Abstract
Objective
Cardiomyopathy (CM) at delivery is increasing in prevalance. The objective of this study was to determine what medical conditions are attributable to this increasing prevalance.
Design
Population prevalence study from 2000 to 2009.
Setting
The Nationwide Inpatient Sample (NIS).
Methods
Pregnant women admitted for delivery were identified in the NIS for the years 2000-2009 and temporal trends in pre-existing medical conditions and medical and obstetric complications at delivery admissions were determined by linear regression. The change in the prevalence of CM among all pregnant women was compared to the change in the prevalance of CM among pregnant women without pre-existing conditions or complications.
Main Outcome Measures
Prevalence of cardiomyopathy.
Results
The prevalence of CM increased from 0.25 per 1000 deliveries in 2000 to 0.43 per 1000 deliveries in 2009 (p<0.0001). Women with chronic hypertension had increased odds of developing CM compared to women without chronic hypertension (odds ratio[OR] 13.2 [95% CI 12.5, 13.7]). The linear increase in chronic hypertension over the ten-year period was the single identified pre-existing medical condition that explained the increasing prevalence of CM at delivery (p=0.005 for the differences in the slopes for linear trend).
Conclusions
Pregnant women with chronic hypertenion are at an increased risk for CM at delivery and the increasing prevalence of chronic hypertension is an important factor associated with the increasing prevalence of CM at the time of delivery. Among women without chronic hypertension, the prevalence of CM at delivery did not change during the time period.
Keywords: cardiomyopathy, hypertension, mortality, nationwide inpatient sample, pregnancy
INTRODUCTION
Pregnancy-associated cardiomyopathy, defined as either peripartum cardiomyopathy or cardiomyopathy with primary causes during pregnancy, is relatively uncommon but is a potentially life-threatening condition. As mortality from other pregnancy conditions, such as hemorrhage and hypertensive disorders, has decreased in developing countries, cardiomyopathy has accounted for an increasing proportion of maternal deaths. For example, in the United States, the proportion of pregnancy-related deaths from cardiomyopathy increased in the time period 1979-1986 to 1991-1997 and up to 11.5% in the period 1998-2005, reaching almost 13% in 2005-2006.(1-5) The proportion of deaths attributable to cardiomyopathy in pregnancy may be even higher in geographic locations with a higher prevalence of this condition. A recent study reviewing preventable causes of maternal death from 1995-1999 found that cardiomyopathy was the most common cause of pregnancy-related death in North Carolina, representing 21% of all cases of maternal mortality.(6) The increasing trends in the reported prevalence of cardiomyopathy complicating pregnancy reported during the time period 1995-2006 are also concerning given that cardiomyopathy is an important contributor to other severe medical complications in pregnancy including cardiac arrest, acute myocardial infarction, pulmonary edema, and acute respiratory distress syndrome.(7)
Pregnancy poses a number of challenges to the maternal cardiovascular system. Blood volume increases by 50%, resulting in an increase in cardiac output by 30%-50% and increased work of the heart.(8-10) Women with underlying heart disease may be at risk for baseline cardiac dysfunction and their cardiac function may not tolerate the additional strain caused by pregnancy. As cardiomyopathy complicating pregnancy has increased over the time period 1995-2006,(7) the objective of this study was to identify pre-existing medical conditions and medical and obstetric complications that might explain the increasing prevalence of cardiomyopathy at the time of delivery.
METHODS
The study was reviewed and declared exempt from review by the Duke University Medical Center Institutional Review Board. We utilized the Nationwide Inpatient Sample (NIS) to conduct a population prevalence study to determine what factors are associated with the increasing prevalence of cardiomyopathy at the time of delivery. The NIS from the Healthcare Cost and Utilization Project of the Agency for Healthcare Research and Quality (AHRQ) for years 2000 to 2009 was queried for all pregnancy-related discharges with a diagnosis of cardiomyopathy. For the years in the study period, the NIS contains data from 7-8 million hospital admissions per year from approximately 1,000 hospitals in 28 (2000) to 44 (2009) states and is the largest all-payer inpatient care database in the United States. The hospitals in the NIS are stratified based on ownership, bed size, teaching status, urban/rural location, and region. Within each stratum, the NIS contains approximately 20% of U.S. hospitals, representing a random sample. The NIS does not include data from rehabilitation hospitals, long-term hospitals, psychiatric hospitals, and alcoholism or chemical dependency treatment facilities, none of which are likely to have delivery admissions. Sampling probabilities are proportional to the number of hospitals in each stratum. The sampling frame comprises 90% of all US hospital discharges and the NIS provides sampling weights that allow for calculation of national estimates. The information included in the NIS is similar to that in a typical administrative discharge abstract with safeguards to protect the privacy of individual patients, physicians, and hospitals. Although the nature of the data is limited to discharge diagnoses and demographic information, the NIS allows for the study of relatively rare conditions such as cardiomyopathy in women during pregnancy.
Using the NIS for each of the years 2000-2009, all records containing a pregnancy-related discharge for delivery were identified. An admission for delivery was defined as any discharge record that included an International Classification of Diseases, Clinical Modification (ICD-9-CM) or Diagnosis-Related Group (DRG) delivery code. ICD-9-CM delivery codes were 74 for cesarean section; 72, 73, and 75 for vaginal delivery; and V27 and 650 for general delivery codes. For the years 2000-2007, DRG codes 370 and 371 were utilized to identify cesarean deliveries and codes 372, 373, 374, and 375 for vaginal deliveries. For the years 2008 and 2009, DRG codes 765 and 766 were utilized to identify cesarean deliveries and codes 767, 768, 774, and 775 for vaginal deliveries.(11-14)
Cardiomyopathy was defined by the ICD-9-CM codes 674.5, the code for peripartum cardiomyopathy, and 425.x, the codes for cardiomyopathy secondary to primary causes.(15) Although all etiologies of cardiomyopathy in pregnancy are not the same, in this study, we elected to combine all types of cardiomyopathy. In a study utilizing a similar design, it was reported that only a minority of cardiomyopathy diagnoses in pregnancy were consistent with peripartum cardiomyopathy, while the majority represented cardiomyopathies secondary to identifiable causes (primary cardiomyopathies).(15) For comorbidities, both the ICD-9-CM code for a particular condition in pregnancy (i.e. 6xx code) and the general ICD-9-CM code for that condition were used. (Table S1 for list of ICD-9-CM codes utilized) The number of deaths occurring during delivery admissions was determined for each study year and were identified by having a discharge status as “DIED” in the NIS. The sampling frame for events was limited to only delivery admissions. By focusing on delivery admissions, since each woman only has one delivery per pregnancy, we were able to estimate outcomes for individuals, rather than hospitalizations, as women could possibly have more than one antepartum or postpartum hospitalization during a single pregnancy, but not more than one hospitalization for delivery.
According to the currently accepted definition, peripartum cardiomyopathy may manifest from the last month of pregnancy to 5 months after delivery. Although the majority of cases of peripartum cardiomyopathy (about 50%) manifests in the first 6-8 weeks postpartum, about one-third of cases are diagnosed during the delivery hospitalization. Thus, only peripartum cardiomyopathies and other (primary) cardiomyopathies that were diagnosed during delivery hospitalization are included in our study.
The analysis accounted for the weighted estimates of hospitalizations that were used by the NIS. Two-way chi-square tests generated cell frequencies and P-values for demographics, pre-existing medical conditions, and medical and obstetric complications among women with and without cardiomyopathy. Logistic regression analyses were used to compute odds ratios with 95% confidence intervals for age, race, pre-existing medical conditions, and medical and obstetric complications among women with cardiomyopathy at delivery compared to those women without cardiomyopathy. The rate of pre-existing medical conditions and medical and obstetric complications among pregnant women with cardiomyopathy at delivery were also calculated for each of the years from 2000 to 2009.
For each pre-existing medical condition and medical or obstetric complication, the rate of the condition or outcome per 1000 deliveries was calculated for each of the 10 years. Linear regression was then utilized to determine if the prevalence of each pre-existing medical condition or incidence of each medical and obstetric complication varied significantly over the 10-year time period. Next, to determine if any pre-existing medical conditions or medical/obstetric complications affected the prevalence of cardiomyopathy at delivery, the slope over the 10-year time period of the prevalence of cardiomyopathy for all subjects with cardiomyopathy during a delivery admission was compared to the slope that was calculated for subjects with cardiomyopathy who did not also have each of the listed pre-existing medical conditions or medical/obstetric complications of interest, and the differences in the two slopes was determined. A significant difference between the two slopes suggests that the condition or complication affects the prevalence of cardiomyopathy. Finally, the population attributable risk proportion for the contribution of chronic hypertension to the development of cardiomyopathy was calculated.(16) Statistical significance for linear regression was assigned as a P value < 0.01.(7) Statistical significance for all other analyses was assigned as a P value < 0.05. All analyses were performed using SAS version 9.3 (SAS Institute Inc, Cary, NC) and GraphPad Prism version 5.0 for Macintosh (GraphPad Software, San Diego, CA).
RESULTS
During the years 2000-2009, there were a weighted estimated 43,226,339 delivery admissions within NIS. The overall age distribution for this time period was 34.8% ages 15-24, 27.0% ages 25-29, 23.4% ages 30-34, and 14.6% ages 35 and older. The age distribution did not vary significantly over this time period except for a slight increase in the proportion of women aged 25-29 from 26.5% in 2000 to 27.8% in 2009 (p<0.001 for age 25-29 and p>0.01 for all other age groups). The racial/ethnic distribution of women during a delivery admission did not vary linearly over the time period, though 24.9% of the entries over the 10-year time period had missing race/ethnicity data. The cesarean delivery rate over the entire time period was 28.6% and increased linearly during the years 2000 to 2009 (p<0.001), from 22.2% in 2000 to 32.5% in 2009. Of the 3309 maternal deaths, 105 (3%) occurred in women with a diagnosis of cardiomyopathy. The maternal mortality rate among all women during a delivery admission did not change with time during the studied time period (p=0.097).
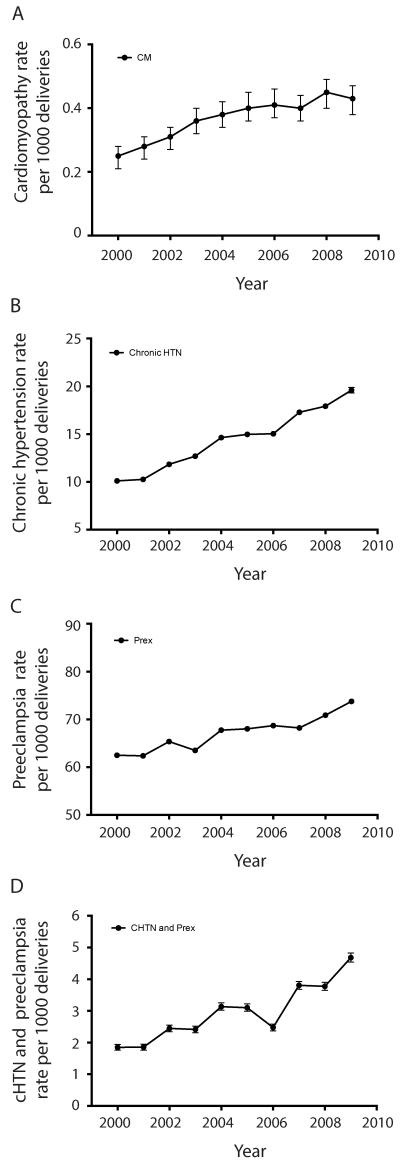
The prevalence of cardiomyopathy at the time of a delivery admission increased during the time period (p<0.001) from 0.25 per 1000 deliveries in 2000 to 0.43 per 1000 deliveries in 2009 (Figure 1A, Table S2). The change in the prevalence of other pre-existing medical and obstetric conditions at delivery admissions was also calculated over the 10-year period. There were significant linear increases (p<0.01) in the prevalence of congenital heart disease, cardiac conduction disorders, history of ischemic heart disease, asthma, disorders of pulmonary circulation, diabetes, thyroid disorders, systemic lupus erythematosus, rheumatoid arthritis, thrombophilia/anti-phospholipid antibody syndrome, thrombocytopenia, anemia, chronic hypertension, chronic renal failure, tobacco use, drug use, gestational diabetes, preeclampsia, and fetal growth restriction during the 10-year study period (Table S2, Figures 1B, 1C and S1). There were also significant linear decreases (p<0.01) in the prevalence of valvular heart disease and fetal death during delivery admissions during the ten-year time period (Table S2, Figure S1).
Figure 1. Trends in the prevalence of cardiomyopathy, chronic hypertension, preeclampsia, and chronic hypertension with preeclampsia at delivery admissions, the 2000 – 2009 Nationwide Inpatient Sample (n = 43,226,239).
Error bars demonstrate 95% confidence intervals. A. There was an increase in the linear trend for cardiomyopathy diagnosed at delivery admissions during the 10-year study period (p<0.001, R2=0.90), increasing from 0.25 cases per 1000 deliveries in 2000 to 0.43 per 1000 deliveries in 2009. B and C. There was an increase in the linear trend for chronic hypertension (B. p<0.001, R2=0.99) and preeclampsia (C. p<0.001, R2=0.90), increasing from 10.1 per 1000 deliveries in 2000 to 19.6 per 1000 deliveries in 2009, and from 62.5 per 1000 deliveries in 2000 to 74.0 per 1000 deliveries in 2009, respectively. D. There was an increase in the linear trend for chronic hypertension with preeclampsia (D. p<0.001, R2=0.84), increasing from 1.8 per 1000 deliveries in 2000 to 4.7 per 1000 deliveries in 2009.
We also estimated trends in medical complications occurring during a delivery admission over the 10-year period. There were significant linear increases (p<0.01) in the incidence of acute respiratory distress syndrome, pulmonary embolism, sepsis, and acute renal failure (Table S3, Figure S2). There were significant linear decreases (p<0.01) in the incidence of pulmonary edema and postpartum bacterial infection (endometritis) during delivery admissions during the 10-year time period (Table S3, Figure S2).
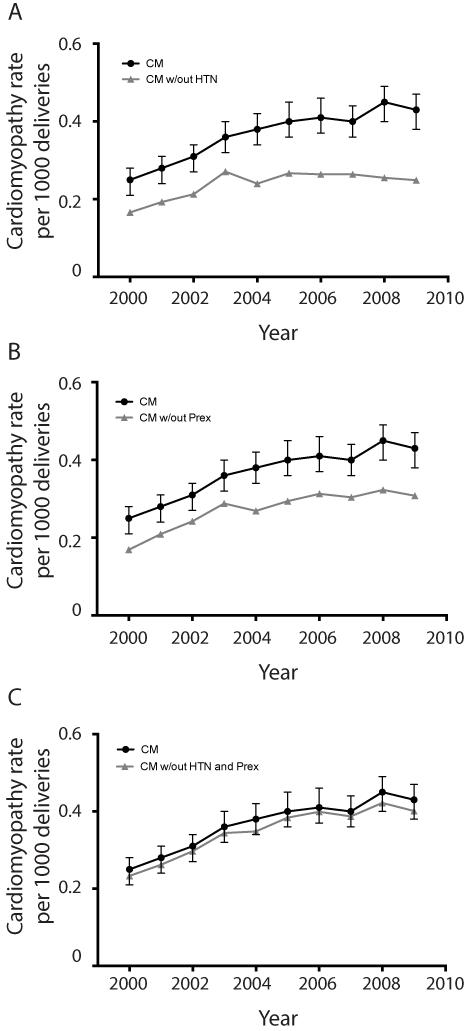
To determine if changes in the prevalence of pre-existing medical conditions or the incidence of medical and obstetric complications were contributing to the increasing prevalence of cardiomyopathy in pregnancy, we compared the slope calculated by linear regression for the change in the prevalence of cardiomyopathy at delivery among all pregnant women over the 10-year time period with the slope calculated by linear regression for cardiomyopathy among subjects not having each of the medical or obstetric conditions/complications of interest. When compared, a significant difference between the two slopes suggested that the tested medical condition was associated, at least in part, with the change in the prevalence of cardiomyopathy during the 10-year period. Of each of the tested pre-existing medical conditions or medical and obstetric complications, only chronic hypertension affected the prevalence of cardiomyopathy in pregnancy (Table 1, Figure 2A, Tables 2 and 3, Figures S3 and S4). For women without chronic hypertension, there was a non-significant linear increase in the prevalence of cardiomyopathy (p=0.015) (Table 1, Figure 2A). Furthermore, when the slope for the change in prevalence of cardiomyopathy among all women was compared to the slope for the change in prevalence of cardiomypathy among women without chronic hypertension, the slopes were significantly different (p=0.005 for differences in slopes) demonstrating that chronic hypertension affected the change in the prevalence of cardiomyopathy (Table 1, Figure 2A).
Table 1.
Comparison of linear trends of CM among all women at a delivery admission to women with CM who did not have chronic hypertension, preeclampsia, or chronic hypertension with preeclampsia during the years 2000 – 2009 by linear regression (significance defined as P-value < 0.01). See Figure 2 for graphical representation.
| Condition | aSlope | R2 | P-value |
bP-value comparing condition slope to CM |
|---|---|---|---|---|
| All women with Cardiomyopathy (CM) | 0.021 | 0.90 | <0.001 | -- |
| CM among those women without chronic hypertension |
0.009 | 0.55 | 0.015 | 0.005 |
| CM among those women without preeclampsia |
0.015 | 0.80 | <0.001 | 0.120 |
| CM among those women without chronic hypertension and preeclampsia |
0.020 | 0.88 | <0.001 | 0.804 |
Slope (Δy/Δx) reported is the change in the listed condition per 1000 deliveries (Δy) per year (Δx) (Figure 2)
Comparison of the slope obtained from the linear regression of the trends for CM among all women to the slope obtained from linear regression for women with CM who did not also have chronic hypertension, preeclampsia, or chronic hypertension with preeclampsia.
Figure 2. Trends in the prevalence of cardiomyopathy in women without hypertensive disorders of pregnancy compared to all women with cardiomyopathy at delivery admissions, the 2000 – 2009 Nationwide Inpatient Sample (n=43,226,239).
Error bars demonstrate 95% confidence intervals. To determine if pre-existing medical conditions or medical and obstetric complications occurring during a delivery admission were accounting for the increased prevalence of cardiomyopathy over the study period, the linear trend for cardiomyopathy among women who also did not have each of the preexisting medical conditions or medical and obstetrics complications listed (Tables 2 and 3) were compared to the linear trend for all women with cardiomyopathy. A. The difference in the slopes for the linear trends of all women with cardiomyopathy compared to women with cardiomyopathy who did not have chronic hypertension were significantly different (p=0.005), suggesting that chronic hypertension was contributing to the increasing prevalence of cardiomyopathy during the study time period. B. and C. In contrast, there were no differences in the slope for linear trend among all women with cardiomyopathy compared to women with cardiomyopathy who did not have preeclampsia (B) or compared to women with cardiomyopathy who did not have both chronic hypertension and preeclampsia (C). (Abbreviations: CM=cardiomyopathy, HTN=hypertension, Prex=preeclampsia)
Table 2.
Comparison of linear trends of CM among all women at a delivery admission to women with CM who did not also have the listed pre-existing medical condition during the years 2000 – 2009 by linear regression (significance defined as P-value < 0.01). See Figure S3 for graphical representation.
| Condition | aSlope | R2 | P-value |
bP-value comparing condition slope to CM |
|---|---|---|---|---|
| Cardiomyopathy (CM) | 0.021 | 0.90 | <0.001 | -- |
| CM without Congenital heart disease | 0.021 | 0.89 | <0.001 | 0.97 |
| CM without Valvular heart disease | 0.021 | 0.93 | <0.001 | 0.91 |
| CM without Conduction disorders | 0.019 | 0.90 | <0.001 | 0.52 |
| CM without History of ischemic heart dz. | 0.021 | 0.89 | <0.001 | 0.97 |
| CM without Asthma | 0.020 | 0.86 | <0.001 | 0.76 |
| CM without Disorders of pulmonary circ. | 0.200 | 0.87 | <0.001 | 0.92 |
| CM without Diabetes | 0.019 | 0.85 | <0.001 | 0.60 |
| CM without Thyroid disorders | 0.021 | 0.90 | <0.001 | 0.95 |
| CM without SLE | 0.021 | 0.89 | <0.001 | 0.93 |
| CM without Rheumatoid arthritis | 0.021 | 0.89 | <0.001 | 0.89 |
| CM without Thrombophilia | 0.021 | 0.88 | <0.001 | 0.98 |
| CM without Thrombocytopenia | 0.021 | 0.89 | <0.001 | 0.99 |
| CM without Anemia | 0.017 | 0.85 | <0.001 | 0.27 |
| CM without Tobacco use | 0.019 | 0.87 | <0.001 | 0.72 |
| CM without Drug use | 0.020 | 0.90 | <0.001 | 0.96 |
| CM without Alcohol use | 0.021 | 0.89 | <0.001 | 0.88 |
| CM without Chronic renal failure | 0.020 | 0.87 | <0.001 | 0.92 |
| CM without Preeclampsia | 0.015 | 0.80 | <0.001 | 0.120 |
| CM without Gestational diabetes | 0.021 | 0.85 | <0.001 | 0.92 |
| CM without Multiple gestation | 0.020 | 0.87 | <0.001 | 0.83 |
| CM without Fetal growth restriction | 0.021 | 0.87 | <0.001 | 0.96 |
| CM without Placental abruption | 0.021 | 0.89 | <0.001 | 0.99 |
| CM without Fetal death | 0.021 | 0.89 | <0.001 | 0.94 |
Slope (Δy/Δx) reported is the change in the listed condition per 1000 deliveries (Δy) per year (Δx) (Figure 2 and Figure S3)
Comparison of the slope obtained from the linear regression of the trends for CM among all women to the slope obtained from linear regression for women with CM who did not also have each of the listed variables (women with CM who do not have the condition listed).
Table 3.
Comparison of linear trends in CM among all women at a delivery admission to women with CM who did not have the listed medical event during the years 2000 – 2009 by linear regression (significance defined as P-value < 0.01). See Figure S4 for graphical representation.
| Condition | aSlope | R2 | P-value |
bP-value comparing condition slope to CM |
|---|---|---|---|---|
| Cardiomyopathy (CM) | 0.021 | 0.90 | <0.001 | -- |
| CM without Myocardial infarction | 0.021 | 0.89 | <0.001 | 0.95 |
| CM without Ventricular fibrillation | 0.021 | 0.89 | <0.001 | 0.94 |
| CM without Cardiac arrest | 0.021 | 0.89 | <0.001 | 0.99 |
| CM without Acute heart failure | 0.015 | 0.92 | <0.001 | 0.053 |
| CM without Pneumonia | 0.020 | 0.89 | <0.001 | 0.83 |
| CM without RDS | 0.020 | 0.87 | <0.001 | 0.84 |
| CM without Pulmonary edema | 0.021 | 0.89 | <0.001 | 0.95 |
| CM without Stoke/CVA | 0.021 | 0.89 | <0.001 | 0.84 |
| CM without Pulmonary embolism | 0.021 | 0.89 | <0.001 | 0.95 |
| CM without DVT | 0.021 | 0.89 | <0.001 | 0.91 |
| CM without Sepsis | 0.021 | 0.88 | <0.001 | 0.98 |
| CM without Influenza | 0.021 | 0.89 | <0.001 | 0.90 |
| CM without Postpartum bacterial infection | 0.021 | 0.90 | <0.001 | 0.76 |
| CM without Acute renal failure | 0.020 | 0.87 | <0.001 | 0.80 |
Slope (Δy/Δx) reported is the change in the listed condition per 1000 deliveries (Δy) per year (Δx) (Figure S4)
Comparison of the slope obtained from the linear regression of the trends in CM among all women to the slope obtained from linear regression for each of the listed variables (women with CM who do not have the condition listed).
As women with chronic hypertension are at increased risk for developing preeclampsia, we analyzed the data to determine the change in prevalence of women who had both chronic hypertension and preeclampsia. There was a significant linear increase in the prevalence of having both chronic hypertension and preeclampsia over the years 2000 to 2009 (p=0.0002, R2=0.835, Figure 1D). Despite this increase in prevalence of having both chronic hypertension and preeclampsia, women with both disorders did not account for the increased prevalence of cardiomyopathy at delivery (p=0.804 for differences in the slopes of all women with cardiomyopathy compared to women with cardiomyopathy who did not have both chronic hypertension and preeclampsia (Table 1, Figure 2C).
When all women with cardiomyopathy were compared to women with cardiomyopathy who did not have each of the other specified pre-existing medical conditions or medical and obstetric complications, the difference in the linear trends was not significant. This suggests that changes in the prevalence of these other pre-existing medical conditions or changes in the incidence of the studied medical or obstetric complications did not affect the prevalence of cardiomyopathy during admission for delivery (p>0.01 for the comparison of slopes, Tables 2 and 3, Figures S3 and S4).
For women with chronic hypertension, the crude relative risk of developing cardiomyopathy at delivery was 13.2 (95% CI 12.5, 13.7) compared to women without chronic hypertension. Based on these estimates, the population attributable risk proportion of cardiomyopathy (the fraction of cases of cardiomyopathy that could theoretically be prevented by eliminating chronic hypertension as a risk factor) was 14.9%. Among all women with chronic hypertension, the risk for developing cardiomyopathy over the entire time period was 4.1 per 1000 at delivery.
DISCUSSION
Main Findings
Based on hospital discharge data, the prevalence of cardiomyopathy at the time of admission for delivery increased during the 10 years from 2000 to 2009. Of the pre-existing medical conditions and medical and obstetric complications tested, the change in prevalence of chronic hypertension among pregnant women was associated with the increasing prevalence of cardiomyopathy at the time of an admission for delivery. Though we acknowledge that this work does not prove that chronic hypertension causes cardiomyopathy, women with chronic hypertension did have increased odds for cardiomyopathy compared to women without chronic hypertension.
Interpretation
Cardiomyopathy in pregnancy is classified as either peripartum cardiomyopathy or cardiomyopathy secondary to an identifiable (primary) cause.(17) Although the majority of cardiomyopathies associated with pregnancy are cardiomyopathies secondary to identifiable primary causes, Whitehead et al demonstrated that among cardiomyopathy-associated pregnancy-related deaths, peripartum cardiomyopathy was a more common diagnosis.(15, 18) Regardless, many risk factors for peripartum cardiomyopathy and cardiomyopathy secondary to identifiable primary causes do overlap. The etiology of peripartum cardiomyopathy is unknown but the classically taught risk factors of multiparity, advanced maternal age, multiple gestation, preeclampsia, and African American race were first reported by Demakis in 1971 in a case series of 27 women. These investigators coined the term peripartum cardiomyopathy and developed diagnostic criteria for the disease.(19) Since then, other case series of women with peripartum cardiomyopathy have found that advanced maternal age, multiparty, hypertension, preeclampsia, use of tocolytic agents (prolonged beta-adrenergic agonist therapy), and multiple gestation continue to be common among women with peripartum cardiomyopathy.(20-25) Conversely, cardiomyopathies secondary to identifiable causes may have more clear risk factors and etiologies. There are numerous etiologies for cardiomyopathies including viral and other infectious disease, myocarditis, ischemic heart disease, hypertension, substance abuse, connective tissue disorders, and infiltrative disorders.(26) As our study does not demonstrate causation, it is unknown how screening and treatment of chronic hypertension among reproductive-age women would affect the prevalence of cardiomyopathy in pregnancy, but chronic hypertension is a potentially modifiable risk factor.
Strengths and Limitations
Using the NIS, we were able to search for associated medical conditions that may be associated with cardiomyopathy presenting in pregnancy, but there are limitations to using these data. The NIS relies on accurate medical coding at discharge. We were unable to determine if changes in other known risk factors for cardiomyopathy-particularly obesity, multiparity, and the use of specific drugs were associated with the increased prevalence.(20, 23, 27) Next, there are wide ranges in reported sensitivity and specificity for medical and obstetric conditions and procedures in large databases that rely on ICD-9 codes.(28-30) Low sensitivity for coding chronic conditions among obstetric patients has been reported.(28) Because of potential low sensitivity for chronic conditions, our reported estimates may be low and may be more representative of those with more severe conditions. Furthermore, it is possible that some medical comorbidities were not coded for subjects during a delivery admission, especially if that condition was not an active aspect of the woman’s admission for delivery. For example, according to the most recently available data, about 5% of reproductive age women in the US reported taking antihypertensive medications while only about 2% of delivery hospital discharge records had an ICD-9-CM code for chronic hypertension.(31-33) Regardless, from our dataset, we were able to demonstrate that chronic hypertension significantly affected the increasing prevalence of cardiomyopathy during pregnancy. It is unknown if milder cases of cardiomyopathy were identified and if not, whether chronic hypertension also is associated with the change in prevalence of these milder forms. Race and ethnicity did not affect the change in prevalence of cardiomyopathy in our study but approximately 25% of NIS entries have race/ethnicity data as not coded. Thus it is unknown if race and ethnicity would play a stronger role in the increasing prevalence of cardiomyopathy if race and ethnicity were better coded in the NIS.
Due to limitations of large databases that rely on identification of subjects using ICD-9 diagnosis codes, we elected to define pregnancy-associated cardiomyopathy as all forms of cardiomyopathy in pregnancy (peripartum cardiomyopathy and cardiomyopathy due to secondary causes). Kuklina et al found that 80% of subjects with the specific peripartum cardiomyopathy ICD-9 code, also had codes for heart disease or chronic conditions that are associated with dilated forms of cardiomyopathy.(15) Because of this lack of specificity in the ICD-9 codes, it is not possible using the NIS or other large databases, to make conclusions about peripartum cardiomyopathy versus cardiomyopathy due to secondary causes. Our conclusions are, therefore, not specific for peripartum cardiomyopathy, but are general to all forms of cardiomyopathy that are present at delivery. Lastly, our data do not establish causality. We identified an association of chronic hypertension with cardiomyopathy at delivery, but it is impossible to know if chronic hypertension causes cardiomyopathy. Regardless, large databases such as the NIS are useful at identifying such associations, especially for rare conditions. Further research utilizing detailed clinical data is needed to expand on the association of chronic hypertension with cardiomyopathy.
Conclusion
In summary, women with chronic hypertension are at increased risk for developing cardiomyopathy during pregnancy, and cardiomyopathy in pregnancy is increasing in prevalence at the time of delivery. The increased prevalence of cardiomyopathy in pregnancy is at least in part due to the increasing prevalence of chronic hypertension among women who become pregnant. Further work is required to identify specific factors among women with pre-existing heart disease or chronic hypertension, which may further increase their risk for cardiomyopathy.
Supplementary Material
Acknowledgements
This work was presented by CAG to the Duke University School of Medicine in partial fulfillment for the degree, Master of Health Sciences in Clinical Research.
Funding
This project was funded in part by the Division of Maternal-Fetal Medicine, Duke University and by the Eunice Kennedy Shriver National Institute of Child Health & Human Development under award number K12-HD-043446 to Chad Grotegut as a Building Interdisciplinary Research Careers in Women’s Health (BIRCWH) Scholar.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
Disclosure of Interests
The authors report no conflict of interest
Contribution of Authorship
CAG and AHJ conceived of the study. AHJ and CAG obtained the data and CAG performed the statistical analysis. CAG, EVK, KJA, RPH, WMC, ERM and AHJ interpreted the data and wrote the paper. KJA, AHJ and ERM served as masters thesis committee members to CAG. RPH provided funding. All authors approved of the final version of the manuscript.
Details of Ethics Approval
This study was reviewed by the Duke University Institutional Review Board and declared as exempt research. (Duke University IRB #: Pro00038300)
REFERENCES
- 1.Berg CJ, Atrash HK, Koonin LM, Tucker M. Pregnancy-related mortality in the United States, 1987-1990. Obstet Gynecol. 1996;88(2):161–7. doi: 10.1016/0029-7844(96)00135-4. [DOI] [PubMed] [Google Scholar]
- 2.Berg CJ, Callaghan WM, Syverson C, Henderson Z. Pregnancy-related mortality in the United States, 1998 to 2005. Obstet Gynecol. 2010;116(6):1302–9. doi: 10.1097/AOG.0b013e3181fdfb11. [DOI] [PubMed] [Google Scholar]
- 3.Berg CJ, Chang J, Callaghan WM, Whitehead SJ. Pregnancy-related mortality in the United States, 1991-1997. Obstet Gynecol. 2003;101(2):289–96. doi: 10.1016/s0029-7844(02)02587-5. [DOI] [PubMed] [Google Scholar]
- 4.Atrash HK, Koonin LM, Lawson HW, Franks AL, Smith JC. Maternal mortality in the United States, 1979-1986. Obstet Gynecol. 1990;76(6):1055–60. [PubMed] [Google Scholar]
- 5.Division of Reproductive Health. National Center for Chronic Disease Prevention and Health Promotion [Accessed November 21, 2012];Pregnancy-related Mortality in the United States. http://www.cdc.gov/reproductivehealth/MaternalInfantHealth/Pregnancy-relatedMortality.htm.
- 6.Berg CJ, Harper MA, Atkinson SM, Bell EA, Brown HL, Hage ML, et al. Preventability of pregnancy-related deaths: results of a state-wide review. Obstet Gynecol. 2005;106(6):1228–34. doi: 10.1097/01.AOG.0000187894.71913.e8. [DOI] [PubMed] [Google Scholar]
- 7.Kuklina E, Callaghan W. Chronic heart disease and severe obstetric morbidity among hospitalisations for pregnancy in the USA: 1995-2006. BJOG. 2011;118(3):345–52. doi: 10.1111/j.1471-0528.2010.02743.x. [DOI] [PubMed] [Google Scholar]
- 8.Pritchard JA. Changes in the Blood Volume during Pregnancy and Delivery. Anesthesiology. 1965;26:393–9. doi: 10.1097/00000542-196507000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Lund CJ, Donovan JC. Blood volume during pregnancy. Significance of plasma and red cell volumes. Am J Obstet Gynecol. 1967;98(3):394–403. [PubMed] [Google Scholar]
- 10.Clark SL, Cotton DB, Lee W, Bishop C, Hill T, Southwick J, et al. Central hemodynamic assessment of normal term pregnancy. Am J Obstet Gynecol. 1989;161(6 Pt 1):1439–42. doi: 10.1016/0002-9378(89)90900-9. [DOI] [PubMed] [Google Scholar]
- 11.Kuklina EV, Whiteman MK, Hillis SD, Jamieson DJ, Meikle SF, Posner SF, et al. An enhanced method for identifying obstetric deliveries: implications for estimating maternal morbidity. Maternal and child health journal. 2008;12(4):469–77. doi: 10.1007/s10995-007-0256-6. [DOI] [PubMed] [Google Scholar]
- 12.Podulka J, Stranges E, Steiner C. Hospitalizations related to childbirth, 2008. Agency for Healthcare Research and Quality; 2011. HCUP Statistical Brief #110. [PubMed] [Google Scholar]
- 13.James AH, Jamison MG, Biswas MS, Brancazio LR, Swamy GK, Myers ER. Acute myocardial infarction in pregnancy: a United States population-based study. Circulation. 2006;113(12):1564–71. doi: 10.1161/CIRCULATIONAHA.105.576751. [DOI] [PubMed] [Google Scholar]
- 14.James AH, Bushnell CD, Jamison MG, Myers ER. Incidence and risk factors for stroke in pregnancy and the puerperium. Obstet Gynecol. 2005;106(3):509–16. doi: 10.1097/01.AOG.0000172428.78411.b0. [DOI] [PubMed] [Google Scholar]
- 15.Kuklina EV, Callaghan WM. Cardiomyopathy and other myocardial disorders among hospitalizations for pregnancy in the United States: 2004-2006. Obstet Gynecol. 2010;115(1):93–100. doi: 10.1097/AOG.0b013e3181c4ee8c. [DOI] [PubMed] [Google Scholar]
- 16.Universtiy of Ottawa. Society, the Individual, and Medicine [Accessed January 7, 2013];Attributable Risk and Population Attributable Risk (PAP) Measures. http://www.med.uottawa.ca/sim/data/PAR_e.htm.
- 17.Pearson GD, Veille JC, Rahimtoola S, Hsia J, Oakley CM, Hosenpud JD, et al. Peripartum cardiomyopathy: National Heart, Lung, and Blood Institute and Office of Rare Diseases (National Institutes of Health) workshop recommendations and review. JAMA. 2000;283(9):1183–8. doi: 10.1001/jama.283.9.1183. [DOI] [PubMed] [Google Scholar]
- 18.Whitehead SJ, Berg CJ, Chang J. Pregnancy-related mortality due to cardiomyopathy: United States, 1991-1997. Obstet Gynecol. 2003;102(6):1326–31. doi: 10.1016/j.obstetgynecol.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 19.Demakis JG, Rahimtoola SH, Sutton GC, Meadows WR, Szanto PB, Tobin JR, et al. Natural course of peripartum cardiomyopathy. Circulation. 1971;44(6):1053–61. doi: 10.1161/01.cir.44.6.1053. [DOI] [PubMed] [Google Scholar]
- 20.Sliwa K, Fett J, Elkayam U. Peripartum cardiomyopathy. Lancet. 2006;368(9536):687–93. doi: 10.1016/S0140-6736(06)69253-2. [DOI] [PubMed] [Google Scholar]
- 21.Elkayam U, Akhter MW, Singh H, Khan S, Bitar F, Hameed A, et al. Pregnancy-associated cardiomyopathy: clinical characteristics and a comparison between early and late presentation. Circulation. 2005;111(16):2050–5. doi: 10.1161/01.CIR.0000162478.36652.7E. [DOI] [PubMed] [Google Scholar]
- 22.Gentry MB, Dias JK, Luis A, Patel R, Thornton J, Reed GL. African-American Women Have a Higher Risk for Developing Peripartum Cardiomyopathy. J Am Coll Cardiol. 2010;55(7):654–9. doi: 10.1016/j.jacc.2009.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lampert MB, Hibbard J, Weinert L, Briller J, Lindheimer M, Lang RM. Peripartum heart failure associated with prolonged tocolytic therapy. Am J Obstet Gynecol. 1993;168(2):493–5. doi: 10.1016/0002-9378(93)90479-3. [DOI] [PubMed] [Google Scholar]
- 24.Demakis JG, Rahimtoola SH. Peripartum cardiomyopathy. Circulation. 1971;44(5):964–8. doi: 10.1161/01.cir.44.5.964. [DOI] [PubMed] [Google Scholar]
- 25.Harper MA, Meyer RE, Berg CJ. Peripartum cardiomyopathy: population-based birth prevalence and 7-year mortality. Obstet Gynecol. 2012;120(5):1013–9. doi: 10.1097/aog.0b013e31826e46a1. [DOI] [PubMed] [Google Scholar]
- 26.Felker GM, Thompson RE, Hare JM, Hruban RH, Clemetson DE, Howard DL, et al. Underlying causes and long-term survival in patients with initially unexplained cardiomyopathy. N Engl J Med. 2000;342(15):1077–84. doi: 10.1056/NEJM200004133421502. [DOI] [PubMed] [Google Scholar]
- 27.Cunningham FG, Pritchard JA, Hankins GD, Anderson PL, Lucas MJ, Armstrong KF. Peripartum heart failure: idiopathic cardiomyopathy or compounding cardiovascular events? Obstet Gynecol. 1986;67(2):157–68. [PubMed] [Google Scholar]
- 28.Yasmeen S, Romano PS, Schembri ME, Keyzer JM, Gilbert WM. Accuracy of obstetric diagnoses and procedures in hospital discharge data. Am J Obstet Gynecol. 2006;194(4):992–1001. doi: 10.1016/j.ajog.2005.08.058. [DOI] [PubMed] [Google Scholar]
- 29.Geller SE, Ahmed S, Brown ML, Cox SM, Rosenberg D, Kilpatrick SJ. International Classification of Diseases-9th revision coding for preeclampsia: how accurate is it? American journal of obstetrics and gynecology. 2004;190(6):1629–33. doi: 10.1016/j.ajog.2004.03.061. discussion 33-4. [DOI] [PubMed] [Google Scholar]
- 30.Parrish KM, Holt VL, Connell FA, Williams B, LoGerfo JP. Variations in the accuracy of obstetric procedures and diagnoses on birth records in Washington State, 1989. American journal of epidemiology. 1993;138(2):119–27. doi: 10.1093/oxfordjournals.aje.a116834. [DOI] [PubMed] [Google Scholar]
- 31.Bateman BT, Shaw KM, Kuklina EV, Callaghan WM, Seely EW, Hernandez-Diaz S. Hypertension in women of reproductive age in the United States: NHANES 1999-2008. PloS one. 2012;7(4):e36171. doi: 10.1371/journal.pone.0036171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuklina EV, Meikle SF, Jamieson DJ, Whiteman MK, Barfield WD, Hillis SD, et al. Severe obstetric morbidity in the United States: 1998-2005. Obstet Gynecol. 2009;113(2 Pt 1):293–9. doi: 10.1097/AOG.0b013e3181954e5b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuklina EV, Ayala C, Callaghan WM. Hypertensive disorders and severe obstetric morbidity in the United States. Obstet Gynecol. 2009;113(6):1299–306. doi: 10.1097/AOG.0b013e3181a45b25. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.