Abstract
The establishment and maintenance of polarized plasma membrane domains is essential for cellular function and proper development of organisms. Epithelial cells polarize along two fundamental axes, the apicobasal and the planar, both depending on finely regulated protein trafficking mechanisms. Newly synthesized proteins destined for either surface domain are processed along the biosynthetic pathway and segregated into distinct subsets of transport carriers emanating from the trans-Golgi network or endosomes. This exocytic trafficking has been identified as essential for proper epithelial polarization. Accumulating evidence now reveals that endocytosis and endocytic recycling play an equally important role in epithelial polarization and the appropriate localization of key polarity proteins. Here, we review recent work in metazoan systems illuminating the connections between endocytosis, postendocytic trafficking, and cell polarity, both apicobasal and planar, in the formation of differentiated epithelial cells, and how these processes regulate tissue dynamics.
Epithelial cells polarize along two axes, the apicobasal and the planar. Endocytic trafficking of key polarity regulators (e.g., Crumbs) helps to establish and reinforce this asymmetry.
ENDOCYTOSIS REGULATES APICOBASAL EPITHELIAL POLARITY
Membrane traffic does not simply reinforce polarity but is critical for the generation of cortical epithelial cell asymmetry. Newly synthesized plasma membrane components sorted in the trans-Golgi network (TGN) use exocytotic pathways to be delivered to specific domains of the plasma membrane in epithelial cells (Rodriguez-Boulan et al. 2005). Then, endocytosed apical and basolateral cargoes are either sorted to late endosomes (LEs) and lysosomes for degradation, or converge in recycling endosomes (REs) and are segregated and recycled back to either membrane domain. Thus, endocytosis and recycling from both surface domains are essential to maintain the composition of the different membrane domains. Recent work has implicated the RE as a polarized sorting center that seems instrumental in the establishment, maintenance, and plasticity of epithelial polarity and separated membrane domains (for review, see Golachowska et al. 2010).
Most of the evidence for the importance of endocytosis in regulating cell polarity has come from several genetic screens in Drosophila (for review, see Shivas et al. 2010). These studies have shown that endocytic pathways are required for general apicobasal polarity in embryonic and different adult Drosophila epithelial tissues. Endocytic transport has been proposed to regulate epithelial polarity through the control of the levels of certain transmembrane proteins that act as “master regulators” of polarized domains such as Crumbs, or signaling events downstream from Crumbs, and the proteins that regulate cell adhesion such as E-cadherin. Additionally, endocytosis and recycling of surface cargo are necessary for polarity because they allow the proper relocation of apical and basolateral proteins that require transcytosis to reach their correct membrane domain. These processes are also important for relocalization of proteins that become wrongly distributed as a consequence of sorting defects or protein diffusion.
Endocytosis Serves to Maintain the Appropriate Levels of Different Surface Proteins
Endocytosis of receptors is a common strategy for regulating the activity of many types of cell-signaling pathways and is thought to sensitively control their kinetics, as well as functioning as biological switches (see Di Fiore and von Zastrow 2014). Similarly, cell polarity requires controlled plasma membrane levels of certain transmembrane proteins that act as master regulators of apicobasal polarity. Endocytosis could function to restrict surface levels of these proteins by mediating their transport to lysosomes for degradation or recycling. Interestingly, polarity regulators have been recently identified as important controllers of endocytosis and postendocytic trafficking (for a recent review, see Shivas et al. 2010).
Polarity regulators are those proteins that show conserved roles in polarizing different cell types. Three key polarity modules are the Crumbs (Crumbs/Stardust/PatJ), Scribble (Scribble/Discs Large/Lethal Giant Larva), and PAR (Par6/Par3/aPKC) modules (St Johnston and Sanson 2011), which in epithelial cells polarize along the apicobasal axis; the PAR and Crumbs complexes localize to the apical domain, whereas the Scribble complex localizes to the basolateral domain. These segregation patterns are maintained both by regulatory interactions between the protein components, such as mutual antagonism (for more information, see St Johnston and Ahringer 2010; McCaffrey and Macara 2011), as well as interaction with additional factors, namely, small GTPases (Iden and Collard 2008) and phosphoinositides (PIs) (Martin-Belmonte and Mostov 2007).
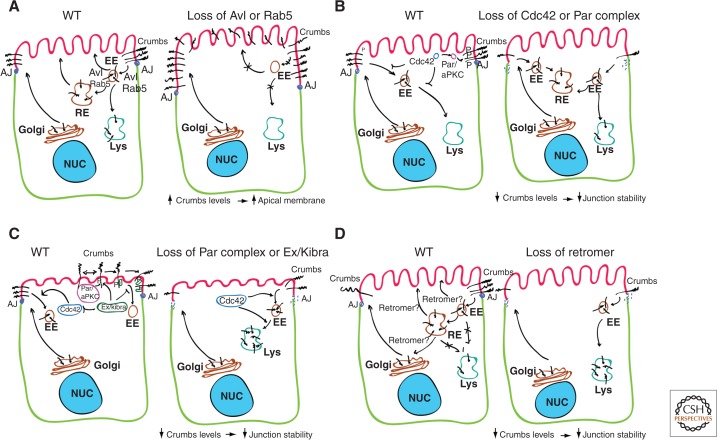
Crumbs was first identified as an apical determinant in Drosophila melanogaster embryonic epithelia, where it is required for the maintenance of apicobasal polarity and promotes the formation of the apical membrane domain (Tepass et al. 1990; Tepass and Knust 1993; Wodarz et al. 1993; Grawe et al. 1996; Tepass 1996). Accumulated evidence suggests that membrane Crumbs is constantly internalized to maintain the level of surface expression that allows appropriate overall apicobasal polarity. Crumbs is endocytosed at the basolateral membrane to avoid apical expansion apparently through an AP2/clathrin pathway regulated by Lgl (Fletcher et al. 2012). However, in order to maintain the proper levels of Crumbs in the apical domain, the endocytic uptake of Crumbs at this domain must be also finely regulated. Almost a decade ago, David Bilder’s laboratory using a mosaic genetic screen showed that inhibition of exocytic machinery has little effect on apicobasal protein localization. However, mutating avalanche (avl), a protein required for apical internalization of Crumbs and Notch, caused a defect in apicobasal polarity in follicle cells similar to that produced by aPKC or Scrib mutations (Lu and Bilder 2005). Interestingly, avl is a syntaxin (homologous to human Stx 7, localized in late endosomes, and Stx 12) and colocalizes with early (Rab5-positive) and recycling (Rab11-positive) endosomes. Furthermore, the Rab5-null deletion mutant showed multilayered, overproliferative phenotypes very similar to that of avl. Thus, apical Crumbs appears to be internalized, via Avl and Rab5, to maintain a level of surface expression that allows appropriate overall apicobasal polarity. Taken together, these data are consistent with a model in which increased Crumbs levels resulting from defective apical endocytosis directly contribute to the avl defect in apicobasal polarity and neoplastic phenotype (Fig. 1A). However, cells also need to prevent an excessive uptake of Crumbs for normal polarization.
Figure 1.
Molecular mechanisms for Crumbs endocytic and recycling regulation in epithelial cells. (A) Apical Crumbs is endocytosed from the apical surface via Avl and Rab5 to the early endosome (EE), to maintain the level of surface expression of Crumbs that allows appropriate overall apicobasal polarity. From the EEs, Crumbs is then recycled back to the plasma membrane or degrades in lysosomes. Biosynthetic transport of Crumbs also contributes to maintain its appropriate levels in the apical surface. When endocytosis is blocked through the loss of Avl or Rab, Crumbs accumulates on the cell surface, leading to mispolarization of the cell. (B) Cdc42 and the Par complex prevent the endocytosis of Crumbs from the apical plasma membrane in the Drosophila neuroectoderm. Loss of Cdc42 or the Par complex induces Crumbs endocytosis and AJs disorganization. Cdc42 also prevents lysosomal degradation of Crumbs. (C) In the Drosophila follicle cell epithelium, Crumbs–Crumbs interaction via the extracellular domain of Crumbs facilitates aPKC phosphorylation and stabilization of the entire apical complex at the plasma membrane by preventing Crumbs endocytosis. Ex and Kibra associate with phosphorylated Crumbs at the FERM domain and function to maintain Crumbs at the plasma membrane. Ex/kibra also inhibits Cdc42, which promotes the lysosomal degradation of Crumbs. (D) Retromer is responsible for sorting Crumbs at the early sorting endosome for recycling, away from the degradative pathway. Crumbs recycling pathways could be from the EE, the recycling endosome (RE), or the trans-Golgi network (TGN).
The small GTPase Cdc42 acting through its effector, the Par complex, seems to play an essential role in preventing the endocytosis of Crumbs and other apical proteins from the plasma membrane in the Drosophila neuroectoderm (Fig. 1) (Harris and Tepass 2008). Loss of Cdc42 caused the endocytosis of Crumbs, a defect that, in turn, causes the disorganization of the AJs of the ventral neuroectoderm of Drosophila. A constitutive active form of aPKC restores the normal phenotype. Recent results, however, suggest that Crumbs regulation could be different in other Drosophila epithelial tissues (Fletcher et al. 2012). Results from this work define an apical positive feedback loop that centers on endocytic regulation of Crumbs, indicating that aPKC phosphorylation is central to stabilizing Crumbs at the plasma membrane in the Drosophila follicle cell epithelium. Indeed, Crumbs is endocytosed when it fails to interact with kinase-active aPKC, and in a Crumbs mutant background, the phosphomutant form of Crumbs localized mainly to endosomes (Fletcher et al. 2012). These results suggest a model of self-recruitment in which Crumbs–Crumbs interaction via the extracellular domain facilitates aPKC phosphorylation and stabilization of the entire apical complex at the plasma membrane by preventing Crumbs endocytosis (Fig. 1C). Expanded (Ex) and Kibra (both in the Hippo pathway) associate with Crumbs at the FERM domain (which is phosphorylated by aPKC) and function to maintain Crumbs at the plasma membrane; thus, their recruitment to the apical membrane is a key element of the positive-feedback loop. Once Crumbs is endocytosed at the apical or basolateral membrane, it could be either degraded in lysosomes or recycled back to the plasma membrane (Fig. 1).
Cdc42 also promotes the degradation of Crumbs and other apical proteins through a mechanism that is still unknown but could be related to the ESCRT machinery, which regulates endocytic sorting for lysosome degradation of signaling membrane receptors (Thompson et al. 2005; Vaccari and Bilder 2005). Interestingly, recent evidence indicates that Crumbs avoids the lysosome due to the action of the retromer machinery (Fig. 1D) (Pocha et al. 2011b). Previous reports showed that transport of Crumbs to the plasma membrane relies on Rab11, the exocyst, and Cdc42 in Drosophila embryonic epithelia (see below). Retromer is responsible for sorting Crumbs at the early sorting endosome for recycling, away from the degradative pathway, thus allowing a high level of control over the amount of cellular Crumbs (Pocha et al. 2011b). It is unclear, however, whether Crumbs transport to the apical domain occurs via the TGN, via recycling endosomes, or through alternative pathways (Fig. 1D). In this study, the investigators propose that Crumbs recycles back to the supra-apical compartment through the TGN. This would suggest an intriguing possibility for the mechanism by which Crumbs specifies and expands the apical membrane, by acting as a transporting chaperone for apically destined proteins. However, the exact trafficking route of Crumbs following recycling by retromer remains to be determined and may depend on the purpose of Crumbs recycling.
Recycling Endosomes as Centers of Protein Trafficking and the Regulation of Cell Polarity
Another important issue is how postendocytic cargo returns to the plasma membrane. Endocytic recycling has emerged as a key mechanism in the dynamic stabilization of cellular polarity. Yet, little is known about the molecules and mechanisms controlling recycling in an epithelium in vivo. This postendocytic traffic must be regulated at different levels: (1) membrane lipids/phosphoinositides (PIs), which confer membrane identity; (2) cargo sorting adaptors that promote vesicle formation; (3) actin filament- and microtubule-associated motors that regulate vesicle translocation; and (4) tethering machinery and SNARE complexes that drive membrane fusion. Cargo entering the early endosomal system can be recycled back to the plasma membrane via two different routes: a fast recycling route from the early endosomes (-EE-, also called sorting endosomes, -SE-) to the plasma membrane, or a slow recycling route through the RE. Accumulated data indicate that the recycling machinery includes small GTPases (Rho, Rab, and Arf families), PI kinases, class V myosins, adaptor proteins, specific SNAREs, and the exocyst (for review, see Golachowska et al. 2010).
Rab11 GTPase has been shown to be a master regulator of protein transport via REs, and many recent studies have focused on the molecular machinery that mediates Rab11-dependent endocytic protein transport in polarized cells. Crumbs is recycled back to the plasma membrane through the action of Rab11 and plays an essential role in adherens junctions (AJ) remodeling during embryogenesis (Roeth et al. 2009). Indeed, disruption of Rab11 function in Drosophila embryos has dramatic effects on epithelial integrity. These defects are associated with fragmentation, and ultimate loss, of AJs, which are preceded by the loss of Crumbs from the apical domain. In contrast, the basolateral distribution of Dlg remains unaffected (Roeth et al. 2009). More recent data have also shown that apically enriched Rab11-positive recycling endosomes (AREs) are important for establishing and maintaining epithelial polarity (Winter et al. 2012). From a genome-wide RNAi screen to identify regulators of epithelial polarity, the researchers in this work found that Par5 (the ortholog of the mammalian protein 14-3-3ζ), together with basolateral Par1 and apical Par3/Par6, controls the subapical positioning of Rab11-positive recycling endosomes in Caenorhabditis elegans intestine, but apparently without controlling the function of this organelle in protein trafficking (Winter et al. 2012).
In addition, current data suggest that transcriptional control of Rab11 expression is also an important regulator of epithelial morphogenesis in development. Indeed, recent results using three-dimensional (3D) in vitro models have shown that several essential modulators of epithelial polarity and morphogenesis are controlled at the transcriptional level (Galvez-Santisteban et al. 2012). The transcription factor Ribbon up-regulates apical Rab11 and maintains apical accumulation of the polarity protein Crumbs during elongation of Drosophila salivary glands (Kerman et al. 2008). Similarly, endocytosis and postendocytic trafficking have a role in the process of tracheal intercalation. The signaling molecule Wingless up-regulates the transcription factor Spalt and inhibits cell intercalation in the dorsal trunk of the trachea. Tracheal cells form tubes, in part, by cell intercalation, but this needs to be restricted to the right time and place. Spalt up-regulates Rab11-mediated recycling of DEcad by regulating levels of the partner dRip11. This elevates surface DEcad, presumably increasing cell adhesion and blocking intercalation specifically in these cells (Shaye et al. 2008). Rab5 has also been proposed to be a master regulator of endosome biogenesis and protein trafficking. Reducing Rab5 below a critical level causes a marked reduction of part of the endosomal system in vivo, including EE, LE, and lysosomes, but does not affect the integrity of the Rab11-positive recycling endosomes (although the RE could be nonfunctional because of lack of supply of endosomal membranes) (Zeigerer et al. 2012). This study emphasizes the multiplicity of trafficking mechanisms used to reach the apical surface in epithelial cells. Apical delivery by transcytosis to the hepatic bile canaliculi is highly affected in Rab5-depleted cells, but not apically transported proteins in the direct biosynthetic route. This suggests that EE, and not only the recycling endosome, could have an essential role in transcytotic transport, at least in hepatic cells (Zeigerer et al. 2012).
The exocyst complex is implicated in the delivery of vesicles to the plasma membrane from the RE and Golgi compartments, and mutations in exocyst proteins have been shown to disrupt recycling endosome morphology (Jafar-Nejad et al. 2005; Langevin et al. 2005). The Exo84-dependent localization of the Crumbs protein to the apical surface is essential to maintain epithelial polarity in the Drosophila embryo. Indeed, when Exo84 is disrupted, Bazooka, Crumbs, aPKC, and dPATJ accumulated in large aggregates at ectopic locations along the apical–basal axis (Blankenship et al. 2007). Myosin motors also play an essential role in recycling. Crumbs interacts with MyoV through the first 14 amino acids of the cytoplasmic domain of Crumbs. This interaction is required for the stabilization of MyoV in Drosophila photoreceptor cells (PRCs), which, in turn, is necessary for the post-Golgi transport of rhodopsin (Rh1) to the rhabdomere (Pocha et al. 2011a). This role promotes the maintenance of rhabdomere function and impedes age-related retinal degeneration, but does not appear to be important for the function of Crumbs in morphogenesis.
Phosphatidyl inositol lipids (PIs) have also been described to play a role in endocytic trafficking and cargo sorting. Phosphatidylinositol 4,5-bis-phosphate [PI(4,5)P2] and phosphatidylinositol 3,4,5-tris-phosphate [PI(3,4,5)P3] function as plasma membrane determinants and organizers in epithelial cells (Gassama-Diagne et al. 2006; Martin-Belmonte and Mostov 2007; Martin-Belmonte et al. 2007). Additionally, different forms of PIs are enriched in specific intracellular organelles to regulate membrane trafficking and confer membrane identity (Di Paolo and De Camilli 2006; Vicinanza et al. 2008). Indeed, PI(3,4,5)P3 might induce the recruitment of the adaptor protein AP1B to the RE membranes to promote lateral segregation of basolateral cargo into a distinct subdomains for basolateral targeting (Fields et al. 2010). AP1B expression might facilitate the recruitment of PI kinases and PI4P as a template for lipid conversion of PI4P to PI(4,5)P2 and PI(3,4,5)P3. In addition, basolateral recycling of E-cadherin depends on AP1B and interaction with phosphatidylinositol 4-phosphate 5-kinase Iγ (PIPKIγ) (Ling et al. 2007). PIPKIγ acts as a scaffold that interacts directly with the μ1 subunits of AP1B and E-cadherin in RE, connecting E-cadherin to the AP1B complex. Inhibition of the interaction between PIPKIγ and either E-cadherin or AP1 mistargets internalized E-cadherin to the apical domain. Basolateral targeting of newly synthesized E-cadherin from Rab11-positive RE is Rab11a-dependent, because Rab11a mutants produce targeting of E-cadherin to the apical domain in MDCK cells (Lock and Stow 2005; Desclozeaux et al. 2008). On the other hand, in polarized epithelial cells, RE membranes are enriched in the apical lipids sphingomyelin, cholesterol, and phosphatidylserine (Lingwood and Simons 2010), which segregate in different microdomains in the RE membranes (Folsch et al. 1999; Thompson et al. 2007). Because local production of PI(4,5)P2 by PIP5KI(α, α′, and β) promotes apically directed trafficking of sphingolipid/cholesterol-rich vesicles (Rozelle et al. 2000), PI(4,5)P2 production in REs might promote apical-directed trafficking.
TRANSCYTOSIS AND APICAL LUMEN MORPHOGENESIS IN POLARIZED EPITHELIAL CELLS
Many different types of epithelial tissues are organized as tubes with an apical lumen. Epithelial lumens can be generated by a diverse set of mechanisms (Lubarsky and Krasnow 2003). One mechanism is de novo lumen generation, in which the transcytosis of vesicles to specific sites of cell–cell contact results in apical membrane and lumen generation (Apodaca et al. 2012). Transcytosis appears to require postendocytic entry into a specialized recycling pathway, in which specific sorting machinery identifies the internalized cargo for apical redelivery rather than basolateral recycling or transport to the lysosome for degradation. In 3D cultures of MDCK cells, this process begins soon after the first cell division (Schluter et al. 2009) and requires the fine control of peripheral actomyosin contractility (Ferrari et al. 2008; Yu et al. 2008; Rodriguez-Fraticelli et al. 2012). This trafficking pathway is initiated by the endocytosis of apical proteins such as podocalyxin, and Crumbs, from the extracellular-free surface. These proteins then accumulate in intracellular vesicles that form a compartment with RE properties (Ferrari et al. 2008; Bryant et al. 2010). Then, apical proteins are delivered to cell–cell contact membranes called the apical membrane initiation site (AMIS) (Bryant et al. 2010). The AMIS, which precedes the formation of a tight-junction-delimited lumen, gives rise to a preapical patch (PAP), which is formed once the lumen is established (Ferrari et al. 2008). Both oriented cell divisions, as well as ion and water influx, regulate lumen expansion resulting in tube-like structures with a central lumen surrounded by polarized epithelial cells (Fig. 2) (Rodriguez-Fraticelli et al. 2011). Interestingly, a similar mechanism is also required in various tissues, including the gut and blood vessels, that undergo de novo lumen formation in vivo (Horne-Badovinac et al. 2001; Bagnat et al. 2007; Herwig et al. 2011; Xu et al. 2011; Apodaca et al. 2012; Zhang et al. 2012).
Figure 2.
Lumen morphogenesis in polarized epithelial cells. (A) De novo lumen generation pathway is initiated by the endocytosis of apical proteins such as podocalyxin and Crumbs, from the extracellular-free surface, which then accumulate in intracellular vesicles that form a compartment with RE properties. (B) Then, apical proteins are delivered to the cell–cell contact membrane in transcytotic vesicles that fuse to this membrane to form the apical membrane initiation site (AMIS). (C) The AMIS, gives rise to a preapical patch (PAP), which is formed once the lumen is established. (D) Spindle-oriented cell divisions and ion and water influx regulate lumen expansion resulting in tube-like structures with a central lumen surrounded by polarized epithelial cells.
Although the mechanisms controlling the initial steps of apical protein transcytosis after endocytosis have not been extensively explored, they could be analogous to the mechanism described for pIgA–pIgR-polarized transcytosis in MDCK cells (Rojas and Apodaca 2002). Indeed, pIgR translocates across the cell with similar kinetics to podocalyxin and Crumbs during MDCK cyst formation (Bryant et al. 2010). After biosynthesis, pIgR is delivered directly from the trans-Golgi network to the basolateral surface, where it encounters dIgA. The pIgR–dIgA complex is then endocytosed through clathrin-coated pits and transported through a series of endosomal compartments across the cell to the apical surface. First, it is delivered to peripherally localized basolateral early endosomes (BEEs), then most of pIgR–dIgA translocates to the Rab17-positive common recycling endosome (CRE), where it is segregated from basolateral recycling cargo, such as TfR. pIgR–dIgA is then transported to the ARE in a microtubule-dependent process (Leung et al. 2000). Interestingly, in cells deficient for AP1B, TfR and other basolateral recycling cargo is transported to the apical domain through a transcytotic pathway regulated by the kinesin KIF16B, which is different from the pIgR transcytosis that is independent of this kinesin (Perez Bay et al. 2013). En route or at the apical surface, pIgR is proteolytically cleaved, and the extracellular binding domain of the receptor that is bound to dIgA is released into the mucosal secretions. This endosomal transport from the BEE to the ARE requires the activity of INF2, a Cdc42-activated formin, which is targeted to subapical recycling endosomes by MAL2, which was previously described to associate with basolateral-to-apical transcytosis (de Marco et al. 2002; Madrid et al. 2010), and Rab3b (van IJzendoorn et al. 2002). Indeed, dIgA binding stimulates pIgR transcytosis through the action of the Rab3b GTPase. Recent evidence has identified that binding of pIgA to pIgR at the basolateral membrane also stimulates transcytosis through the tyrosine kinase Yes, which directly phosphorylates EGF receptor (EGFR). Phosphorylation of EGFR subsequently activates extracellular signal-regulated protein kinase (ERK), which, in turn, phosphorylates Rab11–FIP5 to control the distribution of the ARE and pIgA–pIgR transcytosis (Su et al. 2010).
Endocytosis has also been implicated in the formation of epithelial apical lumens in vivo, both in C. elegans and in zebrafish. Studies in the C. elegans intestine show a crucial function for Rab11 and the clathrin adaptor AP1 in promoting apical polarization (Michaux et al. 2011; Shafaq-Zadah et al. 2012). AP1 is specifically required for the formation of apical Rab11 vesicles and for the apical localization of Cdc42 and the polarity determinant PAR-6, similar to their role in MDCK cell polarization. Loss of AP1 affects the polarized distribution of both apical and basolateral transmembrane proteins. Moreover, it triggers de novo formation of ectopic apical lumens between intestinal cells along the lateral membranes during embryogenesis. These data highlight an unexpected function for AP1 in apical sorting routes at the recycling endosome, controlling apical transport and localization of the Cdc42–Par6 polarity complex. In addition, Rab11-mediated trafficking, functioning downstream from Smoothened (Smo), is required for single lumen formation and lumen resolution in the developing zebrafish gut (M Bagnat, pers. comm.).
Apical proteins are exocytosed to form the AMIS using a recently characterized molecular machinery based on Rab11a and Rab8 present at the recycling endosome, and the small GTPase Cdc42 at the plasma membrane (Fig. 2). First, Rab11a recruits the GEF Rabin8α to activate Rab8a/b (Bryant et al. 2010). Rab11a, and probably Rab8, promotes recruitment of the exocyst subunit Sec15a. Sec15a facilitates exocytosis by promoting binding of exocytic vesicles to the Sec10 exocyst subunit localized at the emerging AMIS. Rab11a recruits the Myo5b actin-based motor to the RE, where it additionally interacts with Rab8a to facilitate transport of these vesicles to the AMIS (Roland et al. 2011). Rab11a also recruits another effector, Rab11Fip5, which, in concert with Snx18, facilitates formation of apically destined carriers to be transported to the lumen from the recycling endosome (Schonteich et al. 2008; Willenborg et al. 2011). Finally, AMIS formation requires the activity of the Cdc42–Par6/aPKC complex (Martin-Belmonte et al. 2007; Horikoshi et al. 2009) in a step possibly mediated by active Rab8 and Rab11a (Bryant et al. 2010). Indeed, Rab11a and Rab8 localize and activate, respectively, Cdc42 at the forming lumen, to stimulate the interaction of this protein with the exocytic carriers (Fig. 2).
The AMIS forms at a previously basolateral cell–cell contact, through conversion of PI (3,4,5)P3 to PI(4,5)P2 via the lipid phosphatase PTEN, which localizes at apical junctions in different types of epithelial cells (Pinal et al. 2006; Martin-Belmonte et al. 2007; Wu et al. 2007). This PI segregation apparently controls lumen formation through the specific binding of PI(4,5)P2-associated proteins with the abovementioned trafficking pathway (Fig. 2). PI (4,5)P2, newly enriched at this site, binds annexin 2, which, in turn, scaffolds activated Cdc42 at the AMIS (Martin-Belmonte et al. 2007). PI(4,5)P2 also seems to be important for the initial recruitment of some of the components of the exocyst complex to the AMIS (Liu et al. 2007). A central question in this process is how apical vesicles are directed to and fuse with the AMIS during lumen biogenesis. Recent analysis shows the involvement of a novel pathway mediated by the synaptotagmin-like proteins Slp2-a/4a in the formation of the AMIS (Galvez-Santisteban et al. 2012). Slp2-a binds to PtdIns(4,5)P2 at the forming lumen and clusters apically destined vesicles to the AMIS in a Rab27a/b-dependent way (Fig. 2). In parallel, Slp4-a bridges Rab27–Rab3b–Rab8 and the apical SNARE syntaxin 3 to promote vesicle fusion exclusively at the AMIS and create a single apical surface and lumen. Therefore, traffic machinery associated with the RE composed of Cdc42, some specific Rab proteins, together with their specific effectors, and certain scaffolding proteins controls the postendocytic vesicle transport that ensures de novo apical membrane biogenesis in epithelial cells.
Thus, endocytosis and endocytic trafficking pathways are essential for de novo lumen formation in epithelial tissues. Much of our understanding of how epithelial tubes form these structures derives from in vitro models, and there are still many gaps in the mechanisms associated with this process. Further work will be needed using in vivo models to fully validate this information and further characterize them during vertebrate organogenesis.
ENDOCYTOSIS AND RECYCLING IN PLANAR POLARIZATION
Endocytosis also plays a critical role in establishing epithelial polarity along a second axis in the plane of the epithelium. Planar polarity (also called tissue polarity) is reflected in the uniform alignment of external structures such as hairs and cilia in many different tissues of vertebrates and invertebrates. The highly conserved Core planar cell polarity (PCP) pathway helps to globally align such structures with respect to the tissue axes.
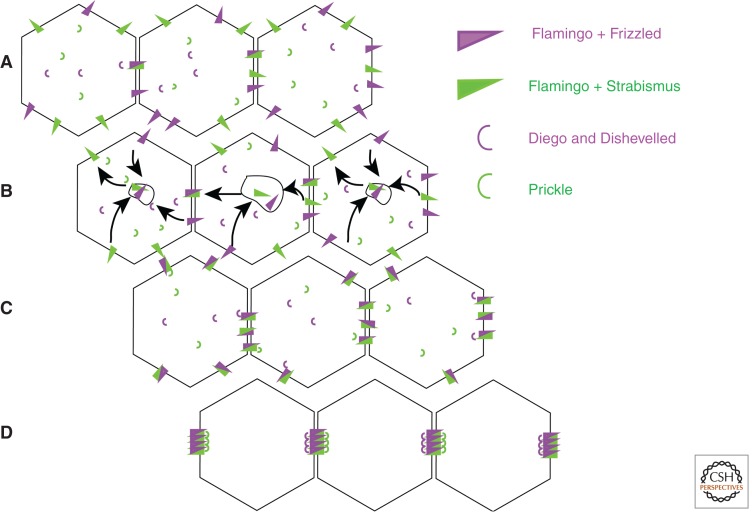
Core PCP proteins localize in a polarized fashion to epithelial AJ, where they form asymmetric complexes that couple the polarity of adjacent cells (Fig. 3). The 7-pass transmembrane cadherin Flamingo (Fmi) engages in homophilic interactions across cell contacts but forms complexes with different proteins on either side. On one side, Fmi recruits Frizzed (Fz), a 7-pass transmembrane protein that can also act as a Wnt receptor, and two peripherally associated proteins: Diego (Dgo) and Dishevelled (Dsh). On the other, Fmi interacts with the transmembrane protein Strabismus (Stbm) and a peripherally associated protein, Prickle (Pk). The polarity of PCP complexes is both locally aligned between adjacent cells and globally aligned with the shape of the tissue.
Figure 3.
A model for the role of endocytosis and recycling in the development of planar polarity. (A) Planar polarity proteins comprise transmembrane proteins (Flamingo, Frizzled, and Strabismus) along with peripherally associated proteins (Diego, Dishevelled, and Prickle). (B) Flamingo can interact with either Frizzled or Strabismus, and heterophilic complexes comprising Flamingo + Frizzled on one side of the cell and Flamingo + Strabismus on the other are more resistant to endocytosis than either complex alone. (C) This results in accumulation of heterophilic complexes of different polarities in the membrane. (D) Clustering of complexes of the same polarity by the peripherally associated proteins results in their segregation to different sides of the cell and local alignment of polarity between cells.
The Drosophila wing has been a particularly powerful system for the study of planar polarity. Global Core PCP patterns arise during the growth of the larval wing disc (Sagner et al. 2012) and undergo two major reorganizations as the wings undergo epithelial remodeling during pupal stages (Aigouy et al. 2010). At the end of this process, shortly before wing hairs form, PCP domains are globally aligned with the proximodistal axis of the wing such that Fz-containing domains on the distal side of each cell are coupled to Stbm domains on the proximal side of the adjacent cell. Proximodistal polarity of PCP domains helps orient distal outgrowth of wing hairs. Interestingly, genetic perturbations of the endocytic pathway in the wing disturb the formation of polarized PCP domains and distal orientation of wing hairs. How does endocytic trafficking support the development of planar polarity?
The development of distal polarity of Core PCP domains throughout the wing is controlled at two levels: (1) a locally acting feedback mechanism based on interactions between PCP proteins that self-organizes intracellular polarity and couples it between neighboring cells; and (2) other mechanisms that globally bias the direction in which polarity develops. Biasing cues include signals from the major organizing centers in the wing (Sagner et al. 2012; Wu et al. 2013), as well as epithelial remodeling itself (Aigouy et al. 2010). Two key features of Core PCP protein interactions underlie their ability to self-organize polarity. First, Fmi/Fz complexes on one side of a cell contact are able to recruit and stabilize Fmi/Stbm complexes on the facing side (and vice versa). Second, the presence of PCP complexes of one polarity on a cell boundary somehow discourages the accumulation of complexes of the opposite polarity (for review, see Strutt and Strutt 2008). Widely divergent types of theoretical models based on these two rules all produce locally aligned polarity in simulations (Amonlirdviman et al. 2005; Burak and Shraiman 2009; Aigouy et al. 2010). These features of the PCP system depend on endocytic turnover of PCP proteins and the precise control of their levels at the junctional region.
Fmi can be detected in endosomes with both Rab5 and Rabenosyn-5, an effector protein that directs postendocytic trafficking toward recycling. Mutations in Rabenosyn-5 cause accumulation of Fmi in late endosomes and disturb polarization of PCP domains (Mottola et al. 2010). PCP domain formation is also perturbed by mutations in fat facets, which encodes a deubiquitinating enzyme that promotes Fmi recycling (Strutt et al. 2013a). Thus, continuing endocytosis and recycling seem to be necessary for intracellular planar polarization. Interestingly, Fmi appears to be more stable against endocytosis when it is incorporated into polarized complexes with Fz and Stbm. Antibody uptake experiments show that Fmi is internalized more rapidly in Fz or Stbm mutant cells. This correlates with a shift in localization from the junctional region to the apical membrane (Strutt and Strutt 2008). Thus, complexes containing Fmi/Fz on one side of the cell boundary and Fmi/Stbm on the other may be selectively stabilized at junctions based on their resistance to endocytosis.
Once formed, Fz/Fmi:Fmi/Stbm complexes of the same polarity are clustered into higher-order domains through the activity of the peripherally associated PCP proteins Pk, Dsh, and Dgo (Aigouy et al. 2010; Strutt et al. 2011). These clusters persist over a time frame of hours and are resistant to endocytosis, and fluorescence recovery after photobleaching (FRAP) experiments show that they contain a Core PCP population that turns over very slowly compared with the more diffusely distributed pool. Clustering can clearly separate complexes of different polarities from each other in the membrane, and it appears to be an important precondition for the longer-range separation of these domains to different sides of the cell; mutating peripheral PCP proteins decreases both clustering and intracellular polarization of transmembrane PCP proteins (Strutt et al. 2011).
How clustering promotes the accumulation of complexes with different polarities on opposite sides of the cell is a matter of some discussion. One possible mechanism is based on the idea of limiting components. If Core PCP proteins are present in limiting amounts, then accumulation of stable Fz/Fmi complexes on one side of the cell might deplete them from the other and favor the formation of Stbm/Fmi complexes there. Such a process would be driven by the much slower diffusion rate of Core PCP proteins within clusters compared with the rapid diffusion rate outside of clusters—this could share features with the phase-separation mechanism responsible for the condensation and enrichment of P-granule material on the posterior side of the C. elegans zygote (Brangwynne et al. 2009). The idea is consistent with the observation that overexpression of any of the Core PCP proteins disturbs the development of intracellular polarity. In this scenario, an important contribution of endocytosis (apart from selectively removing uncomplexed PCP proteins) might be to regulate the total amounts of Core PCP proteins available for interactions. The level of uncomplexed peripheral PCP proteins also appears to be under tight regulation by neddylation and ubiquitinylation-dependent mechanisms that are important for the development of proper intracellular polarity (Strutt et al. 2013a,b).
Some experiments raise the possibility that membrane trafficking may also contribute more instructively to the orientation of domain polarization. The dorsoventral compartment boundary, which forms the wing margin, is responsible for orienting a subset of the PCP pattern in larval and early pupal wings (Sagner et al. 2012). At least part of its effects is mediated by two Wnts, Wingless (Wg) and Wnt4, that are expressed there. Addition of Wg to tissue culture cells expressing Stbm or Fz reduces coaccumulation of these proteins across cell contacts (Wu et al. 2013). The ability of Wnts to block interaction of Fz- and Stbm-containing PCP domains in a graded fashion might account for their polarizing activity in the wing. Because Wnts are ligands for Fz proteins and can induce their internalization, it may be that graded Fz internalization accounts for the orienting effect of Wnts. Consistent with this idea, vertebrate Dishevelled2 (Dvl2) interacts with the µ2-adaptin subunit of AP2 to promote Wnt5-induced internalization of vertebrate Fz4. Mutations that block this interaction blunt the effects of Dvl2 on convergent extension (Yu et al. 2007).
Distal polarization of Fz-containing complexes in the Drosophila wing has also been proposed to depend on oriented endocytosis and recycling guided by planar microtubule polarity. It has been noted that the orientation of microtubules in pupal wing cells is predominantly along the proximodistal axis. Fz-positive vesicles were observed to move more often along this axis with a slight bias toward the distal side, and this slight bias might be amplified by other feedback mechanisms to separate proximal and distal PCP domains (Harumoto et al. 2010).
Endocytosis of Core PCP protein complexes likely has a critical function in tissues undergoing dynamic rearrangements or growth. In Drosophila, oriented tissue flows occur during pupal wing morphogenesis, and these flows shift the global pattern of Core PCP to point distally. As cell boundaries are removed and reformed during tissue flows, Core PCP complexes on cell contacts must turn over. Simulations suggest that the oriented breakdown and reformation of cell contacts can direct the polarity axis, and can even do so in different directions, depending on the rate of Core PCP turnover (Aigouy et al. 2010). In contrast, the endocytosis of Core PCP complexes appears to have an important function in maintaining a stable global polarity pattern in the mammalian skin during mitosis. When cells divide, these complexes are internalized, partitioned equally between daughter cells, and recycled back to the cell surface using neighboring cell polarity as a template (Devenport et al. 2011). This type of trafficking may help to stabilize the global Core polarity pattern in tissues undergoing rapid cell division.
Finally, Core PCP domains may themselves locally affect endocytosis and membrane delivery. Both Rabenosyn-5 and Sec5 localize to the cortex in an Fmi-dependent fashion, and Fmi overexpression recruits these proteins into even larger cortical complexes (Classen et al. 2008; Mottola et al. 2010). Thus, domains of different polarities may organize specific sites for vesicle endocytosis and/or delivery. Interestingly, Rabenosyn-5 is not only recruited by Fmi, it also regulates Fmi trafficking. Fmi accumulates inside the cell in late endosomes in Rabenosyn-5 mutant cells (Mottola et al. 2010). This raises the intriguing possibility of a positive-feedback loop in which Fmi-containing complexes at the plasma membrane organize sites for further delivery, amplifying an initial small bias.
THE REGULATION OF ENDOCYTOSIS IN EPITHELIAL REMODELING
Developing epithelial tissues undergo dramatic shape changes involving oriented cell elongation, cell divisions, and neighbor exchanges. During these processes, apical AJ maintain epithelial integrity while undergoing dynamic turnover that depends on endocytosis. Tissue shape changes depend on the orientation in which junctions break, form, and expand—and, indeed, planar polarity systems like those that control wing hair orientation have also been implicated in a variety of tissue remodeling processes. How these planar polarity systems eventually polarize the endocytic processes necessary for the oriented turnover of cell contacts is an active area of research.
E-cadherin is a core mediator of homophilic adhesion between epithelial cells at AJ. On the cytoplasmic face of the membrane, it interacts with α, β, and p120 catenins, which regulate its endocytosis and recycling and mediate dynamic interaction with the actin cytoskeleton (for review, see Ratheesh and Yap 2012). Endocytosis and recycling of E-cadherin, combined with active cytoskeletal remodeling, are critical for controlled junction assembly and disassembly. E-cadherin turnover and the relative contributions of lateral diffusion, endocytosis, and recycling have been studied in both tissue culture and in living animals using antibody internalization, biotinylation, FRAP, and photoactivation approaches. These experiments show that E-cadherin exists in at least two pools at the membrane—an immobile fraction that coexists along with a more rapidly diffusing pool (Cavey et al. 2008; Canel et al. 2010; Bulgakova et al. 2013). Mature junctions are enriched in a stable pool of E-cadherin that turns over predominantly through endocytosis and recycling, rather than by dissociation and lateral diffusion (de Beco et al. 2009). E-cadherin appears to be internalized in complexes with other junctional proteins (Leibfried et al. 2008). Indeed, endocytosis, rather than dissociation and lateral diffusion, appears to be the major mechanism for the disassembly of adhesive contacts (Troyanovsky et al. 2006; Levayer et al. 2011).
Endocytosis of E-cadherin is mediated by clathrin and dynamin and is promoted by myosin II and RhoGEF activity. The process appears to be regulated by the conserved polarity module comprising Par3/Bazooka, PAR6, aPKC, and CDC42. Once internalized, cadherin passes through Rab5-positive early endosomes. From there it can be directed via a Rab7-mediated pathway to late endosomes and lysosomes for destruction. Alternately, it can be recycled to the cell surface via the Rab11-positive recycling endosome. The Rab11-recycling endosome has also been implicated in the initial targeting of newly synthesized E-cadherin to the basolateral plasma membrane using the exocyst complex, both in cultured mammalian cells and in Drosophila.
Endocytosis and recycling of E-cadherin are particularly important in tissues undergoing remodeling, a process that has been extensively studied in a variety of different Drosophila and vertebrate tissues. During Drosophila germ-band extension, the embryo elongates in the anteroposterior axis, and narrows in the dorsoventral axis. This involves extensive cell rearrangements that are oriented along the anteroposterior axis, separating anteroposterior neighbors and bringing dorsoventral neighbors into apposition (Irvine and Wieschaus 1994; Bertet et al. 2004; Blankenship et al. 2006). The process requires clathrin- and dynamin-dependent endocytosis of E-cadherin from disassembling cell contacts. When dynamin-dependent endocytosis is blocked, cell intercalation fails (Levayer et al. 2011). Disassembly of cell contacts also involves localized tyrosine phosphorylation of β-catenin by c-Abl, a modification that can promote N-cadherin endocytosis (Tai et al. 2007). The orientation of cell rearrangements in the extending germ band is guided by patterns of pair-rule gene expression. These define a series of cell interfaces perpendicular to the anteroposterior body axis that are destined for disassembly (Irvine and Wieschaus 1994). These boundaries accumulate RhoGEF and myosin II (Levayer et al. 2011) and also show increased Abl-dependent phosphorylation of β-catenin (Levayer et al. 2011). Conversely, Bazooka/Par3 accumulates on boundaries with more persistent adhesion (Simoes Sde et al. 2010). However, it is not yet understood how pair-rule-dependent transcriptional patterns eventually recruit RhoGEF, myosin, and Abl to these interfaces.
The Drosophila thorax also changes its shape during pupal development, driven by a complex pattern of cell-shape changes and rearrangements (Bosveld et al. 2012). Blocking the activity of CDC42–aPKC–PAR6 at this time leads to gaps in junctional E-cadherin, and its intracellular accumulation, along with α- and β-catenin, in endosomal structures (Leibfried et al. 2008). The orientation of cell rearrangements in the thorax is guided by a second PCP system, the Fat/Dachsous/Four-jointed planar polarity system, that is molecularly distinct from the Core PCP system (Bosveld et al. 2012). In many tissues, opposing transcriptional gradients of the atypical cadherin Dachsous and the Golgi kinase Four-jointed are translated into intracellular polarization of Fat/Dachsous heterodimers that link adjacent cells. These domains form a global polarity pattern that is oriented down the gradient of Dachsous expression (Ambegaonkar et al. 2012; Bosveld et al. 2012; Brittle et al. 2012). The atypical myosin Dachs becomes enriched on the same side of the cell as Dachsous and increases cell boundary tension (Rogulja et al. 2008; Mao et al. 2011). In the thorax, this polarity is thought to drive boundary disassembly and oriented neighbor exchanges (Bosveld et al. 2012). How Dachs might interface with the mechanisms that promote E-cadherin endocytosis is not yet known.
Oriented cell neighbor exchanges also occur during morphogenesis of the Drosophila pupal wing. During pupal stages, anisotropic stresses along the proximodistal axis of the wing cause cells to elongate, generating tissue shear. Subsequently, wing epithelial cells undergo oriented cell divisions and then oriented neighbor exchanges that relieve cell elongation while preserving the altered tissue shape (Aigouy et al. 2010). These oriented rearrangements also promote the organization of a regular, hexagonal cell-packing geometry (Classen et al. 2008). Blocking either dynamin activity or Rab11-dependent recycling during this remodeling process causes gaps in E-cadherin distribution at the junctional region and eventual disintegration of the epithelium (Classen et al. 2005). Epithelial remodeling in the wing is in part controlled autonomously by the core PCP system (Classen et al. 2005; Warrington et al. 2013). However, it is driven to a large extent by extrinsically generated anisotropic stresses in the plane of the epithelium. Hinge contraction generates proximodistally (PD) oriented stresses in the wing blade such that cell contacts in this direction are under more tension that those lying at other angles. These boundaries lengthen and cells eventually undergo neighbor exchanges that separate cells along the PD axis and result in new contacts along the anteroposterior axis (Aigouy et al. 2010). It is clear in other systems that membrane tension can influence the balance of endocytosis and exocytosis to maintain tension within agreeable limits (Apodaca 2002). It will be interesting to discover whether endocytosis or recycling of junctional assemblies might respond similarly.
Cadherin endocytosis, guided by the Core PCP system, also contributes to tissue remodeling in vertebrate systems. Core PCP regulates convergent extension in both zebrafish and Xenopus and appears to act, at least in part, by regulating cadherin endocytosis (Ulrich et al. 2005; Kraft et al. 2012). Furthermore, rearrangement of endothelial cells during formation of intraluminal valves in the lymphatic system is guided by Core PCP–dependent regulation of VE-cadherin turnover (Tatin et al. 2013).
Thus, polarized trafficking of cadherins, often guided by the Core PCP pathway, is an essential modulator of tissue morphogenesis in both vertebrates and invertebrates.
CONCLUDING REMARKS AND FUTURE PERSPECTIVES
In summary, 3D in vitro models, combined with in vivo studies in Drosophila, C. elegans, and mice have begun to illuminate the endocytic trafficking pathways associated with the formation of apicobasal and PCP polarity in epithelial tissues. Identifying how the endocytic, sorting, and recycling machineries described above are coordinated to generate functional epithelial tissues is an exciting challenge for the next years in the fields of endosomal membrane traffic and epithelial polarity. Moreover, although we have identified many of the regulatory machineries, such as small GTPases, effectors, and polarity complexes that can control this endocytic transport, a further challenge is to understand how these proteins function as a network to generate polarized tubovesicular transport, and more importantly, how they are transcriptionally regulated during epithelial organ development.
ACKNOWLEDGMENTS
We thank Carmen M. Ruiz-Jarabo for comments on the manuscript and members of the Martin-Belmonte and Eaton laboratory for helpful discussions. This work is supported by grants from the Human Frontiers Science Program (HFSP-CDA 00011/2009), MICINN (BFU2011-22622 and CONSOLIDER CSD2009-00016) to F.M.-B., and by a grant from the European Research Council to S.E.
Footnotes
Editors: Sandra L. Schmid, Alexander Sorkin, and Marino Zerial
Additional Perspectives on Endocytosis available at www.cshperspectives.org
REFERENCES
*Reference is also in this collection.
- Aigouy B, Farhadifar R, Staple DB, Sagner A, Roper JC, Julicher F, Eaton S 2010. Cell flow reorients the axis of planar polarity in the wing epithelium of Drosophila. Cell 142: 773–786 [DOI] [PubMed] [Google Scholar]
- Ambegaonkar AA, Pan G, Mani M, Feng Y, Irvine KD 2012. Propagation of Dachsous–Fat planar cell polarity. Curr Biol 22: 1302–1308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amonlirdviman K, Khare NA, Tree DN, Chen W, Axelrod J, Tomlin CJ 2005. Mathematical modeling of planar cell polarity to understand domineering non-autonomy. Science 301: 423–426 [DOI] [PubMed] [Google Scholar]
- Apodaca G 2002. Modulation of membrane traffic by mechanical stimuli. Am J Physiol Renal Physiol 282: F179–F190 [DOI] [PubMed] [Google Scholar]
- Apodaca G, Gallo LI, Bryant DM 2012. Role of membrane traffic in the generation of epithelial cell asymmetry. Nat Cell Biol 14: 1235–1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagnat M, Cheung ID, Mostov KE, Stainier DY 2007. Genetic control of single lumen formation in the zebrafish gut. Nat Cell Biol 9: 954–960 [DOI] [PubMed] [Google Scholar]
- Bertet C, Sulak L, Lecuit T 2004. Myosin-dependent junction remodelling controls planar cell intercalation and axis elongation. Nature 429: 667–671 [DOI] [PubMed] [Google Scholar]
- Blankenship JT, Backovic ST, Sanny JS, Weitz O, Zallen JA 2006. Multicellular rosette formation links planar cell polarity to tissue morphogenesis. Dev Cell 11: 459–470 [DOI] [PubMed] [Google Scholar]
- Blankenship JT, Fuller MT, Zallen JA 2007. The Drosophila homolog of the Exo84 exocyst subunit promotes apical epithelial identity. J Cell Sci 120: 3099–3110 [DOI] [PubMed] [Google Scholar]
- Bosveld F, Bonnet I, Guirao B, Tlili S, Wang Z, Petitalot A, Marchand R, Bardet PL, Marcq P, Graner F, et al. 2012. Mechanical control of morphogenesis by Fat/Dachsous/Four-jointed planar cell polarity pathway. Science 336: 724–727 [DOI] [PubMed] [Google Scholar]
- Brangwynne CP, Eckmann CR, Courson DS, Rybarska A, Hoege C, Gharakhani J, Julicher F, Hyman AA 2009. Germline P granules are liquid droplets that localize by controlled dissolution/condensation. Science 324: 1729–1732 [DOI] [PubMed] [Google Scholar]
- Brittle A, Thomas C, Strutt D 2012. Planar polarity specification through asymmetric subcellular localization of Fat and Dachsous. Curr Biol 22: 907–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant DM, Datta A, Rodriguez-Fraticelli AE, Peranen J, Martin-Belmonte F, Mostov KE 2010. A molecular network for de novo generation of the apical surface and lumen. Nat Cell Biol 12: 1035–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulgakova NA, Grigoriev I, Yap AS, Akhmanova A, Brown NH 2013. Dynamic microtubules produce an asymmetric E-cadherin–Bazooka complex to maintain segment boundaries. J Cell Biol 201: 887–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burak Y, Shraiman BI 2009. Order and stochastic dynamics in Drosophila planar cell polarity. PLoS Comput Biol 5: e1000628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canel M, Serrels A, Anderson KI, Frame MC, Brunton VG 2010. Use of photoactivation and photobleaching to monitor the dynamic regulation of E-cadherin at the plasma membrane. Cell Adh Migr 4: 491–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavey M, Rauzi M, Lenne PF, Lecuit T 2008. A two-tiered mechanism for stabilization and immobilization of E-cadherin. Nature 453: 751–756 [DOI] [PubMed] [Google Scholar]
- Classen AK, Anderson KI, Marois E, Eaton S 2005. Hexagonal packing of Drosophila wing epithelial cells by the planar cell polarity pathway. Dev Cell 9: 805–817 [DOI] [PubMed] [Google Scholar]
- Classen AK, Aigouy B, Giangrande A, Eaton S 2008. Imaging Drosophila pupal wing morphogenesis. Methods Mol Biol 420: 265–275 [DOI] [PubMed] [Google Scholar]
- de Beco S, Gueudry C, Amblard F, Coscoy S 2009. Endocytosis is required for E-cadherin redistribution at mature adherens junctions. Proc Natl Acad Sci 106: 7010–7015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Marco MC, Martin-Belmonte F, Kremer L, Albar JP, Correas I, Vaerman JP, Marazuela M, Byrne JA, Alonso MA 2002. MAL2, a novel raft protein of the MAL family, is an essential component of the machinery for transcytosis in hepatoma HepG2 cells. J Cell Biol 159: 37–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desclozeaux M, Venturato J, Wylie FG, Kay JG, Joseph SR, Le HT, Stow JL 2008. Active Rab11 and functional recycling endosome are required for E-cadherin trafficking and lumen formation during epithelial morphogenesis. Am J Physiol Cell Physiol 295: C545–C556 [DOI] [PubMed] [Google Scholar]
- Devenport D, Oristian D, Heller E, Fuchs E 2011. Mitotic internalization of planar cell polarity proteins preserves tissue polarity. Nat Cell Biol 13: 893–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Di Fiore PP, von Zastrow M 2014. Endocytosis, signaling, and beyond. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a016865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Paolo G, De Camilli P 2006. Phosphoinositides in cell regulation and membrane dynamics. Nature 443: 651–657 [DOI] [PubMed] [Google Scholar]
- Ferrari A, Veligodskiy A, Berge U, Lucas MS, Kroschewski R 2008. ROCK-mediated contractility, tight junctions and channels contribute to the conversion of a preapical patch into apical surface during isochoric lumen initiation. J Cell Sci 121: 3649–3663 [DOI] [PubMed] [Google Scholar]
- Fields IC, King SM, Shteyn E, Kang RS, Folsch H 2010. Phosphatidylinositol 3,4,5-trisphosphate localization in recycling endosomes is necessary for AP-1B-dependent sorting in polarized epithelial cells. Mol Biol Cell 21: 95–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher GC, Lucas EP, Brain R, Tournier A, Thompson BJ 2012. Positive feedback and mutual antagonism combine to polarize Crumbs in the Drosophila follicle cell epithelium. Curr Biol 22: 1116–1122 [DOI] [PubMed] [Google Scholar]
- Folsch H, Ohno H, Bonifacino JS, Mellman I 1999. A novel clathrin adaptor complex mediates basolateral targeting in polarized epithelial cells. Cell 99: 189–198 [DOI] [PubMed] [Google Scholar]
- Galvez-Santisteban M, Rodriguez-Fraticelli AE, Bryant DM, Vergarajauregui S, Yasuda T, Banon-Rodriguez I, Bernascone I, Datta A, Spivak N, Young K, et al. 2012. Synaptotagmin-like proteins control the formation of a single apical membrane domain in epithelial cells. Nat Cell Biol 14: 838–849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassama-Diagne A, Yu W, ter Beest M, Martin-Belmonte F, Kierbel A, Engel J, Mostov K 2006. Phosphatidylinositol-3,4,5-trisphosphate regulates the formation of the basolateral plasma membrane in epithelial cells. Nat Cell Biol 8: 963–970 [DOI] [PubMed] [Google Scholar]
- Golachowska MR, Hoekstra D, van IJzendoorn SC 2010. Recycling endosomes in apical plasma membrane domain formation and epithelial cell polarity. Trends Cell Biol 20: 618–626 [DOI] [PubMed] [Google Scholar]
- Grawe F, Wodarz A, Lee B, Knust E, Skaer H 1996. The Drosophila genes crumbs and stardust are involved in the biogenesis of adherens junctions. Development 122: 951–959 [DOI] [PubMed] [Google Scholar]
- Harris KP, Tepass U 2008. Cdc42 and Par proteins stabilize dynamic adherens junctions in the Drosophila neuroectoderm through regulation of apical endocytosis. J Cell Biol 183: 1129–1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harumoto T, Ito M, Shimada Y, Kobayashi TJ, Ueda HR, Lu B, Uemura T 2010. Atypical cadherins Dachsous and Fat control dynamics of noncentrosomal microtubules in planar cell polarity. Dev Cell 19: 389–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herwig L, Blum Y, Krudewig A, Ellertsdottir E, Lenard A, Belting HG, Affolter M 2011. Distinct cellular mechanisms of blood vessel fusion in the zebrafish embryo. Curr Biol 21: 1942–1948 [DOI] [PubMed] [Google Scholar]
- Horikoshi Y, Suzuki A, Yamanaka T, Sasaki K, Mizuno K, Sawada H, Yonemura S, Ohno S 2009. Interaction between PAR-3 and the aPKC-PAR-6 complex is indispensable for apical domain development of epithelial cells. J Cell Sci 122: 1595–1606 [DOI] [PubMed] [Google Scholar]
- Horne-Badovinac S, Lin D, Waldron S, Schwarz M, Mbamalu G, Pawson T, Jan Y, Stainier DY, Abdelilah-Seyfried S 2001. Positional cloning of heart and soul reveals multiple roles for PKCλ in zebrafish organogenesis. Curr Biol 11: 1492–1502 [DOI] [PubMed] [Google Scholar]
- Iden S, Collard JG 2008. Crosstalk between small GTPases and polarity proteins in cell polarization. Nat Rev Mol Cell Biol 9: 846–859 [DOI] [PubMed] [Google Scholar]
- Irvine KD, Wieschaus E 1994. Cell intercalation during Drosophila germband extension and its regulation by pair-rule segmentation genes. Development 120: 827–841 [DOI] [PubMed] [Google Scholar]
- Jafar-Nejad H, Andrews HK, Acar M, Bayat V, Wirtz-Peitz F, Mehta SQ, Knoblich JA, Bellen HJ 2005. Sec15, a component of the exocyst, promotes notch signaling during the asymmetric division of Drosophila sensory organ precursors. Dev Cell 9: 351–363 [DOI] [PubMed] [Google Scholar]
- Kerman BE, Cheshire AM, Myat MM, Andrew DJ 2008. Ribbon modulates apical membrane during tube elongation through Crumbs and Moesin. Dev Biol 320: 278–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft B, Berger CD, Wallkamm V, Steinbeisser H, Wedlich D 2012. Wnt-11 and Fz7 reduce cell adhesion in convergent extension by sequestration of PAPC and C-cadherin. J Cell Biol 198: 695–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langevin J, Morgan MJ, Sibarita JB, Aresta S, Murthy M, Schwarz T, Camonis J, Bellaiche Y 2005. Drosophila exocyst components Sec5, Sec6, and Sec15 regulate DE-cadherin trafficking from recycling endosomes to the plasma membrane. Dev Cell 9: 365–376 [DOI] [PubMed] [Google Scholar]
- Leibfried A, Fricke R, Morgan MJ, Bogdan S, Bellaiche Y 2008. Drosophila Cip4 and WASp define a branch of the Cdc42–Par6–aPKC pathway regulating E-cadherin endocytosis. Curr Biol 18: 1639–1648 [DOI] [PubMed] [Google Scholar]
- Leung SM, Ruiz WG, Apodaca G 2000. Sorting of membrane and fluid at the apical pole of polarized Madin–Darby canine kidney cells. Mol Biol Cell 11: 2131–2150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levayer R, Pelissier-Monier A, Lecuit T 2011. Spatial regulation of Dia and Myosin-II by RhoGEF2 controls initiation of E-cadherin endocytosis during epithelial morphogenesis. Nat Cell Biol 13: 529–540 [DOI] [PubMed] [Google Scholar]
- Ling K, Bairstow SF, Carbonara C, Turbin DA, Huntsman DG, Anderson RA 2007. Type Iγ phosphatidylinositol phosphate kinase modulates adherens junction and E-cadherin trafficking via a direct interaction with μ1B adaptin. J Cell Biol 176: 343–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingwood D, Simons K 2010. Lipid rafts as a membrane-organizing principle. Science 327: 46–50 [DOI] [PubMed] [Google Scholar]
- Liu J, Zuo X, Yue P, Guo W 2007. Phosphatidylinositol 4,5-bisphosphate mediates the targeting of the exocyst to the plasma membrane for exocytosis in mammalian cells. Mol Biol Cell 18: 4483–4492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lock JG, Stow JL 2005. Rab11 in recycling endosomes regulates the sorting and basolateral transport of E-cadherin. Mol Biol Cell 16: 1744–1755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Bilder D 2005. Endocytic control of epithelial polarity and proliferation in Drosophila. Nat Cell Biol 7: 1232–1239 [DOI] [PubMed] [Google Scholar]
- Lubarsky B, Krasnow MA 2003. Tube morphogenesis: Making and shaping biological tubes. Cell 112: 19–28 [DOI] [PubMed] [Google Scholar]
- Madrid R, Aranda JF, Rodriguez-Fraticelli AE, Ventimiglia L, Andres-Delgado L, Shehata M, Fanayan S, Shahheydari H, Gomez S, Jimenez A, et al. 2010. The formin INF2 regulates basolateral-to-apical transcytosis and lumen formation in association with Cdc42 and MAL2. Dev Cell 18: 814–827 [DOI] [PubMed] [Google Scholar]
- Mao Y, Tournier AL, Bates PA, Gale JE, Tapon N, Thompson BJ 2011. Planar polarization of the atypical myosin Dachs orients cell divisions in Drosophila. Genes Dev 25: 131–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Belmonte F, Mostov K 2007. Phosphoinositides control epithelial development. Cell Cycle 6: 1957–1961 [DOI] [PubMed] [Google Scholar]
- Martin-Belmonte F, Gassama A, Datta A, Yu W, Rescher U, Gerke V, Mostov K 2007. PTEN-mediated apical segregation of phosphoinositides controls epithelial morphogenesis through Cdc42. Cell 128: 383–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaffrey LM, Macara IG 2011. Epithelial organization, cell polarity and tumorigenesis. Trends Cell Biol 21: 727–735 [DOI] [PubMed] [Google Scholar]
- Michaux G, Dyer CE, Nightingale TD, Gallaud E, Nurrish S, Cutler DF 2011. A role for Rab10 in von Willebrand factor release discovered by an AP-1 interactor screen in C. elegans. J Thromb Haemost 9: 392–401 [DOI] [PubMed] [Google Scholar]
- Mottola G, Classen AK, Gonzalez-Gaitan M, Eaton S, Zerial M 2010. A novel function for the Rab5 effector Rabenosyn-5 in planar cell polarity. Development 137: 2353–2364 [DOI] [PubMed] [Google Scholar]
- Perez Bay AE, Schreiner R, Mazzoni F, Carvajal-Gonzalez JM, Gravotta D, Perret E, Lehmann Mantaras G, Zhu YS, Rodriguez-Boulan EJ 2013. The kinesin KIF16B mediates apical transcytosis of transferrin receptor in AP-1B-deficient epithelia. EMBO J 32: 2125–2139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinal N, Goberdhan DC, Collinson L, Fujita Y, Cox IM, Wilson C, Pichaud F 2006. Regulated and polarized PtdIns(3,4,5)P3 accumulation is essential for apical membrane morphogenesis in photoreceptor epithelial cells. Curr Biol 16: 140–149 [DOI] [PubMed] [Google Scholar]
- Pocha SM, Shevchenko A, Knust E 2011a. Crumbs regulates rhodopsin transport by interacting with and stabilizing myosin V. J Cell Biol 195: 827–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocha SM, Wassmer T, Niehage C, Hoflack B, Knust E 2011b. Retromer controls epithelial cell polarity by trafficking the apical determinant Crumbs. Curr Biol 21: 1111–1117 [DOI] [PubMed] [Google Scholar]
- Ratheesh A, Yap AS 2012. A bigger picture: Classical cadherins and the dynamic actin cytoskeleton. Nat Rev Mol Cell Biol 13: 673–679 [DOI] [PubMed] [Google Scholar]
- Rodriguez-Boulan E, Kreitzer G, Musch A 2005. Organization of vesicular trafficking in epithelia. Nat Rev Mol Cell Biol 6: 233–247 [DOI] [PubMed] [Google Scholar]
- Rodriguez-Fraticelli AE, Galvez-Santisteban M, Martin-Belmonte F 2011. Divide and polarize: Recent advances in the molecular mechanism regulating epithelial tubulogenesis. Curr Opin Cell Biol 23: 638–646 [DOI] [PubMed] [Google Scholar]
- Rodriguez-Fraticelli AE, Auzan M, Alonso MA, Bornens M, Martin-Belmonte F 2012. Cell confinement controls centrosome positioning and lumen initiation during epithelial morphogenesis. J Cell Biol 198: 1011–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeth JF, Sawyer JK, Wilner DA, Peifer M 2009. Rab11 helps maintain apical crumbs and adherens junctions in the Drosophila embryonic ectoderm. PLoS ONE 4: e7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogulja D, Rauskolb C, Irvine KD 2008. Morphogen control of wing growth through the Fat signaling pathway. Dev Cell 15: 309–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas R, Apodaca G 2002. Immunoglobulin transport across polarized epithelial cells. Nat Rev Mol Cell Biol 3: 944–955 [DOI] [PubMed] [Google Scholar]
- Roland JT, Bryant DM, Datta A, Itzen A, Mostov KE, Goldenring JR 2011. Rab GTPase–Myo5B complexes control membrane recycling and epithelial polarization. Proc Natl Acad Sci 108: 2789–2794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozelle AL, Machesky LM, Yamamoto M, Driessens MH, Insall RH, Roth MG, Luby-Phelps K, Marriott G, Hall A, Yin HL 2000. Phosphatidylinositol 4,5-bisphosphate induces actin-based movement of raft-enriched vesicles through WASP-Arp2/3. Curr Biol 10: 311–320 [DOI] [PubMed] [Google Scholar]
- Sagner A, Merkel M, Aigouy B, Gaebel J, Brankatschk M, Julicher F, Eaton S 2012. Establishment of global patterns of planar polarity during growth of the Drosophila wing epithelium. Curr Biol 22: 1296–1301 [DOI] [PubMed] [Google Scholar]
- Schluter MA, Pfarr CS, Pieczynski J, Whiteman EL, Hurd TW, Fan S, Liu CJ, Margolis B 2009. Trafficking of Crumbs3 during cytokinesis is crucial for lumen formation. Mol Biol Cell 20: 4652–4663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonteich E, Wilson GM, Burden J, Hopkins CR, Anderson K, Goldenring JR, Prekeris R 2008. The Rip11/Rab11-FIP5 and kinesin II complex regulates endocytic protein recycling. J Cell Sci 121: 3824–3833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafaq-Zadah M, Brocard L, Solari F, Michaux G 2012. AP-1 is required for the maintenance of apico-basal polarity in the C. elegans intestine. Development 139: 2061–2070 [DOI] [PubMed] [Google Scholar]
- Shaye DD, Casanova J, Llimargas M 2008. Modulation of intracellular trafficking regulates cell intercalation in the Drosophila trachea. Nat Cell Biol 10: 964–970 [DOI] [PubMed] [Google Scholar]
- Shivas JM, Morrison HA, Bilder D, Skop AR 2010. Polarity and endocytosis: Reciprocal regulation. Trends Cell Biol 20: 445–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoes Sde M, Blankenship JT, Weitz O, Farrell DL, Tamada M, Fernandez-Gonzalez R, Zallen JA 2010. Rho-kinase directs Bazooka/Par-3 planar polarity during Drosophila axis elongation. Dev Cell 19: 377–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Johnston D, Ahringer J 2010. Cell polarity in eggs and epithelia: Parallels and diversity. Cell 141: 757–774 [DOI] [PubMed] [Google Scholar]
- St Johnston D, Sanson B 2011. Epithelial polarity and morphogenesis. Curr Opin Cell Biol 23: 540–546 [DOI] [PubMed] [Google Scholar]
- Strutt H, Strutt D 2008. Differential stability of Flamingo protein complexes underlies the establishment of planar polarity. Curr Biol 18: 1555–1564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strutt H, Warrington SJ, Strutt D 2011. Dynamics of core planar polarity protein turnover and stable assembly into discrete membrane subdomains. Dev Cell 20: 511–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strutt H, Searle E, Thomas-Macarthur V, Brookfield R, Strutt D 2013a. A Cul-3-BTB ubiquitylation pathway regulates junctional levels and asymmetry of core planar polarity proteins. Development 140: 1693–1702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strutt H, Thomas-Macarthur V, Strutt D 2013b. Strabismus promotes recruitment and degradation of Farnesylated Prickle in Drosophila melanogaster planar polarity specification. PLoS Genet 9: e1003654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su T, Bryant DM, Luton F, Verges M, Ulrich SM, Hansen KC, Datta A, Eastburn DJ, Burlingame AL, Shokat KM, et al. 2010. A kinase cascade leading to Rab11-FIP5 controls transcytosis of the polymeric immunoglobulin receptor. Nat Cell Biol 12: 1143–1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai CY, Mysore SP, Chiu C, Schuman EM 2007. Activity-regulated N-cadherin endocytosis. Neuron 54: 771–785 [DOI] [PubMed] [Google Scholar]
- Tatin F, Taddei A, Weston A, Fuchs E, Devenport D, Tissir F, Makinen T 2013. Planar cell polarity protein Celsr1 regulates endothelial adherens junctions and directed cell rearrangements during valve morphogenesis. Dev Cell 26: 31–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepass U 1996. Crumbs, a component of the apical membrane, is required for zonula adherens formation in primary epithelia of Drosophila. Dev Biol 177: 217–225 [DOI] [PubMed] [Google Scholar]
- Tepass U, Knust E 1993. Crumbs and Stardust act in a genetic pathway that controls the organization of epithelia in Drosophila melanogaster. Dev Biol 159: 311–326 [DOI] [PubMed] [Google Scholar]
- Tepass U, Theres C, Knust E 1990. crumbs encodes an EGF-like protein expressed on apical membranes of Drosophila epithelial cells and required for organization of epithelia. Cell 61: 787–799 [DOI] [PubMed] [Google Scholar]
- Thompson BJ, Mathieu J, Sun HH, Loeser E, Rørth P, Cohen SM 2005. Tumor suppressor properties of the ESCRT-II complex component Vps25 in Drosophila. Dev Cell 9: 711–720 [DOI] [PubMed] [Google Scholar]
- Thompson A, Nessler R, Wisco D, Anderson E, Winckler B, Sheff D 2007. Recycling endosomes of polarized epithelial cells actively sort apical and basolateral cargos into separate subdomains. Mol Biol Cell 18: 2687–2697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troyanovsky RB, Sokolov EP, Troyanovsky SM 2006. Endocytosis of cadherin from intracellular junctions is the driving force for cadherin adhesive dimer disassembly. Mol Biol Cell 17: 3484–3493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich F, Krieg M, Schotz EM, Link V, Castanon I, Schnabel V, Taubenberger A, Mueller D, Puech PH, Heisenberg CP 2005. Wnt11 functions in gastrulation by controlling cell cohesion through Rab5c and E-cadherin. Dev Cell 9: 555–564 [DOI] [PubMed] [Google Scholar]
- Vaccari T, Bilder D 2005. The Drosophila tumor suppressor vps25 prevents nonautonomous overproliferation by regulating Notch trafficking. Dev Cell 9: 687–698 [DOI] [PubMed] [Google Scholar]
- van IJzendoorn SCD, Tuvim MJ, Weimbs T, Dickey BF, Mostov KE 2002. Direct interaction between Rab3b and the polymeric immunoglobulin receptor controls ligand-stimulated transcytosis in epithelial cells. Dev Cell 2: 219–228 [DOI] [PubMed] [Google Scholar]
- Vicinanza M, D’Angelo G, Di Campli A, De Matteis MA 2008. Function and dysfunction of the PI system in membrane trafficking. EMBO J 27: 2457–2470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrington SJ, Strutt H, Strutt D 2013. The Frizzled-dependent planar polarity pathway locally promotes E-cadherin turnover via recruitment of RhoGEF2. Development 140: 1045–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willenborg C, Jing J, Wu C, Matern H, Schaack J, Burden J, Prekeris R 2011. Interaction between FIP5 and SNX18 regulates epithelial lumen formation. J Cell Biol 195: 71–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter JF, Hopfner S, Korn K, Farnung BO, Bradshaw CR, Marsico G, Volkmer M, Habermann B, Zerial M 2012. Caenorhabditis elegans screen reveals role of PAR-5 in RAB-11-recycling endosome positioning and apicobasal cell polarity. Nat Cell Biol 14: 666–676 [DOI] [PubMed] [Google Scholar]
- Wodarz A, Grawe F, Knust E 1993. CRUMBS is involved in the control of apical protein targeting during Drosophila epithelial development. Mech Dev 44: 175–187 [DOI] [PubMed] [Google Scholar]
- Wu H, Feng W, Chen J, Chan LN, Huang S, Zhang M 2007. PDZ domains of Par-3 as potential phosphoinositide signaling integrators. Mol Cell 28: 886–898 [DOI] [PubMed] [Google Scholar]
- Wu J, Roman AC, Carvajal-Gonzalez JM, Mlodzik M 2013. Wg and Wnt4 provide long-range directional input to planar cell polarity orientation in Drosophila. Nat Cell Biol 15: 1045–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K, Sacharidou A, Fu S, Chong DC, Skaug B, Chen ZJ, Davis GE, Cleaver O 2011. Blood vessel tubulogenesis requires Rasip1 regulation of GTPase signaling. Dev Cell 20: 526–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu A, Rual JF, Tamai K, Harada Y, Vidal M, He X, Kirchhausen T 2007. Association of Dishevelled with the clathrin AP-2 adaptor is required for Frizzled endocytosis and planar cell polarity signaling. Dev Cell 12: 129–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Shewan AM, Brakeman P, Eastburn DJ, Datta A, Bryant DM, Fan QW, Weiss WA, Zegers MM, Mostov KE 2008. Involvement of RhoA, ROCK I and myosin II in inverted orientation of epithelial polarity. EMBO Rep 9: 923–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeigerer A, Gilleron J, Bogorad RL, Marsico G, Nonaka H, Seifert S, Epstein-Barash H, Kuchimanchi S, Peng CG, Ruda VM, et al. 2012. Rab5 is necessary for the biogenesis of the endolysosomal system in vivo. Nature 485: 465–470 [DOI] [PubMed] [Google Scholar]
- Zhang H, Kim A, Abraham N, Khan LA, Hall DH, Fleming JT, Gobel V 2012. Clathrin and AP-1 regulate apical polarity and lumen formation during C. elegans tubulogenesis. Development 139: 2071–2083 [DOI] [PMC free article] [PubMed] [Google Scholar]