Abstract
Objective:
1,3,4-oxadiazole ring is a versatile moiety with a wide range of pharmacological properties. The present work deals with the synthesis and evaluation of the anti-inflammatory activity of two novel 2,5-disubstituted-1,3,4-oxadiazoles (OSD and OPD).
Materials and Methods:
Carrageenan-induced rat hind paw edema was employed as an acute model of inflammation. For evaluating sub-acute anti-inflammatory activity, carrageenan-induced inflammation in rat air pouch was employed. Complete Freund's adjuvant-induced arthritis in rats was used as a model of chronic inflammation. To evaluate in vitro anti-inflammatory activity, lipopolysaccharide (LPS)-stimulated RAW264.7 cells were used.
Results:
OSD (100 mg/kg) reduced carrageen-induced paw edema by 60%, and OPD (100 mg/kg) produced a modest 32.5% reduction. OSD also reduced leukocyte influx and myeloperoxidase in carrageenan-induced rat air pouch model. In complete Freund's adjuvant-induced arthritis model, both OSD and OPD (200 mg/kg for 14 days) reduced paw edema and NO levels. In LPS-stimulated RAW264.7 cells, OSD and OPD inhibited formation of nitric oxide and reactive oxygen species, with OPD showing a better activity in comparison to OSD.
Conclusions:
OSD was the better of the two compounds in in vivo models of inflammation. The o-phenol substitution at position 2 of oxadiazole ring in OSD may be responsible for its better in vivo anti-inflammatory activity. The ability of the compounds to inhibit LPS-induced pro-inflammatory mediator release suggests an anti-inflammatory mechanism targeting LPS-TLR4-NF-κB signalling pathway, which needs to be explored in detail. The disparate efficacy in vitro and in vivo also requires in-depth evaluation of the pharmacokinetics of these novel oxadiazoles.
KEY WORDS: Inflammation, myeloperoxidase, oxadiazole
Introduction
Inflammation is central not only to chronic diseases like rheumatoid arthritis and osteoarthritis, but also to atherosclerosis and diabetes. In atherosclerosis, the injury to endothelium causes the accumulation of leukocytes and other inflammatory cells in the intima leading to a state of chronic inflammation. Mediators of inflammation such as cytokines are found in the atheromatic plaques.[1] In diabetes, the hyperglycemia can cause modification of macromolecules by forming advanced glycation end products (AGE). Elevation of inflammatory cytokines and acute-phase proteins like C-reactive protein (CRP) is seen in metabolic syndrome.[2] Besides, many hypoplipidemic drugs have been reported to reduce CRP levels.[3]
The treatment of advanced stages of metabolic syndrome involves the use of many drugs, with a view to controlling the individual risk factors. However, the outcomes are not always satisfactory.[4] If drugs with multiple beneficial effects are developed, that would revolutionize the drug therapy of metabolic syndrome, and the attending morbidity and mortality. Therefore, the discovery and development of new drugs with better therapeutic potential and tolerability assume importance.
1,3,4-oxadiazole ring has been found to be a versatile pharmacophore with a wide range of useful biological activities such as anti-inflammatory,[5] anti-tubercular,[6] and anti-cancer,[7] Research in our laboratory on 1,3,4-oxadiazole derivatives led to the synthesis of 4-[3-acetyl-5-(pyridin-3-yl)-2,3-dihydro-1,3,4-oxadiazol-2-yl] phenyl acetate (NOX1), which has vasorelaxant effect on rat aortic tissue.[8] In the present study, two derivatives, viz., 2-[3-acetyl-5-(pyridin-4-yl)-2,3-dihydro-1,3,4-oxadiazol-2-yl] phenyl acetate (OSD) and 4-[3-acetyl-5-(pyridin-4-yl)-2,3-dihydro-1,3,4-oxadiazol-2-yl] phenyl acetate (OPD), were screened for possible anti-inflammatory activity in rat models of inflammation and macrophage cell-line.
Materials and Methods
General
Melting points were determined in open capillary Toshniwal melting point apparatus (Toshniwal Instruments, Chennai, India). Infrared spectra were recorded on Shimadzu FT-IR-8300/8700 as KBr disc (v/cm-1). Purity of compounds was routinely checked by TLC on silica gel (Merck, Darmstadt, Germany). The mass spectra of OSD and OPD were obtained using Shimadzu GC-MS QP5050. IR spectra were recorded on Shimadzu spectrophotometer with KBr pellets. NMR spectra were taken on spectrometer at 400 MHZ.
λ-Carrageenan, complete Freund's adjuvant (CFA), o-dianisidine hydrochloride, 2’,7’-dichlorofluorescein diacetate (DCFH-DA), lipopolysaccharide from E. coli 0111:B4 (LPS) and Dulbecco's Modified Eagle's Medium (DMEM) were purchased from Sigma-Aldrich Co. LLC., St.Louis, MO, USA. Fetal Bovine Serum (FBS) was purchased from Gibco®, Life Technologies Corporation, NY, USA. Murine macrophage cell-line RAW 264.7 was purchased from National Centre for Cell Science, Pune, MH, India. The various chemicals used in this work were of analytical grade.
Synthesis
The compounds, OSD and OPD were synthesized through well-established synthetic steps.[6] In brief, isoniazid (in ethanol solution) was heated under reflux for 30 min with an equimolar quantity of either salicylaldehyde (for OSD) or p-hydroxybenzaldehyde (for OPD) in ethanol solution, along with 3-4 drops of glacial acetic acid. The Schiff base that separated on cooling the reaction mixture was filtered and purified by recrystallization. The respective schiff base (0.02M) was cyclized to the 1,3,4-oxadiazole derivative by refluxing with acetic anhydride (20 ml) for 4 h. After cooling to room temperature, ice-cold water was added to the flask and stirred for 30 min. The product formed was purified by recrystallization. The scheme of the synthesis is given in Figure 1.
Figure 1.
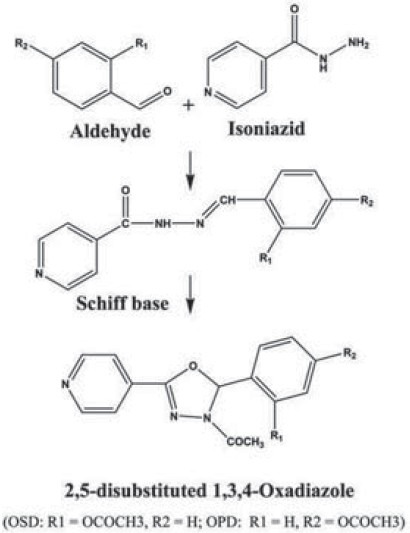
Scheme of synthesis of 1,3,4-oxadiazole derivatives: OSD and OPD
Characterization
OSD
Yield 64%; mp 160°C; IR (KBr) νmax/cm−1 3321-3045 cm− 1(-Ar), 1768 cm− 1 (O-COCH3), 1670 cm− 1(N-COCH3), 1600-1413 cm− 1(-C = N, Pyridine); IH NMR (400 MHz, DMSO-d6, δ, ppm): 2.124 (3H, s, -COCH3), 2.215 (3H, s, -OCOCH3), 7.24 (1H, s, O-CH-N-), 7.20-7.59 (4H, m, Ar-H), 7.71- 8.76 (4H, m, Ar-H of Pyridine); MS-325[M]+; m/z: 283, 223, 106.
OPD
Yield 64%; mp 140°C; IR (KBr) νmax/cm−1 3070 cm− 1 (Ar), 1751 cm− 1 (-OCOCH3), 1680 cm− 1 (N-COCH3), 1598–1411 cm− 1(-C = N, Pyridine); IH NMR (400 MHz, DMSO-d6, δ, ppm): 2.26 (3H, s, -COCH3), 2.28 (3H, s, -OCOCH3), 7.24 (1H, s, O-CH-N-), 7.19–7.57 (4H, m, Ar-H), 7.71–8.80 (4H, m, Ar-H of Pyridine); MS- 325[M]+; m/z: 283, 223, 106.
Experimental Pharmacology
Experimental animals and dose selection
Wistar rats (150-200 g) and Swiss albino mice (25-30 g) bred and maintained in Central Animal Research Facility were used for the study. The animals were fed a standard rodent diet and had access to water ad libitum. The animals were maintained on a 12 h/12 h light-dark cycle at temperature 25 ± 2°C, humidity 45-55% and ventilation 10-12 exchanges/h. They were housed individually in polypropylene cages and were handled gently to avoid too much stress. All the experimental procedures used in this study were approved by the Institutional Animal Ethics Committee and adheres to the guidelines laid down by the Committee for the Purpose of Control and Supervision of Experiments on Animals, Government of India. Acute toxicity study was carried out in mice as per the OECD guidelines (TG-420). Both OSD and OPD were found to be safe up to 2000 mg/kg. Hence, 1/20th (100 mg/kg) and 1/10th (200 mg/kg) of the safe dose were selected as the maximum dose for anti-inflammatory studies. All treatments were administered through the oral route as suspension in 0.25% carboxy methyl cellulose (vehicle).
Anti-inflammatory activity
Acute inflammation-Carrageenan-induced paw oedema in rats.
The method of Winter[9] was employed. Rats were divided into treatment and control groups, and administered ibuprofen (standard-200 mg/kg), OSD (100 mg/kg), OPD (100 mg/kg) and vehicle, orally, 1 h prior to injection of 0.05 ml carrageenan (1%) into the left hind footpad. Paw volume was measured using a digital plethysmometer (Plethysmometer 37140, Ugo Basile Srl, Comerio, VA, Italy) immediately after carrageenan injection (zero hour volume). Paw volume measurement was made 3 h after carrageenan challenge and the increase in paw volume was calculated.
Sub-acute inflammation-Carrageenan-induced inflammation in rat air-pouch
Air pouch was produced using the method of Ellis.[10] Sterile air (20 mL) was injected into the subcutaneous tissue on the back of rats on day 1 and 3. On day 5, the animals were divided into four groups and administered diclofenac (10 mg/kg), OSD (100 mg/kg), OPD (100 mg/kg) and vehicle, respectively. One mL of 1% carrageenan was then injected into the air pouch, except the saline control animals, which were injected with normal saline (1 mL). The animals were sacrificed 6 h after carrageenan challenge and the pouch lavage was obtained by injecting 5 mL of ice-cold saline followed by gentle massage. The total leukocyte and granulocyte counts were determined using veterinary blood cell counter (PCE-210VET, Erma Inc., Tokyo, Japan). Myeloperoxidase (MPO) was determined using the method of Graff.[11] Briefly, 50 μL sample was added to a 96 well microtitre plate, followed by 250 μL O-Dianisidine hydrochloride (ODA) 0.167 mg/ml in 50 mM phosphate buffer (pH 6) containing 0.0005% hydrogen peroxide. After incubation for 15 min in the dark at room temperature, the absorbance was measured using absorbance microplate reader (EL × 800, BioTek Instruments Inc., VT, USA) at 490 nm. The percentage of MPO activity with respect to carrageenan control was calculated and results are expressed as mean % MPO activity ± SEM. The percent of total leukocyte count, granulocyte count and MPO activity with respect to carrageenan control was calculated.
Chronic inflammation-CFA-induced arthritis in rats
Arthritis was induced in rats using the method of Powers[12] with slight modification. Briefly, on day 0, after an initial measurement of paw volume, 0.1 ml CFA was injected into the right hind paw. Paw volumes were measured on days 5, 10, 14, post injection using digital plethysmometer. On day 15, rats with developed arthritis were randomized into treatment and control groups and treated orally, once a day, from day 15-28 [diclofenac (10 mg/kg), OSD (200 mg/kg), OPD (200 mg/kg) and vehicle]. Paw volumes were measured on days 17, 21 and day 28. The persistence of inflammation was measured in terms of paw edema and NO generation locally. On day 28, the animals were sacrificed and the arthritic paws were homogenized and the supernatant was used for the estimation of NO using Griess assay.[13] Briefly, 100 μL sample was added to a 96 well microtitre plate, followed by 100 μL of freshly prepared Griess reagent (0.1% Naphthylethylenediamine dihydrochloride and 1% sulfanilamide in 2.5% phosphoric acid). After incubation for 10 min in the dark at room temperature, the absorbance was measured using Absorbance microplate reader at 540 nm. The concentration of nitrite in pouch exudates (μM) was obtained from a standard curve plotted using sodium nitrite. Results are expressed as mean concentration of nitrite (μM) ± SEM.
In Vitro Anti-Inflammatory Activity
LPS-induced pro-inflammatory mediator release in RAW 264.7 cells
A previously reported method was employed.[14] Briefly, 5 × 104 cells/well were treated with various concentrations of test compounds and LPS (10 μg/ml) for 20 h. Nω-nitro-L-arginine methyl ester hydrochloride (L-NAME hydrochloride) and diphenylene iodonium (DPI) (both from Sigma-Aldrich Co. LLC., St.Louis, MO, USA) were used as standards. The nitric oxide (NO) released into the media was estimated by Griess assay. The intracellular reactive oxygen species (ROS) was estimated using DCFH-DA (100 μM) by fluorescence microplate reader (FL × 800, Biotek Instrument Inc., Winooski, VT, USA) at λex: 485 nm and λem: 530 nm. Two independent experiments were performed. IC50 was calculated.
Statistical Analysis
Results are expressed as mean ± S.E.M, and statistical analysis was performed using Graph Pad Prism Software (Version 5.02-Demo, Graph Pad Software, Inc., San Diego, CA, USA). One-way ANOVA followed by post-hoc Tukey's multiple comparison tests were used.
Results
Carrageenan-induced Paw Oedema in Rats
OSD, OPD and Ibuprofen produced significant inhibition of carrageenan-induced paw edema at the third hour compared to control. At 100 mg/kg, OSD showed comparable activity to ibuprofen [Figure 2].
Figure 2.
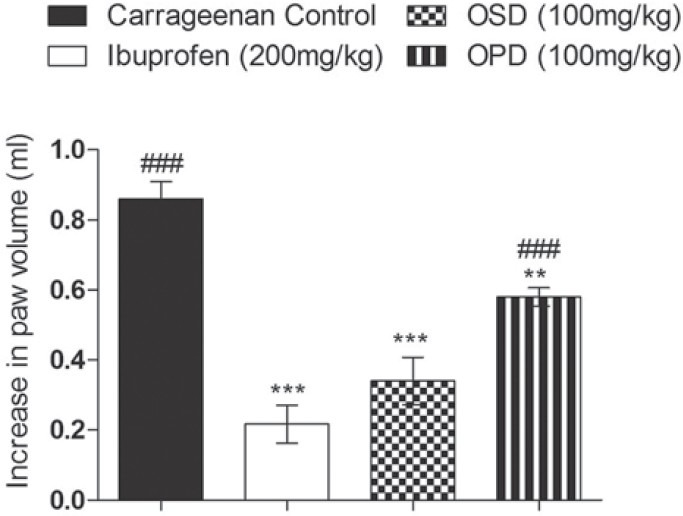
Effect of OSD and OPD in carrageenan-induced paw edema in rats. Data are represented as mean ± S.E.M and **P < 0.01 vs carrageenan control, ***P < 0.001 Vs carrageenan control, ###P < 0.001 vs Ibuprofen; n = 8
Carrageenan-induced inflammation in rat air-pouch
OSD, OPD and diclofenac treated groups had significantly reduced leukocyte count in the exudate collected from the air pouch when compared with the positive control [Figure 3a]. A similar result was obtained with the granulocyte count [Figure 3b]. OSD (100 mg/kg) and OPD (100 mg/kg) treated animals showed significant reduction in percentage MPO activity [Figure 3c]. OSD and OPD showed activity which was comparable to diclofenac (10 mg/kg).
Figure 3.
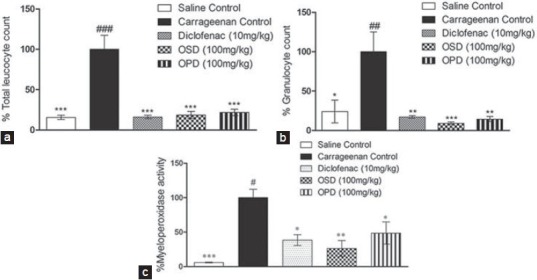
Effect of OSD and OPD in carrageenan-induced inflammation in rat air-pouch (a) total leukocyte count, (b) granulocyte count, (c) MPO levels in air pouch lavage Data are represented as mean ± S.E.M. *P < 0.05 Vs carrageenan control, **P < 0.01 Vs carrageenan control, ***P < 0.001 Vs carrageenan control, #P < 0.05 Vs Diclofenac, ##P < 0.01 Vs Diclofenac, ###P < 0.001 Vs Diclofenac; n = 5
Complete Freund's Adjuvant-induced arthritis in rats
In treatment groups, there was a significant increase in the paw volume between Day 1 (adjuvant injection) and Day 15 (randomization and beginning of treatment), followed by a reduction in paw edema during treatment period [Figure 4a]. On Day 28, there was no significant increase in paw volume in diclofenac (10 mg/kg) and OSD (200 mg/kg) treated groups, while in control and OPD groups there was persistence of paw oedema [Figure 4b]. Diclofenac, OSD and OPD produced significant reduction in NO levels in the arthritic paw compared to control [Figure 4c]. However, no significant difference was found between the drug treated groups. Additionally, we observed that while diclofenac led to gastric ulceration, OSD and OPD were apparently non-toxic (data not shown).
Figure 4.
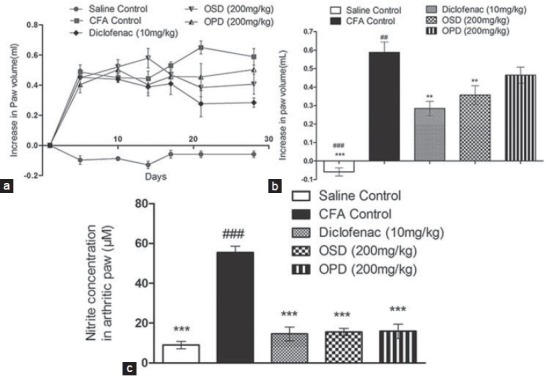
Effect of OSD and OPD in complete Freund's adjuvant-induced arthritis in rats (a) change in paw oedema during study period, (b) increase in paw volume on day 28, (c) NO levels in paw. Data are represented as mean ± S.E.M. **P < 0.01 Vs CFA control, ***P < 0.001 Vs CFA control, ##P < 0.01 Vs Diclofenac, ###P < 0.001 Vs Diclofenac; n = 6
LPS-induced inflammation in RAW264.7 Murine macrophages
OSD and OPD reduced NO and ROS levels in LPS-stimulated macrophages. OPD was found to be more potent than OSD. OSD inhibited NO and ROS production with IC50 of 439.37 ± 20.30 μM and 501.50 ± 112.75 μM respectively, whereas, OPD inhibited NO and ROS production with IC50 of 17.30 ± 2.88 μM and 53.85 ± 9.39 μM, respectively. L-NAME hydrochloride (50 μM), used as standard, showed 16.29 ± 0.81% NO inhibition, and DPI (5 μM), used as standard, showed 65.95 ± 4.45% ROS inhibition.
Discussion
The present study is an exploration of the anti-inflammatory activity of two 1,3,4-oxadiazoles, which were synthesized in our laboratory as part of an on-going program of discovering small molecules with multiple beneficial effects in metabolic syndrome. Metabolic syndrome is a pro-inflammatory state involving a cluster of risk factors which increases the risk of heart disease, diabetes and stroke.[15]
Inflammatory response involves vascular and cellular phases. In an inflammatory reaction, there is extravasation of fluids and plasma proteins followed by migration of leukocytes. Carrageenan-induced rat paw edema is a well-accepted model, which is suited for the rapid evaluation of NSAIDs-like anti-inflammatory agents.[16] Carrageenan induces a biphasic response; an acute phase (first hour) mediated by histamine, 5HT, etc., and a late phase (third hour) involving prostaglandins, proteases, etc.[17] The test drugs (OSD and OPD) showed significant inhibition of paw edema three hours following carrageenan-challenge indicating an NSAID-like anti-inflammatory activity probably targeting COX enzyme. The carrageenan stimulated air pouch lining is similar to the arthritic synovium.[10] Hence, compounds effective in this model may be potential anti-arthritic agents. Neutrophils are the first line of defense in an innate immune response targeted against invading pathogens. MPO is a marker of neutrophil activation. As the test compounds reduced leucocyte infiltration and MPO activiy in the air pouch exudate, it is possible that the test compounds may be targeting neutrophils. In this model, OSD showed comparable activity to diclofenac and was more efficacious than OPD. As the test compounds showed promising results in air pouch model of inflammation, the test compounds were evaluated in a model of chronic inflammation. Adjuvant-induced arthritis induced by Freund's adjuvant is the result of an immunological response that has features of rheumatoid arthritis. The test compounds reduced both paw edema and synovial NO levels. At 200 mg/kg, OSD, but not OPD, showed comparable activity to diclofenac. As in acute inflammation, OSD appeared to show better activity in chronic inflammation also. We also observed that the test compounds did not cause gastric ulcers, whereas diclofenac treatment was ulcerogenic (data not shown). A major shortcoming of NSAIDs-the OTC drugs for inflammation, is ulcerogenicity due to non-specific COX blockade. Thus, the test compounds are promising as they showed anti-inflammatory activity without any apparent signs of ulcerogenicity, even upon dosing (200 mg/kg) for 14 consecutive days.
As the test compounds proved to be good anti-inflammatory agents in acute and chronic models of inflammation, an attempt was made to identify the possible target of action. The pro-inflammatory role of oxidative stress is well documented.[18] The binding of bacterial lipopolysaccharide to TLR4 on macrophages[19] activates NF-KB leading to release of inflammatory mediators.[20,21] In LPS-stimulated macrophages, OSD and OPD reduced pro-inflammatory mediators NO and ROS. An interesting observation was that OPD was more potent in vitro, rather than in vivo. MTT assay for cell viability indicated that IC50 (concentration which resulted in 50% cytotoxicity) for OSD was >1000 μ M and IC50 for OPD was 367.78 ± 7.85 μM. These results confirm that the observed in vitro anti-inflammatory activity is attributed to the compound's efficacy in inhibiting ROS and NO. Thus, the anti-inflammatory activity of test compounds may be the result of an intracellular anti-oxidant activity. As iNOS expression is upregulated by LPS-induced NF-κB translocation into the nucleus,[22] inhibition of NO by test compounds is also suggestive of a mechanism targeting NF-κB and/or iNOS. Thus, the test compounds could be targeting transcription factor NF-κB or COX2, iNOS, etc., in mediating the observed anti-inflammatory activity. Further studies are necessary to identify the exact targets.
OSD and OPD-the two novel 2,5-disubstituted-1,3,4-oxadiazoles showed anti-inflammatory activity in acute and chronic models of inflammation. A look at the structures of OSD and OPD reveals that the former compound has an o-acetyl substitution in the benzene moiety attached to carbon 2 of the oxadiazole ring much like aspirin. On the other hand, OPD has a p-acetyl moiety, which lacks the spatial orientation of the o-acetyl group of the benzene ring. It is tempting to hypothesize that the mechanism of anti-inflammatory effect of OSD may be through inhibition of cyclooxygenase. However, this has to be verified through inhibition studies on arachidonic acid metabolites. Further pharmacological studies on these compounds will look more closely at their mechanisms of action, structure-activity-relationships and pharmacokinetics. Owing to the potential of the test compounds as anti-inflammatory agents, and reported activity of 1,3,4-ozadiazoles on blood glucose[23] and lipid profile,[24] it is definitely worth investigating the effect of the test compounds on the multiple pathologies associated with metabolic syndrome.
Acknowledgements
The authors thank Manipal College of Pharmaceutical Sciences, Manipal for providing the facilities to carry out this study.
Footnotes
Source of Support: Nil
Conflict of Interest: No
References
- 1.Ait-Oufella H, Taleb S, Mallat Z, Tedgui A. Recent advances on the role of cytokines in atherosclerosis. Arterioscler Thromb Vasc Biol. 2011;31:969–79. doi: 10.1161/ATVBAHA.110.207415. [DOI] [PubMed] [Google Scholar]
- 2.Devaraj S, Singh U, Jialal I. Human C-reactive protein and the metabolic syndrome. Curr Opin Lipidol. 2009;20:182–9. doi: 10.1097/MOL.0b013e32832ac03e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prasad K. C-reactive protein (CRP)-lowering agents. Cardiovasc Drug Rev. 2006;24:33–50. doi: 10.1111/j.1527-3466.2006.00033.x. [DOI] [PubMed] [Google Scholar]
- 4.Grundy SM. Drug therapy of the metabolic syndrome: Minimizing the emerging crisis in polypharmacy. Nat Rev Drug Discov. 2006;5:295–309. doi: 10.1038/nrd2005. [DOI] [PubMed] [Google Scholar]
- 5.Akhter M, Husain A, Azad B, Ajmal M. Aroylpropionic acid based 2,5-disubstituted-1,3,4-oxadiazoles: Synthesis and their anti-inflammatory and analgesic activities. Eur J Med Chem. 2009;44:2372–8. doi: 10.1016/j.ejmech.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 6.Kumar H, Javed SA, Khan SA, Amir M. 1,3,4-Oxadiazole/thiadiazole and 1,2,4-triazole derivatives of biphenyl-4-yloxy acetic acid: Synthesis and preliminary evaluation of biological properties. Eur J Med Chem. 2008;43:2688–98. doi: 10.1016/j.ejmech.2008.01.039. [DOI] [PubMed] [Google Scholar]
- 7.Kumar A, D’souza SS, Nagaraj SR, Gaonkar SL, Salimath BP, Rai KM. Antiangiogenic and antiproliferative effects of substituted-1,3,4-oxadiazole derivatives is mediated by down regulation of VEGF and inhibition of translocation of HIF-1alpha in Ehrlich ascites tumor cells. Cancer Chemother Pharmacol. 2009;64:1221–33. doi: 10.1007/s00280-009-0992-y. [DOI] [PubMed] [Google Scholar]
- 8.Bankar GR, Nandakumar K, Nayak PG, Thakur A, Chamallamudi MR, Nampurath GK. Vasorelaxant effect in rat aortic rings through calcium channel blockage: A preliminary in vitro assessment of a 1,3,4-oxadiazole derivative. Chem Biol Interact. 2009;181:377–82. doi: 10.1016/j.cbi.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 9.Winter CA, Risley EA, Nuss GW. Carrageenan-induced edema in hind paw of the rat as an assay for antiiflammatory drugs. Proc Soc Exp Biol Med. 1962;111:544–7. doi: 10.3181/00379727-111-27849. [DOI] [PubMed] [Google Scholar]
- 10.Ellis L, Gilston V, Soo CC, Morris CJ, Kidd BL, Winyard PG. Activation of the transcription factor NF-kappaB in the rat air pouch model of inflammation. Ann Rheum Dis. 2000;59:303–7. doi: 10.1136/ard.59.4.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Graff G, Gamache DA, Brady MT, Spellman JM, Yanni JM. Improved myeloperoxidase assay for quantitation of neutrophil influx in a rat model of endotoxin-induced uveitis. J Pharmacol Toxicol Methods. 1998;39:169–78. doi: 10.1016/s1056-8719(98)00023-9. [DOI] [PubMed] [Google Scholar]
- 12.Powers LJ, Fogt SW, Ariyan ZS, Rippin DJ, Heilman RD, Matthews RJ. Effect of structural change on acute toxicity and antiinflammatory activity in a series of imidazothiazoles and thiazolobenzimidazoles. J Med Chem. 1981;24:604–9. doi: 10.1021/jm00137a022. [DOI] [PubMed] [Google Scholar]
- 13.Marcocei L, Packer L, Droy-Lefaix MT, Sekaki A, Gardès-Albert M. Antioxidant action of Ginko biloba extracts EGb 761. Methods Enzymol. 1994;234:462–75. doi: 10.1016/0076-6879(94)34117-6. [DOI] [PubMed] [Google Scholar]
- 14.Mathew G, Jacob A, Durgashivaprasad E, Reddy ND, Unnikrishnan MK. 6b, 11b-Dihydroxy-6b, 11b-dihydro-7H-indeno[1,2-b] naphtho[2,1-d] furan-7-one (DHFO), a small molecule targeting NF-κB, demonstrates therapeutic potential in immunopathogenic chronic inflammatory conditions. Int Immunopharmacol. 2013;15:182–9. doi: 10.1016/j.intimp.2012.10.028. [DOI] [PubMed] [Google Scholar]
- 15.Paoletti R, Bolego C, Poli A, Cignarella A. Metabolic syndrome, inflammation and atherosclerosis. Vasc Health Risk Manag. 2006;2:145–52. doi: 10.2147/vhrm.2006.2.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whiteley PE, Dalrymple SA. Models of inflammation: Carrageenan-induced paw edema in the rat. Curr Protoc Pharmacol. 2001;54:1–3. doi: 10.1002/0471141755.ph0505s13. [DOI] [PubMed] [Google Scholar]
- 17.Vinegar R, Schreiber W, Hugo R. Biphasic development of carrageenin edema in rats. J Pharmacol Exp Ther. 1969;166:96–103. [PubMed] [Google Scholar]
- 18.Mittal M, Siddiqui MR, Tran K, Reddy SP, Malik AB. Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal. 2014;20:1126–67. doi: 10.1089/ars.2012.5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Monick MM, Powers L, Butler N, Yarovinsky T, Hunninghake GW. Interaction of matrix with integrin receptors is required for optimal LPS-induced MAP kinase activation. Am J Physiol Lung Cell Mol Physiol. 2002;283:L390–402. doi: 10.1152/ajplung.00437.2001. [DOI] [PubMed] [Google Scholar]
- 20.Syahida A, Israf DA, Permana D, Lajis NH, Khozirah S, Afiza AW, et al. Atrovirinone inhibits pro-inflammatory mediator release from murine macrophages and human whole blood. Immunol Cell Biol. 2006;84:250–8. doi: 10.1111/j.1440-1711.2006.01426.x. [DOI] [PubMed] [Google Scholar]
- 21.Tak PP, Firestein GS. NF-kappaB: A key role in inflammatory diseases. J Clin Invest. 2001;107:7–11. doi: 10.1172/JCI11830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arias-Salvatierra D, Silbergeld EK, Acosta-Saavedra LC, Calderon-Aranda ES. Role of nitric oxide produced by iNOS through NF-kappaB pathway in migration of cerebellar granule neurons induced by Lipopolysaccharide. Cell Signal. 2011;23:425–35. doi: 10.1016/j.cellsig.2010.10.017. [DOI] [PubMed] [Google Scholar]
- 23.Girges MM. Synthesis and pharmacological evaluation of novel series of sulfonate ester-containing 1,3,4-oxadiazole derivatives with anticipated hypoglycemic activity. Arzneimittelforschung. 1994;44:490–5. [PubMed] [Google Scholar]
- 24.Idrees GA, Aly OM, Abuo-Rahma Gel D, Radwan MF. Design, synthesis and hypolipidemic activity of novel 2-(naphthalen-2-yloxy) propionic acid derivatives as desmethyl fibrate analogs. Eur J Med Chem. 2009;44:3973–80. doi: 10.1016/j.ejmech.2009.04.026. [DOI] [PubMed] [Google Scholar]


