Abstract
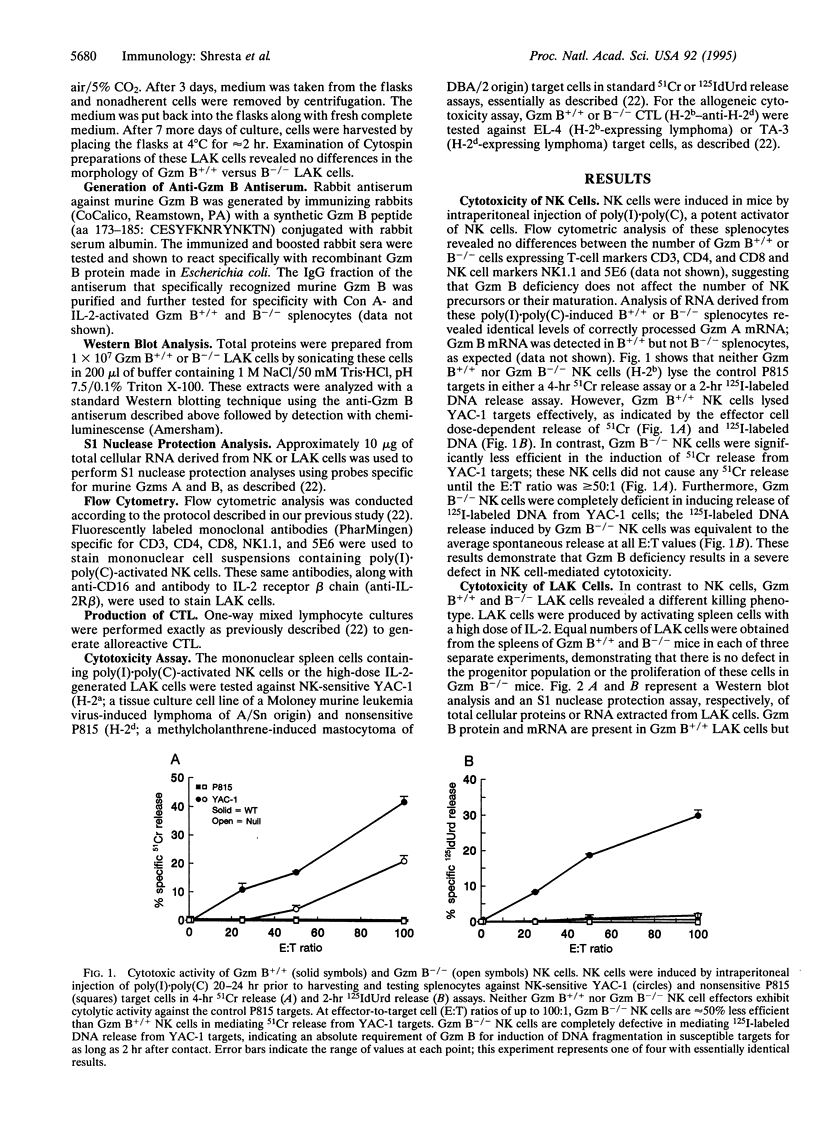
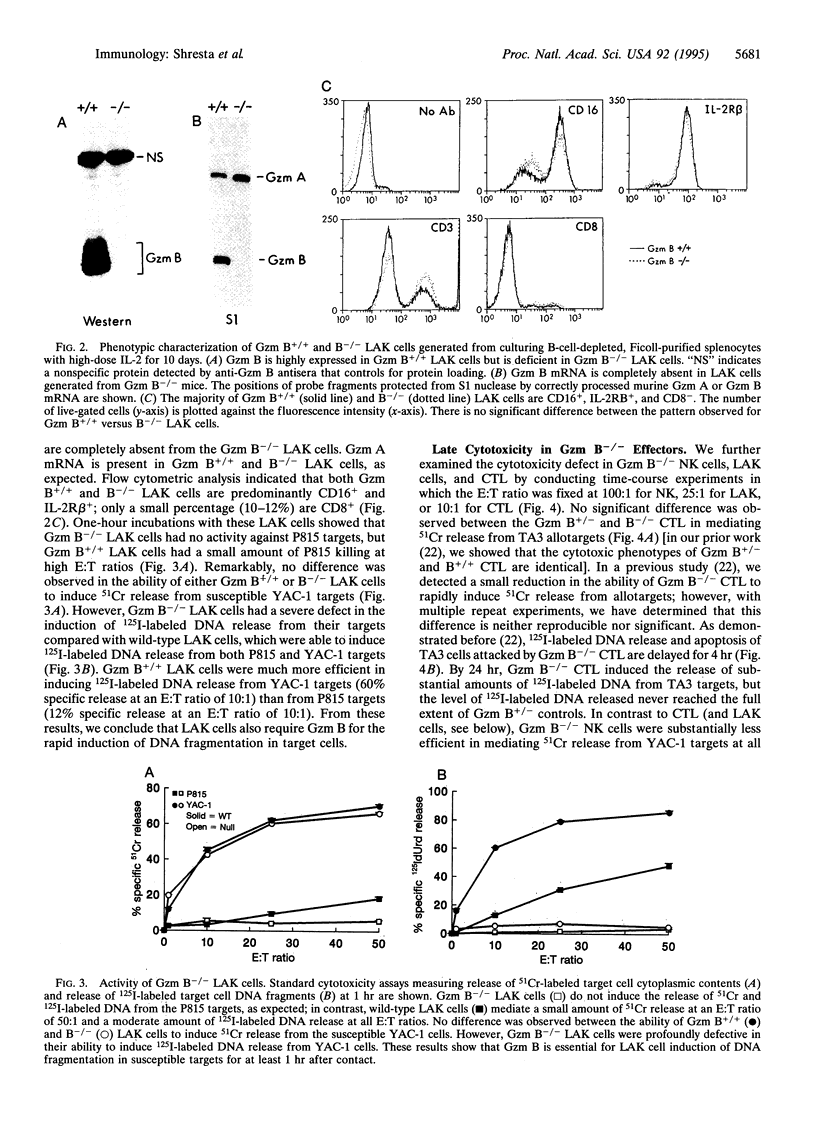
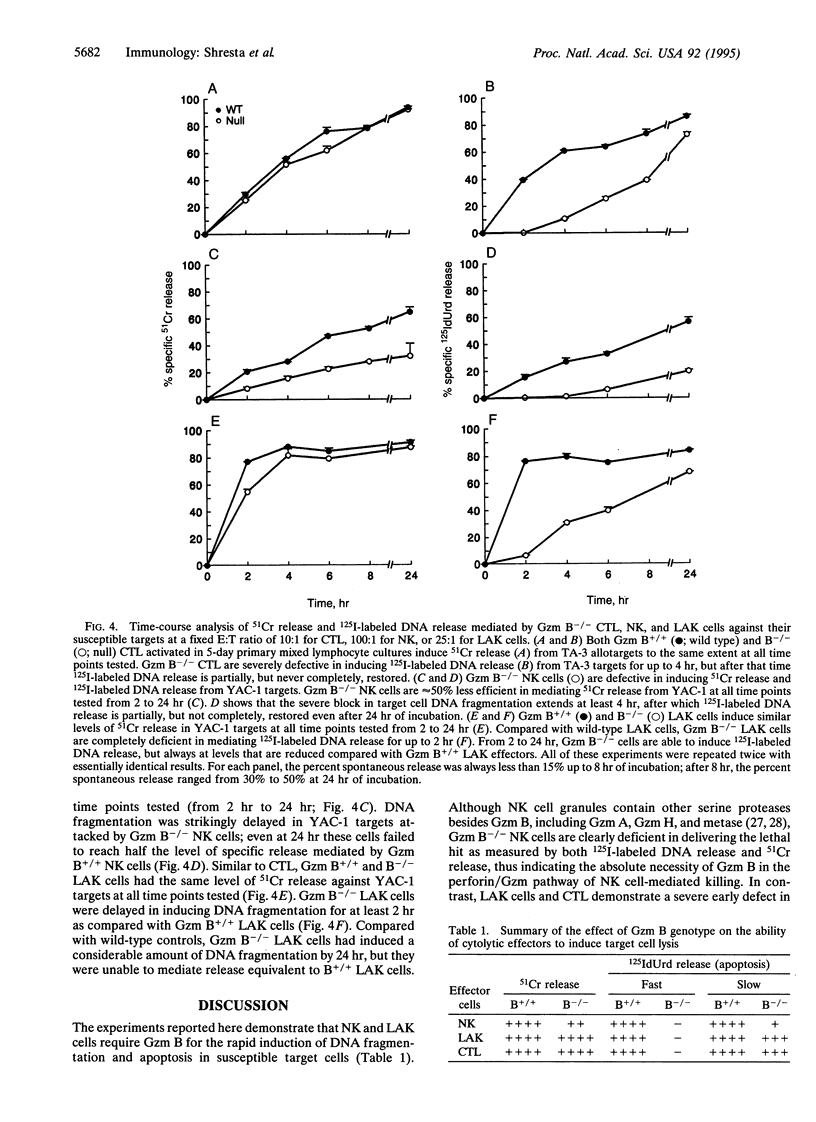
Granzyme (Gzm) B-deficient mice obtained by gene targeting were used to assess the role of Gzm B in the mechanisms used by natural killer (NK) and lymphokine-activated killer (LAK) cells to destroy target cells. Gzm B-/- NK cells, LAK cells, and cytotoxic T lymphocytes (CTL) all are defective in their ability to rapidly induce DNA fragmentation/apoptosis in susceptible target cells. This defect can be partially corrected with long incubation times of effector and target cells. Moreover, Gzm B-/- NK cells (but not CTL or LAK cells) exhibit a defect in 51Cr release from susceptible target cells. This 51Cr release defect in Gzm B-deficient NK cells is also not overcome by prolonged incubation times or high effector-to-target cell ratios. We conclude that Gzm B plays a critical and nonredundant role in the rapid induction of DNA fragmentation/apoptosis by NK cells, LAK cells, and CTL. Gzm B may have an additional role in NK cells (but not in CTL or LAK cells) for mediating 51Cr release.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abo T., Roder J. C., Abo W., Cooper M. D., Balch C. M. Natural killer (HNK-1+) cells in Chediak-Higashi patients are present in normal numbers but are abnormal in function and morphology. J Clin Invest. 1982 Jul;70(1):193–197. doi: 10.1172/JCI110592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleackley R. C., Lobe C. G., Duggan B., Ehrman N., Fregeau C., Meier M., Letellier M., Havele C., Shaw J., Paetkau V. The isolation and characterization of a family of serine protease genes expressed in activated cytotoxic T lymphocytes. Immunol Rev. 1988 Mar;103:5–19. doi: 10.1111/j.1600-065x.1988.tb00746.x. [DOI] [PubMed] [Google Scholar]
- Duke R. C., Persechini P. M., Chang S., Liu C. C., Cohen J. J., Young J. D. Purified perforin induces target cell lysis but not DNA fragmentation. J Exp Med. 1989 Oct 1;170(4):1451–1456. doi: 10.1084/jem.170.4.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewoldt G. R., Winkler U., Powers J. C., Hudig D. Sulfonyl fluoride serine protease inhibitors inactivate RNK-16 lymphocyte granule proteases and reduce lysis by granule extracts and perforin. Mol Immunol. 1992 Jun;29(6):713–721. doi: 10.1016/0161-5890(92)90181-v. [DOI] [PubMed] [Google Scholar]
- Hayes M. P., Berrebi G. A., Henkart P. A. Induction of target cell DNA release by the cytotoxic T lymphocyte granule protease granzyme A. J Exp Med. 1989 Sep 1;170(3):933–946. doi: 10.1084/jem.170.3.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkart P. A. Lymphocyte-mediated cytotoxicity: two pathways and multiple effector molecules. Immunity. 1994 Aug;1(5):343–346. doi: 10.1016/1074-7613(94)90063-9. [DOI] [PubMed] [Google Scholar]
- Henkart P. A. Mechanism of lymphocyte-mediated cytotoxicity. Annu Rev Immunol. 1985;3:31–58. doi: 10.1146/annurev.iy.03.040185.000335. [DOI] [PubMed] [Google Scholar]
- Heusel J. W., Wesselschmidt R. L., Shresta S., Russell J. H., Ley T. J. Cytotoxic lymphocytes require granzyme B for the rapid induction of DNA fragmentation and apoptosis in allogeneic target cells. Cell. 1994 Mar 25;76(6):977–987. doi: 10.1016/0092-8674(94)90376-x. [DOI] [PubMed] [Google Scholar]
- Hudig D., Ewoldt G. R., Woodard S. L. Proteases and lymphocyte cytotoxic killing mechanisms. Curr Opin Immunol. 1993 Feb;5(1):90–96. doi: 10.1016/0952-7915(93)90086-8. [DOI] [PubMed] [Google Scholar]
- Jenne D. E., Tschopp J. Granzymes, a family of serine proteases released from granules of cytolytic T lymphocytes upon T cell receptor stimulation. Immunol Rev. 1988 Mar;103:53–71. doi: 10.1111/j.1600-065x.1988.tb00749.x. [DOI] [PubMed] [Google Scholar]
- Kojima H., Shinohara N., Hanaoka S., Someya-Shirota Y., Takagaki Y., Ohno H., Saito T., Katayama T., Yagita H., Okumura K. Two distinct pathways of specific killing revealed by perforin mutant cytotoxic T lymphocytes. Immunity. 1994 Aug;1(5):357–364. doi: 10.1016/1074-7613(94)90066-3. [DOI] [PubMed] [Google Scholar]
- Kägi D., Ledermann B., Bürki K., Seiler P., Odermatt B., Olsen K. J., Podack E. R., Zinkernagel R. M., Hengartner H. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature. 1994 May 5;369(6475):31–37. doi: 10.1038/369031a0. [DOI] [PubMed] [Google Scholar]
- Kägi D., Vignaux F., Ledermann B., Bürki K., Depraetere V., Nagata S., Hengartner H., Golstein P. Fas and perforin pathways as major mechanisms of T cell-mediated cytotoxicity. Science. 1994 Jul 22;265(5171):528–530. doi: 10.1126/science.7518614. [DOI] [PubMed] [Google Scholar]
- Lowin B., Beermann F., Schmidt A., Tschopp J. A null mutation in the perforin gene impairs cytolytic T lymphocyte- and natural killer cell-mediated cytotoxicity. Proc Natl Acad Sci U S A. 1994 Nov 22;91(24):11571–11575. doi: 10.1073/pnas.91.24.11571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowin B., Hahne M., Mattmann C., Tschopp J. Cytolytic T-cell cytotoxicity is mediated through perforin and Fas lytic pathways. Nature. 1994 Aug 25;370(6491):650–652. doi: 10.1038/370650a0. [DOI] [PubMed] [Google Scholar]
- Masson D., Tschopp J. Isolation of a lytic, pore-forming protein (perforin) from cytolytic T-lymphocytes. J Biol Chem. 1985 Aug 5;260(16):9069–9072. [PubMed] [Google Scholar]
- Nakajima H., Henkart P. A. Cytotoxic lymphocyte granzymes trigger a target cell internal disintegration pathway leading to cytolysis and DNA breakdown. J Immunol. 1994 Feb 1;152(3):1057–1063. [PubMed] [Google Scholar]
- Pasternack M. S., Eisen H. N. A novel serine esterase expressed by cytotoxic T lymphocytes. 1985 Apr 25-May 1Nature. 314(6013):743–745. doi: 10.1038/314743a0. [DOI] [PubMed] [Google Scholar]
- Podack E. R., Young J. D., Cohn Z. A. Isolation and biochemical and functional characterization of perforin 1 from cytolytic T-cell granules. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8629–8633. doi: 10.1073/pnas.82.24.8629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roder J. C., Haliotis T., Klein M., Korec S., Jett J. R., Ortaldo J., Heberman R. B., Katz P., Fauci A. S. A new immunodeficiency disorder in humans involving NK cells. Nature. 1980 Apr 10;284(5756):553–555. doi: 10.1038/284553a0. [DOI] [PubMed] [Google Scholar]
- Roder J., Duwe A. The beige mutation in the mouse selectively impairs natural killer cell function. Nature. 1979 Mar 29;278(5703):451–453. doi: 10.1038/278451a0. [DOI] [PubMed] [Google Scholar]
- Rouvier E., Luciani M. F., Golstein P. Fas involvement in Ca(2+)-independent T cell-mediated cytotoxicity. J Exp Med. 1993 Jan 1;177(1):195–200. doi: 10.1084/jem.177.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J. H. Internal disintegration model of cytotoxic lymphocyte-induced target damage. Immunol Rev. 1983;72:97–118. doi: 10.1111/j.1600-065x.1983.tb01074.x. [DOI] [PubMed] [Google Scholar]
- Russell J. H., Masakowski V., Rucinsky T., Phillips G. Mechanisms of immune lysis. III. Characterization of the nature and kinetics of the cytotoxic T lymphocyte-induced nuclear lesion in the target. J Immunol. 1982 May;128(5):2087–2094. [PubMed] [Google Scholar]
- Sayers T. J., Wiltrout T. A., Smyth M. J., Ottaway K. S., Pilaro A. M., Sowder R., Henderson L. E., Sprenger H., Lloyd A. R. Purification and cloning of a novel serine protease, RNK-Tryp-2, from the granules of a rat NK cell leukemia. J Immunol. 1994 Mar 1;152(5):2289–2297. [PubMed] [Google Scholar]
- Shi L., Kam C. M., Powers J. C., Aebersold R., Greenberg A. H. Purification of three cytotoxic lymphocyte granule serine proteases that induce apoptosis through distinct substrate and target cell interactions. J Exp Med. 1992 Dec 1;176(6):1521–1529. doi: 10.1084/jem.176.6.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L., Kraut R. P., Aebersold R., Greenberg A. H. A natural killer cell granule protein that induces DNA fragmentation and apoptosis. J Exp Med. 1992 Feb 1;175(2):553–566. doi: 10.1084/jem.175.2.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L., Nishioka W. K., Th'ng J., Bradbury E. M., Litchfield D. W., Greenberg A. H. Premature p34cdc2 activation required for apoptosis. Science. 1994 Feb 25;263(5150):1143–1145. doi: 10.1126/science.8108732. [DOI] [PubMed] [Google Scholar]
- Shiver J. W., Su L., Henkart P. A. Cytotoxicity with target DNA breakdown by rat basophilic leukemia cells expressing both cytolysin and granzyme A. Cell. 1992 Oct 16;71(2):315–322. doi: 10.1016/0092-8674(92)90359-k. [DOI] [PubMed] [Google Scholar]
- Smyth M. J., Sayers T. J., Wiltrout T., Powers J. C., Trapani J. A. Met-ase: cloning and distinct chromosomal location of a serine protease preferentially expressed in human natural killer cells. J Immunol. 1993 Dec 1;151(11):6195–6205. [PubMed] [Google Scholar]
- Talento A., Nguyen M., Law S., Wu J. K., Poe M., Blake J. T., Patel M., Wu T. J., Manyak C. L., Silberklang M. Transfection of mouse cytotoxic T lymphocyte with an antisense granzyme A vector reduces lytic activity. J Immunol. 1992 Dec 15;149(12):4009–4015. [PubMed] [Google Scholar]
- Walsh C. M., Matloubian M., Liu C. C., Ueda R., Kurahara C. G., Christensen J. L., Huang M. T., Young J. D., Ahmed R., Clark W. R. Immune function in mice lacking the perforin gene. Proc Natl Acad Sci U S A. 1994 Nov 8;91(23):10854–10858. doi: 10.1073/pnas.91.23.10854. [DOI] [PMC free article] [PubMed] [Google Scholar]




