at 1hr, 2 hrs and 3 hrs of drug administration (P ≤ 0.01) [Table 4].
Table 4.
Percentage change in pain tolerance force (grams) and time (seconds) with Boswellia serrata and placebo after drug administration (n=12)

Compliance was assured as the drugs were administered under supervision. Five subjects received study drug and seven subjects received placebo in the first period. There was no significant difference in pain threshold and pain tolerance between subjects treated with active comparator first versus the subjects who received active drug after the placebo. Both the drugs were well tolerated and none of the subjects reported any adverse events. All safety laboratory investigations (complete blood picture, blood urea, serum creatinine, liver function tests, ECG, RBS and Urine analysis) were done before and after administration of both the medications which were found to be normal.
Discussion
In the present study, we evaluated the analgesic activity of Boswellia serrata by Randall selitto test using Ugo basile analgesymeter for inducing mechanical pressure on the nail bed of non-dominant hand index finger, as it is associated with good precision and smaller interindividual and intraindividual variation.[13,14] This test is having good correlation with the results of the cold stimulation test,[15] which is considered to be sensitive method for evaluation of drugs having central mechanism of action. Randall Selitto test is one of the most predictive of the models of acute pain the other being, the formalin test.[16]
In the present study, the mean pain threshold force and time significantly increased when compared to placebo at 2 hrs and 3 hrs of drug administration and it significantly increased when compared to baseline at 3 hrs of drug administration. The mean percentage change from baseline for pain threshold force and time was significantly increased after 3 hrs of drug administration when compared to placebo. The mean pain tolerance force and time were significantly increased from baseline at 1 hr, 2 hrs and 3 hrs of drug administration, they were significant when compared to placebo at 2 hrs and 3 hrs (for pain tolerance force) and at 2 hrs (for pain tolerance time). The mean percentage change from baseline in pain tolerance force and time were significant at 1 hr, 2 hrs and 3 hrs of drug administration when compared to placebo.
Though to the best of our knowledge, there are very few studies which evaluated the analgesic activity by using pain models in healthy volunteers,[17] some clinical studies were done in patients. In one of the studies, Sengupta et al., demonstrated that 250 mg/day 5-Loxin® (Boswellia serrata) had a significant effect in lowering VAS score by 12.18% (P = 0.02) and Western Ontario and McMaster Universities Arthritis Index (WOMAC) function score by 14.38% (P < 0.01) in OA patients as early as 7 days after the start of treatment. These findings therefore indicate that 5-Loxin® confers prompt and significant pain relief and improvement in physical ability in OA patients.[1] In another 30-day double blind, placebo controlled clinical study, the Aflapin (Boswellia serrata) supplemented group showed statistically significant improvements in all pain scores including Visual Analog Scale (VAS), Lequesne's Algofunctional Index (LFI), WOMAC pain, WOMAC stiffness and WOMAC function scores. Aflapin provided significant reductions in pain scores of VAS and LFI in as early as 5 days.[18] Kimmatkar et al. conducted a randomized double blind placebo controlled crossover study to assess the efficacy, safety and tolerability of BSE in 30 patients of osteoarthritis of knee, 15 each receiving active drug or placebo for eight weeks. All patients receiving drug treatment reported decrease in knee pain, increased knee flexion and increased walking distance.[7]
A randomized, placebo controlled study with a polyherbal combination containing BSE in OA of the knee for 32 weeks showed significantly better efficacy than placebo without any significant adverse effects.[19] Sontakke et al. showed significantly lower (P < 0.001) WOMAC scores with BSE than with valdecoxib at the end of 7 months for all the three parameters.[20] Gupta et al. in a two arm comparative study of 2 months duration, evaluated the efficacy of 500 mg capsule of Shallaki, 6 g per day (in three divided doses) with lukewarm water vs capsule Shallaki as above along with local application of Shallaki ointment on the affected joints observed symptomatic improvement in both the groups at various levels with promising results in the patients of first group.[21] As one of the ingredient in polyherbal formulation, Boswellia serrata significantly reduced knee pain and improved knee function which were equivalent to glucosamine and celecoxib in a 6-month controlled study of knee OA.[22] In another ayurvedic study, a formulation (containing Curcuma longa and Boswellia serrata extracts) at 500 mg administered twice a day demonstrated to be more effective compared to celecoxib 100 mg twice a day for symptom scoring and clinical examination with good safety in OA patients.[23] In the present study, Boswellia serrata also has demonstrated a significant analgesic activity when compared to placebo even when administered as a single dose with good safety and tolerability.
Conclusion
In this study, Boswellia serrata significantly increased pain threshold force and time and pain tolerance force and time when compared to baseline and placebo with good safety and tolerability. Further multiple dose studies are needed to confirm the efficacy of Boswellia serrata.
Acknowledgements
Dr. J. Shravanthi, Ayurvedic physician for her expert advice.
Footnotes
Source of Support: Nil
Conflict of Interest: No
References
- 1.Sengupta K, Alluri KV, Satish AR, Mishra S, Golakoti T, Sarma KV, et al. A double blind, randomized, placebo controlled study of the efficacy and safety of 5-Loxin for treatment of osteoarthritis of the knee. Arthritis Res Ther. 2008;10:R85. doi: 10.1186/ar2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olesen AE, Andresen T, Christrup LL, Upton RN. Translational pain research: Evaluating analgesic effect in experimental visceral pain models. World J Gastroenterol. 2009;15:177–81. doi: 10.3748/wjg.15.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Basch E, Boon H, Davies-Heerema T, Foppo I, Hashmi S, Hasskarl J, et al. Boswellia: An evidence-based systematic review by the Natural Standard Research Collaboration. J Herb Pharmacother. 2004;4:63–83. [PubMed] [Google Scholar]
- 4.Ernst E. Frankincense: Systematic review. BMJ. 2008;337:a2813. doi: 10.1136/bmj.a2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharma S, Thawani V, Hingorani L, Shrivastava M, Bhate VR, Khiyani R. Pharmacokinetic study of 11-Keto beta-Boswellic acid. Phytomedicine. 2004;11:255–60. doi: 10.1078/0944-7113-00290. [DOI] [PubMed] [Google Scholar]
- 6.Anthoni C, Laukoetter MG, Rijcken E, Vowinkel T, Mennigen R, Müller S, et al. Mechanisms underlying the anti-inflammatory actions of boswellic acid derivatives in experimental colitis. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1131–7. doi: 10.1152/ajpgi.00562.2005. [DOI] [PubMed] [Google Scholar]
- 7.Kimmatkar N, Thawani V, Hingorani L, Khiyani R. Efficacy and tolerability of Boswellia serrata extract in treatment of osteoarthritis of knee-a randomized double blind placebo controlled trial. Phytomedicine. 2003;10:3–7. doi: 10.1078/094471103321648593. [DOI] [PubMed] [Google Scholar]
- 8.Ernst E, Pittler MH, Wider B, Boddy K. Complementary therapies for back pain: Is the evidence getting stronger? Clin Rheumatol. 2007;26:736–8. doi: 10.1007/s10067-006-0395-y. [DOI] [PubMed] [Google Scholar]
- 9.Abdel-Tawab M, Werz O, Schubert-Zsilavecz M. Boswellia serrata: An overall assessment of in vitro, preclinical, pharmacokinetic and clinical data. Clin Pharmacokinet. 2011;50:349–69. doi: 10.2165/11586800-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 10.Randall LO, Selitto JJ. A method for measurement of analgesic activity on inflamed tissue. Arch Int Pharmacodyn Ther. 1957;111:409–19. [PubMed] [Google Scholar]
- 11.Beissner F, Brandau A, Henke C, Felden L, Baumgartner U, Treede RD, et al. Quick discrimination of A (delta) and C fiber mediated pain based on three verbal descriptors. PLoS One. 2010;5:e12944. doi: 10.1371/journal.pone.0012944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fernandes ES, Fernandes MA, Keeble JE. The functions of TRPA1 and TRPV1: Moving away from sensory nerves. Br J Pharmacol. 2012;166:510–21. doi: 10.1111/j.1476-5381.2012.01851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luginbuhl M, Schnider TW, Petersen-Felix S, Arendt-Nielsen L, Zbinden AM. Comparison of five experimental pain tests to measure analgesic effects of alfentanil. Anesthesiology. 2001;95:22–9. doi: 10.1097/00000542-200107000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Merskey H, Spear FG. The reliability of the pressure algometer. Br J Soc Clin Psychol. 2011;3:130–6. [Google Scholar]
- 15.Krishnan S, Salter A, Sullivan T, Gentgall M, White J, Rolan P. Comparison of pain models to detect opioid-induced hyperalgesia. J Pain Res. 2012;5:99–106. doi: 10.2147/JPR.S27738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Le Bars D, Gozariu M, Cadden SW. Animal models of nociception. Pharmacol Rev. 2001;53:597–652. [PubMed] [Google Scholar]
- 17.Usharani P, Nalini P, Manjunath NK, Reddy SK. Evaluation of the analgesic activity of standardized aqueous extract of Withania somnifera in healthy human volunteers using Hot Air Pain Model. Res J Life Sci. 2013;1:1–6. [Google Scholar]
- 18.Vishal AA, Mishra A, Raychaudhuri SP. A double blind, randomized, placebo controlled clinical study evaluates the early efficacy of aflapin in subjects with osteoarthritis of knee. Int J Med Sci. 2011;8:615–22. doi: 10.7150/ijms.8.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chopra A, Lavin P, Patwardhan B, Chitre D. A 32-week randomized, placebo-controlled clinical evaluation of RA-11, an ayurvedic drug, on osteoarthritis of the knees. J Clin Rheumatol. 2004;10:236–45. doi: 10.1097/01.rhu.0000138087.47382.6d. [DOI] [PubMed] [Google Scholar]
- 20.Sontakke S, Thawani V, Pimpalkhute S, Kabra P, Babhulkar S, Hingorani L. Open, randomized, controlled clinical trial of Boswellia serrata extract as compared to valdecoxib in osteoarthritis of knee. Indian J Pharmacol. 2007;39:27–9. [Google Scholar]
- 21.Gupta PK, Samarakoon SM, Chandola HM, Ravishankar B. Clinical evaluation of Boswellia serrata (Shallaki) resin in the management of Sandhivata (osteoarthritis) Ayu. 2011;32:478–82. doi: 10.4103/0974-8520.96119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chopra A, Saluja M, Tillu G, Sarmukkaddam S, Venugopalan A, Narsimulu G, et al. Ayurvedic medicine offers a good alternative to glucosamine and celecoxib in the treatment of symptomatic knee osteoarthritis: A randomized, double-blind, controlled equivalence drug trial. Rheumatology (Oxford) 2013;52:1408–17. doi: 10.1093/rheumatology/kes414. [DOI] [PubMed] [Google Scholar]
- 23.Kizhakkedath R. Clinical evaluation of a formulation containing Curcuma longa and Boswellia serrata extracts in the management of knee osteoarthritis. Mol Med Rep. 2013;8:1542–8. doi: 10.3892/mmr.2013.1661. [DOI] [PubMed] [Google Scholar]


