Abstract
Paired vegetable/soil samples from New York City and Buffalo, NY, gardens were analyzed for lead (Pb), cadmium (Cd) and barium (Ba). Vegetable aluminum (Al) was measured to assess soil adherence. Soil and vegetable metal concentrations did not correlate; vegetable concentrations varied by crop type. Pb was below health-based guidance values (EU standards) in virtually all fruits. 47% of root crops and 9% of leafy greens exceeded guidance values; over half the vegetables exceeded the 95th percentile of market-basket concentrations for Pb. Vegetable Pb correlated with Al; soil particle adherence/incorporation was more important than Pb uptake via roots. Cd was similar to market-basket concentrations and below guidance values in nearly all samples. Vegetable Ba was much higher than Pb or Cd, although soil Ba was lower than soil Pb. The poor relationship between vegetable and soil metal concentrations is attributable to particulate contamination of vegetables and soil characteristics that influence phytoavailability.
Keywords: urban vegetable gardening, plant metals uptake, soil particle adherence, lead, cadmium, barium
INTRODUCTION
Urban environments are variably contaminated with substances such as metals and persistent organic pollutants as a result of human activities including transportation, construction, manufacturing, fossil fuel combustion, and incinerator emissions (Alloway, 2004; Biasioli et al., 2007; Bradley et al., 1994; Norm et al., 2001; Peltola and Aström, 2003). Consequently, urban garden soils can be moderately to severely contaminated by one or more metals, with lead (Pb), cadmium (Cd), and mercury (Hg) reported to be most likely to pose some hazard for human health (Alloway, 2004; Chaney et al., 1984; Preer et al., 1980; Stilwell et al., 2008). Elevated barium (Ba) has also been found in urban environments, including soils and airborne particulate matter adjacent to roads (Monaci et al., 2000; Paterson et al., 1996). Because of the widespread use of Ba in manufactured materials such as tiles, automobile clutch and brake linings (ATSDR, 2007), rubber, brick, paint, glass, and other materials, unusually high concentrations of this metal in soils may be a marker for anthropogenic activity, including traffic (Monaci et al., 2000).
Numerous studies have investigated the relationship between metal contamination of urban garden soils and garden-raised foods, particularly for Pb and Cd (Alloway, 2004; Moir and Thornton, 1989; Sánchez-Camazano et al., 1994; Spliethoff et al., 2013). For Pb, the highest levels in vegetables generally occur where soil Pb levels are the highest (Bielinska, 2009; Huang et al., 2012; Jorhem et al., 2000; Moir and Thornton, 1989; Samsøe-Petersen et al., 2002). Some studies using sensitive methods for analyzing vegetable metals concentrations have indicated a near-linear relationship between soil Pb and vegetable Pb concentrations, so that bioconcentration factors (BCFs) could be estimated (e.g., 0.001, 0.002 and 0.05 for lettuce, potato and carrot [with peel], respectively) (Samsøe-Petersen et al., 2002). However, despite some success in linking concentrations of metals in vegetable crops to soil contamination levels, the results overall have been inconsistent, particularly for Pb (Hough et al., 2004; Jorhem et al., 2000; Peris et al., 2007; Samsøe-Petersen et al., 2002; Säumel et al., 2012).
The difficulty in establishing a quantitative relationship between vegetable Pb content and soil Pb content that is frequently (but not always) observed (Hough et al., 2004; Jorhem et al., 2000; Murray et al., 2011) may be in part because the typically low levels of soluble and bioavailable Pb are not simply dependent on the total soil Pb burden, but are subject to control by important soil properties including pH, organic matter content, and dissolved organic matter (Sauvé et al., 2000, 1998). For example, uptake reported for Pb is typically quite low except where soils are strongly acidic because of the strong tendency for Pb to be immobilized in neutral- and higher-pH soils by adsorption and precipitation reactions (Sauvé et al., 2000, 1998; Mosbaek et al., 1989). However, even after accounting for pH and other important soil properties, prediction of Pb uptake by vegetable crops from urban gardens has been generally unsatisfactory (Hough et al., 2004). Jorhem et al. (2000) found a relationship between vegetable Pb content and soil total Pb and pH, but their study sites represented a wider range of soil pH than seen in many studies of garden soils. For most garden soils, crop type has proven to be a stronger determinant of the edible crop metal concentration than soil contamination level (Alexander et al., 2006; Douay et al., 2013; Moir and Thornton, 1989; Samsøe-Petersen et al., 2002).
In order to make some sense of the apparently contradictory nature of Pb uptake results taken as a whole, which Jorhem et al. (2000) referred to as “information with a lack of unanimity,” it is necessary to consider some critical factors that have compromised data and obscured trends in many (particularly older) studies of Pb in food crops:
Insufficient analytical sensitivity to measure low Pb concentrations in vegetables. Market vegetables grown in uncontaminated rural regions have quite low Pb levels (e.g., median Pb concentrations < 0.006 mg/kg (US FDA, 2010, 2007)) that require very sensitive methods such as inductively coupled plasma mass spectrometry (ICP-MS) to measure correctly (McBride, 1998).
External sources of Pb (e.g., deposition from air). This was a major problem decades ago when leaded gasoline was in widespread use and could still be a concern in some urban environments. Many earlier plant uptake studies may have been compromised by aerial contamination of vegetables that obscured impacts of soil Pb on vegetable concentrations (Chamberlain, 1983; Dalenberg and Driel, 1990; Prasad and Nazareth, 2000; Sheppard and Evenden, 1992).
Highly variable physical contamination of vegetables (e.g., from dust, soil splash, traffic-related aerial contamination) related to local conditions, and the type of vegetable (with differing surface area and roughness of plants) (McBride et al., 2012; Nali et al., 2009; Säumel et al., 2012; Uzu et al., 2010).
Highly variable spatial distribution of Pb in soils of urban environments, from the scale of several hundred meters (Säumel et al., 2012; Shinn et al., 2000) to the minute scale of the root zone, soil aggregates and microscopic particles (Tai et al., 2013; Wharton et al., 2012).
The high degree of variability and apparent randomness inherent in data for Pb measured in vegetables is disconcerting, and indicates that even more intensive research will be needed to identify factors that contribute to vegetable contamination by metals such as Pb. Though available data are limited, when the number of environmental and soil variables is reduced, as is done with single-site field studies and greenhouse research with one particular soil containing a range of Pb concentrations, a clearer relationship between vegetable Pb and soil Pb emerges (McBride, 2013; and unpublished results).
The present investigation was undertaken as a follow-up to a study designed to measure trace metals of potential concern in urban community gardens of New York City (NYC). New York State (NYS) Department of Environmental Conservation Soil Cleanup Objectives for residential land use (NYSDEC, 2006) developed for the NYS Environmental Remediation Programs were considered as guidance values for comparison with garden soil metal concentrations in a study of more than 500 garden soil samples (Mitchell et al., 2014), which identified Pb and Ba as metals which commonly exceeded guidance values. Concentrations of arsenic (As), nickel (Ni), copper (Cu), chromium (Cr), and zinc (Zn) rarely exceeded guidance values in the results reported by Mitchell et al. (2014) and so were not included in the present study. Cd is included in the present study because of its potential for uptake into vegetables even at relatively low soil concentrations, and because analytical limitations encountered with inductively coupled plasma optical emission spectrometry (ICP-OES) in the pilot study led to uncertainty about the range of Cd concentrations present in the soils.
The immediate objectives of the present study were to measure: 1. Total Pb, Cd and Ba in the washed edible portion of a range of vegetable types grown in community gardens and farms of urban areas in NYS, 2. These same metals in soil samples collected from the exact location of vegetable collection, and 3. Aluminum (Al) in the vegetables. Al is quite insoluble and unavailable for plant uptake at the near-neutral pH levels generally found in urban garden soils, and its presence in vegetable samples can be taken as an indicator of the physical presence of soil particles either in or on the washed vegetable samples (McBride et al., 2012). For example, washed vegetables were found to contain from 0.07 to 4.88% soil on a dry-weight basis (using immobile soil elements such as Al as indicators of vegetable contamination) (Cary et al., 1994).
The overall goals of the study were: 1. To determine the degree to which the concentrations of these metals in vegetables could be linked to metals concentrations in soils, 2. To compare metals concentrations in urban garden produce with concentrations found in market-basket produce, and 3. To determine whether consumption of the urban garden produce could represent a significant health hazard based on comparison with available health-based guidance values.
MATERIALS AND METHODS
Vegetable samples (80 fruiting vegetables, 67 leafy, 16 herb and 32 roots) were collected over the growing seasons in 2011 and 2012 from seven community gardens in NYC and ten gardens and urban farms (hereafter referred to as “gardens”) in Buffalo, NY. Each vegetable sample was collected simultaneously with a paired surface soil sample (0–15 cm) from the same location in the garden plot. A total of 195 pairs of soil and vegetable samples were processed (160 from NYC and 35 from Buffalo). The number of soil/vegetable pairs collected from a particular garden varied widely depending on sample availability. In some cases, samples of multiple crop types were harvested from the same garden bed. Sampling locations were not pre-screened for soil contaminant level, as the intent was to collect samples from a range of soils. Upon arrival at Cornell University, vegetable samples were washed thoroughly under tap water, scrubbed with a vegetable brush when necessary to remove visible soil (e.g., for all root crops), and blotted dry with paper towels. Root crops were not peeled. The vegetables were cut into small pieces, placed into labeled brown paper bags (most vegetables) or open-topped glass jars (for juicy vegetables such as tomatoes), and dried in an oven at 70°C for several days to a week until the samples appeared dry based on visual inspection. Once dry, samples were ground into a coarse powder using a coffee grinder, and stored in sealed labeled Whirl-Pak™ bags for later digestion and analysis. The grinder was cleaned of all plant residue between samples using a pressurized air stream to prevent cross-contamination.
Soil samples were prepared by air-drying in a laboratory hood for several days and passing through a 2 mm plastic sieve, and were subsequently stored in closed cardboard containers. Soil pH was determined by weighing out 10 g of each soil sample from the cardboard containers into small glass jars, adding 20 mL of distilled water, mixing the soil-water slurry, allowing it to stand for 30 min, and determining the pH of the supernatant using a glass electrode.
All samples underwent microwave digestion with HNO3 (US EPA SW-846 Method 3051, (US EPA, 2012) prior to metals analysis. Soil samples were analyzed for Pb, Cd, and Ba either by a commercial laboratory (H2M Labs, Inc.) certified by the NYS Environmental Laboratory Approval Program using ICP- MS (US EPA SW-846 Method 6020) or by the Cornell Nutrient Analysis Laboratory (CNAL) using ICP-OES (US EPA SW-846 Method 6010) (US EPA, 2012). 39 soils were analyzed by H2M, and 156 by CNAL. The majority of vegetable samples were analyzed for Pb, Cd, Ba, and Al by H2M using ICP-MS; a small number of samples (N=30) were analyzed by CNAL using ICP-MS for Pb and Cd, and ICP-OES for Al and Ba. Standard protocols for quality assurance were followed. Procedures used to ensure precision and accuracy in the measurements of metals in the vegetables and soils included the use of certified plant and soil standards as well as laboratory internal standards in the sample sets, along with duplicate samples and blanks in each sample set. Interlaboratory comparisons of metal analysis results from sample subsets verified acceptable analytical agreement between the two labs. The commercial laboratory analyzed a single digest from each plant sample for Pb, Cd, Ba and Al simultaneously. The relatively high concentrations of Al and Ba required that the extracts be significantly diluted prior to analysis. This dilution resulted in fairly high detection limits for tissue Pb (0.1 or 0.6 mg kg−1) and Cd (0.25 mg kg−1). (By comparison, CNAL reported lower vegetable detection limits near 0.04 mg kg−1 for Pb and 0.02 mg kg−1 for Cd, as this laboratory separately analyzed Al and Ba in the plant digests by ICP-OES.)
Because all vegetable sample analyses are reported on a d.w. basis, the plant tissue metal concentrations were converted to fresh weight (f.w.) concentrations using reported moisture contents of the different vegetable types (US EPA, 1997) to facilitate comparison to market-basket concentrations (US FDA, 2010, 2007) and guidance values based on European Union (EU) food standards (EC, 2006). Non-parametric Mann-Whitney U tests were used to compare metals concentrations among pairs of vegetable types, as vegetable results were not normally distributed. Spearman correlation coefficients were calculated using SAS software (v. 9.3) (SAS Institute, Inc., 2010) to evaluate relationships between paired soil and vegetable metals results.
RESULTS AND DISCUSSION
Soil Results
The frequency distributions of Pb, Cd and Ba concentrations measured in the garden bed soil samples are described in Table 1. The data are not normally distributed (Kolmogorov-Smirnov test, p < 0.010), with median (50th percentile) metal concentrations lower than mean concentrations. The widest range of soil metal concentration was for Pb (about 200-fold), with less variation for Ba (39-fold), and the least for Cd (14-fold, with just one sample below the detection limit).
Table 1.
Pb, Cd and Ba concentrations and pH in sampled urban garden soils in NYC and Buffalo, NY (n = 195).
| Percentile
|
|||||||
|---|---|---|---|---|---|---|---|
| Metal | Mean | Min | 25th | 50th | 75th | 95th | Max |
| Pb | 292 | 17.5 | 100 | 158 | 303 | 934 | 3580 |
| Cd | 0.87 | <0.25 | 0.58 | 0.76 | 1.00 | 1.70 | 3.67 |
| Ba | 144 | 30.9 | 70.1 | 93.7 | 162 | 418 | 1210 |
| pH | 6.9 | 5.5 | 6.7 | 6.9 | 7.2 | 7.8 | 8.2 |
Pb, Cd and Ba are reported in mg kg−1. The one Cd result below the detection limit was considered equal to half the detection limit in calculating mean values.
The fractions of sampled garden soils that exceeded health-based guidance values (residential Soil Cleanup Objectives used in NYS Environmental Remediation Programs; NYSDEC, 2006) for Pb (400 mg kg−1), Cd (2.5 mg kg−1) and Ba (350 mg kg−1) were 13.3%, 2.6% and 6.2%, respectively.
The pH in the garden bed soils averaged 6.94, with only 24 of the 195 soils collected below pH 6.5 and 2 below pH 6.0. The near-neutral pH combined with relatively high organic matter contents of these soils (Mitchell et al. (2014) reported carbon contents ranging from 1% – 27%, with a median of 4.9 %, in an earlier study of 508 NYC community garden beds) is anticipated to greatly limit the plant availability of Pb and Cd in particular, both of which are strongly adsorbed by organic matter under non-acidic conditions (Basta et al., 1993; Guo et al., 2006).
Vegetable Results
Vegetables were grouped into 4 types (leafy greens, herbs, roots, and fruits) for statistical analysis, as trace metals are known to be accumulated to different degrees in different plant tissues (Douay et al., 2013). Herbs were grouped separately from leafy greens because preliminary results showed a greater tendency for Pb to accumulate in garden-grown herbs than in salad greens such as lettuce, and because gardeners may be likely to consume herbs to a smaller degree than most other leafy greens. The fruit grouping for the purpose of this study is broad, consisting predominantly of vegetable fruits (e.g., tomato, pepper, cucumber, green beans), but also including non-vegetable fruits (e.g., grapes, raspberries).
A summary of the results of vegetable analyses for Pb, Cd, Ba and Al is provided in Table 2, where all metal concentrations have been converted to a f.w. basis for ease of comparison to health-based guidance values (EC, 2006) and concentrations in market vegetables (US FDA, 2010, 2007). Because this study did not measure vegetable moisture contents, vegetable metals concentrations were converted to f.w. concentrations using a published mean moisture content (US EPA, 1997) for each crop, which adds a degree of uncertainty to the results presented in Table 2. Comparisons between vegetable and soil results were done using metals concentrations measured in dried vegetables, avoiding the added uncertainty associated with conversion to f.w. concentrations (see all d.w. concentrations in Supplementary Table S).
Table 2.
Concentrations of Pb, Cd, Ba and Al in vegetables sampled from urban gardens in NYC and Buffalo, NY.
| Metal | Veg type | Measured Values
|
Market Basket Comparisonc
|
Guidance Value (EC, 2006) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Meana | Min | Mediana | Max | %ND | Min | Media n | 95th pct. | Max | |||
| Pb | Fruit | 0.018 | 0.0023b | 0.012 | 0.21 | 46% | <0.005 | <0.005 | 0.009 | 0.04 | 0.1 |
| Leafy | 0.099 | 0.010b | 0.059 | 0.59 | 7% | <0.004 | <0.004 | 0.022 | 0.14 | 0.3 | |
| Herb | 0.44 | 0.085 | 0.25 | 2.1 | 0% | N/A | N/A | N/A | N/A | N/A | |
| Root | 0.20 | 0.014b | 0.089 | 1.9 | 9% | <0.006 | <0.006 | 0.011 | 0.033 | 0.1 | |
|
| |||||||||||
| Cd | Fruit | 0.014 | 0.0021b | 0.0076 | 0.36 | 81% | <0.001 | 0.006 | 0.026 | 0.053 | 0.05 |
| Leafy | 0.028 | <0.013 | 0.019 | 0.20 | 40% | 0.002 | 0.039 | 0.21 | 0.52 | 0.2 | |
| Herb | 0.023 | <0.029 | 0.015 | 0.060 | 75% | N/A | N/A | N/A | N/A | 0.2 | |
| Root | 0.022 | <0.013 | 0.015 | 0.074 | 63% | <0.002 | 0.015 | 0.038 | 0.081 | 0.1 | |
|
| |||||||||||
| Ba | Fruit | 0.24 | 0.028 | 0.11 | 1.5 | 0% | N/A | N/A | N/A | N/A | N/A |
| Leafy | 3.7 | 0.37 | 2.4 | 22 | 0% | 0.16 | 0.59 | 3.2 | 3.7 | N/A | |
| Herb | 4.7 | 0.54 | 4.4 | 14 | 0% | N/A | N/A | N/A | N/A | N/A | |
| Root | 2.5 | 0.32 | 2.3 | 8.9 | 0% | 1.1 | 2.4 | 3.7 | 4.1 | N/A | |
|
| |||||||||||
| Al | Fruit | 0.30 | 0.079 | 0.25 | 1.7 | 0% | N/A | N/A | N/A | N/A | N/A |
| Leafy | 2.4 | 0.17 | 1.3 | 17 | 0% | N/A | N/A | N/A | N/A | N/A | |
| Herb | 5.2 | 0.88 | 2.9 | 39 | 0% | N/A | N/A | N/A | N/A | N/A | |
| Root | 0.89 | 0.36 | 0.64 | 3.6 | 0% | N/A | N/A | N/A | N/A | N/A | |
All concentrations are reported in mg kg−1 f.w. Sample sizes for each category: fruit (80), leafy (67), herb (16), and root (32).
Results below the detection limit were considered equal to half the detection limit in determining mean and median values.
Minimum detected concentration. Some samples in which Pb and/or Cd were not detected had a detection limit higher than this concentration. Detection limits ranged up to 0.038 mg kg−1 f.w. for Pb and 0.021 mg kg−1 f.w. for Cd.
Market basket concentrations for Pb and Cd are based on graphite furnace atomic absorption spectroscopy analytical results reported by the US FDA for the Total Diet Study. Results were for 384 fruit, 280 leafy, and 360 root vegetable samples (US FDA, 2010, 2007). We measured market-basket concentrations for Ba in vegetables (6 root and 9 leafy samples) purchased at three markets in the Ithaca, NY, area.
Lead
The average concentration of Pb in the garden-grown vegetables varied by type as shown in Table 2 (f.w) and Figure 1 (d.w.). On a fresh weight basis, herbs had the highest concentrations (> leafy and fruit, p < 0.0001), followed by root crops (> leafy and fruit, p < 0.0001), with fruits having the lowest concentrations (< leafy, root, and herb, p < 0.0001). Although vegetable type was clearly an important predictor of vegetable Pb concentration, individual paired soil Pb concentrations were not. For fruits, herbs, and root crops, there was no significant (p > 0.05) relationship of crop Pb to soil Pb; even for leafy greens, there was only a weak tendency for crops grown in higher Pb soils to be more Pb-contaminated. Linear regression analysis of log-transformed data showed soil Pb to account for only about 10% of the variability in leafy vegetable Pb content, as shown in Figure 2. Thus, the wide range of Pb concentration within each crop type (from 26-fold in herbs to 140-fold in roots) was largely unexplained by the soil Pb concentration. There was also no significant relationship between Pb concentrations in vegetables and soil pH.
Figure 1.
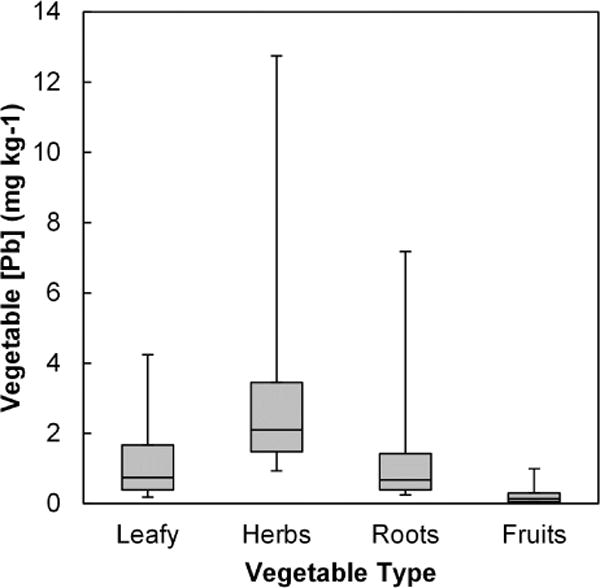
Pb concentrations in garden-grown vegetables by crop type (mg kg−1 d.w.). Boxes represent 25th, 50th and 75th percentiles. Whiskers extend to 5th and 95th percentiles.
Figure 2.
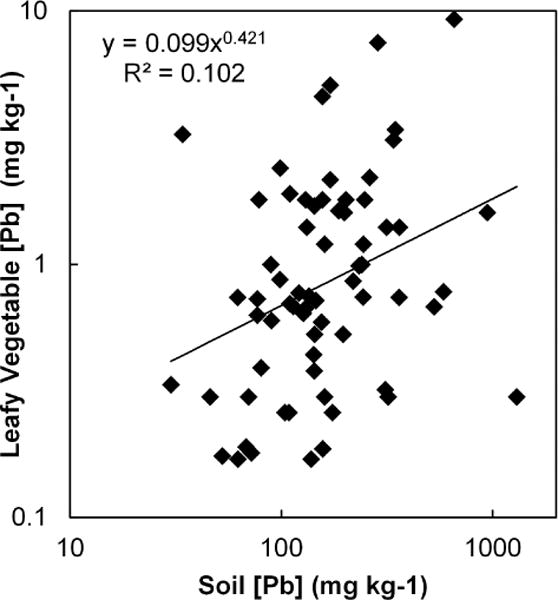
Relationship of leafy vegetable Pb concentrations (mg kg−1 d.w.) to soil total Pb concentration.
There are no accepted health-based standards in the United States with which to compare the measured Pb in vegetables. EU standards, which vary by vegetable type and limit contaminant concentrations in foods that may be sold commercially (EC, 2006), were used as guidance values. Only one of 80 fruit samples exceeded the 0.1 mg kg−1 fruit guidance value, but 15 root crops (nearly half; mostly carrots) exceeded the 0.1 mg kg−1 root guidance value, and 6 leafy greens (9%) exceeded the 0.3 mg kg−1 guidance value for leafy vegetables. There is no guidance value (i.e., EU standard) for Pb in herbs. Vegetable Pb concentrations were elevated compared to concentrations measured in market-basket samples, with more than half of garden vegetable results exceeding the 95th percentile of market-basket concentrations (US FDA, 2010, 2007).
The poor ability of soil Pb to predict plant Pb is perhaps understood in view of the fact that vegetable Al content was a strong predictor of vegetable Pb for leafy greens and herbs, and to lesser extent for fruit crops (all regressions were statistically significant with p < 0.01). Only the root crops did not have a statistically significant (p > 0.05) relationship of vegetable Pb to vegetable Al. The relationship is shown in Figure 3 for leafy greens and herbs only (samples for these two crop types were combined because of similar morphology/physiology). The Al content of the crop, which indicates the extent of physical contamination of plant tissue by soil particles (either adhered to plant surfaces or incorporated into plant tissue (Uzu et al., 2010), explains about 44% of the variability of Pb concentration in the sampled leafy greens and herbs, much more than soil Pb concentration. Soil particles containing mineral Al are associated with Pb, and processes such as splash and wind contaminate plant surfaces with these particles. An even higher percentage of the variability (66%) of Pb concentration in the leafy greens and herbs can be explained by an indicator of the adhered soil lead, estimated by calculating the product of the tissue Al concentration (as an indicator of adhered soil) and the soil Pb concentration (regressions not shown). However, this indicator of particulate Pb contamination did not explain a higher percentage of Pb concentration variability in fruits than Al alone, nor did it raise the regression r-value to a level of significance in root crops.
Figure 3.
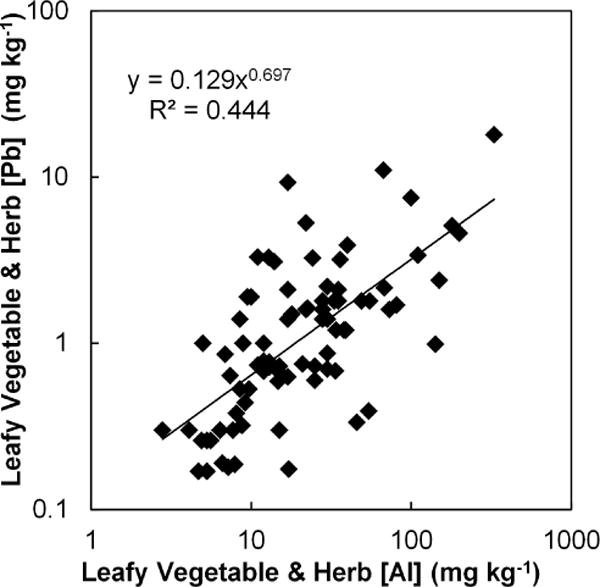
Relationship of Pb concentrations (mg kg−1 d.w.) in leafy vegetables and herbs to Al concentrations in leafy vegetables and herbs.
The fact that vegetable Pb content was correlated to vegetable Al more strongly than to soil Pb is an indication that particulate contamination of vegetables from the soil or from aerosols is more important than plant uptake via roots in transferring Pb into the crop. This is supported by Mosbaek et al. (1989), who found that plant root (physiological) uptake of Pb from non-acid soils is low (0.02 – 0.10 mg/kg d.w.) with the exception of carrots (up to 0.3 mg/kg d.w.); most of the Pb they measured in plants (1–10 mg/kg in many ag crops) was from airborne contamination. Since urban aerosols as well as soil particles can have high Al/Pb ratios (Smichowski et al., 2004), the results found here do not exclude the possibility that atmospheric aerosols from local or non-local sources are contributing to garden vegetable Pb contamination. The variability of individual soil particle composition, the random nature of soil particle adherence and incorporation into plant tissues, and the possible contribution of non-local urban aerosols may have contributed to the very weak relationship between soil Pb and vegetable Pb concentrations.
Recent research has shown that the local settings of urban gardens affected trace metal contamination of vegetable crops, with Pb concentrations being higher in leafy vegetables, fruits and roots grown in gardens with higher traffic burdens (Säumel et al., 2012), even in places where the sale of leaded gasoline has long been banned, as it has been in the U.S. since 1996. This trend could indicate that particle dispersal and aerial redeposition over relatively short distances is contributing to vegetable contamination, and obscuring the effect of soil Pb concentration in the garden beds.
Cadmium
The Cd concentrations in the vegetables, summarized in Figure 4, were with few exceptions quite low compared to Pb. Overall, leafy green vegetables contained the highest Cd concentrations; roots, herbs and fruits had the lower concentrations (< leafy; p < 0.0001), although the high percentage of results below the detection limit makes statistical comparisons among the latter three categories less meaningful. The range of measured Cd concentrations within each vegetable grouping was as high as 168-fold (in fruits), and as low as 3-fold (in herbs); however, given the large proportion of samples with Cd below the detection limit, the actual ranges may have been greater. As noted below, this variation could not be explained by soil Cd levels.
Figure 4.
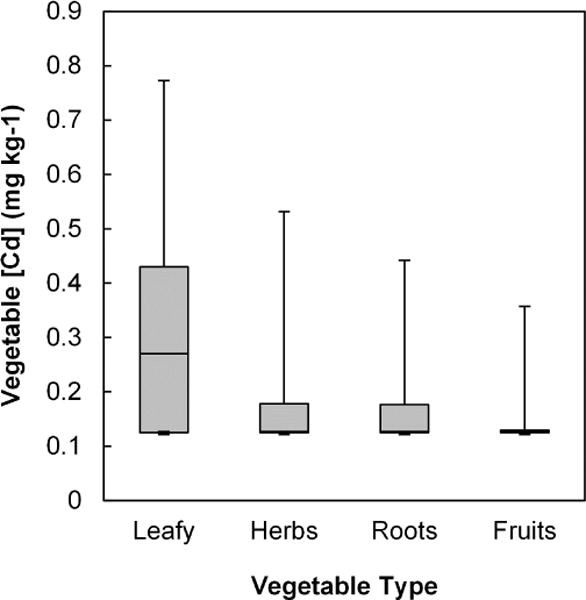
Cd concentrations in garden-grown vegetables by crop type (mg kg−1 d.w.). Boxes represent 25th, 50th and 75th percentiles. Whiskers extend to 5th and 95th percentiles. Cd was detected in < 25% of leafy vegetables, < 50% of herbs and roots, and < 75% of fruits.
Uncontaminated rural and agricultural soils in the Northeastern USA generally have low total Cd concentrations, in the range of 0.1–0.3 mg/kg (US EPA, 2005; Holmgren et al., 1993; McBride, 2011). Conversely, Cd in soils of urban community gardens averaged substantially higher than this rural background, sometimes exceeding 1.0 mg/kg (Mitchell et al., 2014). Linear regression analysis (not shown) indicated no significant positive relationship between vegetable and soil Cd concentration. Furthermore, there was no significant relationship of Cd concentration in any of the vegetable types to soil pH.
There are no accepted health-based standards in the United States with which to compare the measured Cd in vegetables. A comparison to EU standards, which vary by vegetable type and were used as guidance values, shows that no leafy greens, herbs or root crops exceeded the Cd guidance values (0.2 mg kg−1 for leafy greens and herbs and 0.1 mg kg−1 for roots (f.w.)). One vegetable fruit (okra) exceeded the 0.05 mg kg−1 (f.w.) Cd guidance value. This exceedance could be explained by unusually high soil Cd as described above. Concentrations of Cd in garden vegetable samples were similar to market-basket concentrations (US FDA, 2010, 2007). The fact that Cd concentrations in vegetables from the urban gardens were not higher than concentrations measured in market vegetables may reflect the importance of soil pH and organic matter in limiting soil Cd phytoavailability. The urban garden bed soils were on average more alkaline (median 6.9; range 5.5–8.2) and much higher in organic matter than most agricultural soils where vegetables are grown commercially. For example, a previous study that included some of the same gardens as this investigation reported carbon contents ranging from 1% to 27%, with a median of 4.9% (Mitchell et al., 2014); typically, agricultural soils contain levels of organic carbon ranging from 1 to 2% (Brady, 1990). Since almost all (87%) of the garden soils had pH > 6.5, the low Cd concentrations in leafy greens, which are known as strong accumulators for this metal (McBride, 2003), can be attributed to low Cd phytoavailability in the soils. Cd concentrations in vegetables were not correlated to soil Cd concentrations in this study, probably because the near-neutral pH of most soils would act to strongly limit plant uptake. Assessment of the relationship between Cd and Al was complicated by the low rate of Cd detection in vegetables. Among vegetables with detectable Cd, there was only a weak relationship between Cd and Al concentrations.
Barium
Barium concentration in the vegetables followed the order of: leafy greens and herbs > roots >> fruits, as shown in Figure 5. Concentrations in fruits were significantly lower than in herbs, leafy greens and roots (p < 0.0001). Concentrations of Ba in all vegetables were much higher than those of Pb or Cd. However, the ranges of Ba concentrations within vegetable groupings (from a 26-fold variation for herbs to a 59-fold variation for leafy greens) were smaller than for Pb or Cd. This variation could not be explained by soil Ba concentrations, as there was no significant correlation between total soil Ba and crop Ba for any of the vegetable types. There was also no significant relationship between Ba in vegetables and soil pH. There was a weak statistically significant relationship between vegetable Ba and vegetable Al for leafy greens and herbs (r2 = 0.122, p < 0.01) and for fruits (r2=0.084, p < 0.084), but not for root crops. Because these relationships of vegetable Ba to Al are weaker than those of vegetable Pb, it is unlikely that physical contamination accounts for most of the Ba content of the vegetables, and plant uptake via roots appears to be the predominant process of soil-plant transfer.
Figure 5.
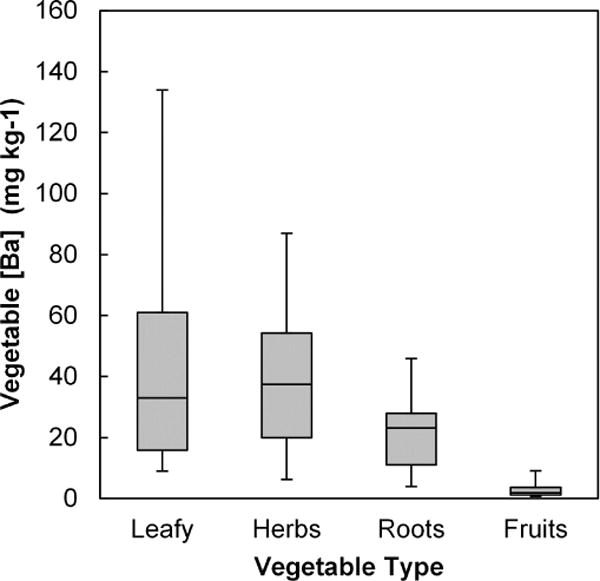
Ba concentrations in garden-grown vegetables by crop type (mg kg−1 d.w.). Boxes represent 25th, 50th and 75th percentiles. Whiskers extend to 5th and 95th percentiles.
Because very few data are reported for Ba in vegetable crops, we conducted a limited survey of supermarket vegetables. We purchased leafy green (n=9) and root vegetables (n=6) from three markets near Ithaca, NY. Ba concentrations in the leafy vegetables were variable (d.w. mean 11.9 mg kg−1, range 2.7 – 33 mg kg−1), with somewhat higher and less variable concentrations in the root crops (d.w. mean 19.1 mg kg−1, range 9.4 – 32 mg kg−1). A comparison of these data with the average Ba measured in the urban garden leafy greens (45.7 mg kg−1 d.w.) and root crops (21.5 mg kg−1 d.w.) suggests that the garden leafy vegetables are higher in Ba than market vegetables, but garden root crops have Ba concentrations similar to those from the market. However, because no health-based standards or guidance values exist for Ba in food crops, the results reported here are not interpreted in the context of risk.
The Ba concentrations in leafy vegetables and root crops were much higher than Pb or Cd, and are much too high to be explained mainly by physical contamination when it is considered that soil total Ba concentrations are lower than soil Pb concentrations (see Table 1). Recent research has indicated a potential for substantial Ba uptake into leafy crops in particular, with concentrations reaching 100 mg kg−1 (d.w.) or higher (Lamb et al., 2013; Nabulo et al., 2012). One greenhouse study demonstrated Ba uptake into vegetables grown in soils spiked with barium sulfate (Coscione and Berton, 2009). Thus, the Ba contents of these urban garden vegetables can be reasonably attributed to plant uptake via roots, with minimal transfer into the fruiting parts of the vegetables. Despite the evidence for physiological uptake, soil total Ba failed to predict crop Ba content, probably because the solubility of Ba in soils is low and dependent on a number of soil properties (Lamb et al., 2013). Our limited survey suggests that similarly high Ba concentrations in leafy green and root vegetables purchased at the market are commonly encountered (Table 2).
Aluminum
Aluminum concentrations varied considerably among vegetable types, with herbs having the highest concentrations (> root and fruiting vegetables; p < 0.0001), followed by leafy (> fruits; p < 0.0001), root, and fruiting vegetables (< leafy, root and herb; p < 0.0001). Leafy vegetables had the biggest range of Al concentrations (100-fold), while root crop had the smallest (10-fold).
Tissue aluminum is generally an indicator of adhered or physically incorporated soil (Cary et al., 1994), and the lack of published estimates of soil adherence on vegetables was recently identified by US EPA in their Technical Review Workgroup Recommendations Regarding Gardening and Reducing Exposure to Lead-Contaminated Soils (US EPA, 2013) as a significant data gap that affects the ability to assess garden-related Pb exposure. Vegetable Al concentrations from our study can be used in conjunction with estimates of soil aluminum concentrations to calculate the approximate amount of soil adhered to or physically incorporated into vegetables. Although Al was not measured in this study’s soil samples, an earlier study of 564 soil samples from 54 NYC community gardens found Al concentrations ranging from 1,620 to 12,500 mg/kg, with a median concentration of 5,680 mg/kg (Mitchell et al., 2014). Dividing the median Al concentration for each vegetable type (Table 2) by the median Al concentration in soil yields soil adherence estimates (as a percentage of vegetables’ fresh weight) of 0.004% for fruits, 0.011% for roots, 0.023% for leafy vegetables and 0.058% for herbs. Considering a wider range of Al concentrations in soil (25th – 75th percentile) leads to estimated ranges of 0.004%0.005% for fruits, 0.009% to 0.014% for roots, 0.019% – 0.027% for leafy vegetables, and 0.048% to 0.070% for herbs. Based on these estimates, as a percentage of weight, herbs, with their smaller leaves and greater surface area relative to other crops, have approximately twice as much adhered soil by mass as leafy vegetables, and can have more than ten times as much adhered soil as fruiting vegetables, which tend to be least affected by physical contamination by soil particles.
A number of factors contribute to uncertainty in these estimates of soil adherence. For instance, particles that adhere to plant surfaces may have a different size distribution than that of bulk soil. Al may be enriched in the smaller particle-size fraction of bulk soil (Luo et al., 2011); thus, preferential adherence of smaller particles to vegetables would result in inflated estimates of soil adherence. Additionally, the sample digestion method used in this study may not extract all the Al in bulk soil (Chen and Ma, 1998). Estimates of soil adherence could also be inflated if the method resulted in a higher extraction efficiency for the smaller soil particles adhered to vegetables.
Additional uncertainty could be associated with the assumption that Al is not substantially taken up by vegetable crops. However, almost all crop plants are non-accumulators which exclude Al from above-ground tissues (Marschner, 1995).
Implications
Based on the concentrations of Pb, Ba and Cd reported here in urban garden soils, Pb is the metal most likely to exceed health-based guidance values in NYC and Buffalo community garden soils, followed by Ba, with Cd having the lowest likelihood of exceeding soil guidance values based on residential Soil Cleanup Objectives for Environmental Remediation projects in NYS (NYSDEC, 2006). This result is in agreement with a larger-scale study of community garden soils conducted earlier (Mitchell et al., 2014).
Contaminant levels found in urban garden produce were generally below health-based guidance values and consistent with levels associated with foods that can be sold legally (i.e., in Europe); therefore consumption of urban garden produce should not present a significant health hazard.
The lack of correlation between vegetable and paired soil concentrations for Pb, Cd and Ba can be attributed to several important factors, including the strong effect that soil pH, organic matter content and other soil properties have on metal solubility and bioavailability. Patterns of plant growth may also influence metals concentrations; for instance, it is possible that the perennial nature of many herb crops may contribute to this crop type having the highest measured mean concentrations of Pb and Ba. Most critically, much of the vegetable content of Pb appears to be due to physical contamination by soil particles or aerosols, a process that is highly random by nature. Finally, our studies have shown that metals such as Pb and Ba are not distributed homogeneously in these urban soils, but are concentrated in “nuggets” (Wharton et al., 2012), a characteristic that complicates the assessment of metal bioavailability and its relationship to metal concentration in soils. The particularly high Pb concentrations in some garden beds present a risk, but also a need and opportunity for intervention and management to reduce exposure. Although our findings support the gardener exposure reduction practice of growing fruit crops in preference to leafy and other crop types, the application of other best practices to minimize gardener exposures to vegetable contaminants is complicated by the variability in contaminant levels seen in garden vegetables, difficulties in quantifying the relationship between soil and vegetable concentrations, and evidence for adhered soil or aerosols that cannot be easily removed by washing.
Supplementary Material
Highlights.
We analyzed metals (Pb, Ba and Cd) in paired urban garden vegetable/soil samples.
Soil and vegetable metal concentrations did not correlate.
Metals levels were highest in leafy/herb and lowest in fruiting vegetables.
Some vegetable Pb exceeded market-basket levels and guidance values (EU standards).
Soil particle adherence increases metal concentrations in vegetables.
Acknowledgments
Funding for this research was provided by the National Institute of Environmental Health Sciences (NIEHS), Award Number R21ES017921. The content is solely the responsibility of the authors and does not necessarily represent the official views of NIEHS or the National Institutes of Health. We greatly appreciate the contributions of members of the Healthy Soils, Healthy Communities project team, gardeners, and others who facilitated sample collection and analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander PD, Alloway BJ, Dourado AM. Genotypic variations in the accumulation of cadmium, copper, lead and zinc exhibited by six commonly grown vegetables. Environ Pollut. 2006;144:736–745. doi: 10.1016/j.envpol.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Alloway BJ. Contamination of soils in domestic gardens and allotments: a brief overview. Land Contam Reclam. 2004;12:179–187. doi: 10.2462/09670513.658. [DOI] [Google Scholar]
- ATSDR. Toxicological profile for Barium. U.S. Department of Health and Human Services, Public Health Service; Atlanta, GA: 2007. [Google Scholar]
- Basta NT, Pantone DJ, Tabatabai MA. Path Analysis of Heavy Metal Adsorption by Soil. Agron J. 1993;85:1054–1057. doi: 10.2134/agronj1993.00021962008500050018x. [DOI] [Google Scholar]
- Biasioli M, Grcman H, Kralj T, Madrid F, Díaz-Barrientos E, Ajmone-Marsan F. Potentially toxic elements contamination in urban soils: a comparison of three European cities. J Environ Qual. 2007;36:70–79. doi: 10.2134/jeq2006.0254. [DOI] [PubMed] [Google Scholar]
- Bielinska EJ. Influence of the root layer on the content of cadmium and lead in soils and vegetable plants in regions with diverse anthropogenic impact. J Res Appl Agric Eng. 2009;54:16–20. [Google Scholar]
- Bradley LJN, Magee BH, Allen SL. Background levels of polycyclic aromatic hydrocarbons (PAH) and selected metals in new England urban soils. J Soil Contam. 1994;3:349–361. doi: 10.1080/15320389409383475. [DOI] [Google Scholar]
- Brady NC. The Nature and Properties of Soils. 10. MacMillan; New York: 1990. [Google Scholar]
- Cary EE, Grunes DL, Dallyn SL, Pearson GA, Peck NH, Hulme RS. Plant Fe, Al, and Cr concentrations in vegetables as influenced by soil inclusion. J Food Qual. 1994;17:467–476. [Google Scholar]
- Chamberlain AC. Fallout of lead and uptake by crops. Atmospheric Environ. 1983;17:693–706. doi: 10.1016/0004-6981(83)90416-X. 1967. [DOI] [Google Scholar]
- Chaney RL, Sterrett SB, Mielke HW. Proceedings of the Symposium on Heavy Metals in Urban Gardens. Agricultural Experiment Station, University of the District of Columbia; Washington: 1984. The potential for heavy metal exposure from urban gardens and soils; pp. 37–84. [Google Scholar]
- Chen M, Ma LQ. Comparison of Four USEPA Digestion Methods for Trace Metal Analysis Using Certified and Florida Soils. J Environ Qual. 1998;27:1294–1300. doi: 10.2134/jeq1998.00472425002700060004x. [DOI] [Google Scholar]
- Coscione AR, Berton RS. Barium extraction potential by mustard, sunflower and castor bean. Sci Agric. 2009;66:59–63. doi: 10.1590/S0103-90162009000100008. [DOI] [Google Scholar]
- Dalenberg JW, van Driel W. Contribution of atmospheric deposition to heavy-metal concentrations in field crops. Neth J Agric Sci. 1990;38:369–379. [Google Scholar]
- Douay F, Pelfrêne A, Planque J, Fourrier H, Richard A, Roussel H, Girondelot B. Assessment of potential health risk for inhabitants living near a former lead smelter. Part 1: metal concentrations in soils, agricultural crops, and homegrown vegetables. Environ Monit Assess. 2013;185:3665–3680. doi: 10.1007/s10661-012-2818-3. [DOI] [PubMed] [Google Scholar]
- EC. Commission Regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off J Eur Union L. 2006;364:06–24. [Google Scholar]
- Guo X, Zhang S, Shan XQ, Luo LEI, Pei Z, Zhu YG, Liu T, Xie YN, Gault A. Characterization of Pb, Cu, and Cd adsorption on particulate organic matter in soil. Environ Toxicol Chem SETAC. 2006;25:2366–2373. doi: 10.1897/05-636r.1. [DOI] [PubMed] [Google Scholar]
- Holmgren GGS, Meyer MW, Chaney RL, Daniels RB. Cadmium, lead, zinc, copper and nickel in agricultural soils of the United States of America. J Environ Qual. 1993:335–348. [Google Scholar]
- Hough RL, Breward N, Young SD, Crout NMJ, Tye AM, Moir AM, Thornton I. Assessing potential risk of heavy metal exposure from consumption of home-produced vegetables by urban populations. Environ Health Perspect. 2004;112:215–221. doi: 10.1289/ehp.5589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang ZY, Chen T, Yu J, Qin DP, Chen L. Lead contamination and its potential sources in vegetables and soils of Fujian, China. Environ Geochem Health. 2012;34:55–65. doi: 10.1007/s10653-011-9390-6. [DOI] [PubMed] [Google Scholar]
- Jorhem L, Engman J, Lindeström L, Schröder T. Applications in food quality and environmental contamination: Uptake of lead by vegetables grown in contaminated soil. Commun Soil Sci Plant Anal. 2000;31:2403–2411. doi: 10.1080/00103620009370594. [DOI] [Google Scholar]
- Lamb DT, Matanitobua VP, Palanisami T, Megharaj M, Naidu R. Bioavailability of Barium to Plants and Invertebrates in Soils Contaminated by Barite. Environ Sci Technol. 2013 doi: 10.1021/es302053d. [DOI] [PubMed] [Google Scholar]
- Luo X, Yu S, Li X. Distribution, availability, and sources of trace metals in different particle size fractions of urban soils in Hong Kong: Implications for assessing the risk to human health. Environ Pollut. 2011;159:1317–1326. doi: 10.1016/j.envpol.2011.01.013. [DOI] [PubMed] [Google Scholar]
- Marschner H. Mineral Nutrition of Higher Plants. 2. Academic Press; New York: 1995. [Google Scholar]
- McBride MB. Growing food crops on sludge-amended soils: Problems with the U.S. Environmental Protection Agency method of estimating toxic metal transfer. Environ Toxicol Chem. 1998;17:2274–2281. doi: 10.1002/etc.5620171118. [DOI] [Google Scholar]
- McBride MB. Cadmium Concentration Limits in Agricultural Soils: Weaknesses in USEPA’s Risk Assessment and the 503 Rule. Hum Ecol Risk Assess Int J. 2003;9:661–674. doi: 10.1080/713609960. [DOI] [Google Scholar]
- McBride MB. A comparison of reliability of soil cadmium determination by standard spectrometric methods. J Environ Qual. 2011;40:1863–1869. doi: 10.2134/jeq2011.0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride MB. Arsenic and lead uptake by vegetable crops grown on historically contaminated orchard soils. Appl Environ Soil Sci. 2013 doi: 10.1155/2013/283472. 2013, ID 283742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride MB, Simon T, Tam G, Wharton S. Lead and Arsenic Uptake by Leafy Vegetables Grown on Contaminated Soils: Effects of Mineral and Organic Amendments. Water Air Soil Pollut. 2012;224:1–10. doi: 10.1007/s11270-012-1378-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell RG, Spliethoff HM, Ribaudo LN, Lopp DM, Shayler HA, Marquez-Bravo LG, Lambert VT, Ferenz GS, Russell-Anelli JM, Stone EB, McBride MB. Lead (Pb) and other metals in New York City community garden soils: Factors influencing contaminant distributions. Environ Pollut. 2014;187:162–169. doi: 10.1016/j.envpol.2014.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moir AM, Thornton I. Lead and cadmium in urban allotment and garden soils and vegetables in the United Kingdom. Environ Geochem Health. 1989;11:113–119. doi: 10.1007/BF01758660. [DOI] [PubMed] [Google Scholar]
- Monaci F, Moni F, Lanciotti E, Grechi D, Bargagli R. Biomonitoring of airborne metals in urban environments: new tracers of vehicle emission, in place of lead. Environ Pollut. 2000;107:321–327. doi: 10.1016/S0269-7491(99)00175-X. [DOI] [PubMed] [Google Scholar]
- Mosbaek H, Tjell JC, Hovmand MF. Atmospheric lead input to agricultural crops in Denmark. Chemosphere. 1989;19:1787–1799. [Google Scholar]
- Murray H, Pinchin TA, Macfie SM. Compost application affects metal uptake in plants grown in urban garden soils and potential human health risk. J Soils Sediments. 2011;11:815–829. doi: 10.1007/s11368-011-0359-y. [DOI] [Google Scholar]
- Nabulo G, Black CR, Craigon J, Young SD. Does consumption of leafy vegetables grown in peri-urban agriculture pose a risk to human health? Environ Pollut. 2012;162:389–398. doi: 10.1016/j.envpol.2011.11.040. [DOI] [PubMed] [Google Scholar]
- Nali C, Balducci E, Frati L, Paoli L, Loppi S, Lorenzini G. Lettuce plants as bioaccumulators of trace elements in a community of central Italy. Environ Monit Assess. 2009;149:143–149. doi: 10.1007/s10661-008-0189-6. [DOI] [PubMed] [Google Scholar]
- Norm S, Weber A, Kramar U, Stüben D. Mapping of trace metals in urban soils. J Soils Sediments. 2001;1:77–97. doi: 10.1007/BF02987713. [DOI] [Google Scholar]
- NYSDEC. 6 NYCRR Part 375 – Environmental Remediation Programs. New York State Department of Environmental Conservation; 2006. [Google Scholar]
- Paterson E, Sanka M, Clark L. Urban soils as pollutant sinks — a case study from Aberdeen, Scotland. Appl Geochem. 1996;11:129–131. doi: 10.1016/0883-2927(95)00081-X. [DOI] [Google Scholar]
- Peltola P, Aström M. Urban geochemistry: a multimedia and multielement survey of a small town in northern Europe. Environ Geochem Health. 2003;25:397–419. doi: 10.1023/b:egah.0000004553.56489.0c. [DOI] [PubMed] [Google Scholar]
- Peris M, Micó C, Recatalá L, Sánchez R, Sánchez J. Heavy metal contents in horticultural crops of a representative area of the European Mediterranean region. Sci Total Environ. 2007;378:42–48. doi: 10.1016/j.scitotenv.2007.01.030. [DOI] [PubMed] [Google Scholar]
- Prasad LR, Nazareth B. Contamination of allotment soil with lead: managing potential risks to health. J Public Health. 2000;22:525–530. doi: 10.1093/pubmed/22.4.525. [DOI] [PubMed] [Google Scholar]
- Preer JR, Sekhon HS, Weeks J, Stephens BR. Heavy metals in garden soil and vegetables in Washington, D.C. Trace Substances in Environmental Health; XIV: Proceedings of University of Missouri’s 14th Annual Conference on Trace Substances in Environmental Health Held at Memorial Union, University of Missouri-Columbia; Columbia, Missouri. June 2, 3, 4 and 5, 1980; University of Missouri-Columbia; 1980. pp. 516–521. [Google Scholar]
- Samsøe-Petersen L, Larsen EH, Larsen PB, Bruun P. Uptake of trace elements and PAHs by fruit and vegetables from contaminated soils. Environ Sci Technol. 2002;36:3057–3063. doi: 10.1021/es015691t. [DOI] [PubMed] [Google Scholar]
- Sánchez-Camazano M, Sánchez-Martín MJ, Lorenzo LF. Lead and cadmium in soils and vegetables from urban gardens of Salamanca (Spain) Sci Total Environ. 1994:146–147. doi: 10.1016/0048-9697(94)90233-x. [DOI] [PubMed] [Google Scholar]
- SAS Institute, Inc. Version 9.3 of the SAS System for Windows. SAS Institute, Inc; Cary, NC: 2010. Copyright © 2003 – 2010. [Google Scholar]
- Säumel I, Kotsyuk I, Hölscher M, Lenkereit C, Weber F, Kowarik I. How healthy is urban horticulture in high traffic areas? Trace metal concentrations in vegetable crops from plantings within inner city neighbourhoods in Berlin, Germany. Environ Pollut. 2012;165:124–132. doi: 10.1016/j.envpol.2012.02.019. [DOI] [PubMed] [Google Scholar]
- Sauvé S, Hendershot W, Allen HE. Solid-solution partitioning of metals in contaminated soils: Dependence on pH, total metal burden, and organic matter. Environ Sci Technol. 2000;34:1125–1131. doi: 10.1021/es9907764. [DOI] [Google Scholar]
- Sauvé S, McBride M, Hendershot W. Soil Solution Speciation of Lead(II): Effects of Organic Matter and pH. Soil Sci Soc Am J. 1998;62:618–621. doi: 10.2136/sssaj1998.03615995006200030010x. [DOI] [Google Scholar]
- Sheppard SC, Evenden WG. Concentration enrichment of sparingly soluble contaminants (U, Th and Pb) by erosion and by soil adhesion to plants and skin. Environ Geochem Health. 1992;14:121–131. doi: 10.1007/BF01783487. [DOI] [PubMed] [Google Scholar]
- Shinn NJ, Bing-Canar J, Cailas M, Peneff N, Binns HJ. Determination of spatial continuity of soil lead levels in an urban residential neighborhood. Environ Res. 2000;82:46–52. doi: 10.1006/enrs.1999.4004. [DOI] [PubMed] [Google Scholar]
- Smichowski P, Gomez DR, Dawidowski L, Gine MF, Sanchez Bellato AC, Reich SL. Monitoring trace metals in urban aerosols from Buenos Aires city. J Environ Monit. 2004;6:286–294. doi: 10.1039/b312446k. [DOI] [PubMed] [Google Scholar]
- Spliethoff HM, Mitchell RG, Ribaudo LN, Taylor O, Shayler HA, Greene V, Oglesby D. Lead in New York City community garden chicken eggs: influential factors and health implications. Environ Geochem Health. 2013 doi: 10.1007/s10653-013-9586-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stilwell DE, Rathier TM, Musante CL, Ranciato JF. Lead and Other Heavy Metals in Community Garden Soils in Connecticut (No. Bulletin 1019) The Connecticut Agricultural Experiment Station 2008 [Google Scholar]
- Tai Y, McBride MB, Li Z. Evaluating specificity of sequential extraction for chemical forms of lead in artificially-contaminated and field-contaminated soils. Talanta. 2013;107:183–188. doi: 10.1016/j.talanta.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US EPA. National Center for Environmental Assessment. US Environmental Protection Agency; Washington, DC: 1997. Exposure Factors Handbook: 1997 Edition. [Google Scholar]
- US EPA. OSWER Directive 92857-65. US EPA office of Solid Waste and Emergency Response; Washington, D.C. 20460: 2005. Ecological soil screening levels for cadmium. Interim final. [Google Scholar]
- US EPA. SW-846 On-line [WWW Document]. Waste – Hazard. Waste – Test Methods. 2012 URL http://www.epa.gov/osw/hazard/testmethods/sw846/online/index.htm (accessed 1.25.13)
- US EPA. Technical Review Workgroup Recommendations Regarding Gardening and Reducing Exposure to Lead-Contaminated Soils (No OSWER 9200.2-142) US Environmental Protection Agency; 2013. [Google Scholar]
- US FDA. Total Diet Study Statistics on Element Results Revision 4.1, Market Baskets 1991–3 through 2005–4. US Food and Drug Administration; College Park, MD: 2007. [Google Scholar]
- US FDA. Total Diet Study Statistics on Element Results Market Baskets 2006–1 through 2008–4. US Food and Drug Administration, Center for Food Safety and Applied Nutrition; College Park, MD: 2010. [Google Scholar]
- Uzu G, Sobanska S, Sarret G, Muñoz M, Dumat C. Foliar lead uptake by lettuce exposed to atmospheric fallouts. Environ Sci Technol. 2010;44:1036–1042. doi: 10.1021/es902190u. [DOI] [PubMed] [Google Scholar]
- Wharton SE, Shayler HA, Spliethoff HM, Marquez-Bravo LG, Ribaudo L, McBride MB. A comparison of screening tests for soil Pb. Soil Sci. 2012;177:650–654. doi: 10.1097/SS.0b013e318277718b. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


