Abstract
Objective:
To study the relation between N-terminal pro-brain natriuretic peptide (NT-proBNP) levels, used as a marker of heart failure in clinical practice, blood pressure (BP), and cognitive decline in the oldest old.
Methods:
In 560 participants of the Leiden 85-plus Study, we measured NT-proBNP levels and BP at age 85 years, at baseline, and global cognitive function (Mini-Mental State Examination [MMSE]) annually during the follow-up of 5 years.
Results:
Subjects in the highest tertile of NT-proBNP levels scored 1.7 points lower on the MMSE at age 85 years than subjects in the lowest tertile (p = 0.004), and had a 0.24-point-steeper decline in MMSE score per year (p = 0.021). The longitudinal association disappeared after full adjustment for possible confounders (0.14-point-steeper decline, p = 0.187). Subjects in the category “highest tertile of NT-proBNP and the lowest tertile of systolic BP” had a 3.7-point-lower MMSE score at baseline (p < 0.001) and a 0.49-point-steeper decline in MMSE score per year (p < 0.001) compared with subjects in the other categories.
Conclusions:
In the oldest old, high NT-proBNP levels are associated with lower MMSE scores. The combination of high NT-proBNP levels and low systolic BP is associated with worst global cognitive function and the steepest cognitive decline. Possibly, a failing pump function of the heart results in lower BP and lower brain perfusion with resultant brain dysfunction.
Cardiovascular diseases, such as myocardial infarction, generalized atherosclerosis, and stroke have been associated with increased dementia risk and cognitive decline.1–4 Hypertension, however, is only a risk factor for dementia when present in middle age, whereas most studies show that dementia and cognitive decline in the oldest old associate with lower blood pressures.5 In the oldest old, blood pressures decrease over time,6,7 and stronger declines are related to the presence of dementia and cognitive decline.8,9 One possible explanation for the blood pressure decline in the oldest old might be the increasing prevalence of (subclinical) congestive heart failure.10 Despite this dynamic correlation between blood pressure and cardiac function in relation to cognitive function in old age, previous studies have mainly focused on either the association of blood pressure or cardiac function with cognitive impairment.
Congestive heart failure has been associated with an increased risk of cognitive impairment.11–13 A frequently used serum marker of heart failure is the N-terminal pro-brain natriuretic peptide (NT-proBNP), the inactive fragment of the proBNP hormone.14 NT-proBNP has proven to be a quantitative marker of acute heart failure in clinical practice, with high sensitivity and reasonable specificity,15 although elevated NT-proBNP levels are not specific for heart failure in the general population. Several studies have shown that higher NT-proBNP levels associate with lower cognitive function.16,17 However, the relation among NT-proBNP levels, blood pressure, and cognitive function over time in the oldest old has not been studied until now.
In this study, we examined the associations among NT-proBNP levels, blood pressure, and cognitive function in the Leiden 85-plus Study, a population-based follow-up study in subjects aged 85 years at baseline. We hypothesized that in the oldest old, high NT-proBNP levels and low systolic blood pressures associate with worse cognitive function, and combined high NT-proBNP and low systolic blood pressure puts the oldest old subjects at highest risk of cognitive decline.
METHODS
Participants.
van der Wiel et al.18 described the participants of the Leiden 85-plus Study: “Between September 1, 1997, and September 1, 1999, a total of 705 inhabitants of the community of Leiden, the Netherlands, reached the age of 85 years. Among all of these 85-year-old persons, we initiated a follow up study to investigate determinants of successful aging. Fourteen inhabitants died before they could be enrolled. The response rate was 87%; a total of 599 subjects (397 women and 202 men) participated.” Of the 599 participants in the cohort, 38 refused to provide a blood sample, and NT-proBNP measurement failed in one participant, yielding a total number of 560 participants for this study. Participants were visited within 1 month after their 85th birthday at their home for face-to-face interviews and neuropsychological testing. Each year they were revisited until age 90 years. Information on the missing follow-up data has been published before.19
Standard protocol approvals, registrations, and participant consents.
The Medical Ethical Committee of the Leiden University Medical Centre approved the study, and informed consent was obtained from all participants.
Plasma levels of NT-proBNP.
Nonfasted blood samples were taken at the first visit early in the morning. Blood samples were kept frozen at −80°C. Citrated plasma levels of NT-proBNP were measured in one batch in 2011 using the NT-proBNP assay, developed by Roche Diagnostics (Mannheim, Germany) on a Roche Modular E-170 automated immunoanalyzer.
Blood pressure.
Blood pressure was measured each year, using a mercury sphygmomanometer. During each home visit, 2 blood pressure measurements were taken in seated position, approximately 90 minutes apart, and after at least 5 minutes of rest and no vigorous exercise in the preceding 30 minutes. These were then averaged.
Global cognitive function.
At age 85 years and then annually, global cognitive function was assessed in participants using the Mini-Mental State Examination (MMSE).20
Other covariates.
The level of education of each participant was defined by less than 7 years of schooling for low education, whereas high education was defined by more than 6 years of schooling. The burden of vascular disease at baseline was determined by the number of cardiovascular and cerebrovascular pathologies (myocardial infarction, angina pectoris or myocardial ischemia, claudicatio intermittens, arterial surgery, stroke, and TIA). Information on the presence of cardiovascular and cerebrovascular pathologies was provided by general practitioners or treating physicians. Information on use of antihypertensive medication was obtained from pharmacist records, or from questionnaires, filled out by the treating physician for institutionalized participants. Participants were classified as having diabetes according to previously described criteria.21 Information on smoking was obtained from questionnaires. Body mass index was calculated by dividing the weight in kilograms by the square of length in meters (kg/m2). Creatine clearance was estimated with the Cockroft-Gault formula. Prevalence of atrial fibrillation was determined by automated Minnesota Coding (Minnesota Code 8-3-1) of ECGs, which were recorded on a Siemens Sicard 440 (Erlangen, Germany), and were transmitted to the ECG Core Laboratory in Glasgow Royal Infirmary.
Statistical analyses.
Characteristics of the study participants are reported as means (SDs) for continuous variables and numbers (percentages) for categorical variables in tertiles of NT-proBNP levels.
The associations of both NT-proBNP levels and systolic blood pressure with MMSE score at baseline and during follow-up were analyzed using linear mixed models. Linear mixed models use all available data during follow-up and account for repeated measurements, thereby handling missing data more appropriately than traditional models.22 In the primary analyses, categorical NT-proBNP levels and systolic blood pressure were the independent variable, whereby NT-proBNP levels and systolic blood pressure were divided in tertiles. For continuous analyses, log-transformed NT-proBNP levels were used. In a multivariate model, all analyses were corrected for level of education, use of antihypertensives, smoking status, prevalence of diabetes, body mass index, renal function, prevalence of atrial fibrillation, and finally, the number of cardiovascular and cerebrovascular pathologies at baseline. In a final analysis, a new variable consisting of 4 categories, using combinations of the lowest and highest tertile of NT-proBNP levels and the lowest and highest tertile of systolic blood pressure, was used as an independent variable with MMSE scores as the outcome variable. All calculations were performed using SPSS software (version 20.0.1; SPSS Inc., Chicago, IL).
RESULTS
Baseline analyses.
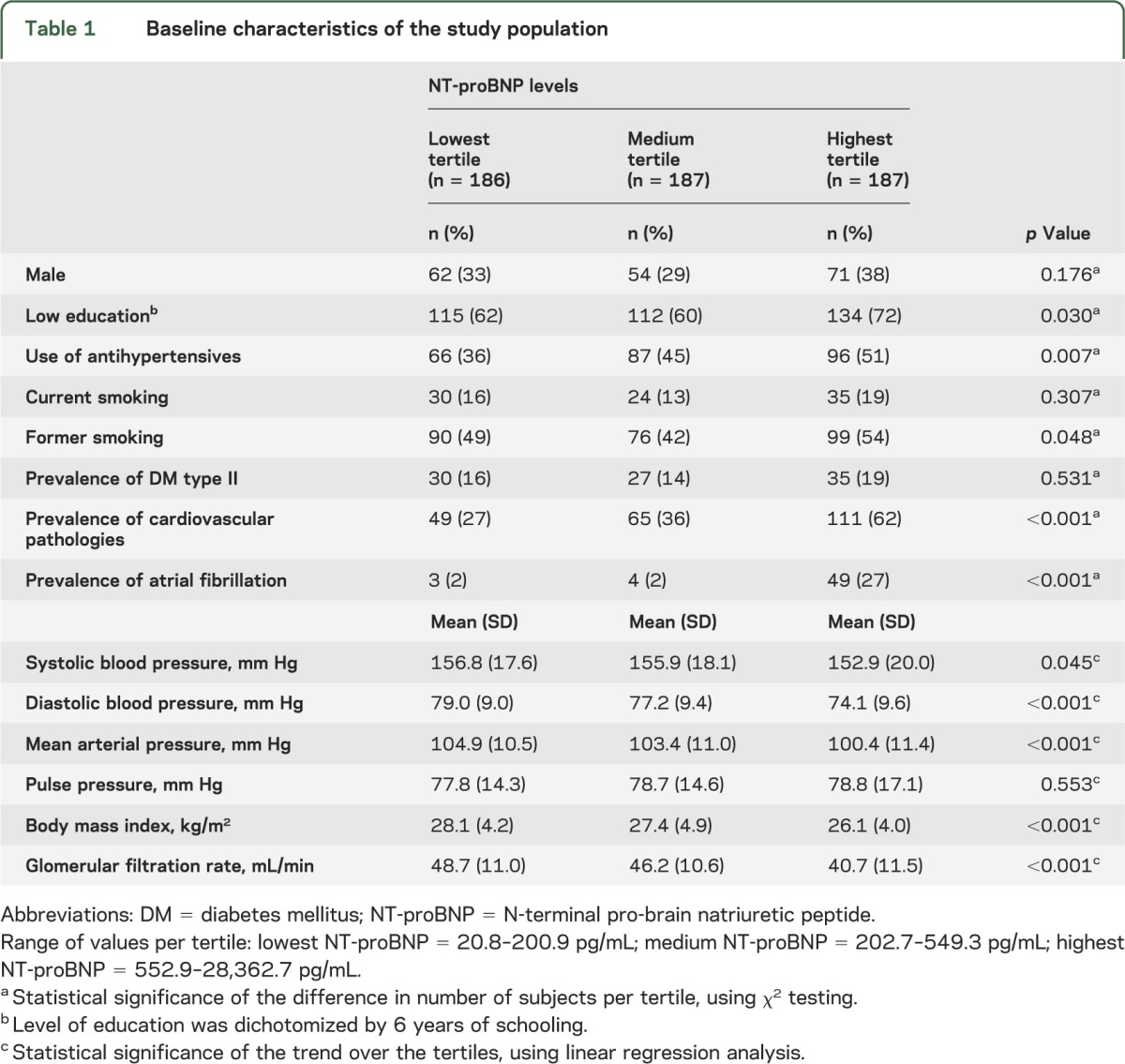
Table 1 shows baseline characteristics of all 560 study participants in tertiles of NT-proBNP levels. Compared with subjects in the lowest tertile, subjects in the highest tertile of NT-proBNP had a lower educational level, lower systolic and diastolic blood pressures, lower body mass index, and lower renal function. Furthermore, they were more likely to use antihypertensives, to have a history of smoking, and they had a higher prevalence of cardiovascular pathologies and atrial fibrillation.
Table 1.
Baseline characteristics of the study population
NT-proBNP levels, systolic blood pressure, and MMSE scores from age 85 to 90 years.
Figure 1A graphically shows the association between NT-proBNP levels and MMSE scores for participants aged 85 to 90 years. At age 85 years, subjects in the highest tertile of NT-proBNP had a 1.7-point-lower MMSE score than subjects in the lowest tertile (p = 0.004). Moreover, during the 5-year follow-up, subjects in the highest tertile of NT-proBNP had a 0.24-point-steeper decline in MMSE score per year compared with subjects in the lowest tertile (p = 0.021). Table 2 shows that after additional adjustment for smoking, prevalence of diabetes mellitus and atrial fibrillation, use of antihypertensives, body mass index, and renal function, subjects in the highest tertile of NT-proBNP still had a 1.7-point-lower MMSE score at baseline than subjects in the lowest tertile, but not a significant steeper decline in MMSE score per year (0.16-point-steeper decline). Further adjustment for the number of cardiovascular pathologies at baseline slightly decreased the baseline association between NT-proBNP levels and MMSE score. There was also an association between systolic blood pressure and MMSE score (figure 1B). Subjects in the lowest tertile of systolic blood pressure had a 2.8-point-lower MMSE score at age 85 years than subjects in the highest tertile (p < 0.001), and they also had a 0.39-point-steeper decline in MMSE score per year (p < 0.001). Table 2 shows that additional adjustment in the fully adjusted model did not materially change the associations.
Figure 1. MMSE score from age 85 to 90 years dependent on tertiles of NT-proBNP and tertiles of systolic blood pressure.
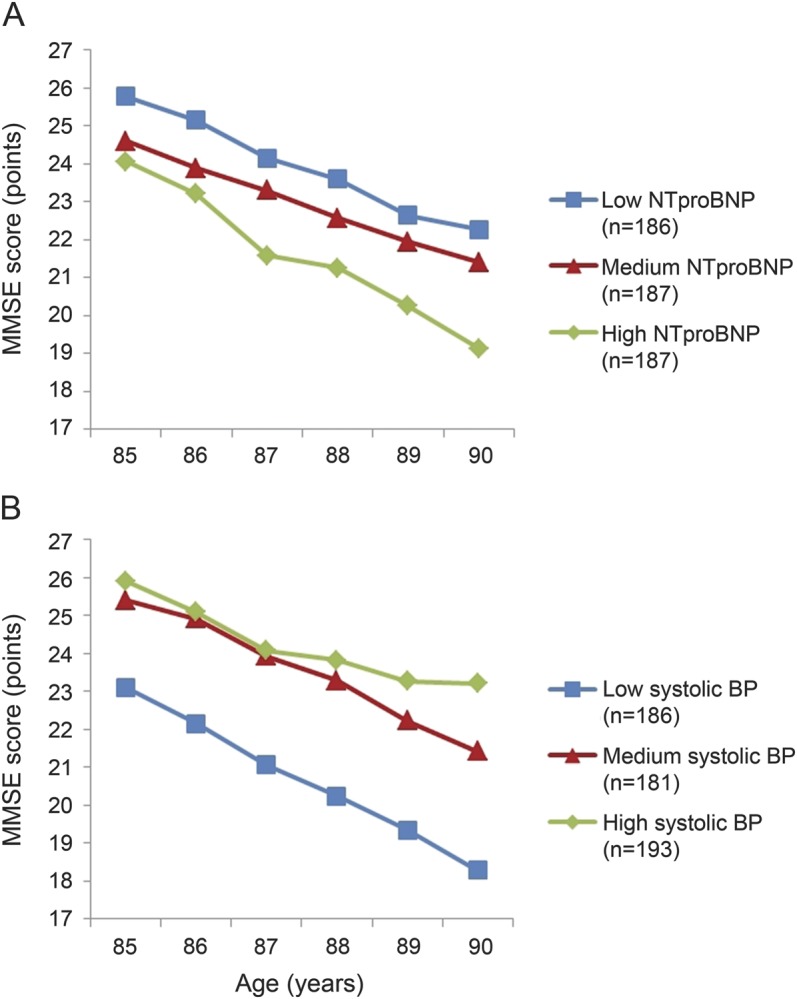
NT-proBNP (A) and systolic blood pressure (B) were divided in equal tertiles. Data points represent estimates, calculated using linear mixed models, with adjustment for sex and level of education. Range of values per tertile: low NT-proBNP = 20.8–200.9 pg/mL; medium NT-proBNP = 202.7–549.3 pg/mL; high NT-proBNP = 552.9–28,362.7 pg/mL; low systolic blood pressure = 110.0–146.5 mm Hg; medium systolic blood pressure = 147.0–161.5 mm Hg; high systolic blood pressure = 162.0–215.0 mm Hg. BP = blood pressure; MMSE = Mini-Mental State Examination; NT-proBNP = N-terminal pro-brain natriuretic peptide.
Table 2.
MMSE score at age 85 years and change in MMSE score from age 85 to 90 years dependent on tertiles of NT-proBNP and tertiles of systolic blood pressure
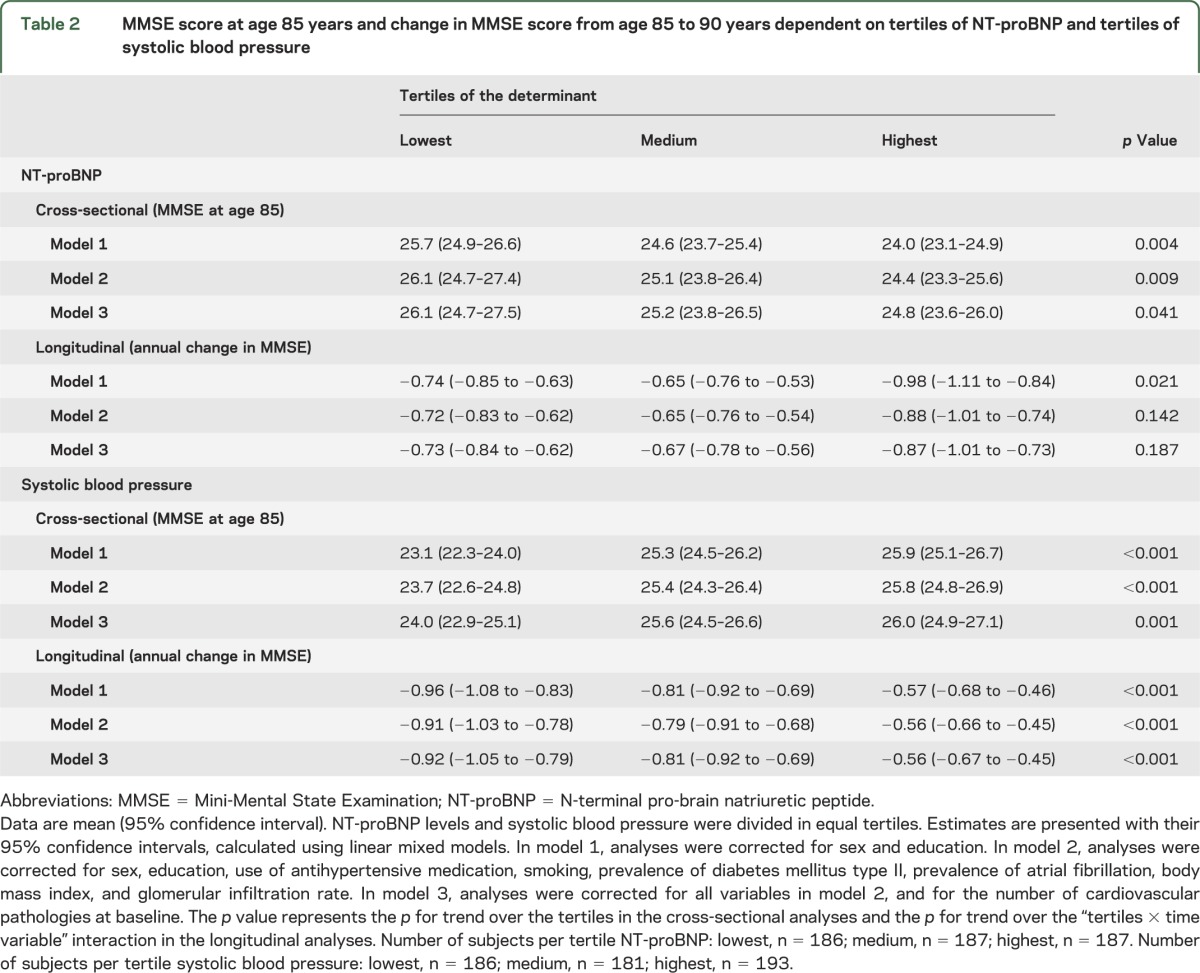
When analyzing these associations with NT-proBNP levels and systolic blood pressure as continuous variables in the model, similar associations were found. In analyses adjusted for sex and level of education, higher NT-proBNP levels were associated with both lower MMSE scores at baseline (−0.57 points MMSE per 1 increase in ln NT-proBNP, p = 0.007) and with steeper annual declines in MMSE score from age 85 to 90 years (−0.084 points MMSE per year per 1 increase in ln NT-proBNP, p = 0.012). However, additional adjustment in a fully adjusted model showed a diminished effect size and no significant association (baseline: −0.36 points, p = 0.131; longitudinal: −0.056 points/y, p = 0.092). Higher systolic blood pressures associated strongly with higher MMSE scores at baseline and with lower annual declines in MMSE score, also in fully adjusted models (all p < 0.001).
NT-proBNP levels and systolic blood pressures combined.
Finally, to test for the combined effect of NT-proBNP levels and systolic blood pressure on MMSE scores, a new variable was created, including combinations of the lowest and highest tertiles of NT-proBNP levels and systolic blood pressure. Table 3 shows the baseline characteristics of the subjects in the 4 different categories. Figure 2 shows that subjects in the category “high NT-proBNP and low systolic blood pressure” had the lowest MMSE score at baseline and had the steepest decline in MMSE score from age 85 to 90 years. Compared with the other 3 categories, subjects in the category “high NT-proBNP and low systolic blood pressure” had a 3.7-point-lower MMSE score at age 85 years (95% confidence interval 2.1–5.3, p < 0.001), and a 0.49-point-steeper decline in MMSE score per year from age 85 to 90 years (95% confidence interval 0.24–0.75, p < 0.001). In the fully adjusted model, these associations remained, although attenuated (baseline: −3.0 points, p = 0.001; longitudinal: −0.32 points/y, p = 0.014) (table e-1 on the Neurology® Web site at Neurology.org). When restricting the analyses to a subset of subjects who did not use antihypertensives at baseline, similar associations were found (table e-2). When using diastolic blood pressure, mean arterial pressure, and pulse pressure in the models, similar associations were found at baseline (all p ≤ 0.001) and during follow-up for pulse pressure (p < 0.001), but not for diastolic blood pressure (p = 0.580) and mean arterial pressure (p = 0.089) (figure e-1). Formal testing for interaction between NT-proBNP levels and systolic blood pressure in relation to MMSE score, both in cross-sectional and longitudinal analyses, showed no interaction in fully adjusted models (all p > 0.100).
Table 3.
Characteristics of the study population in categories of tertiles of NT-proBNP and tertiles of systolic blood pressure
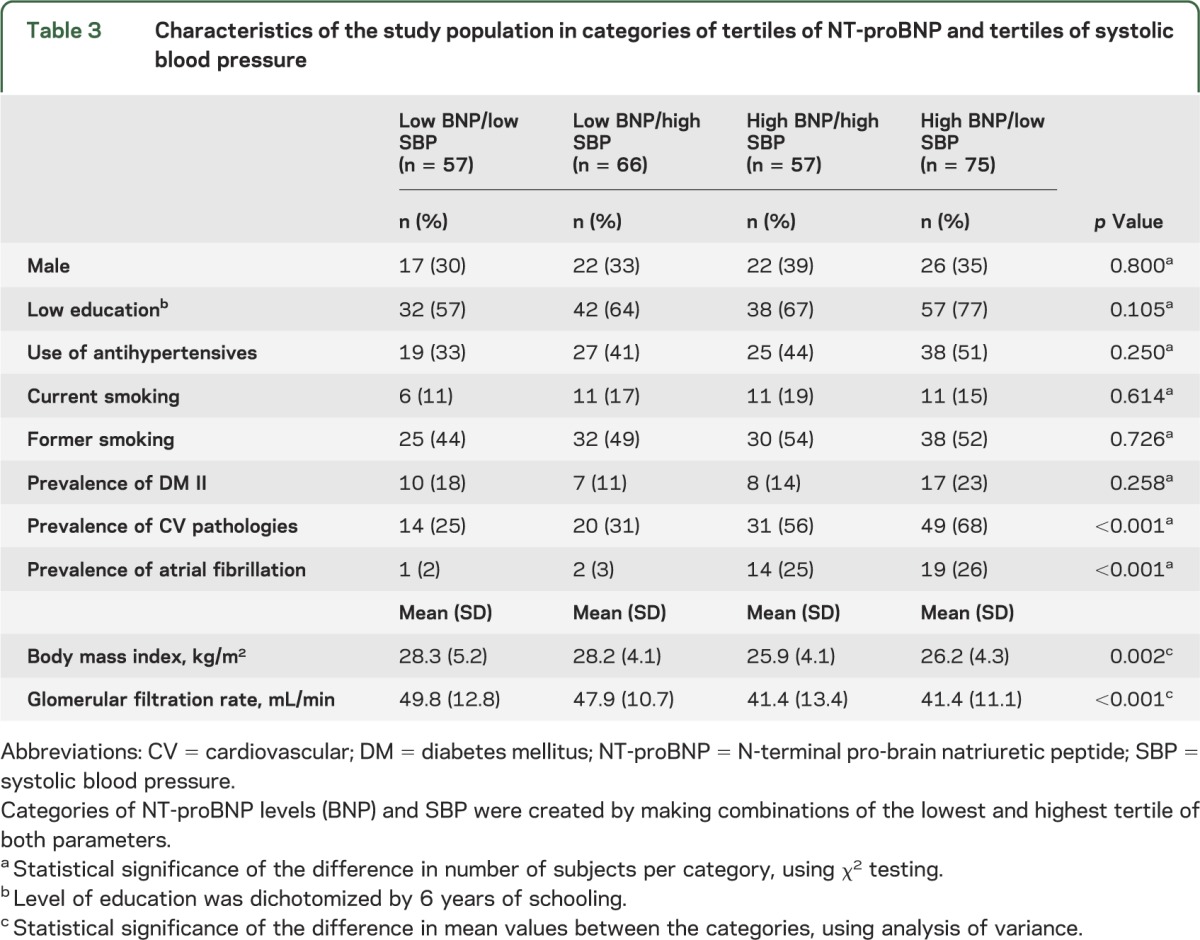
Figure 2. MMSE score from age 85 to 90 years dependent on categories of tertiles of NT-proBNP and tertiles of systolic blood pressure.
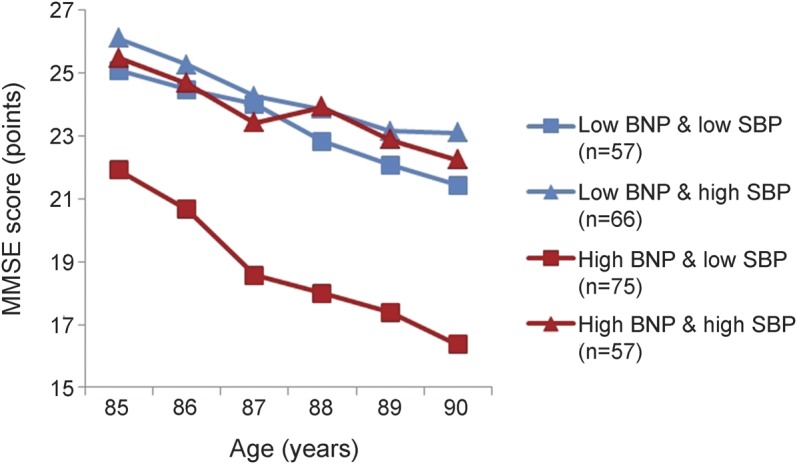
Categories of NT-proBNP and systolic blood pressure were created by making tertiles, and combining the highest and the lowest tertile of each parameter. Data points represent estimates, calculated using linear mixed models, with adjustment for sex and level of education. MMSE = Mini-Mental State Examination; NT-proBNP = N-terminal pro-brain natriuretic peptide; SBP = systolic blood pressure.
DISCUSSION
The main finding of our study is that higher NT-proBNP levels are associated with worse global cognitive function at baseline in the oldest old. Moreover, lower systolic blood pressures were associated with worse global cognitive function at baseline and with steeper cognitive decline during follow-up. When combining NT-proBNP levels and systolic blood pressures in a new variable, subjects with both the highest NT-proBNP levels and lowest systolic blood pressures had the worst global cognitive function and the steepest cognitive decline compared with all other subjects.
Our finding of higher NT-proBNP levels associating with worse cognitive function is in accordance with previous studies. Both in the Rancho Bernardo Study and a recently published study of elderly patients with type 2 diabetes, subjects with high NT-proBNP levels had worse cognitive function compared to subjects with low NT-proBNP levels.16,17 However, these studies reported only on cross-sectional associations. In the Hoorn Study, higher NT-proBNP levels at baseline were associated with worse cognitive function measured after 5 to 8 years.23 There was no information on cognitive function at baseline, and therefore conclusions on cognitive decline could not be drawn from these results. In the only study reporting on longitudinal associations, high NT-proBNP levels were associated with both steeper declines in MMSE score, measured at baseline and after 5 years of follow-up, and with an increased risk of dementia.24 Information on blood pressure was not reported.
NT-proBNP has emerged as an important biomarker of cardiac function and is frequently used in clinical practice for the evaluation of congestive heart failure.25 It is the biologically inactive N-terminal fragment of the prohormone proBNP,26 which is secreted predominantly by cardiac myocytes of the ventricles in response to stretching and distension of the cardiac wall and has a natriuretic and diuretic effect.27,28 This might explain the observed association between high NT-proBNP levels and lower blood pressures in our study. However, this contradicts with results from previous studies, in which high NT-proBNP levels were associated with higher blood pressures, albeit in younger populations.29,30 An explanation for these opposing results might be that initially, as a consequence of hypertension, higher filling pressures of the ventricles result in an increased release of NT-proBNP. However, with longer-standing hypertension, ventricular enlargement and dysfunction may develop, leading to heart failure with lower cardiac output, subsequently resulting in lower blood pressures.
The use of NT-proBNP levels in the elderly for the evaluation of cardiac disease is under current debate, because cutoff values in prediction models show an age-dependent effect. In our study, subjects in the highest tertile of NT-proBNP had levels >550 pg/mL. When comparing these values to suggested cutoff values from studies on the use of NT-proBNP levels for cardiovascular risk prediction in elderly subjects, they are in the high range. In a study in subjects with a mean age of 77 years, a cutoff value of 450 pg/mL was used in the prediction of cardiovascular death.31 Another study calculated ideal cutoff points in the prediction of cardiac dysfunction in subjects aged 80 years and older in different strata of identified confounders.32 Generally, a cutoff value of 400 pg/mL was found. This however does not necessarily mean that all subjects in the highest tertile have a severe cardiovascular status.
Because low NT-proBNP levels reliably exclude heart failure and high NT-proBNP levels are related to heart failure, our results may suggest that heart failure associates with worse cognitive function and cognitive decline in the oldest old. The relation between cognitive function and heart failure, irrespective of NT-proBNP levels, has been reviewed before.33,34 The authors conclude that there is a strong cross-sectional association between heart failure and cognitive impairment, but that very few longitudinal data are available. Possible pathophysiologic mechanisms explaining the association between heart failure and cognitive impairment may be found in direct and indirect pathways. An indirect pathway may be the presence of common risk factors that associate with both heart failure and cognitive impairment. Hypertension at midlife for instance is a risk factor for late-life cognitive impairment,5 but is also a well-known risk factor for heart failure up to old age.35 Similar common risk factors are a history of smoking and prevalence of atherosclerotic disease.36–38 Specifically, the prevalence of atherosclerotic disease may explain the found associations for a substantial part, because correction for this variable in the multivariate model diminished the cross-sectional and longitudinal association between NT-proBNP levels and MMSE scores. However, these indirect pathways may not explain all of the observed associations between NT-proBNP levels and cognitive function in our study. Another likely pathophysiologic explanation might be found in the direct effect of heart failure on cerebral perfusion. Heart failure is characterized by a decreased cardiac pump function, with resultant lower cardiac output. This eventually may result in cerebral hypoperfusion with concomitant decreased cerebral function. In an older population of patients with heart failure, ejection fractions below 30% were indeed associated with worse memory function.13 In further support of this possible pathophysiologic mechanism, we showed in a recently performed meta-analysis that both Alzheimer disease and vascular dementia are associated with decreased cerebral blood flow velocities.39
The worst global cognitive function and strongest cognitive decline were observed in subjects who had both high NT-proBNP levels and lower systolic blood pressures, as opposed to all other subjects. This suggests that specifically the combination of the 2 is detrimental. A possible explanation for this finding might be that high NT-proBNP levels reflect a different pathomechanism in subjects with lower than in subjects with higher blood pressures. In subjects with lower blood pressures, high NT-proBNP levels may be the result of longer existing heart failure, which results in cardiac dysfunction, lower cardiac output, and lower blood pressures. This then eventually may result in cerebral hypoperfusion. In subjects with higher blood pressure, high NT-proBNP levels may be the result of cardiac stress caused by hypertension, which does not necessarily have a direct negative impact on cerebral function. This reasoning corresponds with previous findings, in which lower diastolic blood pressure in old age was shown to associate with increased mortality risk.40 In the Leiden 85-plus Study, we previously showed that in the oldest old, declines in blood pressure during follow-up associate with increased mortality risk.6,41
A limitation of our study is that information on cardiac output and cerebral perfusion is not available. Echocardiography was only performed in a convenience sample of 90 year olds, and unfortunately not at baseline. With additional information of these 2 parameters, the possible pathophysiologic mechanisms could have been further elucidated. Moreover, the effect of unmeasured confounding, such as unmeasured cardiovascular risk factors, cannot be ruled out. Given that information on blood pressure measurements before age 85 years was not available, it was not possible to evaluate the influence of blood pressure trajectories on our results. A final limitation is that the MMSE only gives a broad estimate of cognitive function and, although used often in clinical practice for the assessment of global cognitive function, does not cover all cognitive domains.
Supplementary Material
GLOSSARY
- BP
blood pressure
- MMSE
Mini-Mental State Examination
- NT-proBNP
N-terminal pro-brain natriuretic peptide
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
P.v.V. and B.S.: study design, statistical analysis, drafting the manuscript. L.W.W.: drafting the manuscript, critical review and revision of the manuscript. R.K.E.P. and S.P.M.: critical review and revision of the manuscript. W.d.R.: data acquisition, critical review and revision of the manuscript. J.G.: critical review and revision of the manuscript. A.J.M.d.C. and R.G.J.W.: study design, critical review and revision of the manuscript.
STUDY FUNDING
This study was funded in part by an unrestricted grant from the Dutch Ministry of Health, Welfare and Sports.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Aronson MK, Ooi WL, Morgenstern H, et al. Women, myocardial infarction, and dementia in the very old. Neurology 1990;40:1102–1106 [DOI] [PubMed] [Google Scholar]
- 2.Haan MN, Shemanski L, Jagust WJ, Manolio TA, Kuller L. The role of APOE epsilon4 in modulating effects of other risk factors for cognitive decline in elderly persons. JAMA 1999;282:40–46 [DOI] [PubMed] [Google Scholar]
- 3.Vinkers DJ, Stek ML, van der Mast RC, et al. Generalized atherosclerosis, cognitive decline, and depressive symptoms in old age. Neurology 2005;65:107–112 [DOI] [PubMed] [Google Scholar]
- 4.De Ronchi D, Palmer K, Pioggiosi P, et al. The combined effect of age, education, and stroke on dementia and cognitive impairment no dementia in the elderly. Dement Geriatr Cogn Disord 2007;24:266–273 [DOI] [PubMed] [Google Scholar]
- 5.Qiu C, Winblad B, Fratiglioni L. The age-dependent relation of blood pressure to cognitive function and dementia. Lancet Neurol 2005;4:487–499 [DOI] [PubMed] [Google Scholar]
- 6.van Vliet P, Oleksik AM, van Heemst D, de Craen AJ, Westendorp RG. Dynamics of traditional metabolic risk factors associate with specific causes of death in old age. J Gerontol A Biol Sci Med Sci 2010;65:488–494 [DOI] [PubMed] [Google Scholar]
- 7.Lernfelt B, Svanborg A. Change in blood pressure in the age interval 70–90: late blood pressure peak related to longer survival. Blood Press 2002;11:206–212 [DOI] [PubMed] [Google Scholar]
- 8.Qiu C, von Strauss E, Winblad B, Fratiglioni L. Decline in blood pressure over time and risk of dementia: a longitudinal study from the Kungsholmen Project. Stroke 2004;35:1810–1815 [DOI] [PubMed] [Google Scholar]
- 9.van Vliet P, Westendorp RG, van Heemst D, de Craen AJ, Oleksik AM. Cognitive decline precedes late-life longitudinal changes in vascular risk factors. J Neurol Neurosurg Psychiatry 2010;81:1028–1032 [DOI] [PubMed] [Google Scholar]
- 10.van Bemmel T, Holman ER, Gussekloo J, Blauw GJ, Bax JJ, Westendorp RG. Low blood pressure in the very old, a consequence of imminent heart failure: the Leiden 85-plus Study. J Hum Hypertens 2009;23:27–32 [DOI] [PubMed] [Google Scholar]
- 11.Zuccala G, Onder G, Pedone C, et al. Hypotension and cognitive impairment: selective association in patients with heart failure. Neurology 2001;57:1986–1992 [DOI] [PubMed] [Google Scholar]
- 12.Trojano L, Antonelli Incalzi R, Acanfora D, Picone C, Mecocci P, Rengo F. Cognitive impairment: a key feature of congestive heart failure in the elderly. J Neurol 2003;250:1456–1463 [DOI] [PubMed] [Google Scholar]
- 13.Festa JR, Jia X, Cheung K, et al. Association of low ejection fraction with impaired verbal memory in older patients with heart failure. Arch Neurol 2011;68:1021–1026 [DOI] [PubMed] [Google Scholar]
- 14.Palazzuoli A, Gallotta M, Quatrini I, Nuti R. Natriuretic peptides (BNP and NT-proBNP): measurement and relevance in heart failure. Vasc Health Risk Manag 2010;6:411–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Richards AM, Troughton RW. Use of natriuretic peptides to guide and monitor heart failure therapy. Clin Chem 2012;58:62–71 [DOI] [PubMed] [Google Scholar]
- 16.Daniels LB, Laughlin GA, Kritz-Silverstein D, et al. Elevated natriuretic peptide levels and cognitive function in community-dwelling older adults. Am J Med 2011;124:670–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feinkohl I, Sattar N, Welsh P, et al. Association of N-terminal pro-brain natriuretic peptide with cognitive function and depression in elderly people with type 2 diabetes. PLoS ONE 2012;7:e44569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van der Wiel AB, van Exel E, de Craen AJ, et al. A high response is not essential to prevent selection bias: results from the Leiden 85-plus Study. J Clin Epidemiol 2002;55:1119–1125 [DOI] [PubMed] [Google Scholar]
- 19.Mooijaart SP, van Vliet P, van Heemst D, et al. Plasma levels of apolipoprotein E and cognitive function in old age. Ann NY Acad Sci 2007;1100:148–161 [DOI] [PubMed] [Google Scholar]
- 20.Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198 [DOI] [PubMed] [Google Scholar]
- 21.van den Berg E, de Craen AJ, Biessels GJ, Gussekloo J, Westendorp RG. The impact of diabetes mellitus on cognitive decline in the oldest of the old: a prospective population-based study. Diabetologia 2006;49:2015–2023 [DOI] [PubMed] [Google Scholar]
- 22.Gueorguieva R, Krystal JH. Move over ANOVA: progress in analyzing repeated-measures data and its reflection in papers published in the Archives of General Psychiatry. Arch Gen Psychiatry 2004;61:310–317 [DOI] [PubMed] [Google Scholar]
- 23.van den Hurk K, Reijmer YD, van den Berg E, et al. Heart failure and cognitive function in the general population: the Hoorn Study. Eur J Heart Fail 2011;13:1362–1369 [DOI] [PubMed] [Google Scholar]
- 24.Kerola T, Nieminen T, Hartikainen S, Sulkava R, Vuolteenaho O, Kettunen R. B-type natriuretic peptide as a predictor of declining cognitive function and dementia: a cohort study of an elderly general population with a 5-year follow-up. Ann Med 2010;42:207–215 [DOI] [PubMed] [Google Scholar]
- 25.Jessup M, Abraham WT, Casey DE, et al. 2009 focused update: ACCF/AHA Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation 2009;119:1977–2016 [DOI] [PubMed] [Google Scholar]
- 26.Levin ER, Gardner DG, Samson WK. Natriuretic peptides. N Engl J Med 1998;339:321–328 [DOI] [PubMed] [Google Scholar]
- 27.Magga J, Marttila M, Mantymaa P, Vuolteenaho O, Ruskoaho H. Brain natriuretic peptide in plasma, atria, and ventricles of vasopressin- and phenylephrine-infused conscious rats. Endocrinology 1994;134:2505–2515 [DOI] [PubMed] [Google Scholar]
- 28.Cheung BM, Kumana CR. Natriuretic peptides: relevance in cardiovascular disease. JAMA 1998;280:1983–1984 [DOI] [PubMed] [Google Scholar]
- 29.Olsen MH, Hansen TW, Christensen MK, et al. N-terminal pro brain natriuretic peptide is inversely related to metabolic cardiovascular risk factors and the metabolic syndrome. Hypertension 2005;46:660–666 [DOI] [PubMed] [Google Scholar]
- 30.Mayer O, Jr, Simon J, Plaskova M, Cifkova R, Trefil L. N-terminal pro B-type natriuretic peptide as prognostic marker for mortality in coronary patients without clinically manifest heart failure. Eur J Epidemiol 2009;24:363–368 [DOI] [PubMed] [Google Scholar]
- 31.Daniels LB, Laughlin GA, Clopton P, Maisel AS, Barrett-Connor E. Minimally elevated cardiac troponin T and elevated N-terminal pro-B-type natriuretic peptide predict mortality in older adults: results from the Rancho Bernardo Study. J Am Coll Cardiol 2008;52:450–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vaes B, Gruson D, Van Pottelbergh G, et al. The impact of confounders on the test performance of natriuretic peptides for cardiac dysfunction in subjects aged 80 and older. Peptides 2012;38:118–126 [DOI] [PubMed] [Google Scholar]
- 33.Vogels RL, Scheltens P, Schroeder-Tanka JM, Weinstein HC. Cognitive impairment in heart failure: a systematic review of the literature. Eur J Heart Fail 2007;9:440–449 [DOI] [PubMed] [Google Scholar]
- 34.Pressler SJ. Cognitive functioning and chronic heart failure: a review of the literature (2002–July 2007). J Cardiovasc Nurs 2008;23:239–249 [DOI] [PubMed] [Google Scholar]
- 35.Butler J, Kalogeropoulos A, Georgiopoulou V, et al. Incident heart failure prediction in the elderly: the Health ABC Heart Failure Score. Circ Heart Fail 2008;1:125–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peters R, Poulter R, Warner J, Beckett N, Burch L, Bulpitt C. Smoking, dementia and cognitive decline in the elderly, a systematic review. BMC Geriatr 2008;8:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leening MJ, Elias-Smale SE, Kavousi M, et al. Coronary calcification and the risk of heart failure in the elderly: the Rotterdam Study. JACC Cardiovasc Imaging 2012;5:874–880 [DOI] [PubMed] [Google Scholar]
- 38.Breteler MM, Claus JJ, Grobbee DE, Hofman A. Cardiovascular disease and distribution of cognitive function in elderly people: the Rotterdam Study. BMJ 1994;308:1604–1608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sabayan B, Jansen S, Oleksik AM, et al. Cerebrovascular hemodynamics in Alzheimer's disease and vascular dementia: a meta-analysis of transcranial Doppler studies. Ageing Res Rev 2012l;11:271–277 [DOI] [PubMed] [Google Scholar]
- 40.Kagiyama S, Takata Y, Ansai T, et al. Does decreased diastolic blood pressure associate with increased mortality in 80-year-old Japanese? Clin Exp Hypertens 2009;31:639–647 [DOI] [PubMed] [Google Scholar]
- 41.Poortvliet RK, de Ruijter W, de Craen AJ, et al. Blood pressure trends and mortality: the Leiden 85-plus Study. J Hypertens 2013;31:63–70 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


