Abstract
Thalidomide developed in 1954 for morning sickness had proven to be a teratogen and hence was withdrawn from market. Resurgence of thalidomide began as an immunomodulator when it was shown to be effective in the management of multiple myeloma and many conditions like erythema nodosum leprosum, graft versus host disease, recurrent aphthous ulcers etc. We report a case of Stevens Johnson syndrome-toxic epidermal necrolysis developing in an elderly male who was prescribed thalidomide after being diagnosed with multiple myeloma.
KEY WORDS: Multiple myeloma, SJS-TEN overlap, thalidomide
Introduction
Thalidomide, an oral agent with antiangiogenic and immunomodulatory properties, is being investigated extensively in the management of advanced cancer. Multiple myeloma is a B-cell malignancy characterized by an excess of monotypic plasma cells in the bone marrow. Multiple studies with large numbers of patients have proven that this drug has significant activity in multiple myeloma.[1] On May 26, 2006, the U.S. Food and Drug Administration (US-FDA) granted accelerated approval for thalidomide in combination with dexamethasone for the treatment of newly diagnosed multiple myeloma patients.[2]
Stevens-Johnson syndrome (SJS) is an immune-complex-mediated hypersensitivity complex that typically involves the skin and the mucous membranes with less than 10% body surface area (BSA) detachment. The more severe forms of the SJS spectrum are SJS-TEN overlap (10-30% BSA detachment) and toxic epidermal necrolysis (TEN) with > 30% detachment of BSA.
We report a case of SJS-TEN overlap in a patient of multiple myeloma treated with thalidomide.
Case Report
A 69-year-old normotensive non-diabetic male patient was newly diagnosed with multiple myeloma (56% plasmacytosis, gamma M1 = 3.2 in bone marrow aspirate) and was started on thalidomide monotherapy (200 mg once daily orally). Three days later, he developed fever, stinging eyes, and pain upon swallowing; along with skin lesions which appeared first on the trunk, spreading to the neck, face and proximal upper extremities. Later on, the entire body was involved along with erosion of mucous membrane. The skin lesions were tender, and the mucosal erosions were very painful. Over the next two days the erythema increased and some areas showed peeling of the skin as well. A local physician started paracetamol, antihistamine (cetirizine), proton pump inhibitor (pantoprazole) and topical application of calamine; and referred the patient to our tertiary care centre. On admission the cutaneous examination revealed erythematous, dusky red, purpuric macules of irregular sizes and shapes with a tendency to coalesce and involved approximately 25% of BSA [Figure 1]. Flaccid blisters with positive Asboe-Hansen sign were also noted in various parts of the body. Skin could be easily peeled away by tangential pressure, revealing large areas of raw and bleeding dermis beneath, thus conforming to positive Pseudo-Nikolsky sign. Mucous membrane involvement which had preceded the skin eruption, was characterized by erythema and painful erosions of the lips [Figure 2], buccal, ocular, and genital mucosa; leading to impaired food intake, photophobia, and painful micturition. Painful hemorrhagic erosions were coated by grayish white pseudomembranes and lips showed crusting. The case was diagnosed as SJS-TEN overlap. This past history revealed that he had taken first line anti-tubercular drugs for one month due to a possible diagnosis of tuberculosis and splenic abscess. These medications were stopped when the patient was diagnosed as multiple myeloma and the specific therapy for multiple myeloma (with thalidomide) began three weeks after diagnosis. Laboratory investigations showed he had acute kidney injury (serum creatinine 4 mg/dl), raised liver enzymes, anemia, leucocytosis, and thrombocytopenia.
Figure 1.
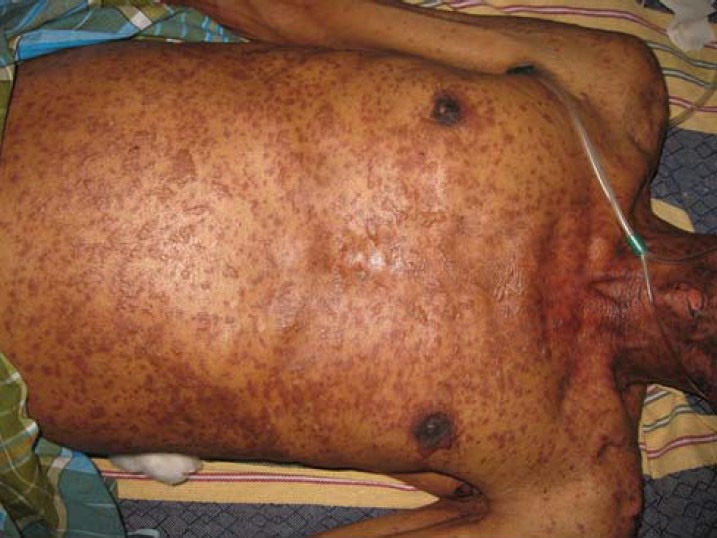
Purpuric macules (some surmounted by vesicles) scattered on the trunk and coalescing at areas to form sheets of necrotic epidermis
Figure 2.
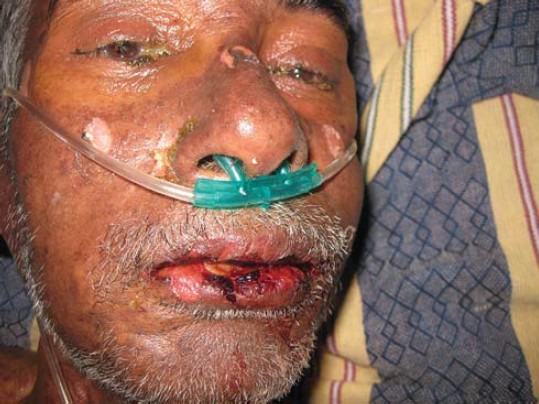
Erosion and crusting of lips with areas of peeling of necrotic epidermis on the bridge of nose and malar areas
Causality assessment was carried out using WHO-Uppsala monitoring centre (UMC) criteria and Naranjo's Scale. Both the algorithms labelled the reaction as “probable” (Naranjo's score = 5) relationship between the drug and development of SJS. Severity assessment by the Hartwig scale showed the reaction as severe (Level 5).
The patient was withdrawn from all the drugs he was having then (apart from thalidomide also those which were started later, that is, paracetamol, cetirizine, and pantoprazole). Apart from this, he was given supportive management, including intravenous fluids with a meticulous monitoring of intake-output chart, moist oxygen inhalation, maintenance of temperature chart. Blood sample was sent for serum electrolytes (Na+, K+, Cl- and HCO3-), culture and sensitivity. History was obtained from the patient that he had taken beta-lactum antibiotics in the past with no adverse reaction, hence intravenous injections of piperacillin-tazobactam combinations was started along with Condy's compress for the oozy lesions. Emollients were advised on lips and antibiotic eye ointment and artificial tears prescribed for eyes. In spite of the vigorous management, our patient did not show any signs of improvement manifested by deteriorating Glasgow-Coma score (GCS), reduction in urine output, increasing breathlessness and tachycardia; features suggestive of progress of the patient towards Acute Respiratory Distress Syndrome (ARDS) and unfortunately, he succumbed to the ongoing disease process.
Discussion
Thalidomide and immunomodulatory drugs (IMiDs) have now been shown to block several pathways important for disease progression in multiple myeloma. First established as agents with antiangiogenic properties, thalidomide and IMiDs inhibit the production of interleukin (IL)-6, which is a growth factor for the proliferation of myeloma cells. In addition, they activate apoptotic pathways through caspase 8-mediated cell death.
Thalidomide due to its immunomodulatory and antiangiogenic action had been considered as a possible treatment for SJS-TEN.[3] However, the only trial available on thalidomide usage did not show any benefit from the treatment compared to placebo, but highlighted increased chances of mortality in the active intervention group.[4]
Thalidomide can cause neurological, haematological, immunologic gastrointestinal, genitourinary, and dermatological side effects. Incomplete SJS and TEN in HIV-seropositive patients have been mentioned in the product information. However, extensive Pubmed search of relation between “Thalidomide, SJS” did not reveal a single report. There has been one report in which thalidomide caused TEN in a patient of glioblastoma.[5] In the above mentioned case report, the authors observed that although thalidomide-induced dermatologic disorders rarely were reported before thalidomide was administered to patients positive for the human immunodeficiency virus (HIV), hypersensitivity reactions including rash are the agent's major dose-limiting toxicities in them. As it is prescribed for other immunosuppressed patients, such as those with malignancies, the frequency of dermatologic reactions (including TEN) may increase. In our case, the patient had multiple myeloma, which compromised his immune status. Reports on a related drug, lenalidomide showed that it had caused SJS in patients of plasma cell leukemia, multiple myeloma.[6] However, whether this adverse drug reaction is a class effect has not been clearly delineated yet.
This case is the first reported case of SJS due to thalidomide. A drug which was researched into being the cure has caused the very disease it was intended to cure. Thus, caution regarding its usage in immunosuppressed patients is justified.
Footnotes
Source of Support: Nil
Conflict of Interest: No
References
- 1.Eleutherakis-Papaiakovou V, Bamias A, Dimopoulos AM. Thalidomide in cancer medicine. Ann Oncol. 2004;15:1151–60. doi: 10.1093/annonc/mdh300. [DOI] [PubMed] [Google Scholar]
- 2.FDA Approves Thalomid (thalidomide) to Treat Multiple Myeloma: U.S. Food and Drug Administration [homepage on the Internet] [Last updated on 2009 Nov 05, Last cited on 2014 Jun 6]. Available from: http://www.fda.gov/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/CDER/ucm095651.htm .
- 3.Namazi MR. Increased mortality in toxic epidermal necrolysis with thalidomide: Corroborating or exonerating the pathogenetic role of TNF-alpha? Br J Dermatol. 2006;155:842–3. doi: 10.1111/j.1365-2133.2006.07436.x. [DOI] [PubMed] [Google Scholar]
- 4.Wolkestein P, Latarjet J, Roujeau JC, Duguet C, Boudeau S, Vaillant L, et al. Randomised comparison of thalidomide versus placebo in toxic epidermal necrolysis. Lancet. 1998;352:1586–9. doi: 10.1016/S0140-6736(98)02197-7. [DOI] [PubMed] [Google Scholar]
- 5.Horowitz SB, Stirling AL. Thalidomide-induced toxic epidermal necrolysis. Pharmacotherapy. 1999;19:1177–80. doi: 10.1592/phco.19.15.1177.30571. [DOI] [PubMed] [Google Scholar]
- 6.Wäsch R, Jakob T, Technau K, Finke J, Engelhardt M. Stevens-Johnson/toxic epidermal necrolysis overlap syndrome following lenalidomide treatment for multiple myeloma relapse after allogeneic transplantation. Ann Hematol. 2012;91:287–9. doi: 10.1007/s00277-011-1235-y. [DOI] [PubMed] [Google Scholar]


