Abstract
Background
Only limited data exist on the relationship of lung function to patients with extreme obesity. To assess the relationship between lung function tests and clinical characteristics in a cohort of morbidly obese patients undergoing evaluation for bariatric procedures in a university hospital in the United States.
Methods
Consecutive patients undergoing clinical evaluation were reviewed. The variables included demographic, anthropometric, clinical, and pulmonary function data.
Results
A total of 229 patients underwent a standardized preoperative evaluation. Of these 229 patients, 136 (59%) had evaluable data and 102 (75%) were women. The mean ± standard deviation age was 45 ± 10 years, the mean weight was 164 ± 42 kg, and the mean body mass index was 57 ± 13 kg/m2. Smoking or asthma was reported in 38% and 24% of patients, respectively. The mean forced vital capacity and forced expiratory volume in 1 s was 80% ± 17% of predicted and 76% ± 19% of predicted, respectively. Of the 136 patients, 29% had a measured forced expiratory volume in 1 s/forced vital capacity of ≥.08 below the predicted ratio. The mean total lung capacity was 86% ± 14% of predicted; 26% of subjects had a total lung capacity < 80% of predicted. Multivariate logistic regression analysis demonstrated an association of obstructive ventilatory defects with male gender (odds ratio [OR] 2.35, 95% confidence interval [CI] 1.00–5.50) and current or previous smoking (OR 2.41, 95% CI 1.10–5.30), but not body mass index. Restrictive defects were associated with body mass index (OR 1.06, 95% CI 1.01–1.10), in particular, obesity hypoventilation syndrome (OR 3.7, 95% CI 1.2–11.1).
Conclusion
The mean preoperative spirometry, lung volumes, and gas exchange values were within the established reference ranges. Restrictive ventilatory defects were less common than obstructive ventilatory patterns and were most prominently associated with obesity hypoventilation syndrome.
Keywords: Morbid obesity, Lung volume measurements, Spirometry, Blood gas analysis, Obstructive sleep apnea, Pulmonary diffusing capacity
The well-described effects of obesity on the respiratory system include an increased incidence of obstructive sleep apnea (OSA), hypercarbia, and restrictive ventilatory defects [1–4]. The results of spirometry, lung volume and/or diffusing capacity, arterial blood gas analysis, and assessment for OSA on individuals with a wide range of weights have been reported [5,6]. The most complete of these consisted of data from 43 patients (weighing 103–237 kg) collected during 2 decades and did not address the issues of surgical intervention or OSA [7]. They found that vital capacity, maximal minute ventilation, and total lung capacity (TLC) became abnormal only in those individuals whose height/weight ratio (weight in kilograms/height in centimeters) was greater than a threshold of 1.0. Another important series evaluated another 43 morbidly obese patients (weight 140–227 kg) and found that the forced vital capacity (FVC), forced expiratory volume in 1 s (FEV1), and expiratory reserve volume were all reduced in this population with preserved diffusing capacity [8].
The purpose of this study was to delineate the pulmonary function tests and respiratory physiology of morbidly obese subjects who were heavier than those previously described. Previously published case series did not simultaneously evaluate the demographic, clinical, and physiologic data for their entire subject population. Our primary hypothesis was that the frequently cited effect of morbid obesity in producing restrictive ventilatory defects would be found chiefly in patients with a body mass index (BMI) greater than a threshold value [5,6]. The secondary goals were to delineate the associations among the clinical features, degree of obesity, and lung function.
Methods
Study subjects
Patients undergoing bariatric surgery at the University of Washington Medical Center (UWMC) enter a standardized preoperative protocol, including a detailed assessment of co-morbid conditions and lung function testing. All patients who underwent bariatric operations after evaluation at UWMC were reviewed. The inclusion criteria required that the subject had been evaluated in the Bariatric Surgery Clinic at the UWMC and had undergone spirometry before surgery. A total of 229 patients were seen between January 1, 2000 and August 31, 2004. The institutional review board of the University of Washington approved the study.
Study design
The study was a cross-sectional retrospective analysis of obese and morbidly obese patients. The study data (demographic and anthropometric data) were abstracted from the clinical chart by a single reviewer (J.A.S.) using a standardized evaluation form. Pertinent co-morbid conditions were ascertained from clinical documentation and included OSA, asthma, venous thromboembolic disease, pulmonary hypertension, chronic obstructive lung disease (COPD), coronary artery disease, venous stasis ulcers, and diabetes. Weight was assessed using a calibrated digital scale. The explicit criteria for diagnosis were determined a priori for sleep apnea and asthma. The criteria for the sleep apnea diagnosis required explicit physician documentation and evidence of a prescription or use of appropriate therapy (i.e., continuous positive airway pressure or bilevel positive airway pressure therapy). The ascertainment of asthma was based on explicit physician documentation or inclusion in the patient’s medication list of typical asthma therapy, specifically, inhaled corticosteroids and/or inhaled beta2-agonists (short or long acting). The diagnosis of COPD was determined from a physician diagnosis recorded in the chart. The diagnosis of obesity hypoventilation syndrome (OHS) was inferred by the presence of resting hypoxemia (partial pressure arterial oxygen <70 mm Hg) and/or resting hypercarbia (partial pressure of carbon dioxide ≥45 mm Hg) in the setting of a BMI >30 kg/m2 according to previously recommended diagnostic criteria [9–11].
Pulmonary function testing, including spirometry, lung volume, diffusing capacity measurement, and arterial blood gas analysis, was ascertained from either the UWMC or reports from referring centers. Spirometry and lung volume measurements were reinterpreted in accordance with the prevailing American Thoracic Society (ATS) guidelines [12] at study entry using reference equations from Crapo et al. [13] with the patients in a sitting position. Obstruction was deemed present when the measured FEV1 divided by FVC (FEV1/FVC) was ≥.08 U less than the ratio calculated from the predicted FEV1/FVC. The lung volume was not uniformly obtained for all subjects. The lung volume, assessed using the nitrogen washout technique or body pleth-ysmography, was available for 111 subjects; the diffusing capacity for carbon monoxide data were available for 51 subjects. Restriction was deemed present when the TLC decreased to less than the fifth percentile of the normal distribution, in accordance with the most recent ATS guidelines. This was defined by using the lower limit of the 90% confidence interval (CI) for the predicted value (equates to the lower fifth percentile of the predicted values, consistent with the recent ATS/European Respiratory Society Standardization of Lung Function Testing) using data from Crapo et al. [14] and Pellegrino et al. [15]. Arterial blood gases were obtained with the subject breathing room air and were analyzed in a calibrated ABL 520 blood gas analyzer (Radiometer, Copenhagen, Denmark) and were evaluated for compatibility with steady-state measurements based on a pH > 7.35. The data points with a pH suggestive of uncompensated respiratory acidosis were censored; those compatible with acute respiratory alkalosis were kept.
In describing our findings, we included the weight/height ratios to facilitate comparisons with older data sets. A weight/height ratio > 1.0 has been cited as a cutoff point pertinent to the effect of obesity on respiratory function, particularly in older data [2,5,6,16,17].
Statistical analysis
Descriptive statistics are presented as the mean, standard deviation, median, and interquartile range, as appropriate. Normally distributed continuous variables were compared between groups using Student’s 2-sample t test; variables that were not normally distributed were analyzed using the Mann-Whitney rank sum test. Chi-square tests were used to compare the nominal data; and Fisher’s exact test was used to assess significantly imbalanced 2 × 2 contingency tables with < 10 expected outcomes in any cell. A 2-sided P value <.05 was considered significant.
Linear regression models were used to assess the relationship among age, gender, clinical diagnoses, BMI, lung function abnormalities, and hypercarbia. The nonlinearity of associations was assessed initially by visual inspection. The addition of second order or greater polynomials to the regression model was assessed using reduced and full model comparisons with the partial F statistic. Additional regression diagnostics included assessment of the standardized residuals and outliers.
Logistic regression models were used to assess the effect of the covariates on airway obstruction and restriction. Univariate logistic regression analysis was performed to analyze the association of each covariate with the presence of obstruction and/or restriction. Those variables showing a significant odds ratio (OR) on univariate analysis (P ≤.2) were then evaluated in a multivariate model. Only variables that, when included, resulted in a ≥ 10% change in the point estimate of the final odds ratio of the primary variable of interest were kept in the final model. Analyses were performed with Stata, version 8.0 (Stata-Corp, College Station, TX).
Results
The 229 screened subjects had been evaluated at the Bariatric Surgery Clinic of UWMC between January 2000 and August 2004. Of the 229 patients, 136 had undergone planned pulmonary function testing, and the results of these tests were included in the clinic chart (Fig. 1).
Fig. 1.
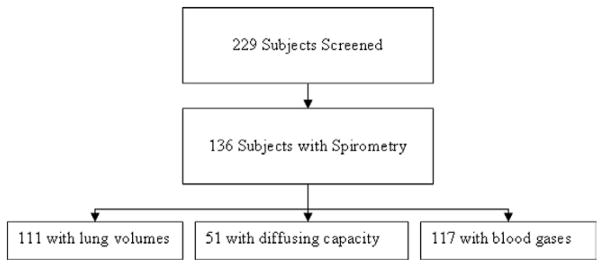
Cohort description.
The population was predominantly women (n = 102, 75%), with a mean age of 45.4 ± 9.7 years (Table 1). The mean weight in the women and men was 152.9 ± 33.5 and 196.5 ± 47.6 kg, respectively. The mean BMI was 55.7 ± 12.1 and 59.7 ± 13.8 kg/m2 in the women and men, respectively. Of the 136 patients, 95% had extreme or class III obesity, defined as a BMI >40 kg/m2 [18] and 65% were classified as severely obese (BMI ≥ 50 kg/m2). Of the 136 patients, 50 (37%) had a weight/height ratio >1.0; 37 were asthmatic, 2 had COPD, and 3 had OHS documented in the chart; and 54 patients were former smokers and 6 current smokers. The pack-years were recorded for 45 current or former smokers (75%), and the duration of cessation for 30 former smokers (56%). The mean duration of cessation was 9 ± 7 years. OSA was noted in 117 of the cohort (51%).
Table 1.
Demographic, anthropometric, and clinical description of study population
| Characteristic | All patients (n = 136) | Women (n = 102) | Men (n = 34) |
|---|---|---|---|
| Age (y) | 45.4 ± 9.7 (19–73) | 45.0 ± 10.2 (19–73) | 46.6 ± 7.8 (34–64) |
| Height (cm) | 169 ± 10 (152–200) | 165 ± 7 (152–180) | 180 ± 7 (1.65–2.00)* |
| Weight (kg) | 164 ± 41.9 (95–295) | 152.9 ± 33.5 (95–240) | 196.5 ± 47.6 (103–295)* |
| Weight/height (kg/cm) | 0.96 ± 0.22 (0.59–1.59) | 0.92 ± 0.20 (0.59–1.44) | 1.08 ± 0.25 (0.6–1.59)* |
| BMI (kg/m2) | 56.7 ± 12.6 (35–93) | 55.7 ± 12.1 (35–93) | 59.7 ± 13.8 (35–86) |
| Asthma (%) | 27 (n = 37) | 27 (n = 27) | 26 (n = 10) |
| Smokers (%) | 44 (n = 60) | 43 (n = 44) | 47 (n = 16) |
| Pack-years | 20 ± 10–31 (2–100) | 15 ± 10–30 (2–100) | 30 ± 20–40 (12–40) |
| OSA (%) | 57 (n = 78) | 54 (n = 55) | 68 (n = 23) |
BMI = body mass index; OSA = obstructive sleep apnea.
Data presented as mean ± standard deviation, with ranges in parentheses, unless otherwise noted.
P < .05 for difference comparing genders.
Spirometry
The mean FVC and FEV1 was 83% and 79% of predicted in the women and 71% and 65% of predicted in the men, respectively (Table 2). Of the 136 patients, 24 women (24%) and 15 men (44%) displayed obstructive physiologic features (P= .029); 38% of the smokers and 38% of the asthmatics were obstructed (Table 3).
Table 2.
Results of spirometry, lung volumes, and gas exchange parameters
| Parameter | Women | Men |
|---|---|---|
| Spirometry | n = 102 | n = 34 |
| FVC (L) | 2.97 ± 0.69 (1.51–4.75) | 3.67 ± 0.79 (1.79–5.08)* |
| FVC (% predicted) | 83 ± 17 (42–119) | 71 ± 15 (33–96)* |
| FEV1 (L) | 2.32 ± 0.63 (0.77–3.98) | 2.73 ± 0.73 (1.27–4.43)* |
| FEV1 (% predicted) | 79 ± 19 (29–126) | 65 ± 16 (30–99)* |
| FEV1/FVC | 0.77 ± 0.08 (0.50–1.11) | 0.73 ± 0.08 (0.51–0.99)* |
| Lung volumes | n = 83 | n = 28 |
| TLC (L) | 4.54 ± 0.73 (2.50–6.00) | 5.88 ± 1.19 (3.73–8.03)* |
| TLC (% predicted) | 87 ± 14 (48–123) | 82 ± 16 (49–116) |
| RV (L) | 1.58 ± 0.60 (0.37–4.03) | 2.16 ± 0.80 (0.79–3.56)* |
| RV (% predicted) | 85 ± 27 (26–143) | 107 ± 37 (47–179)* |
| ERV (L) | 0.41 ± 0.27 (0.06–1.33) | 0.38 ± 0.19 (0.15–0.90) |
| ERV (% predicted) | 35 ± 24 (5–107) | 22 ± 12 (9–56)* |
| IC (L) | 2.52 ± 0.55 (1.3–3.91) | 3.25 ± 0.71 (1.55–4.36)* |
| IC (% predicted) | 109 ± 26 (65–247) | 92 ± 23 (44–125)* |
| FRC (L) | 2.09 ± 0.62 (1.02–4.61) | 2.56 ± 0.72 (1.04–3.89)* |
| FRC (% predicted) | 74 ± 25 (34–171) | 74 ± 18 (32–119) |
| Diffusing capacity | n = 35 | n = 16 |
| DLCO (mL/mm Hg/min) | 22.2 ± 5.1 (8.5–30.9) | 30.1 ± 6.3 (18.6–40.8)* |
| DLCO (% predicted) | 81 ± 17 (35–123) | 79 ± 19 (48–121) |
| Arterial blood gases | n = 89 | n = 28 |
| pH | 7.41 ± 0.03 (7.35–7.49) | 7.41 ± 0.02 (7.38–7.46) |
| PaCO2 (mm Hg) | 40.9 ± 5.8 (28.0–70.2) | 46.2 ± 5.5 (36.0–90.0)* |
| PaO2 (mm Hg) | 83.0 ± 11.7 (40.2–110.0) | 69.0 ± 13.2 (36.0–90.0)* |
| HCO3 (mEq) | 25.8 ± 3.5 (17.0–42.5) | 28.1 ± 2.7 (24.4–34.0)* |
FVC = forced vital capacity; FEV1 = forced expiratory volume in 1 s; TLC = total lung capacity; RV = reserve volume; ERV = expiratory reserve volume; IC = inspiratory capacity; FRC = functional reserve capacity; DLCO = arterial pressure of diffusing capacity for carbon monoxide; PaCO2 = carbon dioxide; PaO2 = arterial pressure of oxygen; HCO3 = bicarbonate.
P < .05 for difference comparing genders.
Table 3.
Rates of obstruction stratified by smoking and asthma status
| No asthma | Asthma | Total (by smoking, any asthma status) | |
|---|---|---|---|
| Nonsmoker | 9/53 (17)* | 7/23 (30) | 16/76 (21)† |
| Smoker | 16/46 (35)* | 7/14 (50) | 23/60 (38)† |
| Total (by asthma, any smoking status) | 25/99 (25) | 14/37 (38) | 39/136 (29) |
Data presented as proportion of subjects with obstruction categorized by smoking and asthma; obstruction increased in frequency with smoking and showed a trend toward increased frequency with asthma diagnosis; trend also seen for additive effects.
All other P values nonsignificant.
P = .042, chi-square test and P = .063, Fisher’s exact test for smoker versus nonsmoker.
P = .036, Fisher’s exact test, P = .027, chi-square test for smoker versus nonsmoker.
On univariate logistic regression analysis, male gender (OR 2.56, 95% CI 1.13–5.80, P= .02), BMI (OR 1.03, for a 1-unit increase in BMI, 95% CI 1.001–1.06, P= .04), and smoking (OR for current or past smoking 2.33, 95% CI 1.09–4.98, P= .03) were associated with obstruction; however, patient age, asthma, and OSA were not. In a parsimonious multivariate logistic regression model, the association of BMI with airflow obstruction was not significant (OR 1.03 for a 1-unit increase in BMI, 95% CI 0.998–1.06, P= .067); however, male gender (OR 2.35, 95% CI 1.00–5.50, P= .048) and smoking history (OR 2.41, 95% CI 1.10–5.30, P= .028) were significantly associated with obstruction.
Lung volumes
The lung volumes were in the low-normal range according to the reference values of Crapo et al. [13]. The mean TLC was 87% and 82% of predicted in the women and men, respectively; 27 women (32%) and 9 men (32%; P = 1.0) demonstrated restriction (Table 2). In 99 patients with the method of lung volume measurement documented, 94 (95%) underwent plethysmography, and the remainder underwent nitrogen washout.
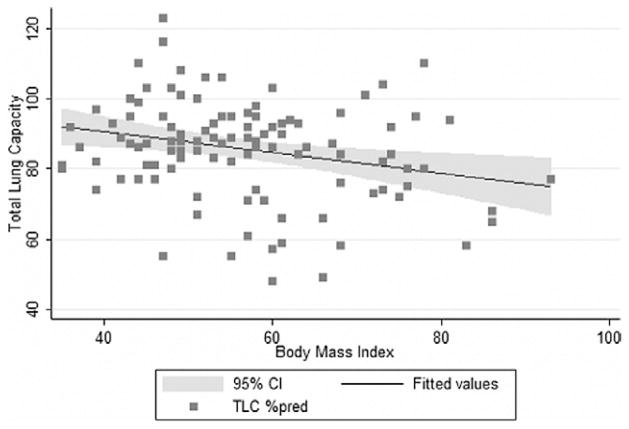
The TLC decreased with increasing BMI. A 1-kg/m2 increase in BMI was associated with a decrease in TLC (percentage of predicted) of .29% (95% CI –.50 to −.08, P = .006; Fig. 2). An analysis of the sensitivity to the outlying BMI data points was performed by repeating the linear regression analysis for those with a BMI of ≤ 80 kg/m2. The effect of the BMI on the TLC was no longer significant (−.24, 95% CI −.48 to .01, P= .059).
Fig. 2.
Body habitus and TLC correlated poorly. TLC expressed as percentage of predicted value. Shaded curve represents 95% confidence intervals for fitted values.
On univariate logistic regression analysis, restrictive pathophysiologic features were associated with BMI (OR 1.04, 95% CI 1.01–107, P = .02). Age, gender, asthma diagnosis, a diagnosis of OSA, and smoking status were not significantly associated with restriction. In a multivariate model adjusted for gender and age, BMI was still associated with restriction, with an OR of 1.04 for each 1-unit increase in BMI (95% CI 1.01–1.08, P = .01).
An evaluation for an association of restriction with a threshold degree of obesity was performed by assessment of logistic regression models incorporating a variable representing a threshold corresponding to a BMI >40 kg/m2. A BMI >40 kg/m2 was not associated with a significantly greater risk of restriction.
Gas exchange
The diffusing capacity of the lung for carbon monoxide was measured in 51 patients. The mean diffusing capacity of the lung for carbon monoxide percentage of predicted was at the low end of the normal range at 79% ± 19% (Table 2). Arterial blood gas data were available for 122 subjects. Of these 122 patients, 5 (4%) had incompletely compensated respiratory acidosis and were censored (mean pH 7.31 and mean arterial pressure of carbon dioxide [PaCO2] of 54 mm Hg). The mean PaCO2 was 42 mm Hg. Of the remaining 117 patients, 70 had a PaCO2 >40 mm Hg, and 37 of these 70 had a PaCO2 >45 mm Hg (Table 2).
On univariate linear regression analysis, PaCO2 was associated with gender, BMI, FEV1, FVC, measured FEV1/FVC, TLC, and OSA status, but not age, asthma diagnosis, or smoking history. In a multivariate model, only BMI and gender remained associated with PaCO2. These associations were best modeled by a quadratic equation [Y = β0 + β1 (BMI) + β2 (BMI)2 + β3 (gender); R2 = 0.46; Fig. 3]. The coefficient of regression for this model was−.88 (95% CI −1.5 to .29, P= .004) for the linear term (β1) and .0092 (95% CI .0044–.014, P <. 001) for the quadratic term (β2). The coefficient of regression for the linear term for gender (β3) was 4.32 (95% CI 2.2–6.4, P <. 001). The model demonstrated that male gender was associated with a mean PaCO2 that was 4 mm Hg greater than that for female gender, regardless of BMI; an increase in BMI (regardless of gender) from 50 to 60 kg/m2 was associated with a 1-mm Hg increase in PaCO2 from 40 to 41 mm Hg, and an additional increase in BMI from 60 to 70 kg/m2 was associated with a 4-mm Hg increase to 45 mm Hg. This association was robust to the effects of outlying data points (no change in the coefficients in patients with a BMI <80 kg/m2). Repeating the regression analyses with censoring of the data points with a pH >7.45 to assess the potential effect of the inclusion of subjects with possible primary metabolic alkalosis and secondary hypercarbia [19] did not produce significant differences.
Fig. 3.
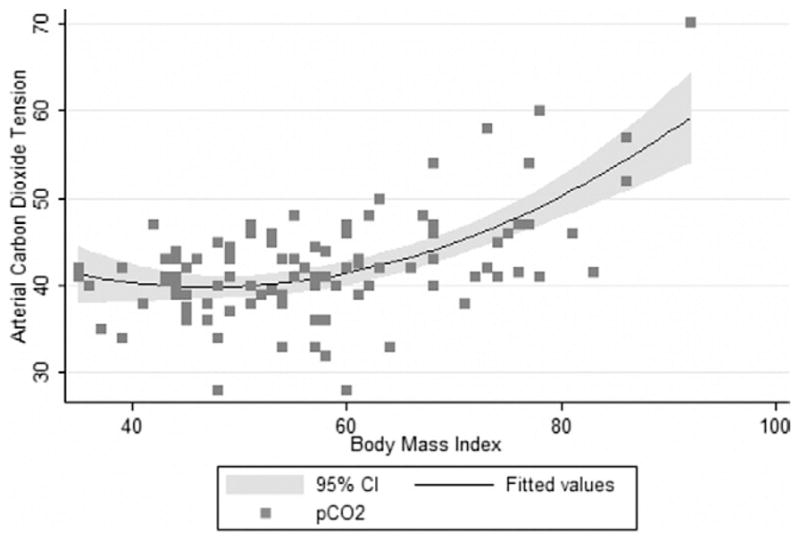
Arterial carbon dioxide tension and BMI were related. PaCO2 expressed in millimeters of mercury. Shaded curve represents 95% confi-dence intervals for fitted values for polynomial regression model, including BMI, BMI*BMI, and gender.
Obesity hypoventilation syndrome
We performed a subset analysis on all subjects who fulfilled our diagnosis of OHS, even if they did not have that clinical diagnosis on their chart. A total of 42 subjects (36%) with available blood gas data fulfilled our criteria for OHS. As might be expected, these subjects differed from the remaining 75 who had had blood gas determinations. They were heavier (191 kg versus 148 kg, P <.001), had a greater BMI (64.1 kg/m2 versus 55.5 kg/m2, P< .001), and had a greater height/weight ratio (1.10 versus 0.88 kg/cm, P <.001). Lung function also differed between these subjects, with the mean FEV1 percentage of predicted and FVC percentage of predicted both lower (62% versus 84%, P <.001; and 68% versus 88%, P <.001, respectively). The TLC percentage of predicted was lower in those with OHS, but the mean remained within the normal range (83% versus 90%, P <.01). The expiratory reserve volume was also lower in the patients with OHS (24% versus 35%, P = .02). Patients with OHS were more likely to fulfill the criteria for restriction (31% versus 11%, P = .01), with a markedly increased risk of having restriction (OR 3.7, 95% CI 1.2–11.1). A linear regression model did not find a significant relationship between the BMI and the TLC percentage of predicted (P = .27) in this subpopulation, but this might have been because of the limited sample size in this subset population.
Assessment of bias
Patients with available pulmonary function data (n = 136) differed from those without these data (n = 93). Patients with such data were more obese (BMI 56.7 ± 12.6 kg/m2 versus 50.6 ± 11.5 kg/m2; P = .0002) and were more likely to have OSA (57% versus 42%, P = .023) with a trend toward greater rates of former smokers (44% versus 31%, P = .054).
Discussion
The major findings of our study were that airflow and restriction obstruction were found in a high proportion of patients; the restriction, when present, was not associated with a threshold weight value, as has been previously reported; an elevated PaCO2 value was associated with the BMI and male gender; and the finding of restriction was associated with the presence of OHS.
Most subjects demonstrated spirometry, lung volumes, and gas exchange measurements within the normal ranges [13,20]. Our finding of frequent obstructive ventilatory defects (29%) was unexpected. Airflow obstruction, defined by an FEV1/FVC less than the 95% CI, was rarely noted in previous studies (4 [4.9%] of 81) [6]. The investigators found no relationship between body size and spirometry. Other groups have described evidence for increased peripheral airway resistance in obesity [21–25], but they likewise found normal values for FEV1/FVC [26,27]. In our analyses, obstruction was associated with smoking and male gender; an absence of an association with asthma is reasonable given that normal baseline spirometry is common in those with asthma [28]. The associations of obesity with spirometry in our study appeared confined to mild symmetric reductions in FEV1 and FVC, similar to that in previous studies [5,6]. The observed prevalence of asthma in our cohort supports the notion that asthma is overrepresented in the obese [29–32]. The inclusion of former and recent cigarette smokers and patients with asthma in our study was useful in delineating the range of typical pulmonary function test findings in obese patients with these common co-morbidities.
Restriction was found in one third of our patients, although the mean TLC was within the normal range. We, as have others [5,6], found that an increasing BMI was associated with a decrease in the TLC. In our cohort, a 1-kg/m2 increase in BMI was associated with a decrease in TLC of .29%. Ray et al. [6] found that TLC measurements less than the 95% CI were distributed evenly throughout the weight groups, except for in the largest group (weight/height > 1.0). Their accompanying figure suggests a relative preservation of the TLC at weight/height ratios of <1.0, with a distinct threshold effect at a weight/height of 1.0, above which the TLC progressively decreases [6]. We found no threshold size above which restriction was disproportionately likely to be present; however, we were able to clearly demonstrate a linear relationship between increasing body size and TLC. Our conclusions might differ from those of older data owing to the greater number of more severely obese subjects we studied. However, our data are consistent with previous reports from Sugerman et al. [33] noting pulmonary restriction in the subset of patients with OHS.
PaCO2 was elevated in nearly 32% of our patients with resting blood gas measurements. We found that PaCO2 was only associated with male gender and BMI. The 4-mm Hg greater PaCO2 observed in men was slightly greater than the gender difference of 1.3 mm Hg described in previously referenced values [34]. The relation of PaCO2 to BMI was nonlinear and best modeled as a quadratic function. Our model described a closer correlation of body size with PaCO2 than was found by Crapo et al. [34], who reported a statistically insignificant correlation coefficient for weight and PaCO2. The inclusion of the quadratic term, the use of the BMI rather than weight as a measure of body size, and the wider distribution of weights in our cohort might have contributed to the observed association. Other investigators have found hypercapnia in obese patients to be associated with the severity of OSA [35–37]; increasing weight, decreasing upper airway size, or alcohol use [38]; decreased FVC [37]; and decreased FEV1 [36]. However, most of these studies included only patients with OSA and obesity. Given the limited sample of patients in our study who carried the diagnosis of OSA, we were unable to confirm these findings.
Our findings regarding OHS are similar to those reported by Sugerman et al. [33,39]. They noted a clear relationship between hypercapnia and restriction in those patients with OHS. Importantly, they noted an improvement in gas exchange and lung function after gastric bypass surgery in the setting of OHS [33]. Long-term follow-up noted sustained improvement in gas exchange and lung function [39]. The effect on lung function could partly be explained by the increased intra-abdominal pressure associated with OHS; the degree of central obesity as measured by the sagittal diameter has been associated with increasing intra-abdominal pressure in morbidly obese patients [40]. Identifying patients with OHS is important given published reports suggesting that this group has a markedly increased operative mortality (4% mortality rate compared with an overall mortality rate of .7% in those without respiratory insuffi-ciency undergoing gastric surgery for weight reduction) [39]. Given the increased perioperative mortality of this patient subset, the identification of abnormal gas exchange values preoperatively is critical. The current preoperative recommendations from the American Association of Clinical Endocrinologists, the Obesity Society, and the American Society for Metabolic and Bariatric Surgery include chest radiography for all patients. Patients “with intrinsic lung disease or disordered sleep patterns should have a formal pulmonary evaluation, including arterial blood gas measurement and sleep polysomnography, when knowledge of the results would alter patient care” [41]. Our current clinical practice is to obtain full lung function tests for patients with known lung disease (i.e., asthma or COPD) or pulmonary symptoms. This recommendation is consistent with that of Crapo et al. [42]. On the basis of their review of spirometry in 114 morbidly obese patients being considered for bariat-ric surgery in 1983, routine spirometry was not recommended. The distribution of spirometry in their population was consistent with the normal distribution of the nonobese population. Their study did not include blood gases or lung volume assessments and studied patients with a lesser degree of obesity (mean BMI 42 kg/m2). Our current practice is to obtain routine blood gas measurements on all patients either at screening or just before induction for surgery. Blood gas assessment permits appropriate ventilator management during the operation and in the immediate postoperative period. Operative anesthesia management permits appropriate levels of ventilation for patients with diminished central drive to breathe (i.e., OHS). The high rate of airflow obstruction and carbon dioxide retention found in our cohort could influence the postoperative management. An assessment of the relationship of preoperative lung function and blood gas assessment with the development of postoperative complications is necessary to clarify whether strong predictors of poor postoperative outcome are available.
The limitations of this study included a selection bias in that the population undergoing pulmonary function testing differed from those not tested. Overrepresentation of co-morbidities in the group undergoing testing would be expected to bias the results toward increased pulmonary function abnormalities. The near-normal results described suggest that selection bias was not a significant limitation. Any resultant bias would limit the generalization of our findings but not the internal validity. Although we were unable to be certain that all pulmonary function testing performed outside our institution met the ATS criteria [12], this should have limited effects given the standardized performance of spirometry and accreditation of laboratories. Finally, an ascertainment bias of co-morbid illnesses and habits was possible with the chart and medication review. However, this scheme was clinically relevant and has been used in other studies in this area [27].
Conclusion
Even in extreme obesity, a substantial proportion of patients have normal spirometry, lung volumes, and gas exchange parameters. Although increased size is associated with an increasing risk of restriction and with increasing PaCO2, no threshold effect of BMI on these measures was identified. Restriction was associated with OHS when OHS was determined by a definition using room air blood gas. Obstructive and restrictive ventilatory defects were both common in this population.
Acknowledgments
Supported by grants 1 K23 HL72017-01 and HL07287 from the National Heart, Lung and Blood Institute.
Footnotes
Disclosures
The authors claim no commercial associations that might be a conflict of interest in relation to this article.
References
- 1.Barrera F, Reidenberg MM, Winters WL. Pulmonary function in the obese patient. Am J Med Sci. 1967;254:785–96. doi: 10.1097/00000441-196712000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Luce JM. Respiratory complications of obesity. Chest. 1980;78:626–31. doi: 10.1378/chest.78.4.626. [DOI] [PubMed] [Google Scholar]
- 3.Koenig SM. Pulmonary complications of obesity. Am J Med Sci. 2001;321:249–79. doi: 10.1097/00000441-200104000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Unterborn J. Pulmonary function testing in obesity, pregnancy, and extremes of body habitus. Clin Chest Med. 2001;22:759–67. doi: 10.1016/s0272-5231(05)70064-2. [DOI] [PubMed] [Google Scholar]
- 5.Biring MS, Lewis MI, Liu JT, Mohsenifar Z. Pulmonary physiologic changes of morbid obesity. Am J Med Sci. 1999;318:293–7. doi: 10.1097/00000441-199911000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Ray CS, Sue DY, Bray G, Hansen JE, Wasserman K. Effects of obesity on respiratory function. Am Rev Respir Dis. 1983;128:501–6. doi: 10.1164/arrd.1983.128.3.501. [DOI] [PubMed] [Google Scholar]
- 7.Ray CS, Sue DY, Bray G, Hansen JE, Wasserman K. Effects of obesity on respiratory function. Am Rev Respir Dis. 1983;128:501–6. doi: 10.1164/arrd.1983.128.3.501. [DOI] [PubMed] [Google Scholar]
- 8.Biring MS, Lewis MI, Liu JT, Mohsenifar Z. Pulmonary physiologic changes of morbid obesity. Am J Med Sci. 1999;318:293–7. doi: 10.1097/00000441-199911000-00002. [DOI] [PubMed] [Google Scholar]
- 9.American Academy of Sleep Medicine Task Force. Sleep-related breathing disorders in adults: recommendations for syndrome defini-tion and measurement techniques in clinical research—the report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 10.Mokhlesi B, Tulaimat A. Recent advances in obesity hypoventilation syndrome. Chest. 2007;132:1322–36. doi: 10.1378/chest.07-0027. [DOI] [PubMed] [Google Scholar]
- 11.Mokhlesi B, Tulaimat A, Faibussowitsch I, Wang Y, Evans AT. Obesity hypoventilation syndrome: prevalence and predictors in patients with obstructive sleep apnea. Sleep Breath. 2007;11:117–24. doi: 10.1007/s11325-006-0092-8. [DOI] [PubMed] [Google Scholar]
- 12.American Thoracic Society. Standardization of spirometry, 1994 update. Am J Respir Crit Care Med. 1995;152:1107–36. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 13.Crapo RO, Morris AH, Gardner RM. Reference spirometric values using techniques and equipment that meet ATS recommendations. Am Rev Respir Dis. 1981;123:659–64. doi: 10.1164/arrd.1981.123.6.659. [DOI] [PubMed] [Google Scholar]
- 14.Crapo RO, Morris AH, Clayton PD, Nixon CR. Lung volumes in healthy nonsmoking adults. Bull Eur Physiopathol Respir. 1982;18:419–25. [PubMed] [Google Scholar]
- 15.Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26:948–68. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 16.Barker AF. Bronchiectasis. N Engl J Med. 2002;346:1383–93. doi: 10.1056/NEJMra012519. [DOI] [PubMed] [Google Scholar]
- 17.Bates DV. Respiratory function in disease. 3. Philadelphia: WB Saunders; 1989. [Google Scholar]
- 18.Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: the evidence report. Bethesda: National Institutes of Health; 1998. [PubMed] [Google Scholar]
- 19.Nowbar S, Burkart KM, Gonzales R, et al. Obesity-associated hy-poventilation in hospitalized patients: prevalence, effects, and outcome. Am J Med. 2004;116:1–7. doi: 10.1016/j.amjmed.2003.08.022. [DOI] [PubMed] [Google Scholar]
- 20.Crapo RO, Jensen RL, Wanger JS. Single-breath carbon monoxide diffusing capacity. Clin Chest Med. 2001;22:637–49. doi: 10.1016/s0272-5231(05)70057-5. [DOI] [PubMed] [Google Scholar]
- 21.Douglas FG, Chong PY. Influence of obesity on peripheral airways patency. J Appl Physiol. 1972;33:559–63. doi: 10.1152/jappl.1972.33.5.559. [DOI] [PubMed] [Google Scholar]
- 22.Pelosi P, Croci M, Ravagnan I, Vicardi P, Gattinoni L. Total respiratory system, lung, and chest wall mechanics in sedated-paralyzed postoperative morbidly obese patients. Chest. 1996;109:144–51. doi: 10.1378/chest.109.1.144. [DOI] [PubMed] [Google Scholar]
- 23.Pelosi P, Croci M, Ravagnan I, et al. The effects of body mass on lung volumes, respiratory mechanics, and gas exchange during general anesthesia. Anesth Analg. 1998;87:654–60. doi: 10.1097/00000539-199809000-00031. [DOI] [PubMed] [Google Scholar]
- 24.Watson RA, Pride NB. Postural changes in lung volumes and respiratory resistance in subjects with obesity. J Appl Physiol. 2005;98:512–7. doi: 10.1152/japplphysiol.00430.2004. [DOI] [PubMed] [Google Scholar]
- 25.Yap JC, Watson RA, Gilbey S, Pride NB. Effects of posture on respiratory mechanics in obesity. J Appl Physiol. 1995;79:1199–205. doi: 10.1152/jappl.1995.79.4.1199. [DOI] [PubMed] [Google Scholar]
- 26.Rubinstein I, Zamel N, DuBarry L, Hoffstein V. Airflow limitation in morbidly obese, nonsmoking men. Ann Intern Med. 1990;112:828–32. doi: 10.7326/0003-4819-112-11-828. [DOI] [PubMed] [Google Scholar]
- 27.Sin DD, Jones RL, Man SF. Obesity is a risk factor for dyspnea but not for airflow obstruction. Arch Intern Med. 2002;162:1477–81. doi: 10.1001/archinte.162.13.1477. [DOI] [PubMed] [Google Scholar]
- 28.Expert Panel Report 2: Guidelines for the Diagnosis and Management of Asthma. Bethesda, MD: National Institutes of Health, National Heart, Lung and Blood Institute; 1997. [Google Scholar]
- 29.Young SY, Gunzenhauser JD, Malone KE, McTiernan A. Body mass index and asthma in the military population of the northwestern United States. Arch Intern Med. 2009;61:1605–11. doi: 10.1001/archinte.161.13.1605. [DOI] [PubMed] [Google Scholar]
- 30.Shore SA, Fredberg JJ. Obesity, smooth muscle, and airway hyper-responsiveness. J Allergy Clin Immunol. 2005;115:925–7. doi: 10.1016/j.jaci.2005.01.064. [DOI] [PubMed] [Google Scholar]
- 31.Camargo CA, Jr, Weiss ST, Zhang S, Willett WC, Speizer FE. Prospective study of body mass index, weight change, and risk of adult-onset asthma in women. Arch Intern Med. 1999;159:2582–8. doi: 10.1001/archinte.159.21.2582. [DOI] [PubMed] [Google Scholar]
- 32.Ford ES. The epidemiology of obesity and asthma. J Allergy Clin Immunol. 2005;115:897–909. doi: 10.1016/j.jaci.2004.11.050. [DOI] [PubMed] [Google Scholar]
- 33.Sugerman HJ, Fairman RP, Baron PL, Kwentus JA. Gastric surgery for respiratory insufficiency of obesity. Chest. 1986;90:81–6. doi: 10.1378/chest.90.1.81. [DOI] [PubMed] [Google Scholar]
- 34.Crapo RO, Jensen RL, Hegewald M, Tashkin DP. Arterial blood gas reference values for sea level and an altitude of 1,400 meters. Am J Respir Crit Care Med. 1999;160(5 Pt 1):1525–31. doi: 10.1164/ajrccm.160.5.9806006. [DOI] [PubMed] [Google Scholar]
- 35.Berger KI, Ayappa I, Chatr-Amontri B, et al. Obesity hypoventilation syndrome as a spectrum of respiratory disturbances during sleep. Chest. 2001;120:1231–8. doi: 10.1378/chest.120.4.1231. [DOI] [PubMed] [Google Scholar]
- 36.Krieger J, Sforza E, Apprill M, Lampert E, Weitzenblum E, Rato-maharo J. Pulmonary hypertension, hypoxemia, and hypercapnia in obstructive sleep apnea patients. Chest. 1989;96:729–37. doi: 10.1378/chest.96.4.729. [DOI] [PubMed] [Google Scholar]
- 37.Leech JA, Onal E, Baer P, Lopata M. Determinants of hypercapnia in occlusive sleep apnea syndrome. Chest. 1987;92:807–13. doi: 10.1378/chest.92.5.807. [DOI] [PubMed] [Google Scholar]
- 38.Chan CS, Grunstein RR, Bye PT, Woolcock AJ, Sullivan CE. Obstructive sleep apnea with severe chronic airflow limitation: comparison of hypercapnic and eucapnic patients. Am Rev Respir Dis. 1989;140:1274–8. doi: 10.1164/ajrccm/140.5.1274. [DOI] [PubMed] [Google Scholar]
- 39.Sugerman HJ, Fairman RP, Sood RK, Engle K, Wolfe L, Kellum JM. Long-term effects of gastric surgery for treating respiratory insuffi-ciency of obesity. Am J Clin Nutr. 1992;55(2 suppl):597S–601S. doi: 10.1093/ajcn/55.2.597s. [DOI] [PubMed] [Google Scholar]
- 40.Sugerman H, Windsor A, Bessos M, Wolfe L. Intra-abdominal pressure, sagittal abdominal diameter and obesity comorbidity. J Intern Med. 1997;241:71–9. doi: 10.1046/j.1365-2796.1997.89104000.x. [DOI] [PubMed] [Google Scholar]
- 41.Mechanick JI, Kushner RF, Sugerman HJ, et al. Executive summary of the recommendations of the American Association of Clinical Endocrinologists, the Obesity Society, and American Society for Metabolic and Bariatric Surgery medical guidelines for clinical practice for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient. Endocr Pract. 2008;14:318–36. doi: 10.4158/EP.14.3.318. [DOI] [PubMed] [Google Scholar]
- 42.Crapo RO, Kelly TM, Elliott CG, Jones SB. Spirometry as a preop-erative screening test in morbidly obese patients. Surgery. 1986;99:763–8. [PubMed] [Google Scholar]