Abstract
Objective:
To assess the cardiovascular safety of two commonly prescribed atypical antipsychotics risperidone (RSP) and olanzapine (OZP) in schizophrenic patients, using electrocardiography (ECG) and Blood Pressure (BP).
Materials and Methods:
This was a 10-week prospective open label, observational study, carried out in a newly diagnosed 64 schizophrenic patients receiving either RSP or OZP. RSP (n = 32) was started with dose of 2 mg/day and increased to 4 mg/day after 2 weeks, whereas OZP (n = 32) was started at a dose of 5 mg/day and was increased to 10 mg/day after 2 weeks. Heart rate (HR), ECG parameters (PR, RR, QRS, QT intervals and QTc and QTd) and BP (systolic and diastolic in supine and standing position) were recorded at baseline (before drug therapy)) and during follow-up visits at 2(I), 6(II) and 10(III) weeks.
Results:
In the RSP group, at II and III follow-ups, a significant increase in the HR (P = 0.018, P = 0.011 respectively) as well as in QTc (P = 0.025, P = 0.015, respectively) was observed when compared to the basal values. In the OZP group, diastolic BP was significantly decreased in standing position at II and III follow-ups (P = 0.045 and P = 0.024, respectively) compared to the basal values. When the two groups were compared with each other, no significant differences were observed in the changes of HR, PR, QRS, QT, RR, QT, QTd and SBP (supine and standing position); and DBP (supine position). However, DBP in standing position showed a significant decrease in the OZP group at II and III follow-up (P = 0.036 and P = 0.016, respectively) compared to the RSP group.
Conclusions:
Patients treated with OZP are at higher risk to develop postural hypotension as compared with RSP; hence RSP could be better tolerated by patients taking antihypertensive drugs as compared with OZP whereas OZP would have a safer cardiac profile.
KEY WORDS: ECG changes, heart rate, olanzapine, QTc, QTd, risperidone
Introduction
Schizophrenia is a major mental illness affecting about 7/1000 in adult population, majority in the age group of 15-35 years. Antipsychotic drugs have a central role in treating this illness and are associated with a variety of adverse effects, out of which cardiac adverse effects being the most concerned. Cardiac adverse effects are mostly reported with typical (first generation) antipsychotics and for the same reason - thioridazine in June 2005 and Sertindole in 1998 were discontinued and withdrawn.
Sudden unexplained deaths in individuals with mental health problems were first described in 1849 and a link with antipsychotic drugs was postulated.[1] Cohort studies[2,3] in schizophrenic patients on antipsychotics treatment has reported a persistent excess of mortality related to cardiovascular diseases. Antipsychotics have been suspected to increase the risk of serious ventricular arrhythmias and sudden death.[4]
As cardiac arrhythmias are difficult to predict, it is important to understand the susceptibility of heart to develop arrhythmias, which may reflect as specific changes in electrocardiographic parameters. The changes in parameters like heart rate, the PR, QRS, and QT intervals, can be used to evaluate cardio-toxicity.[5,6] Out of these, QTc interval is considered to be a marker of the arrhythmogenic potential of a drug, specifically linked to an increased risk of ventricular arrhythmia.[7]
Atypical (second-generation) antipsychotics have replaced traditional/typical (first generation) antipsychotics in clinical practice because of their relatively better safety profile, but cardiac safety of atypical antipsychotics continues to be a concern. Several observational studies suggest that patients treated with atypical antipsychotics have a higher risk than expected rate of electrocardiogram (ECG) changes, like prolongation of the heart rate-corrected QT interval (QTc).[8,9] Most of the atypical antipsychotics prolong the QTc interval in overdose, but some prolong it even at therapeutic doses. Risperidone (RSP) and olanzapine (OZP) are the most commonly prescribed atypical antipsychotics and studies have shown prolongation of QTc in experimental models.[10]
The cardiovascular safety of RSP[11] and OZP[12,13] has been studied in different countries and different age group but their status regarding Indian population is not known.
Hence, we decided to study the cardiovascular safety of RSP and OZP with respect to their effects on heart rate (HR), ECG parameters and blood pressure (BP).
Materials and Methods
The study protocol was approved by the Institutional Ethics Committee and the study was conducted in accordance with the Declaration of Helsinki.
Patient Population
Newly diagnosed 64 patients of schizophrenia [based on Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, (DSM-IV-TR) criteria] attending psychiatry outpatient department of Mahatma Gandhi Institute of Medical Sciences (MGIMS), Sevagram, of age 18–50 years of either gender who were prescribed either risperidone (RSP group, n = 32) olanzapine (OZP group, n = 32) and who gave written informed consent were enrolled. Each drug group involved 16 male and 16 female patients.
Patients with cardiovascular co-morbid illness and patients with history (past/present) of medications that affect the ECG parameters, history of substance abuse within a year were excluded from the study. Patients with abnormal cardiac rate and rhythm, QTc interval more than 450 ms in the ECG performed at baseline,[14] were also excluded.
Study Design
The study was an open label, observational, non-interventional, prospective longitudinal study. During the entire study period of 10 weeks, the patients were assessed 4 times as follows;
Baseline: On the day of consultation at psychiatry OPD when they were prescribed either RSP or OZP
Follow-up I: After 2 weeks
Follow-up II: After 6 weeks
Follow-up III: After 10 weeks
Vital Signs
Physical examination and vital signs (blood pressure and heart rate) of every patient were recorded at each visit. Blood pressure [Diastolic (DBP) and systolic (SBP)] were measured in supine position followed by standing position with 15 minutes rest in between. All tests were performed during routine Outpatient Department during the hours i.e. 10 am to 1 pm.
ECG Recording and Calculations
The ECGs were obtained by a standard 12-lead resting ECG procedure in the supine position and the PR, QRS, RR and QT intervals were evaluated at each visit. The QT interval was assessed in long lead II, due to the higher variability of measurements in precordial leads.
QTc Interval
Corrected QT (QTc) interval was calculated using various corrections like:
Fridericia's correction: QTc = QT/RR0.33
Bazett's correction: QTc = QT/RR0.5 i.e., QTc = QT/√(R-R)
Corrections based on linear regression techniques.
Bazett's correction was frequently used in clinical practice[14] for correction, in our study.
QTd (QT dispersion)
It is the measure of variability of the QT interval duration between different leads of ECG, calculated simply as difference between maximum and minimum values of QT interval irrespective of lead of measurement. It helps to distinguish homogeneous myocardium from myocardium that displays in- homogeneity. Hence, it is proposed as an index of the abnormality of repolarization[15] i.e. spatial dispersion of the ventricular recovery times.[16]
Drug Administration
The initial dose of RSP was 2 mg/day and the dose was increased to 4 mg/day at the end of the 2 weeks and then continued with the same dose. Olanzapine was started at 5 mg/day and increased to 10 mg/day at the end of the 2nd week of the treatment, and then continued with the same dose for rest of study duration.
Data Analysis
Statistical analyses were performed using Graphpad- Quickcalcs. Descriptive statistics were calculated and the measure of variability was given as Mean ± standard deviation. The value of each ECG parameter before drug administration was used as its own control.
Paired and unpaired t-test was used to assess differences in the variables within the group and between the groups, respectively. For all tests, a value of ‘P’ <0.05 was considered statistically significant.
Results
A total of 64 patients (32 in each group) were enrolled in the study and evaluated for ECG parameters and BP changes. There were no significant differences between the groups in terms of age, gender and basal values of heart rate, BP and ECG parameters.
Heart rate was significantly increased in the RSP group at II and III follow up (P = 0.018, P = 0.011 respectively) [Table 1], whereas it was not significantly affected in the OZP group. Since heart rate holds an inverse relationship with R-R interval, the R-R interval was found to be significantly decreased at II follow up (P = 0.0057) and III follow up (P = 0.0037) in RSP group [Table 1], whereas it was not significantly affected in the OZP group [Table 2].
Table 1.
Comparison of effects of Risperidone on ECG parameters of patients measured during follow up with those at baseline (n=32)
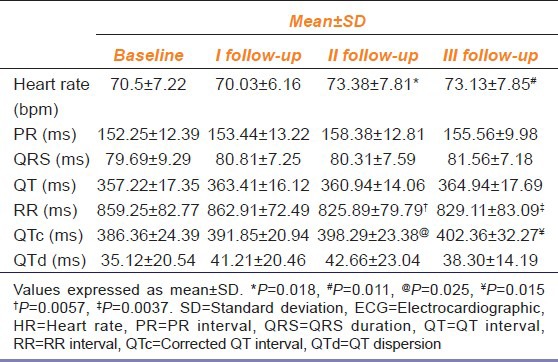
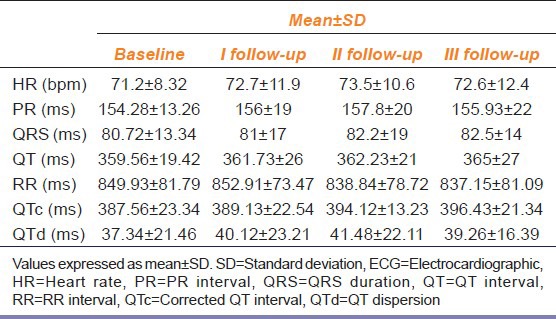
Table 2.
Comparison of effects of olanzapine on ECG parameters of patients measured during follow up visits with those at baseline (n=32)
The QTc interval was significantly prolonged at II and III follow-up (P = 0.025, P = 0.015 respectively) in the RSP group [Table 1].
HR and QTc interval were not significantly affected in OZP group while other ECG parameters did not demonstrate a significant difference in the OZP group, throughout the study [Table 2].
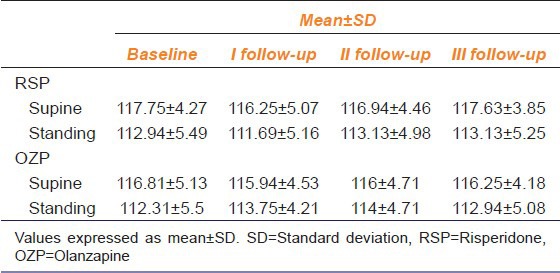
SBP (supine and standing position) did not significantly differ in the RSP as well as in OZP group when compared with the basal values [Table 3].
Table 3.
Effects of risperidone and olanzapine on systolic blood pressure of patients measured in supine and standing position during follow up visits compared with baseline values (n=32)
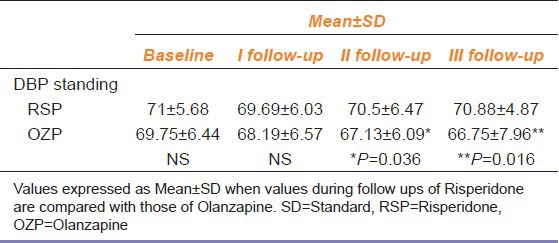
DBP in standing position in the OZP group was significantly decreased at IInd follow up (P = 0.045) and IIIrd follow-up (P = 0.024) compared with the basal values [Table 4] whereas DBP in supine position was not significantly affected.
Table 4.
Effect of risperidone and olanzapine on diastolic blood pressure measured in supine and standing position during follow up visits compared with baseline values (n=32)
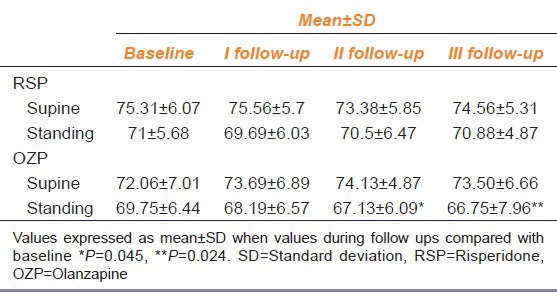
While in the RSP group, DBP both in supine and in standing position did not show a significant change compared with the basal values for the same.
When the two groups were compared, no significant differences were found in terms of changes in heart rate, PR, QRS, QT, RR, QT, QTd and SBP (supine and standing position); and DBP (supine position).
However, DBP in standing position showed a significant decrease in the olanzapine group at II and III follow-up (P = 0.036 and P = 0.016, respectively) compared with the RSP group [Table 5].
Table 5.
Comparison of effects of risperidone on diastolic blood pressure of patients in standing position during the follow-up visits with olanzapine (n=32)
Discussion
The RSP and OZP are the most commonly prescribed atypical antipsychotic drugs because of their relatively better safe profile like less extra pyramidal symptoms.
The main finding of this study is that RSP has a more impact on heart rate and ECG parameters, whereas OZP significantly affects DBP in standing position.
The accelerated heart rate in RSP group at II and III follow-ups in this study may have been related to the dose increase in RSP after I follow-up. Although the accelerated HR is within the normal range of heart rate (60-100), much higher values of HR might be observed at higher doses of RSP, whereas significant changes in heart rate with OZP was not observed. Both these agents act through blocking dopamine, serotonin, muscarinic, α, adrenergic, and histamine receptors with different affinity for these receptors [Table 6].[17]
Table 6.
Difference in binding affinities of risperidone and olanzapine to different receptors[17]
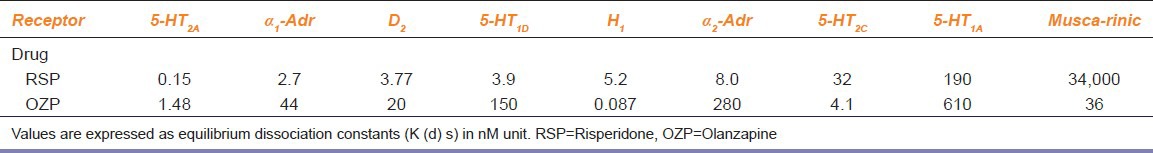
Accelerated heart rate in RSP group can be attributed to the sympathomimetic activity of RSP. Because the affinity of RSP for muscarinic receptor (blocking) is very high as compared with OZP.[17]
None of the patients in the study population had tachycardia (>110 bpm).
QTc was prolonged significantly in RSP group while change in QTc was not significant in OZP group.
Research suggests that RSP acts similar to class III antiarrhythmics, causing concentration-dependent blockage of the rapid component of the delayed rectifier K + current (Ikr) and possibly explaining the QTc prolongation in patients.[18]
The QTc interval is considered a marker of the arrhythmogenic potential of a drug, specifically linked to an increased risk of ventricular tachycardia.[7] For men, QTc values over 0.45 sec and for women, values over 0.47 sec are considered to be prolonged.[11] A QTc interval value over 0.50 sec is associated with an increased risk for torsades de pointes that may be fatal in some cases[8] and a QTc interval greater than 0.50 sec has been suggested as a clinically significant cut-off.[19] Hence, the prolongation of QTc in this study might not be considered clinically significant based on the ranges observed i.e. (0.38-0.40 sec).
Olanzapine has not shown any significant QTc prolongation in this study, which is in line with a previous study.[13]
In the present study, none of the treatments affected SBP, but DBP (standing) was significantly decreased at II and III follow-up in OZP group. This may have been related to the dose increase in OZP after I follow-up. This might be attributed to high α1and2 adrenergic and 5 HT1A and 2A blocking action[16] leading to inhibition of vasoconstriction normally [Table 6] initiated by the baroreceptor reflex upon postural change and the subsequent drop in pressure.[20] Lower values of DBP (standing) could have been observed at higher doses of OZP.
Studies performed in different countries and in different age groups of patients treated with RSP[11] or OZP[12,13] have not clinically revealed significant changes in cardiovascular parameters and blood pressure, which is similar to our study.
Limitations
The small sample size is a major limitation of the study. One cannot exclude the influence of other factors, like subjective stress caused by the experimental setting, which could have influenced the measurements. The doses of drugs administered in this study may also be considered low.
In the present study, the measured QT intervals were corrected by Bazett's formula; however, guidelines[14] state that “Bazett's correction overcorrects at elevated heart rates and under corrects at heart rates below 60 bpm and hence is not an ideal correction formula”. However, in our study, we could not find heart rates in elevated or lower range and hence, Bazett's correction would be considered ideal for our study readings.
Lastly, the values altered in both the group were within normal reference ranges; hence were considered clinically non-significant.
Impact of the Study
While considering those patients with baseline cardiovascular co-morbidities, these drugs could have affected those parameters more significantly and could be fatal, this clinicians showed take care while prescribing these drugs to patients with co-morbid cardiovascular conditions.
Recommendations
Various guidelines are available that suggestg monitoring the health of patients who are prescribed antipsychotics.[21]
Any past history of syncope, sudden unexpected cardiac death or arrhythmias in family; medication history of drugs that increase QTc or any factor, which cause torsades de pointes[22,23] should be documented.
A baseline ECG is recommended initiating RSP or OZP.[24,25] The follow-up ECG should be obtained after a steady state is established to ascertain that the ECG intervals remain in the acceptable range. Additional ECG recording is indicated if there is a change in medication or dose of drug.
Conclusion
The risk of tachycardia is more with RSP; however, OZP has potential risk for postural hypotension. RSP and OZP are used widely and cardiovascular adverse effects are unusual but potentially life-threatening consequences of these drugs. It is important that patients and healthcare professionals understand these potentially lethal adverse effects.
Footnotes
Source of Support: Nil
Conflict of Interest: No
References
- 1.Newman SC, Bland RC. Mortality in a cohort of patients with schizophrenia: A record linkage study. Can J Psychiatry. 1991;36:239–45. doi: 10.1177/070674379103600401. [DOI] [PubMed] [Google Scholar]
- 2.Jackson T, Ditmanson L, Phibbs B. Torsades de pointes and low dose oral Haloperidol. Arch Inern Med. 1997;157:2013–5. [PubMed] [Google Scholar]
- 3.Ravin DS, Levenson JW. Fatal cardiac events following initiation of risperidone therapy. Ann Pharmacother. 1997;31:867–70. doi: 10.1177/106002809703100712. [DOI] [PubMed] [Google Scholar]
- 4.Mortensen PB, Juel K. Mortality and causes of death in schizophrenic patients in Denmark. Acta Psychiatr Scand. 1990;81:372–7. doi: 10.1111/j.1600-0447.1990.tb05466.x. [DOI] [PubMed] [Google Scholar]
- 5.Morita H, Wu J, Zipes DP. The QT syndromes: Long and short. Lancet. 2008;372:750–63. doi: 10.1016/S0140-6736(08)61307-0. [DOI] [PubMed] [Google Scholar]
- 6.Mozaffarian D, Prineas RJ, Stein PK, Siscovick DS. Dietary fish and n–3 fatty acid intake and cardiac electrocardiographic parameters in humans. J Am Coll Cardiol. 2006;48:478–84. doi: 10.1016/j.jacc.2006.03.048. [DOI] [PubMed] [Google Scholar]
- 7.Haddad PM, Anderson IM. Antipsychotic-related QTc prolongation, torsade de pointes and sudden death. Drugs. 2002;62:1649–71. doi: 10.2165/00003495-200262110-00006. [DOI] [PubMed] [Google Scholar]
- 8.Stollberger C, Huber JO, Finsterer J. Antipsychotic drugs and QT prolongation. Int Clin Psychopharmacol. 2005;20:243–51. doi: 10.1097/01.yic.0000166405.49473.70. [DOI] [PubMed] [Google Scholar]
- 9.Vieweg WV. New Generation Antipsychotic Drugs and QTc Interval Prolongation. Prim Care Companion J Clin Psychiatry. 2003;5:205–15. doi: 10.4088/pcc.v05n0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drici MD, Wang WX, Liu XK, Woosley RL, Flockhart DA. Prolongation of QT interval in isolated feline hearts by antipsychotic drugs. J Clin Psychopharmacol. 1998;18:477–81. doi: 10.1097/00004714-199812000-00011. [DOI] [PubMed] [Google Scholar]
- 11.Margari L, Matera E, Craig F, Petruzzelli MG, Palmieri VO, Pastore A, et al. Tolerability and safety profile of risperidone in a sample of children and adolescents. Int Clin Psychopharmacol. 2013;28:177–83. doi: 10.1097/YIC.0b013e328362497b. [DOI] [PubMed] [Google Scholar]
- 12.Woo YS, Kim W, Chae JH, Yoon BH, Bahk WM. Blood pressure changes during clozapine or olanzapine treatment in Korean schizophrenic patients. World J Biol Psychiatry. 2009;10:420–5. doi: 10.1080/15622970801910399. [DOI] [PubMed] [Google Scholar]
- 13.Czekalla J, Beasley CM, Jr, Dellva MA, Berg PH, Grundy S. Analysis of the QTc interval during Olanzapine treatment of patients with schizophrenia and related psychosis. J Clin Psychiatry. 2001;62:191–8. doi: 10.4088/jcp.v62n0310. [DOI] [PubMed] [Google Scholar]
- 14.FDA. The clinical evaluation of QT/QTc interval prolongation and pro-arrhythmic potential for nonantiarrhythmic drugs. Preliminary concept paper. FDA 3956B1.2002. [Google Scholar]
- 15.Malik M, Batchvarov VN. Measurement, interpretation and clinical potential of QT dispersion. J Am Coll Cardiol. 2000;36:1749–66. doi: 10.1016/s0735-1097(00)00962-1. [DOI] [PubMed] [Google Scholar]
- 16.Day CP, McComb JM, Campbell RW. QT dispersion: An indication of arrhythmia risk in patients with long QT intervals. Br Heart J. 1990;63:342–4. doi: 10.1136/hrt.63.6.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Richelson E, Souder T. Binding of antipsychotic drugs to human brain receptors focus on newer generation compounds. Life Sci. 2000;68:29–39. doi: 10.1016/s0024-3205(00)00911-5. [DOI] [PubMed] [Google Scholar]
- 18.Magyar J, Bányász T, Bagi Z, Pacher P, Szentandrássy N, Fülöp L, et al. Electrophysiological effects of Risperidone in mammalian cardiac cells. Naunyn Schmiedebergs Arch Pharmacol. 2002;366:350–6. doi: 10.1007/s00210-002-0595-1. [DOI] [PubMed] [Google Scholar]
- 19.Glassmann AH, Bigger JT., Jr Antipsychotic drugs: Prolonged QTc interval, torsades de pointes, and sudden death. Am J Psychiatry. 2001;158:1774–82. doi: 10.1176/appi.ajp.158.11.1774. [DOI] [PubMed] [Google Scholar]
- 20.Dickinson JG. FDA wants label change for Novartis NDA. Med Mark Media. 2000;35:34. [Google Scholar]
- 21.Marder SR, Essock SM, Miller AL, Buchanan RW, Casey DE, Davis JM, et al. Physical health monitoring of patients with schizophrenia. Am J Psychiatry. 2004;161:1334–49. doi: 10.1176/appi.ajp.161.8.1334. [DOI] [PubMed] [Google Scholar]
- 22.Woosley R. Drugs that prolong QT interval and/or induce torsades de pointes. Available from: https://www.crediblemeds.org/everyone/composite-list-all-qtdrugs .
- 23.Haverkamp W, Breithardt G, Camm AJ, Janse MJ, Rosen MR, Antzelevitch C, et al. The potential for QT prolongation and proarrhythmia by non-antiarrhythmic drugs: Clinical and regulatory implications. Report on a policy conference of the European Society of Cardiology. Eur Heart J. 2000;21:1216–31. doi: 10.1053/euhj.2000.2249. [DOI] [PubMed] [Google Scholar]
- 24.Jacobs ES, Dickstein DP, Liebelt EL. Novel psychotropic medications in children: New toxicities to master. Pediatr Emerg Care. 2001;17:226–31. doi: 10.1097/00006565-200106000-00020. [DOI] [PubMed] [Google Scholar]
- 25.Gury C, Canceil C, Iaria P. Antipsychotic drugs and cardiovascular safety: Current studies of prolonged QT interval and risk of ventricular arrhythmia. Encephale. 2000;26:62–72. [PubMed] [Google Scholar]


