Abstract
Objectives
Cerebrotendinous xanthomatosis (CTX) is a rare genetic disorder of bile acid (BA) synthesis that can cause progressive neurological damage and premature death. Blood (normally serum or plasma) testing for CTX is performed by a small number of specialized laboratories, routinely by gas chromatography-mass spectrometry (GC-MS) measurement of elevated 5α- cholestanol. We report here on a more sensitive biochemical approach to test for CTX particularly useful for confirmation of CTX in the case of a challenging diagnostic sample with 5α-cholestanol that, although elevated, was below the cut-off used for diagnosis of CTX (10 μg/ml or 1.0 mg/dL).
Design and Methods
We have previously described liquid chromatography-electrospray ionization-tandem mass spectrometry (LC-ESI-MS/MS) methodology utilizing keto derivatization to enable the sensitive quantification of plasma ketosterol BA precursors that accumulate in CTX. We have expanded this methodology to perform isotope dilution LC-ESI-MS/MS quantification of a panel of plasma ketosterol BA precursors, with internal standards readily generated using isotopically-enriched derivatization reagent.
Results
Quantification of plasma ketosterol BA precursors (7α-hydroxy-4-cholesten-3-one, 7α,12α-dihydroxy-4-cholesten-3-one and 7α,12α-dihydroxy-5β-cholestan-3-one) in a single LC- ESI/MS/MS test provided better discrimination between a CTX-positive and negative samples analyzed (n=20) than measurement of 5α-cholestanol alone.
Conclusions
Quantification of plasma ketosterol BA precursors provides a more sensitive biochemical approach to discriminate between CTX negative and positive samples. A multiplexed LC-ESI-MS/MS test quantifying a panel of plasma ketosterols, with simple sample preparation, rapid analysis time and readily available internal standards, can be performed by most clinical laboratories. Wider availability of testing will benefit those affected with CTX.
Keywords: Cerebrotendinous xanthomatosis, CYP27A1, bile acids, ketosterols, cholestanol
1. Introduction
Cerebrotendinous xanthomatosis (CTX; OMIM#213700) is an autosomal recessive neurodegenerative disorder associated with deficient sterol 27-hydroxylase (CYP27A1), a mitochondrial enzyme important in conversion of cholesterol to bile acids (BA). Childhood-onset symptoms can include diarrhea and juvenile cataracts. Adolescent to adult-onset symptoms can include tendon and cerebral xanthomas associated with neurological symptoms. CTX is difficult to diagnose, often there are many years between the age of first symptom onset and the age at diagnosis, which often occurs only after significant neurological involvement. As the disorder progresses, affected individuals can become incapacitated with motor dysfunction with premature death occurring due to advancing neurological deterioration. Although only around three hundred cases of CTX have been described worldwide [1], relatively large series of patients has been described by physicians with experience in recognizing the disorder.
An effective oral therapy for CTX is available in the form of chenodeoxycholic acid (CDCA), the main BA deficient in CTX. Treatment with CDCA has been shown to normalize the biochemical phenotype and halt progression of disease [2,3]. In many cases treatment of patients with advanced neurological disease does not reverse the impairment [3], therefore it is essential to diagnose and treat CTX as early as possible.
Biochemical tests for CTX include screening blood samples using gas chromatography- mass spectrometry (GC-MS) measurement of elevated 5α-cholestanol [4,5]. The upper normal range for plasma 5α-cholestanol has been reported to range from 7-11 μg/ml [4,5]. Diagnostic confirmation is routinely performed using fast atom bombardment (FAB)-MS measurement of more specific markers for CTX, bile alcohol glucuronides in urine [6]. We have utilized keto- moiety derivatization to enable sensitive liquid chromatography-electrospray ionization-tandem MS (LC-ESI-MS/MS) measurement of ketosterol BA precursors that accumulate in CTX [7-9]. Free (non-hydrolyzed) plasma 7α-hydroxy-4-cholesten-3-one (7αC4) possessed improved utility over 5α-cholestanol as a marker for CTX [7]. We describe here quantification of a panel of ketosterols in a plasma sample from a CTX affected individual with a plasma 5α-cholestenol concentration above the concentration range we determined for normal samples (n=20) but below our cut-off for diagnosis of CTX (10 μg/ml).
2. Materials and methods
2.1 Human subject research considerations
Blood was obtained from participants enrolled in studies at OHSU under Institutional Review Board (IRB) approved protocols. Informed consent was obtained from all study participants. De-identified plasma samples submitted to the OHSU diagnostic laboratory for CTX testing using GC-MS measurement of elevated plasma 5α-cholestanol were used with IRB approval. For CTX positive samples diagnostic confirmation was by molecular genetic testing.
2.2 Chemicals and reagents
7αC4, 7α, 12α-dihydroxy-4-cholesten-3-one (7α12αC4) and 7α,12α-dihydroxy-5β- cholestan-3-one (7α12αC5β) were from Toronto Research Chemicals (Toronto, Ontario). 5α- Cholestanol was from Steraloids (Newport, RI) and epicoprostanol from Sigma-Aldrich (St. Louis, MO). BSTFA reagent was from Thermo Scientific (Bellefonte, PA). Human plasma and double charcoal stripped (DCS) plasma were from Golden West Bio (Temecula, CA). Methanol and water (GC-MS grade) were from Burdick and Jackson (Muskegon, MI). Formic acid (90%) was J.T.Baker brand, and glacial acetic acid (99.99%) was from Aldrich. Quaternary amonoxy (QAO) reagent (O-(3-trimethylammoniumpropyl) hydroxylamine) bromide is commercially available as Amplifex™ Keto reagent from http://www.sciex.com. The QAO-d3 reagent was provided by AB SCIEX.
2.3 Preparation of calibrators and samples for GC-MS measurement of 5α-cholestanol
GC-MS measurement of elevated plasma 5α-cholestanol has been described [7,9]. In brief, internal standard (epicoprostanol) was added to plasma samples or calibrants generated using 5α-cholestanol. Sterols were saponified by the addition of ethanol/KOH and the aqueous phase was extracted with hexane. Dried sterols were derivatized with BSTFA and the trimethylsilyl ether derivative of 5α-cholestanol was measured using GC (splitless injection) performed with a ZB1701 column (30m, 0.25mmID, 0.25μm film thickness, Phenomenex, Torrance, CA) coupled to a mass spectrometer (Agilent GC 6890N and MS 5975; Santa Clara, CA). Mass spectra were collected in selected ion mode (with m/z= 355 and 370 ions monitored for epicoprostanol and m/z= 306 and 305 ions for 5α-cholestanol).
2.4 Preparation of calibrators and samples for LC-MS/MS measurement of ketosterols
We have previously described the approach used for LC-ESI-MS/MS measurement of elevated plasma ketosterols [9]. In brief, method calibrators were generated using dilutions of authentic standard in methanol spiked into DCS plasma. QAO reagent solution (210 μg in methanol plus 5% acetic acid, v/v) was added to calibrators or plasma samples (4 μl). After 2 hours at RT QAO derivatization was complete. Previously prepared QAO-d3 tagged ketosterol internal standards (300 pg) in 10 μl methanol were added [9].
2.5 LC-ESI-MS/MS method
LC-ESI-MS/MS analyses were performed using a QTRAP® 5500 triple-quadrupole hybrid mass spectrometer with linear ion trap functionality (AB SCIEX, Framingham, MA), equipped with a TurboIonSpray® ESI source. The ionization interface was operated in the positive mode and multiple reaction monitoring (MRM) transitions monitored for quantification of ketosterols were as follows: QAO 7αC4 m/z 515.7→152.2, for QAO 7α12αC4 m/z 531.7→152.2, for QAO 7α12αC5β m/z 533.7→145.0, for QAO-d3 7αC4 m/z 518.7→152.3, for QAO-d3 7α12αC4 m/z 534.7→152.1 and for QAO-d3 7α12αC5β m/z 536.7→145.0. The QTRAP® 5500 was coupled to a Shimadzu UPLC system (Columbia, MD) composed of a SIL- 20ACXR auto-sampler and two LC-20ADXR LC pumps. QAO derivatives were resolved using a 50×2.1(i.d.) mm, 5.0 μm Luna C8-HPLC column with guard (Phenomenex; Torrance, CA). The gradient mobile phase was delivered at a flow rate of 0.8 ml/min and the water:acetonitrile:0.1% formic acid mobile phase [9]. The column temperature was kept at 35°C using a Shimadzu CTO-20AC column oven. The sample injection volume was 10 μl.
2.6 Data analysis and method performance
Calibration curves were generated by performing a least-squares linear regression for peak area ratios (QAO-d0 ketosterol analyte/QAO-d3 ketosterol internal standard) plotted against specified calibrant concentration in plasma (ng/ml) [9]. The lower limit of quantification (LLOQ) was determined as the lowest spiked concentration in matrix for which the signal-to- noise (S/N) ratio was ≥5 and the within- and between-day reproducibility of the peak area was ≤20% relative standard deviation (RSD). Potential method interference was evaluated by examination of the peak shape, peak shoulder, and peak area ratio of two MRM transitions (quantifier and qualifier) acquired for each analyte and by analysis of possible method interferents. The matrix effect was determined by comparing the QAO ketosterol peak area for matrix-based against non-matrix calibrators.
3. Results
As we have previously described, DCS plasma calibration curves for QAO tagged 7αC4 and 7α12αC4, with QAO-d3 tagged 7αC4 and 7α12αC4 internal standards respectively, demonstrated acceptable linearity (correlation coefficients across the range 20-250 ng/ml possessed r2 values >0.990) [9]. This was also the case for a novel ketosterol marker for CTX we quantified with isotope dilution LC-ESI-MS/MS, 7α12αC5β. DCS plasma calibration curves for QAO tagged 7α12αC5β with QAO-d3tagged 7α12αC5β internal standard demonstrated acceptable linearity (correlation coefficients across the range 20-250 ng/ml possessed r2 values >0.990). Satisfactory between and within-run accuracy and precision data was also obtained for calculated concentrations of 7α12αC5β (<20% RSD at the LLOQ and <15% RSD between 50 and 250 ng/ml). The LLOQ for all ketosterols in plasma (including 7α12αC5β) was 20 ng/ml.
The sensitivity of ketosterols as plasma markers for CTX compared to 5α-cholestanol was highlighted by analysis of a plasma sample from a possible CTX-affected individual with a clinical history consistent with the disorder (see Figure 1). GC-MS analysis revealed a plasma 5α-cholestanol concentration of 8.4 g/ml, close to reported upper normal concentrations of 7- 11 g/ml [4,5]. The plasma ketosterol concentrations determined with LC-ESI-MS/MS were 795, 1548 and 1004 ng/ml for 7αC4, 7α12αC4 and 7α12αC5β respectively (with upper normal concentrations of 22 and 0.8, and 29 ng/ml determined; for reference ranges see Table 1). Molecular genetic testing confirmed the individual possessed a homozygous R474Q CYP27A1 gene mutation, previously reported to be a causative mutation for CTX [10].
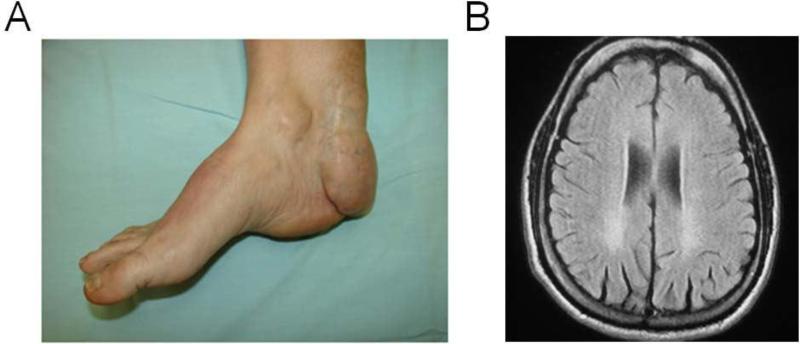
Figure 1.
the plasma sample analyzed was from the daughter of a non-consanguineous couple, with two possibly affected siblings that died at 24 and 55 years old with neurological symptoms suggestive of CTX. The daughter had developed cataracts at age 22 years old that were surgically corrected and Achilles tendon xanthomas from age 13 (left panel) that could not be alleviated with surgery, resulting in difficulty walking and intense pain in the feet and knees. There was spastic gait present (pyramidal tract syndrome) with no ataxic manifestations, extrapyramidal signs or dementia, although an MRI of the brain showed bilateral occipital periventricular white matter signal hyperintensitiy on T2 and Flair (right panel).
Table 1.
Plasma ketosterol concentrations
| 7α-hydroxy-4- cholesten-3-onea,b (ng/ml) |
7α12α-dihydroxy-4- cholesten-3-onea,b (ng/ml) |
7α12α-dihydroxy-5β- cholestan-3-onea (ng/ml) |
5α- cholestanolb,c (μg/ml) |
|
|---|---|---|---|---|
| CTX-case described plasma | 795 | 1548 | 1004 | 8.4 |
| Untreated CTX-affected adult plasma (n=10) | 1174 ± 711d [204-1828] | 1545 ± 1144d [57-2420] | 498 ± 310d [100-1004] | 31 ± 19 [8.4-66] |
| Unaffected adult plasma (n=20) | 6.4 ± 5.3e [1.6-22] | 0.4 ± 0.3e [0.1-0.8] | 10 ± 5.5e [0-29] | 1.3 [0.8-1.8]f |
The mean concentration ± S.D. and [range of results] are given
non-hydrolyzed free sterol.
this data has been reported previously [9].
hydrolyzed total sterol; quantification by GC-MS.
CTX plasma samples with concentrations >250 ng/ml were diluted and re-analyzed.
Calculated outside the quantifiable range.
4. Discussion
5α-Cholestanol can be elevated in a number of liver diseases and concerns have been raised regarding the specificity of this disease marker for CTX [11]. Although not routinely used for diagnosis, other markers elevated in CTX include cholesterol precursors such as 7- dehydrocholesterol and 8-dehydrocholesterol [12]. Ketosterol BA precursors and bile alcohol glucuronides also accumulate in CTX [7-9,13-15]. We have previously described an approach using keto derivatization to incorporate a permanent charge and improve sensitivity for LC-ESI- MS/MS detection of ketosterol BA precursors [7-9]. We report here on expansion of this methodology to allow isotope dilution quantification of a panel of plasma ketosterol BA precursor markers to test for CTX. The sensitivity of the panel to detect disease was highlighted by analysis of a CTX-positive sample that possessed a 5α-cholestanol concentration below the cut-off used for diagnosis of CTX (10 μg/ml). Although GC-MS analysis of the sample indicated elevated 7- and 8-dehydrocholesterol suggestive of CTX [12], these sterols are not ideal disease markers as their quantification can be problematic. They are relatively unstable, and stable-isotope labeled internal standard analogues are not readily available. Isotope dilution LC- ESI-MS/MS quantification of plasma ketosterols allowed for ready discrimination between the sample we describe here and CTX negative samples (n=20), such that it serves as an improved blood test for CTX. The availability of LC-ESI-MS/MS methodology utilizing QAO derivatization with simple and rapid sample preparation amenable to automation, analysis times of 4-6 min using conventional LC instrumentation and buffers, and readily available stable-isotope labeled internal standard analogues, allows performance by most clinical laboratories. Wider availability of testing will benefit those affected with this disorder.
Highlights.
Elevated cholestanol is used to test for cerebrotendinous xanthomatosis (CTX)
We describe a useful test for a case with cholestanol below the diagnostic cut-off
Plasma ketosterols allowed for discrimination between the case sample and controls
Rapid isotope-dilution LC-ESI-MS/MS testing can be performed by most laboratories
Wider availability of plasma testing will benefit those affected with CTX
Acknowledgements
The authors would like to thank the Bioanalytical Shared Resource at OHSU for providing technical assistance and access to analytical instrumentation. AED has been supported as a KL2 awardee by the Oregon Clinical and Translational Research Institute (OCTRI), grant number (KL2TR000152) from the National Center for Advancing Translational Sciences (NCATS) at the National Institutes of Health (NIH) and also as a training grant awardee by the Sterol and Isoprenoid Diseases (STAIR) consortium. STAIR is part of the NIH Rare Diseases Clinical Research Network (RDCRN). Funding and/or programmatic support for this project has been provided by a grant (1U54HD061939) from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the NIH Office of Rare Diseases Research (ORDR).
Abbreviations
- CTX
cerebrotendinous xanthomatosis
- BA
bile acid
- GC-MS
gas chromatography- mass spectrometry
- ESI- MS/MS
liquid chromatography-electrospray ionization-tandem MS
- CDCA
chenodeoxycholic acid
- FAB
fast atom bombardment
- 7αC4
7α-hydroxy-4- cholesten-3-one
- IRB
Institutional Review Board
- 7α12αC5β
α,12α-dihydroxy-4-cholesten-3-one (7α12αC4) and 7α,12α-dihydroxy-5α-cholestan-3-one
- DCS
double charcoal stripped
- QAO
quaternary amonoxy
- MRM
multiple reaction monitoring
- LLOQ
limit of quantification
- S/N
signal-to-noise
- RSD
relative standard deviation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Gallus GN, Dotti MT, Mignarri A, Rufa A, Da PP, Cardaioli E, Federico A. Four novel CYP27A1 mutations in seven Italian patients with CTX. Eur J Neurol. 2010;17:1259–1262. doi: 10.1111/j.1468-1331.2010.03002.x. [DOI] [PubMed] [Google Scholar]
- 2.Berginer VM, Salen G, Shefer S. Long-term treatment of cerebrotendinous xanthomatosis with chenodeoxycholic acid. N Engl J Med. 1984;311:1649–1652. doi: 10.1056/NEJM198412273112601. [DOI] [PubMed] [Google Scholar]
- 3.Mondelli M, Sicurelli F, Scarpini C, Dotti MT, Federico A. Cerebrotendinous xanthomatosis: 11-year treatment with chenodeoxycholic acid in five patients. An electrophysiological study. J Neurol Sci. 2001;190:29–33. doi: 10.1016/s0022-510x(01)00563-9. [DOI] [PubMed] [Google Scholar]
- 4.Leitersdorf E, Safadi R, Meiner V, Reshef A, Bjorkhem I, Friedlander Y, Morkos S, Berginer VM. Cerebrotendinous xanthomatosis in the Israeli Druze: molecular genetics and phenotypic characteristics. Am J Hum Genet. 1994;55:907–915. [PMC free article] [PubMed] [Google Scholar]
- 5.Salen G. Cholestanol deposition in cerebrotendinous xanthomatosis. A possible mechanism. Ann Intern Med. 1971;75:843–851. doi: 10.7326/0003-4819-75-6-843. [DOI] [PubMed] [Google Scholar]
- 6.Egestad B, Pettersson P, Skrede S, Sjovall J. Fast atom bombardment mass spectrometry in the diagnosis of cerebrotendinous xanthomatosis. Scand J Clin Lab Invest. 1985;45:443–446. doi: 10.3109/00365518509155241. [DOI] [PubMed] [Google Scholar]
- 7.DeBarber AE, Connor WE, Pappu AS, Merkens LS, Steiner RD. ESI-MS/MS quantification of 7alpha-hydroxy-4-cholesten-3-one facilitates rapid, convenient diagnostic testing for cerebrotendinous xanthomatosis. Clin Chim Acta. 2010;411:43–48. doi: 10.1016/j.cca.2009.09.036. [DOI] [PubMed] [Google Scholar]
- 8.DeBarber AE, Sandlers Y, Pappu AS, Merkens LS, Duell PB, Lear SR, Erickson SK, Steiner RD. Profiling sterols in cerebrotendinous xanthomatosis: Utility of Girard derivatization and high resolution exact mass LC-ESI-MS(n) analysis. J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879:1384–1392. doi: 10.1016/j.jchromb.2010.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeBarber AE, Luo J, Star-Weinstock M, Purkayastha S, Geraghty MT, Chiang J, Merkens LS, Pappu AS, Steiner RD. J Lipid Res. 2013 doi: 10.1194/jlr.P043273. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuriyama M, Fujiyama J, Yoshidome H, Takenaga S, Matsumuro K, Kasama T, Fukuda K, Kuramoto T, Hoshita T, Seyama Y. Cerebrotendinous xanthomatosis: clinical and biochemical evaluation of eight patients and review of the literature. J Neurol Sci. 1991;102:225–232. doi: 10.1016/0022-510x(91)90073-g. [DOI] [PubMed] [Google Scholar]
- 11.Koopman BJ, van der Molen JC, Wolthers BG, de Jager AE, Waterreus RJ, Gips CH. Capillary gas chromatographic determination of cholestanol/cholesterol ratio in biological fluids. Its potential usefulness for the follow-up of some liver diseases and its lack of specificity in diagnosing CTX (cerebrotendinous xanthomatosis) Clin Chim Acta. 1984;137:305–315. doi: 10.1016/0009-8981(84)90119-0. [DOI] [PubMed] [Google Scholar]
- 12.de Sain-van der Velden MG, Verrips A, Prinsen BH, de BM, Berger R, Visser G. Elevated cholesterol precursors other than cholestanol can also be a hallmark for CTX. J Inherit Metab Dis. 2008;2:S387–393. doi: 10.1007/s10545-008-0963-1. [DOI] [PubMed] [Google Scholar]
- 13.Bjorkhem I, Oftebro H, Skrede S, Pedersen JI. Assay of intermediates in bile acid biosynthesis using isotope dilution--mass spectrometry: hepatic levels in the normal state and in cerebrotendinous xanthomatosis. J Lipid Res. 1981;22:191–200. [PubMed] [Google Scholar]
- 14.Clayton PT, Verrips A, Sistermans E, Mann A, Mieli-Vergani G, Wevers R. Mutations in the sterol 27-hydroxylase gene (CYP27A) cause hepatitis of infancy as well as cerebrotendinous xanthomatosis. J Inherit Metab Dis. 2002;25:501–513. doi: 10.1023/a:1021211520034. [DOI] [PubMed] [Google Scholar]
- 15.Honda A, Salen G, Matsuzaki Y, Batta AK, Xu G, Leitersdorf E, Tint GS, Erickson SK, Tanaka N, Shefer S. Differences in hepatic levels of intermediates in bile acid biosynthesis between Cyp27(−/−) mice and CTX. J Lipid Res. 2001;42:291–300. [PubMed] [Google Scholar]