Abstract
Background: Micronutrient deficiencies and in utero exposure to HIV may impair infant neurodevelopment.
Objective: To evaluate the effect of daily multivitamin supplementation on the cognitive, language and motor development of HIV-exposed Tanzanian infants.
Methods: A total of 2387 infants were randomized to receive daily oral supplementation of multivitamins (B-complex, C and E) or placebo from age 6 weeks for 24 months. The cognitive, language and motor scales of the Bayley Scales of Infant and Toddler Development, third edition, were administered to a subset of 206 infants at age 15 months.
Results: Multivitamin supplementation did not improve measures of cognitive development, expressive or receptive language or gross motor capabilities. There was a trend toward improved fine motor skills among infants randomized to the multivitamin group (difference in mean score = 0.38; 95% CI = −0.01, 0.78, p = 0.06).
Conclusion: Daily provision of multivitamins to HIV-exposed infants does not substantially improve developmental outcomes at age 15 months.
Keywords: Multivitamins, infant development, HIV
Introduction
Every year, more than 200 million children living in developing countries fail to reach their developmental potential [1]. The first 2 years of life are particularly crucial for healthy brain development, and a multitude of factors can heighten the risk of developmental delay during this critical period. Micronutrient deficiencies are among the risk factors, and yet are completely preventable. Many vitamins and minerals play an important role in infant development, and studies have shown deficiencies in early life can have long-lasting adverse effects on motor, mental and socio-emotional development [2–5].
While micronutrient deficiencies are widespread in many developing countries, their effects on infant development may be amplified in the context of HIV/AIDS. Children born to HIV-infected women represent a particularly vulnerable subgroup for several reasons. Households affected by HIV/AIDS are often food insecure, which may limit the quality and quantity of food the infant receives and increase the risk of micronutrient deficiencies [6]. Conditions of poverty and socioeconomic stress often require both parents to work outside the home and may prevent optimal caregiving and feeding practices. Higher rates of infectious disease-related morbidity may also lower appetite and interfere with nutrient absorption [7]. Furthermore, if the infant is infected with HIV, the virus can invade the central nervous system and destroy neuronal tissue, directly impairing brain development [8].
Interventions to improve micronutrient status in the context of HIV/AIDS may be an effective way of improving child development in sub-Saharan Africa. Although several trials have demonstrated the benefits of multiple micronutrient supplementation or fortification on developmental outcomes in HIV-negative populations [9–13], little research has explored their effects among HIV-exposed infants. We previously reported a reduced risk of delayed motor development among infants born to HIV-infected women who received multivitamins during pregnancy and lactation [14]. However, the effects of providing multivitamin supplements directly to HIV-exposed infants have not yet been evaluated. We therefore conducted a randomized placebo-controlled trial to examine the effect of daily multivitamin supplementation (B vitamins, vitamin C and vitamin E) on the cognitive, language and motor development of HIV-exposed infants in Dar es Salaam, Tanzania.
Materials and Methods
Subjects in this study were part of a randomized, double-blind, placebo-controlled trial designed to examine whether daily administration of multivitamins to infants born to HIV-infected women from 6 weeks of age for 24 months reduced the risk of mortality and infectious disease morbidity, compared with placebo [15]. A secondary endpoint of the study was to evaluate the effect of the supplement on infant neurodevelopment. Women 18 years of age and older who tested HIV-positive at the 32nd week of gestation or earlier in one of eight antenatal clinics in Dar es Salaam, Tanzania, were invited to participate. Written informed consent for participation was obtained from all women while still pregnant. Eligibility for infant participation in the trial included the following: (i) singleton birth and (ii) 5-7 weeks of age at randomization. Infants with serious congenital anomalies or other conditions that would have interfered with study procedures were excluded. Infants were then randomly assigned to receive a daily oral dose of multivitamins or placebo from enrollment at 6 weeks of age for 24 months. A study biostatistician in Boston prepared a randomization list from 1 to 2400 with the use of permuted blocks of size 20. The list was provided to the pharmacy department in Dar es Salaam, with each number corresponding to a code denoting one of the two treatment arms. On-site study pharmacists stored the coded randomization list in a locked file cabinet and concealed allocation by covering the numeric regimen code on each blister pack with a sticker. Infants enrolled at the study clinic were provided with the next consecutive number in series. Study physicians, research nurses and participants were unaware of the treatment groups.
From age 6 weeks–6 months, infants in the multivitamin arm received one capsule containing 60 mg of vitamin C, 8 mg of vitamin E, 0.5 mg of thiamine, 0.6 mg of riboflavin, 4 mg of niacin, 0.6 mg of vitamin B6, 130 µg of folate and 1 mg of vitamin B12. From 7 months of age to the end of follow-up, two capsules were given daily. This mix of vitamins was chosen based on our earlier results [16], and all mothers were provided with oral multivitamins from enrollment until the end of follow-up. Maternal multivitamin doses were generally several times the recommended daily allowance (RDA) for B vitamins, vitamin C and vitamin E; women who were initiated on ARV therapy were changed to single recommended daily allowance multivitamin doses.
Mothers and children were asked to return to the study clinics for monthly follow-up visits. As part of standard medical care, all children received growth monitoring, immunizations, routine medical care for illnesses and large doses of vitamin A at 9, 15 and 21 months of age. Length-for-age, weight-for-length and weight-for-age Z-scores were calculated using the 2006 WHO Child Growth Standards. Stunting, wasting and underweight were defined as binary outcomes based on a Z-score <−2. Mothers were counseled on the risks and benefits of exclusive breastfeeding. Nevirapine prophylaxis for mother-to-child transmission of HIV was provided to mothers at the onset of labor and to the infant within 72 h of birth. Part way through the trial, the availability of antiretroviral medication increased, and mothers and children in the study were screened for eligibility according to the guidelines by Tanzania’s Ministry of Health. All children were tested for HIV infection at age 6 weeks using Amplicor HIV-1 DNA PCR assay version 1.5 (Roche Molecular Systems, Branchburg, NJ, USA) and at 18 months using ELISAs, followed by Enzygnost anti-HIV-1/2 Plus (Dade Behring). If a child tested positive at 18 months of age, stored blood samples were back-tested to better estimate the time of transmission.
The cognitive, language and motor scales of the Bayley Scales of Infant and Toddler Development, -third edition (BSID-III), were used to assess the developmental functioning of a subset of 206 children at 15 months of age (range = 14–17 months). Owing to personnel and logistic constraints, it was only possible to include a sample of children attending one of the three research clinics in this subgroup. We chose to assess the children at 15 months, as most of the early developmental milestones should have been passed by this age. Furthermore, the risk of confounding by intercurrent illness and home environment is likely to increase as the time between nutritional deficiency in infancy and early childhood and developmental assessment increases. A specialist in child neurology (D.C.B.) traveled to Dar es Salaam to train the BSID-III test administrators and conducted didactic lectures and observed testing as part of quality control. Tests were administered in Kiswahili by one of two trained Tanzanian nurses at the start of the child’s clinic visit, in a spacious and well-ventilated room. Children who were acutely ill were not tested, but their mothers were asked to return them for testing once they had recovered. Children who were found to have developmental delay were referred to a pediatrics clinic for further evaluation and follow-up, and caregivers were provided with appropriate counseling.
The BSID-III includes separate raw scores that reflect the infant’s cognitive functioning, expressive and receptive language skills, as well as fine and gross motor capabilities. While it is possible to calculate composite BSID-III scores that are age-standardized based on a reference population of US infants, we did not include these composite scores in our analysis for two reasons: (i) all study infants were approximately the same age at the time of developmental assessment, eliminating the need for age-standardized scores; and (ii) because the BSID-III has not been validated in the Tanzanian context, it may not be appropriate to compare our study population’s composite scores with the US reference population.
Statistical analyses
Descriptive statistics were used to summarize baseline characteristics of the study population. Frequencies were reported for categorical variables and the mean ± SD was reported for continuous variables. All BSID-III raw scores were adjusted for the infant’s gestational age at birth. We reported mean ± SD scores in the multivitamin and placebo arms and compared them using two-sided t-tests. We then constructed multivariate linear regression models that controlled for test administrator and infant sex to calculate the adjusted difference in mean scores between study arms. We introduced interaction terms between the treatment variable and infant sex, low birth weight, preterm birth and maternal CD4 into each of the models to evaluate potential effect modification. We also estimated the odds ratio of having a score <25th percentile (based on the distribution within our study population) using logistic regression models that also adjusted for test administrator and infant sex. Children who were HIV-positive at the time of developmental assessment (N = 14) were excluded from the initial analyses because the number of HIV-positive children per arm was not equivalent and also because HIV-positive children are known to have impaired neurological development [17]. We calculated that with ∼100 children per group and an alpha of 0.05, we would have 80% power to detect a difference of 1.2 in the mean raw cognitive score between groups. All analyses were performed using SAS software (version 9.2; SAS Institute). Values of p < 0.05 were considered statistically significant.
Ethics
Institutional approval was granted by the Harvard School of Public Health Human Subjects Committee, the Muhimbili University of Health and Allied Sciences Committee of Research and Publications, the Tanzanian National Institute of Medical Research and the Tanzanian Food and Drugs Authority. A Data Safety Monitoring Board met twice annually during the course of the study.
Results
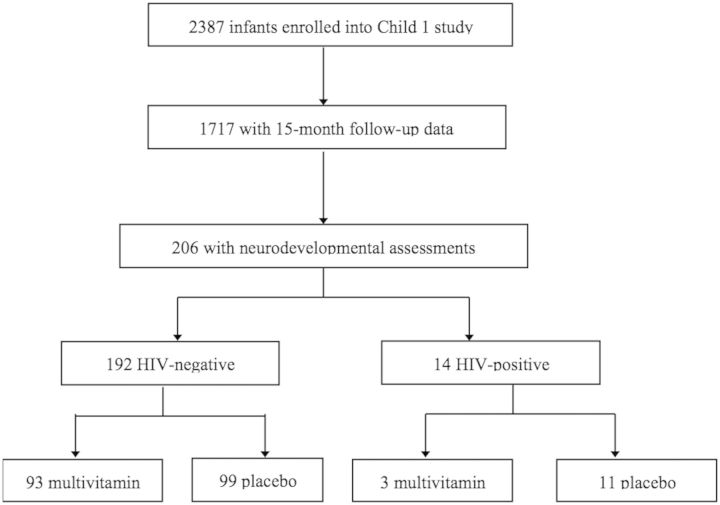
The flow of subjects from the main randomized trial to this sub-study is shown in Fig. 1. Of the 2387 infants who were randomized between August 2004 and November 2007, 1717 (71.9%) attended a follow-up visit at 15 months of age, and 206 (12.0%) of these infants were selected for developmental testing (Fig. 1). Fourteen (7.3%) infants were HIV positive and were excluded from the initial analyses. Of the 192 remaining infants, 93 (48.4%) had been randomized to the multivitamin arm and 99 (51.6%) randomized to the placebo arm. Measures of maternal demographics and WHO disease stage were comparable between groups (Table 1). More than 70% of mothers had 1–7 years of education; approximately two-thirds were housewives without any income of their own; and more than one-quarter received antiretroviral medication during pregnancy. Infants in the two study groups also had similar characteristics. Just >15% were born preterm, <10% had a birth weight <2500 g and the mean duration of exclusive breastfeeding was 3.3 months.
Fig. 1.
Flowchart of selection for study population.
Table 1.
Baseline characteristics of HIV-infected mothers and their HIV-negative children according to supplement assignment
| Placebo |
Multivitamin |
|
|---|---|---|
| N (%) or mean ± SD (N = 99) | N (%) or mean ± SD (N = 93) | |
| Maternal characteristics | ||
| Age, years | 28.9 ± 5.2 | 28.0 ± 5.2 |
| Formal education, years | ||
| 0 | 7 (7.1) | 7 (7.5) |
| 1–7 | 73 (73.7) | 66 (71.0) |
| ≥8 | 19 (19.2) | 20 (21.5) |
| Employment | ||
| Housewife without income | 64 (64.7) | 63 (67.7) |
| Housewife with income | 26 (26.3) | 23 (24.7) |
| Other | 9 (9.1) | 7 (7.5) |
| Marital status | ||
| Married | 55 (55.6) | 56 (60.2) |
| Single | 44 (44.4) | 37 (39.8) |
| Hemoglobina, g/dl | 11.8 ± 1.1 | 11.5 ± 1.3 |
| Received ARVs during pregnancy | 26 (26.3) | 34 (36.6) |
| WHO disease stage ≥3 | 13 (15.5) | 8 (10.8) |
| CD4 count, cells/mm3 | ||
| <200 | 5 (5.4) | 5 (5.9) |
| 200–349 | 20 (21.5) | 14 (16.5) |
| ≥350 | 68 (73.1) | 66 (77.7) |
| Child characteristics | ||
| Male sex | 47 (47.5) | 54 (58.1) |
| Born <37 weeks gestational age | 15 (15.2) | 16 (17.2) |
| Birth weight <2500 g | 7 (7.1) | 3 (3.2) |
| Apgar score ≤7 at 5 min after birth | 4 (4.3) | 1(1.2) |
| Duration of exclusive breastfeeding, months | 3.3 ± 1.8 | 3.2 ± 2.0 |
| Length-for-age Z-scorea | −0.30 ± 1.1 | −0.39 ± 1.1 |
| Weight-for-length Z-scorea | 0.09 ± 1.0 | −0.07 ± 1.0 |
| Weight-for-age Z-scorea | −0.24 ± 1.1 | −0.44 ± 1.0 |
| Head circumference, cm | 34.3 ± 2.1 | 34.4 ± 1.3 |
aMissing values: N = 13 for maternal hemoglobin; N = 34 for maternal disease stage; N = 14 for maternal CD4 count; N = 5 for infant length-for-age Z-score; N = 7 for infant weight-for-length Z-score and weight-for-age Z-score.
At 15 months of age, 18 (9.5%) and 10 (5.3%) children were stunted and wasted, respectively. The mean ± SD raw cognitive, expressive language, receptive language, fine motor and gross motor scores among all 192 HIV-exposed uninfected children was 51.4 ± 3.06, 18.7 ± 1.74, 18.3 ± 1.76, 35.4 ± 1.65 and 47.7 ± 1.91, respectively. As shown in Table 2, there were no significant differences in the mean cognitive, expressive language, receptive language or gross motor scores between the multivitamin and placebo groups. Adjustment for infant sex and test administrator did not substantially alter these results. However, there was a trend toward slightly improved fine motor scores among infants in the multivitamin group. In adjusted analyses, their mean fine motor score was 0.38 (95% CI: −0.01, 0.78, p = 0.06) points higher than the mean score among infants in the placebo group.
Table 2.
Effect of multivitamin supplementation on mean BSID-III raw scores at 15 months of age
| BSID-III Raw Score | Placebo | Multivitamin | p |
|---|---|---|---|
| Cognitive | |||
| N | 99 | 93 | |
| Mean ± SDa | 51.1 ± 2.9 | 51.6 ± 3.21 | 0.3 |
| Multivariable, mean difference (95% CI)b | Reference | 0.35 (−0.49, 1.18) | 0.42 |
| Expressive language | |||
| N | 98 | 93 | |
| Mean ± SDa | 18.7 ± 1.67 | 18.7 ± 1.82 | 0.76 |
| Multivariable, mean difference (95% CI)b | Reference | −0.09 (−0.58, 0.39) | 0.7 |
| Receptive language | |||
| N | 98 | 91 | |
| Mean ± SDa | 18.2 ± 1.80 | 18.4 ± 1.71 | 0.48 |
| Multivariable, mean difference (95% CI)b | Reference | 0.15 (−0.35, 0.64) | 0.56 |
| Fine motor | |||
| N | 97 | 91 | |
| Mean ± SDa | 35.2 ± 1.82 | 35.6 ± 1.41 | 0.05 |
| Multivariable, mean difference (95% CI)b | Reference | 0.38 (−0.01, 0.78) | 0.06 |
| Gross motor | |||
| N | 98 | 93 | |
| Mean ± SDa | 47.5 ± 1.90 | 47.9 ± 1.93 | 0.21 |
| Multivariable, mean difference (95% CI)b | Reference | 0.27 (−0.26, 0.80) | 0.32 |
ap value obtained from two-sided t-test assuming unequal variances.
bMean difference, 95% CI, and p value obtained from a linear regression model that adjusted for test administrator and infant sex.
When we compared the odds of having a raw BSID-III score below the 25th percentile according to treatment group, we found no statistically significant differences for cognitive, expressive language, receptive language, fine motor or gross motor developments (Table 3). Our analyses examining potential effect modification revealed that the effect of multivitamins on each BSID-III score did not significantly vary by infant sex, birth weight, gestational age at birth or maternal CD4 count (p values of all interaction terms were >0.10).
Table 3.
Effect of multivitamin supplementation on the odds of a raw BSID-III score <25th percentile at 15 months of age
| BSID-III Raw Score | Placebo | Multivitamin | pa |
|---|---|---|---|
| Cognitive | |||
| N (%) with score <25th percentile | 21 (29.3) | 21 (18.3) | |
| Odds ratio (95% CI) | 1 | 0.57 (0.29, 1.14) | 0.11 |
| Expressive language | |||
| N (%) with score <25th percentile | 21 (21.4) | 21 (22.6) | |
| Odds ratio (95% CI) | 1 | 1.10 (0.55, 2.21) | 0.78 |
| Receptive language | |||
| N (%) with score <25th percentile | 16 (16.3) | 12 (13.2) | |
| Odds ratio (95% CI) | 1 | 0.84 (0.37, 1.91) | 0.67 |
| Fine motor | |||
| N (%) with score <25th percentile | 15 (15.5) | 7 (7.69) | |
| Odds ratio (95% CI) | 1 | 0.49 (0.17, 1.38) | 0.18 |
| Gross motor | |||
| N (%) with score <25th percentile | 10 (10.2) | 6 (6.45) | |
| Odds ratio (95% CI) | 1 | 1.10 (0.55, 2.21) | 0.78 |
aOdds ratio, 95% CI and p values obtained from logistic regression models that adjusted for test administrator and infant sex.
Discussion
Multiple micronutrient deficiencies are common in low-income countries and may be increasingly prevalent among HIV-exposed children [18]. While several studies have illustrated detrimental effects of micronutrient deficiencies on infant and child development [19–21], results of micronutrient supplementation trials have been mixed and evidence from HIV-exposed populations is particularly limited. In an earlier study, we examined the effects of supplementing HIV-infected mothers with multivitamins during the pre- and post-natal periods on infant neurodevelopment and found that multivitamins significantly improved measures of psychomotor development [14]. Our current study is the first to assess the impact of providing a daily supplement of B vitamins, vitamin C and vitamin E directly to HIV-exposed infants from 6 weeks of age. At 15 months of age, infant multivitamin supplementation was not associated with any significant differences in mean cognitive, receptive language, expressive language or gross motor scores compared with placebo. There was, however, a non-significant trend toward improved fine motor scores among HIV-exposed uninfected infants randomized to the multivitamin group.
Our finding of a potentially beneficial effect of multivitamins on infant motor development has also been observed in studies of maternal supplementation during pregnancy. In our previous study, maternal multivitamin supplementation was also associated with gains in infant psychomotor, but not mental development [14]. Prado et al. [22] also observed improvements in motor ability and visual attention among preschool-aged Indonesian children of undernourished mothers who were supplemented with multiple micronutrients during pregnancy and until 3 months post-partum. Similarly, in rural Nepal, supplementation of pregnant women with iron, folic acid and vitamin A was associated with improvements in the fine motor functioning of their offspring at seven to nine years of age in comparison with vitamin A alone [23]. However, the effects of maternal micronutrient supplementation on infant mental development outcomes are less clear. Although Li et al. [24] have reported improvements in the mental development scores of 12-month-old infants in rural China whose mothers received a daily multiple micronutrient supplement from 14 weeks gestation until delivery, a recent systematic review found no conclusive evidence that maternal micronutrient supplementation resulted in improved mental development [25].
The results of infant or child micronutrient supplementation trials on neurodevelopment are also varied. A study of 221 presumably HIV-negative infants in rural Bangladesh reported improvements in psychomotor development after weekly administration of a multiple micronutrient supplement containing iron and zinc from 6 to 12 months of age [10]. However, findings from two recent studies in rural Nepal appear to contradict these results. The first showed that after a year of daily supplementation, neither zinc nor iron-folic acid improved the attainment of motor or language milestones of infants at 12 months of age [26]. A separate analysis of 732 Nepalese children revealed that iron and folic acid or zinc supplementation during the preschool years did not affect motor functioning at 9 years of age [27].
Methodological differences in the composition of the micronutrient supplement, frequency and duration of supplementation and duration of follow-up make the comparison of results across studies difficult. In contrast to many studies involving HIV-negative children, we did not include iron or zinc in the micronutrient regimen, as their roles in immune function and disease progression in HIV-exposed children are uncertain [28, 29]. Furthermore, the safety of routine iron supplementation of children living in malaria-endemic areas is questionable [30]. In studies such as ours that provide supplementation to both mothers and children, it is particularly difficult to identify the window during which supplementation provides the greatest benefit. Because our previous studies demonstrated beneficial effects of multivitamin supplementation during pregnancy and lactation [31], all mothers in the current study were provided with multivitamins. Thus, our inability to detect substantial improvements in the neurodevelopment of infants who received multivitamins themselves could mean that maternal supplementation is a sufficient means of enhancing infant micronutrient and neurodevelopment status [32]. Christian et al. [33] arrived at a similar conclusion after finding that iron-folic acid supplementation with or without zinc during the preschool years conferred no additional benefit in cognitive outcomes among school-aged children whose mothers received iron and folic acid supplementation during pregnancy.
Findings from our study are particularly novel, as they are based in the context of HIV-exposure. As Filteau points out, the increasing availability of antiretroviral treatment and the growing success of interventions to prevent mother-to-child transmission of HIV means that very large numbers of HIV-exposed uninfected children are being born in sub-Saharan Africa [34]. However, a number of limitations to our study deserve mention and caution against premature generalizations of our findings to other populations of HIV-exposed children. First, we assessed infant development only at 15 months of age, which prevented the detection of possible improvements at younger ages or evaluation of changes in developmental status over time. Second, although we included >200 children in the study, the sample size may have been insufficient to detect significant differences and/or to fully assess potential effect modification. Because multiple vitamins were provided in the supplement, it is also not possible to attribute any improvements to a particular nutrient. As mentioned earlier, it is difficult to disentangle the effects of maternal supplementation from infant supplementation. Finally, the use of the BSID-III may not be ideally suited to measure developmental outcomes in African infants.
In conclusion, daily supplementation of B vitamins and vitamins C and E to HIV-exposed children whose mothers also received multivitamin supplementation did not substantially improve cognitive, language or gross motor development at 15 months of age. However, there was an indication of a slight improvement in fine motor skills, a finding whose clinical significance is difficult to determine. Larger studies, as well as trials of alternate micronutrient regimens and varying supplementation periods, are called for before recommendations can be made.
Funding
National Institute of Child Health and Development [R01 HD043688-01, K24HD058795].
Acknowledgements
The authors would like to thank James Okuma for his assistance in conducting the preliminary analysis. The opinions in this article are those of the authors and may not reflect official UNICEF policies.
References
- 1.Grantham-McGregor S, Cheung YB, Cueto S, et al. Developmental potential in the first 5 years for children in developing countries. Lancet. 2007;369:60–70. doi: 10.1016/S0140-6736(07)60032-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beard J. Recent evidence from human and animal studies regarding iron status and infant development. J Nutr. 2007;137:S524–30. doi: 10.1093/jn/137.2.524S. [DOI] [PubMed] [Google Scholar]
- 3.Lozoff B. Perinatal iron deficiency and the developing brain. Pediatr Res. 2000;48:137–9. doi: 10.1203/00006450-200008000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Graham SM, Arvela OM, Wise GA. Long-term neurologic consequences of nutritional vitamin B12 deficiency in infants. J Pediatr. 1992;121(5 Pt 1):710–4. doi: 10.1016/s0022-3476(05)81897-9. [DOI] [PubMed] [Google Scholar]
- 5.von Schenck U, Bender-Gotze C, Koletzko B. Persistence of neurological damage induced by dietary vitamin B-12 deficiency in infancy. Arch Dis Child. 1997;77:137–9. doi: 10.1136/adc.77.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ivers LC, Cullen KA, Freedberg KA, et al. HIV/AIDS, undernutrition, and food insecurity. Clin Infect Dis. 2009;49:1096–102. doi: 10.1086/605573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scrimshaw NS, SanGiovanni JP. Synergism of nutrition, infection, and immunity: an overview. Am J Clin Nutr. 1997;66:S464–77. doi: 10.1093/ajcn/66.2.464S. [DOI] [PubMed] [Google Scholar]
- 8.Wachsler-Felder JL, Golden CJ. Neuropsychological consequences of HIV in children: a review of current literature. Clin Psychol Rev. 2002;22:443–64. doi: 10.1016/s0272-7358(01)00108-8. [DOI] [PubMed] [Google Scholar]
- 9.Faber M, Kvalsvig JD, Lombard CJ, et al. Effect of a fortified maize-meal porridge on anemia, micronutrient status, and motor development of infants. Am J Clin Nutr. 2005;82:1032–9. doi: 10.1093/ajcn/82.5.1032. [DOI] [PubMed] [Google Scholar]
- 10.Black MM, Baqui AH, Zaman K, et al. Iron and zinc supplementation promote motor development and exploratory behavior among Bangladeshi infants. Am J Clin Nutr. 2004;80:903–10. doi: 10.1093/ajcn/80.4.903. [DOI] [PubMed] [Google Scholar]
- 11.Olney DK, Pollitt E, Kariger PK, et al. Combined iron and folic acid supplementation with or without zinc reduces time to walking unassisted among Zanzibari infants 5- to 11-mo old. J Nutr. 2006;136:2427–34. doi: 10.1093/jn/136.9.2427. [DOI] [PubMed] [Google Scholar]
- 12.Chen CM, Wang YY, Chang SY. Effect of in-home fortification of complementary feeding on intellectual development of Chinese children. Biomed Environ Sci. 2010;23:83–91. doi: 10.1016/S0895-3988(10)60036-0. [DOI] [PubMed] [Google Scholar]
- 13.Adu-Afarwuah S, Lartey A, Brown KH, et al. Randomized comparison of 3 types of micronutrient supplements for home fortification of complementary foods in Ghana: effects on growth and motor development. Am J Clin Nutr. 2007;86:412–20. doi: 10.1093/ajcn/86.2.412. [DOI] [PubMed] [Google Scholar]
- 14.McGrath N, Bellinger D, Robins J, et al. Effect of maternal multivitamin supplementation on the mental and psychomotor development of children who are born to HIV-1-infected mothers in Tanzania. Pediatrics. 2006;117:e216–25. doi: 10.1542/peds.2004-1668. [DOI] [PubMed] [Google Scholar]
- 15.Duggan C, Manji KP, Kupka R, et al. Multiple micronutrient supplementation in Tanzanian infants born to HIV-infected mothers: a randomized, double-blind, placebo-controlled clinical trial. Am J Clin Nutr. 2012;96:1437–46. doi: 10.3945/ajcn.112.044263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fawzi WW, Msamanga GI, Spiegelman D, et al. randomized trial of multivitamin supplements and HIV disease progression and mortality. N Engl J Med. 2004;351:23–32. doi: 10.1056/NEJMoa040541. [DOI] [PubMed] [Google Scholar]
- 17.McGrath N, Fawzi WW, Bellinger D, et al. The timing of mother-to-child transmission of human immunodeficiency virus infection and the neurodevelopment of children in Tanzania. Pediatr Infect Dis J. 2006;25:47–52. doi: 10.1097/01.inf.0000195638.80578.e0. [DOI] [PubMed] [Google Scholar]
- 18.Monteiro JP, Freimanis-Hance L, Faria LB, et al. Both human immunodeficiency virus-infected and human immunodeficiency virus-exposed, uninfected children living in Brazil, Argentina, and Mexico have similar rates of low concentrations of retinol, beta-carotene, and vitamin E. Nutr Res. 2009;29:716–22. doi: 10.1016/j.nutres.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Black MM. Micronutrient deficiencies and cognitive functioning. J Nutr. 2003;133(11 Suppl 2):S3927–31. doi: 10.1093/jn/133.11.3927S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walker SP, Wachs TD, Grantham-McGregor S, et al. Inequality in early childhood: risk and protective factors for early child development. Lancet. 2011;378:1325–38. doi: 10.1016/S0140-6736(11)60555-2. [DOI] [PubMed] [Google Scholar]
- 21.Grantham-McGregor SM, Ani CC. The role of micronutrients in psychomotor and cognitive development. Br Med Bull. 1999;55:511–27. doi: 10.1258/0007142991902583. [DOI] [PubMed] [Google Scholar]
- 22.Prado EL, Ullman MT, Muadz H, et al. The effect of maternal multiple micronutrient supplementation on cognition and mood during pregnancy and postpartum in Indonesia: a randomized trial. PLoS One. 2012;7:e32519. doi: 10.1371/journal.pone.0032519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Christian P, Murray-Kolb LE, Khatry SK, et al. Prenatal micronutrient supplementation and intellectual and motor function in early school-aged children in Nepal. JAMA. 2010;304:2716–23. doi: 10.1001/jama.2010.1861. [DOI] [PubMed] [Google Scholar]
- 24.Li Q, Yan H, Zeng L, et al. Effects of maternal multimicronutrient supplementation on the mental mevelopment of infants in rural western China: follow-up evaluation of a double-blind, randomized, controlled trial. Pediatrics. 2009;123:e685–92. doi: 10.1542/peds.2008-3007. [DOI] [PubMed] [Google Scholar]
- 25.Leung BM, Wiens KP, Kaplan BJ. Does prenatal micronutrient supplementation improve children's mental development? A systematic review. BMC Pregnancy Childbirth. 2011;11:12. doi: 10.1186/1471-2393-11-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Surkan PJ, Siegel EH, Patel SA, et al. Effects of zinc and iron supplementation fail to improve motor and language milestone scores of infants and toddlers. Nutrition. 2013;29:542–8. doi: 10.1016/j.nut.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murray-Kolb LE, Khatry SK, Katz J, et al. Preschool micronutrient supplementation effects on intellectual and motor function in school-aged Nepalese children. Arch Pediatr Adolesc Med. 2012;166:404–10. doi: 10.1001/archpediatrics.2012.37. [DOI] [PubMed] [Google Scholar]
- 28.Villamor E, Aboud S, Koulinska IN, et al. Zinc supplementation to HIV-1-infected pregnant women: effects on maternal anthropometry, viral load, and early mother-to-child transmission. Eur J Clin Nutr. 2006;60:862–9. doi: 10.1038/sj.ejcn.1602391. [DOI] [PubMed] [Google Scholar]
- 29.Kupka R, Fawzi W. Zinc nutrition and HIV infection. Nutr Rev. 2002;60:69–79. doi: 10.1301/00296640260042739. [DOI] [PubMed] [Google Scholar]
- 30.Sazawal S, Black RE, Ramsan M, et al. Effects of routine prophylactic supplementation with iron and folic acid on admission to hospital and mortality in preschool children in a high malaria transmission setting: community-based, randomised, placebo-controlled trial. Lancet. 2006;367:133–43. doi: 10.1016/S0140-6736(06)67962-2. [DOI] [PubMed] [Google Scholar]
- 31.Fawzi WW, Msamanga GI, Spiegelman D, et al. Randomised trial of effects of vitamin supplements on pregnancy outcomes and T cell counts in HIV-1-infected women in Tanzania. Lancet. 1998;351:1477–82. doi: 10.1016/s0140-6736(98)04197-x. [DOI] [PubMed] [Google Scholar]
- 32.Baylin A, Villamor E, Rifai N, et al. Effect of vitamin supplementation to HIV-infected pregnant women on the micronutrient status of their infants. Eur J Clin Nutr. 2005;59:960–8. doi: 10.1038/sj.ejcn.1602201. [DOI] [PubMed] [Google Scholar]
- 33.Christian P, Morgan ME, Murray-Kolb L, et al. Preschool iron-folic acid and zinc supplementation in children exposed to iron-folic acid in utero confers no added cognitive benefit in early school-age. J Nutr. 2011;141:2042–8. doi: 10.3945/jn.111.146480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Filteau S. The HIV-exposed, uninfected African child. Trop Med Int Health. 2009;14:276–87. doi: 10.1111/j.1365-3156.2009.02220.x. [DOI] [PubMed] [Google Scholar]