Abstract
Objective:
Tongxinluo (TXL) is a traditional Chinese medicine (TCM). It is used to treat coronary heart disease and atherosclerosis. We investigated the effects of TXL on the neointima formation and expression of inflammatory cytokines in rats after carotid artery balloon injury.
Materials and Methods:
Male Sprague-Dawley rats were randomly divided into four groups: sham operation group (Sham, n = 15), balloon injury group treated with vehicle (Control, n = 15), TXL low-dose group treated with TXL of 0.5 g/kg/d (TXL-L, n = 15), and TXL high-dose group treated with TXL of 1.0 g/kg/d (TXL-H, n = 15). TXL was given by gavage daily. 14 days after injury’, the levels of serum nitric oxide (NO), endothelin-1 (ET-1), monocyte chemoattractant protein-1 (MCP-1), and soluble intercellular adhesion molecule-1 (sICAM-1) were evaluated. The morphology of carotid artery tissue was observed with hematoxylin-eosin staining. Expressions of MCP-1 and ICAM-1 in the artery were detected by real-time polymerase chain reaction (RT-PCR) and western blotting.
Results:
14 days after injury, a significant increase in concentrations of serum ET-1, MCP-1, and sICAM-1 (P < 0.05), as well as a significant decrease in NO serum level were observed in rats subjected to artery injury compared to the sham rats (P < 0.05). TXL significantly decreased ET-1, MCP-1 and sICAM-1 serum levels (P < 0.05), whereas significantly increased NO serum level compared with the control (P < 0.05). TXL significantly reduced the neointimal thickening at day14 after injury (P < 0.05). In addition, TXL significantly reduced mRNA and protein expressions of ICAM-1 and MCP-1 in injured artery (P < 0.05).
Conclusions:
This study demonstrates that TXL is effective in improving endothelial function, attenuating neointimal formation of artery after balloon injury, and reducing expression of inflammatory cytokine MCP-1 and ICAM-1. It may be a useful agent for protecting the artery against injury.
KEY WORDS: Carotid artery injury, inflammatory cytokine, neointima formation, tongxinluo
Introduction
Neointima formation is a major pathological feature of atherosclerosis and restenosis after percutaneous coronary intervention (PCI). However, the mechanism of neointima formation has not been fully understood. Chemokines play a crucial role in initiating and progressing neointima formation by controlling vascular remodelling in response to stimuli.[1] Among several chemokines, monocyte chemoattractant protein-1 (MCP-1) is receiving increasing attention. Eliminating MCP-1 gene or blocking MCP-1 signaling decreases neointima hyperplasia after balloon- and stent- induced injury in several animal models.[2,3,4] Elevated circulating level of MCP-1 was observed in patients with restenosis after coronary angioplasty.[5] In addition, intercellular adhesion molecule-1 (ICAM-1) was upregulated in neointimal and medial smooth muscle cells (SMCs) after vascular injury, and an anti-ICAM-1 neutralizing antibody prevented neointima formation.[6] These findings suggest that anti-inflammatory treatment may be an effective approach for prevention of neointima formation.
Tongxinluo (TXL), as a traditional Chinese medicine (TCM), is used for treating cardiovascular diseases such as atherosclerosis. TXL may stabilize the vulnerable plaques in artery.[7,8] TXL has been proved to protect coronary artery from spasm and endothelial dysfunction in both clinical and experimental trials.[9,10] The serum level of nitric oxide (NO) increases after TXL treatment.[9,10,11] In addition, it is reported that TXL has an anti-inflammatory action by way of attenuating the myocardial inflammatory reaction in myocardial infarction rats.[12] Thus, these data suggested that TXL may play a role in preventing neointimal formation. Thus, in this study, we investigated the effects of TXL on the neointima formation and expression of inflammatory cytokine using an animal model of rat carotid artery balloon injury.
Materials and Methods
Animal Preparation
Adult male Sprague-Dawley (SD) rats (16-week-old) were obtained from the Animal Experimental Center of Shanghai. All animals received humane care in accordance with the “Guide for the Care and Use of Laboratory Animals”. All experimental procedures were approved by the Institutional Animal Ethics Committee of Fujian medical university. All rats received a normal diet (The feed was of the granular shape. All the rats were free of food, and had unrestricted access to water.). Rats were randomized into four groups: Sham operation group (Sham, n = 15), balloon injury group treated with vehicle (Control, n = 15), TXL low-dose group treated with TXL of 0.5 g/kg/d (TXL-L, n = 15), and TXL high-dose group treated with TXl of 1.0 g/kg/d (TXL-H, n = 15). TXL powder (Shijiazhuang Yiling Pharmaceutic Co. Ltd., Hebei, China) mixed with 2 ml water or saline was administered via direct gastric gavage daily for 14 days.
Balloon Injury Model
The rats were anesthetized with sodium pentobarbital (i.p. 50 mg/kg), and then balloon injury of the left carotid artery was performed as described previously.[6,13] The rats in the sham group underwent the same operation without balloon insertion.
Evaluation of NO, ET-1, MCP-1, and sICAM-1 Serum Levels
The concentrations of serum nitric oxide (NO), Endothelin-1 (ET-1), monocyte chemoattractant protein-1 (MCP-1), and soluble intercellular adhesion molecule-1 (sICAM-1) were determined using enzyme linked immunosorbent assay (ELISA) kits according to the manufacturer's instructions (ELISAs, Nanjing Jiancheng Bioengineering Institute).
Morphometric Analysis of Neointimal Hyperplasia
The effect of TXL on neointimal formation was measured as described previously.[14] Briefly, the rats were euthanized by lethal injection of sodium pentobarbital (i.p. 100 mg/kg body weight) on fourteenth day after balloon injury, and then perfused with saline followed by 10% formalin at physiological pressure. For morphometric analysis, arteries were fixed in 100% methanol overnight, and the middle one third of the common carotid artery was then cut into four segments and embedded in paraffin. Specimens were cross-sectioned at a thickness of 3 μm and stained with hematoxylin-eosin (HE). Intimal and medial cross-sectional areas of four cross-sections of the artery obtained from each rat were measured. The intima/media cross-sectional area ratios were determined with a computerized apparatus and NIH Image software (version 1.57).
RT-PCR Analysis
After the rats were euthanized and perfused with ice-cold PBS, the carotid arteries were removed and snap frozen in dry ice/acetone and stored at − 80°C until use. Frozen samples of the carotid arteries were homogenized in TRIzol (Invitrogen Corp). Total RNA was isolated and reverse-transcribed as described previously.[15] Polymerase chain reaction (PCR) was performed with cDNA according to the manufacturer's instructions. The primers used to amplify MCP-1 and ICAM-1 are listed in Table 1. 18S ribosomal RNA was amplified as an internal control. PCR was performed according to the profiles shown in Table 2. PCR was performed in a DNA thermal cycler (GeneAmp PCR System 2700; Applied Biosystems, Foster City, CA). The quality and concentration of amplified PCR products were determined with an Agilent 2100 Bioanalyzer (Agilent, Palo Alto, CA).
Table 1.
The primers used to amplify MCP-1 and ICAM-1 and product sizes
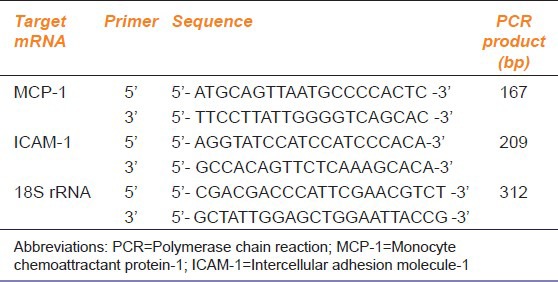
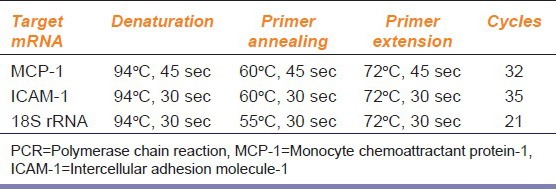
Table 2.
Thermal cycle profiles for PCR
Western-Blotting Analysis
Rat carotid artery was crushed into powder and resuspended in lysis buffer (50 mM Tris-HCl [pH 8.0], 150 mM NaCl, 0.02% sodium azide, 100 mg/ml phenylmethylsulfonyl fluoride (PMSF), 1 mg/ml aprotinin, 1% Triton X-100). Total proteins were extracted, heated at 95°C for 5 min, subjected to 10% SDS-polyacrylamide gel electrophoresis (SDS-PAGE), and electroblotted onto PVDF membranes (Amersham Biosciences, Uppsala, Sweden). Blots were incubated with goat polyclonal anti-MCP-1 antibody or anti-ICAM-1 antibody (1:200, Santa Cruz Biotechnology, Inc.), or mouse monoclonal antibody specific for α-tubulin (1:2000, Sigma) as an internal control and then with anti-goat IgG or anti-mouse IgG (1:2000, Bio-Rad Laboratories, Hercules, CA), respectively, as secondary antibodies. Bound antibodies were detected by enhanced chemiluminescence (ECL Kit, RPN2106, Amersham) and exposure to X-ray films. SDS–PAGE molecular weight markers (Prestained SDS–PAGE Standard Broad Range, Bio-Rad) were run to calibrate the gel. Bands were scanned and quantitated by densitometry with NIH Image Software (NIH Image 1.63f).
Statistical Analyses
Values are reported as mean ± SEM. A student's t test was used for unpaired data. Two-way ANOVA was also used for the counting data. P <0.05 was considered statistically significant.
Results
Effect of TXL on NO, ET-1, MCP-1, and sICAM-1 Serum Levels
The serum level of NO was significantly elevated in TXL-treated rats at fourteenth day after artery injury versus the control rats (P < 0.05). There was a significant decrease in level of ET-1 in TXL-treated rats compared to the control rats (P < 0.05). MCP-1 level was also found to be significantly decreased in TXL-administered rats versus the control group (P < 0.05). Similarly, sICAM-1 level was also significantly decreased in TXL-treated rats compared to the control group (P < 0.05) [Figure 1].
Figure 1.
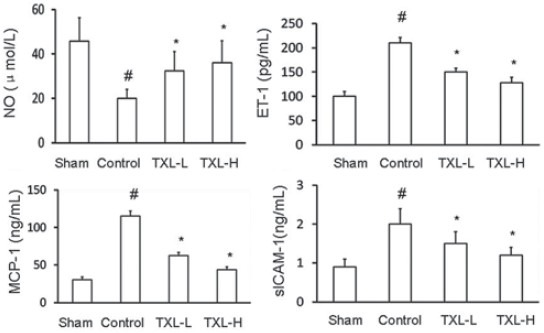
Effect of Tongxinluo (TXL) on serum NO, ET-1, MCP-1, and sICAM-1 levels in rats subjected to carotid artery balloon injury, and treated without (control) or with low-dose Tongxinluo (TXL-L) or high-dose Tongxinluo (TXL-H). Data are presented as mean ± SEM (n = 7-8). * P < 0.05 vs. control group; # P < 0.05 vs. sham group. NO, nitric oxide; ET-1, endothelin-1; MCP-1, monocyte chemoattractant protein-1; sICAM-1, soluble intercellular adhesion molecule-1
Effects of TXL Treatment on Neointimal Hyperplasia
To determine the effect of TXL treatment on the limitation of neointimal hyperplasia, a rat carotid arterial injury model was used. Significant neointimal hyperplasia was observed in injured arteries 14 days after injury [Figure 2a]. Two doses of TXL treatment caused a significant reduction of neointima formation by 41% (TXL-L group) and 62% (TXL-H group), respectively, at day 14 compared with the control rats (P < 0.05) [Figure 2b]. The intima and media remained intact in the carotid artery of the sham rats. Medial area was not affected by both artery injury and TXL treatment.
Figure 2.
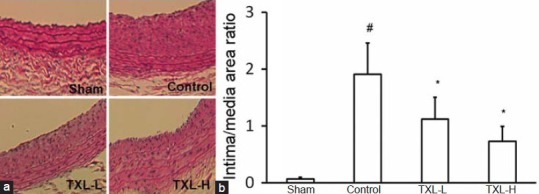
Effect of Tongxinluo (TXL) on neointimal hyperplasia in rat carotid arteries at day 14 after injury. (a) Photomicrographs showing the effect of TXL on neointima formation in rat carotid arteries 14 days after balloon injury (Magnification × 200). Hematoxylin-eosin (HE) staining of cross section of the specimens treated without (control) or with low-dose Tongxinluo (TXL-L) or high-dose Tongxinluo (TXL-H). (b) The intimal to medial area ratio of the arteries were determined 14 days after injury. Data are expressed as mean ± SEM (n = 5). * P < 0.05 vs. control group; # P < 0.05 vs. sham group
Effect of TXL on Expression of MCP-1 and ICAM-1 mRNAs in Injured Artery
In the sham rats, expressions of MCP-1 and ICAM-1 mRNA levels were undetectable. Expression of MCP-1 and ICAM-1 mRNA was significantly (P < 0.05) increased in injured artery (Control group) compared to non-injured artery (Sham group). Treatment with 2 doses of TXL significantly (P < 0.05) reduced expression of these mRNAs compared to the control group [Figure 3].
Figure 3.
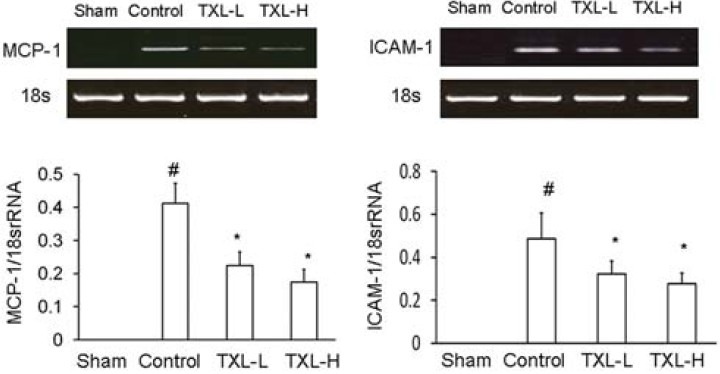
Effect of Tongxinluo (TXL) on expression of MCP-1 and ICAM-1 mRNAs in rat carotid artery 14 days after balloon injury. After the artery injury rats were treated without (control) or with lowdose Tongxinluo (TXL-L) or high-dose Tongxinluo (TXL-H). At day 14 after balloon injury, total RNA was extracted and mRNA expression was evaluated by RT-PCR assay. Ratio of mRNA to 18S rRNA was evaluated. Data are mean ± SEM (n = 5). * P < 0.05 vs. control group; # P < 0.05 vs. sham group. MCP-1, monocyte chemoattractant protein-1; ICAM-1,intercellular adhesion molecule-1
Effect of TXL on Expressions of MCP-1 and ICAM-1 Protein in Injured Artery
Expressions of MCP-1 and ICAM-1 protein were undetectable in the sham group. Expression of MCP-1 and ICAM-1 protein was significantly (P < 0.05) increased in the control group compared to the sham group. Two doses of TXL treatment significantly (P < 0.05) decreased abundances of MCP-1 and ICAM-1 protein compared to the control rats [Figure 4].
Figure 4.
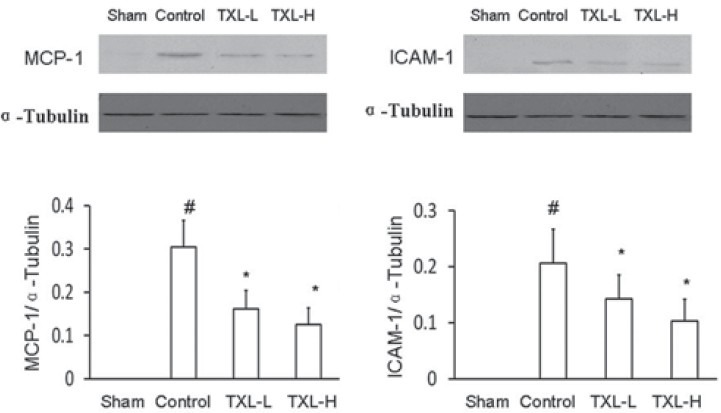
Western blot analysis of expression of MCP-1 and ICAM-1 protein in rat carotid artery 14 days after balloon injury. After the injury rats were treated without (control) or with low-dose Tongxinluo (TXL-L) or high-dose Tongxinluo (TXL-H) for 14 days. Ratio of protein to ƒ¿-Tubulin was evaluated. Data are mean ± SEM (n = 5). * P < 0.05 vs. control group; # P < 0.05 vs. sham group. MCP-1, monocyte chemoattractant protein-1; ICAM-1, intercellular adhesion molecule-1
Discussion
Neointima formation is a major pathological feature of atherosclerosis and restenosis after percutaneous coronary intervention (PCI). However, the mechanism of neoinitma formation has not been fully understood. The primary event in the development of neoinitma formation is the injury to the endothelium, which induces SMC migration from the media to the intima and subsequent proliferation.[16] In addition, chemokines play a crucial role in initiating and progressing neointima formation by controlling vascular remodelling in response to the stimuli.[1]
In the present study, an increase in ET-1 serum concentration, as well as an decrease in NO serum level were observed in rats subjected to artery injury. Treatment with TXL caused effective inhibition of ET-1 serum level and significant increase of NO serum concentration, suggesting that TXL may be effective in protecting functions of endothelial cells in the artery after injury. Neointima formation is a major pathological feature of atherosclerosis and restenosis after PCI.[16] The primary event in the development of neoinitma formation is the injury to the endothelium, which induces SMC migration from the media to the intima and subsequent proliferation.[17] TXL, which is a TCM, has been proved to protect coronary artery from endothelial dysfunction in both clinical and experimental trials.[9,10] The serum level of NO was increased after treatment with TXL.[9,10,11] Thus, it is suggested that TXL may contribute to the inhibition of neoinitma formation via protecting functions of endothelial cells.
After artery injury, the rat carotid artery develops neointima formation, the neointima formation contributes to the restenosis after artery angioplasty. Monocyte chemoattractant protein-1 (MCP-1) is implicated in these processes and the source of this chemokine is include the major cells in the injured arteries, such as endothelial cells, SMCs, and macrophages.[18] Increased levels of circulating MCP-1 in animals subjected to vascular injury are in keeping with an active role for this cytokine in tissue pathogenesis and correlate with epidemiological evidence showing higher MCP-1 plasma levels associated with restenosis.[5,19] MCP-1 activity is in part due to recruitment of monocytes/macrophages that are responsible for local production of cytokines, but MCP-1 may also directly induce SMC proliferation and migration in the artery.[20,21,22] Eliminating MCP-1 gene or blocking MCP-1 signaling decreases neointima hyperplasia after balloon- and stent-induced injury in several animal models.[2,3,4] Elevated circulating levels of MCP-1 were observed in patients with restenosis after coronary angioplasty.[5] ICAM-1 was upregulated in neointimal and medial SMCs after vascular injury, and an anti-ICAM-1 neutralizing antibody prevented neointima formation.[6] All of these findings suggest that anti-inflammatory treatment may be an effective approach for prevention of neointima formation.
In the present study, sham operation of artery did not affect neointimal formation, while neointimal hyperplasia was markedly increased in the artery subjected to the balloon injury. Expression of MCP-1 and ICAM-1 mRNA and protein was low in the non-injury artery, whereas expression of MCP-1 and ICAM-1 mRNA and protein was significantly increased after balloon injury, suggesting that increases in these molecules are associated with induction of inflammatory response in the injured artery. Treatment with TXL effectively attenuated neointimal formation of artery after injury and significantly inhibited MCP-1 and ICAM-1 expression in injured artery, suggesting that this agent inhibits neointimal formation via reducing of inflammatory cytokines production. TXL was reported to have anti-inflammatory action by way of attenuating the myocardial inflammatory reaction in myocardial infarction rats.[12] MCP-1 is a potent chemotactic factor of monocytes,[23,24] and is produced by activated SMCs or other type of cells.[25] Inhibition of MCP-1 results in a significant attenuation of neointimal hyperplasia.[2] Previous study showed that ICAM-1 was upregulated in neointimal and medial SMCs after vascular injury and that an anti-ICAM-1 neutralizing antibody prevented neointima formation.[6] These findings suggest an important role of MCP-1 and ICAM-1 in the neointima formation after vascular injury. Thus MCP-1 or ICAM-1 is a target for the treatment of restenosis, and TXL may be an effective approach to inhibit restenosis after artery injury. Taken together, TXL may attenuate intimal hyperplasia through inhibiting adhesion molecules expression and chemotactic activity of injured vessels for neutrophils. This might partially explain the beneficial effects of the TXL on the suppression of neointimal hyperplasia.
In conclusion, this study demonstrates that TXL, a TCM, is effective in improving endothelial function, attenuating neointima formation of artery after balloon injury, and reducing expression of inflammatory cytokine MCP-1 and ICAM-1. It may be a useful agent for protecting the artery against injury, and for treating the vascular diseases such as atherosclerosis and restenosis of artery after angioplasty.
Acknowledgments
This work was supported by the Medical Innovation Project of Fujian Province, China (Grant No. 2012-CX-19).
Footnotes
Source of Support: Nil
Conflict of Interest: No
References
- 1.Ando H, Fukuda N, Kotani M, Yokoyama S, Kunimoto S, Matsumoto K, et al. Chimeric DNA-RNA hammerhead ribozyme targeting transforming growth factor-h1 mRNA inhibits neointima formation in rat carotid artery after balloon injury. Eur J Pharmacol. 2004;483:207–14. doi: 10.1016/j.ejphar.2003.10.035. [DOI] [PubMed] [Google Scholar]
- 2.Charo IF, Taubman MB. Chemokines in the pathogenesis of vascular disease. Circ Res. 2004;95:858–66. doi: 10.1161/01.RES.0000146672.10582.17. [DOI] [PubMed] [Google Scholar]
- 3.Cipollone F, Marini M, Fazia M, Pini B, Iezzi A, Reale M, et al. Elevated circulating levels of monocyte chemoattractant protein-1 in patients with restenosis after coronary angioplasty. Arterioscler Thromb Vasc Biol. 2001;21:327–34. doi: 10.1161/01.atv.21.3.327. [DOI] [PubMed] [Google Scholar]
- 4.Egashira K, Koyanagi M, Kitamoto S, Ni W, Kataoka C, Moroshita R, et al. Anti-monocyte chemoattractant protein-1 therapy inhibits vascular remodeling in rats: Blockade of MCP-1 activity after intramuscular transfer of a mutant gene inhibits vascular remodeling induced by chronic blockade of NO synythesis. FASEB J. 2000;14:1974–8. doi: 10.1096/fj.00-0141com. [DOI] [PubMed] [Google Scholar]
- 5.Egashira K, Nakano K, Ohtani K, Funakoshi K, Zhao G, Ihara Y, et al. Local delivery of anti-monocyte chemoattractant protein-1 by gene-eluting stents attenuates in-stent stenosis in rabbits and monkeys. Arterioscler Thromb Vasc Biol. 2007;27:2563–8. doi: 10.1161/ATVBAHA.107.154609. [DOI] [PubMed] [Google Scholar]
- 6.Furukawa Y, Matsumori A, Ohashi N, Shioi T, Ono K, Harada A, et al. Anti-monocyte chemoattractant protein-1/monocyte chemotactic and activating factor antibody inhibits neointimal hyperplasia in injured rat carotid arteries. Circ Res. 1999;84:306–14. doi: 10.1161/01.res.84.3.306. [DOI] [PubMed] [Google Scholar]
- 7.Jia Z, Gu F, Xue Y. Effect of tongxinluo capsule in treating variant angina pectoris patients and its influence on endothelial function. Zhongguo Zhong Xi Yi Jie He Za Zhi. 1999;19:651–2. [PubMed] [Google Scholar]
- 8.Kong Q, Yang XC, Liu XL. Effects of tongxinluo on the myocardial inflammatory reaction and expression of tumor necrosis factor-alpha in rats with myocardial infarction. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2006;26:545–9. [PubMed] [Google Scholar]
- 9.Li Z, Yang YJ, Qin XW, Ruan YM, Chen X, Meng L, et al. Effects of Tongxinluo and Simvastatin on the stabilization of vulnerable atherosclerotic plaques of aorta in aortic atherosclerosis and molecular mechanism thereof: A comparative study with rabbits. Zhonghua Yi Xue Za Zhi. 2006;86:3146–50. [PubMed] [Google Scholar]
- 10.Martinovic I, Abegunewardene N, Seul M, Vosseler M, Horstick G, Buerke M, et al. Elevated monocyte chemoattractant protein-1 serum levels in patients at risk for coronary artery disease. Circ J. 2005;69:1484–9. doi: 10.1253/circj.69.1484. [DOI] [PubMed] [Google Scholar]
- 11.Massberg S, Vogt F, Dickfeld T, Brand K, Page S, Gawaz M. Activated platelets trigger an inflammatory response and enhance migration of aortic smooth muscle cells. Thromb Res. 2003;110:187–94. doi: 10.1016/s0049-3848(03)00342-6. [DOI] [PubMed] [Google Scholar]
- 12.Mintz GS, Popma JJ, Hong MK, Pichard AD, Kent KM, Satler LF, et al. Intravascular ultrasound to discern device-specific effects and mechanisms of restenosis. Am J Cardiol. 1996;78:18–22. doi: 10.1016/s0002-9149(96)00493-6. [DOI] [PubMed] [Google Scholar]
- 13.Parenti A, Bellik L, Brogelli L, Filippi S, Ledda F. Endogenous VEGF-A is responsible for mitogenic effects of MCP-1 on vascular smooth muscle cells. Am J Physiol Heart Circ Physiol. 2004;286:H1978–84. doi: 10.1152/ajpheart.00414.2003. [DOI] [PubMed] [Google Scholar]
- 14.Robinson EA, Yoshimura T, Leonard EJ, Tanaka S, Griffin PR, Shabanowitz J, et al. Complete amino acid sequence of a human monocyte chemoattractant, a putative mediator of cellular immune reactions. Proc Natl Acad Sci U S A. 1989;86:1850–4. doi: 10.1073/pnas.86.6.1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rollins BJ, Stier P, Ernst T, Wong GG. The human homolog of the JE gene encodes a monocyte secretory protein. Mol Cell Biol. 1989;9:4687–95. doi: 10.1128/mcb.9.11.4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schober A. Chemokines in vascular dysfunction and remodeling. Arterioscler Thromb Vasc Biol. 2008;28:1950–9. doi: 10.1161/ATVBAHA.107.161224. [DOI] [PubMed] [Google Scholar]
- 17.Schwartz RS, Holmes DR, Jr, Topol EJ. The restenosis paradigm revisited: An alternative proposal for cellular mechanisms. J Am Coll Cardiol. 1992;20:1284–93. doi: 10.1016/0735-1097(92)90389-5. [DOI] [PubMed] [Google Scholar]
- 18.Seki Y, Kai H, Shibata R, Nagata T, Yasukawa H, Yoshimura A, et al. Roles of JAK/STAT pathway in rat carotid artery remodeling after vascular injury. Circ Res. 2000;87:12–8. doi: 10.1161/01.res.87.1.12. [DOI] [PubMed] [Google Scholar]
- 19.Selzman CH, Miller SA, Zimmerman MA, Gamboni-Robertson F, Harken AH, Banerjee A. Monocyte chemotactic protein-1 directly induces human vascular smooth muscle proliferation. Am J Physiol Heart Circ Physiol. 2002;283:H1455–61. doi: 10.1152/ajpheart.00188.2002. [DOI] [PubMed] [Google Scholar]
- 20.Tahira Y, Fukuda N, Endo M, Suzuki R, Ikeda Y, Takagi H, et al. Transforming growth factor-beta expression in cardiovascular organs in stroke-prone spontaneously hypertensive rats with the development of hypertension. Hypertens Res. 2002;25:911–8. doi: 10.1291/hypres.25.911. [DOI] [PubMed] [Google Scholar]
- 21.Tang YS, Cui X. The effect of Tong Xin Luo on vascular remodeling after angioplasty in rabbits. Chin J Cardio (Chin) 2002;32:1147–9. [Google Scholar]
- 22.Valente AJ, Graves DT, Vialle-Valentin CE, Delgado R, Schwartz CJ. Purification of a monocyte chemotactic factor secreted by nonhuman primate vascular cells in culture. Biochemistry. 1988;27:4162–8. doi: 10.1021/bi00411a039. [DOI] [PubMed] [Google Scholar]
- 23.Wang HJ, Huang YW, Sun J. Effect of Tongxinluo capsule on function of vascular endothelium in patients with unstable angina pectoris. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2003;23:587–9. [PubMed] [Google Scholar]
- 24.Yasukawa H, Imaizumi T, Matsuoka H, Nakashima A, Morimatsu M. Inhibition of intimal hyperplasia after balloon injury by antibodies to intercellular adhesion molecule-1 and lymphocyte function-associated antigen-1. Circulation. 1997;98:1515–22. doi: 10.1161/01.cir.95.6.1515. [DOI] [PubMed] [Google Scholar]
- 25.Zhao JL, Yang YJ, You SJ, Jin Z, Wu Y, Yang W, et al. Effect of Tongxinluo on endothelin-1 in the mini-swine model of acute myocardial infarction and reperfusion. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2005;25:902–6. [PubMed] [Google Scholar]


