Abstract
miRNAs have emerged as important regulators of lipoprotein metabolism. Work over the past few years has demonstrated that miRNAs control the expression of most of the genes associated with high-density lipoprotein (HDL) metabolism, including the ATP transporters, ABCA1 and ABCG1, and the scavenger receptor SRB1. These findings strongly suggest that miRNAs regulate HDL biogenesis, cellular cholesterol efflux, and HDL cholesterol (HDL-C) uptake in the liver, thereby controlling all of the steps of reverse cholesterol transport. Recent work in animal models has demonstrated that manipulating miRNA levels including miR-33 can increase circulating HDL-C. Importantly, antagonizing miR-33 in vivo enhances the regression and reduces the progression of atherosclerosis. These findings support the idea of developing miRNA inhibitors for the treatment of dyslipidaemia and related cardiovascular disorders such as atherosclerosis. This review article focuses on how HDL metabolism is regulated by miRNAs and how antagonizing miRNA expression could be a potential therapy for treating cardiometabolic diseases.
Keywords: MiRNAs, Cholesterol metabolism, ABCA1 and SRB1
1. Introduction
Cholesterol is a major component of the plasma membrane in mammalian cells. In addition to its structural requirement, cholesterol is important for other cell functions such as cell proliferation and bile acid and hormone biosynthesis.1,2 Despite its pivotal role in controlling multiple physiological processes, abnormal levels of cholesterol can trigger a number of cardiometabolic diseases, including atherosclerosis and type-II diabetes.3 Because mammalian cells cannot degrade cholesterol, cholesterol removal is indispensable in order to prevent cholesterol accumulation in cells. Excess cholesterol must be removed and transported from the peripheral tissues to the liver for reutilization and excretion into feces in a physiological process traditionally known as reverse cholesterol transport (RCT).4 During RCT, plasma high-density lipoprotein (HDL) is thought to function as a sterol transporter that facilitates the movement of sterols from the peripheral cells to the liver. HDL integrates a heterogeneous class of lipoproteins with a density > 1.063 g/mL.5 HDL particles contain various apolipoproteins of which ApoA1 and ApoA2 are quantitatively the most abundant.6,7
HDL formation occurs in the liver and intestine. The interaction between lipid poor ApoA1 with the ATP binding cassette (ABC) A1 mediates this first step in HDL formation (Figure 1).6 ABCA1 is a member of the ABC family of membrane transporters that promotes phospholipid and cholesterol transfer from cells to poorly lipidated ApoA1. Even though the mechanism by which ABCA1 regulates this process is not fully characterized, it is thought that poorly lipidated ApoA1 binds to ABCA1 in the membrane surface leading to increased stability and activity of the transporter in the plasma membrane. In response to ATP hydrolysis, ABCA1 promotes the trans-bilayer transport of phospholipids from the inner to outer leaflet of the plasma membrane. The uneven phospholipid packing in the plasma membrane bilayer leads to the formation of extravesiculated lipid domains. Lipid poor ApoA1 binds to this phospholipid and cholesterol-rich domain and promotes the spontaneous solubilization to form pre β-HDL particles.8
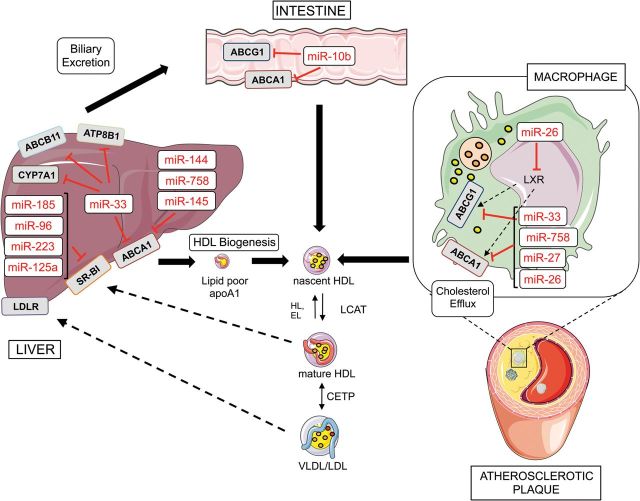
Figure 1.
miRNAs regulate reverse cholesterol transport (RCT). ABCA1 is regulated by a number of miRNAs that reduce the cholesterol efflux to lipid-poor ApoA1 that originates nascent HDL particles. miR-33 also inhibits the expression of bile acid transporters (ABCB11 and ATP8B1) in the liver, thereby regulating the last step of the RCT. ABCA1 is also regulated by miRNAs in the intestine and in the macrophages accumulated in atherosclerotic plaques. Free cholesterol in the nascent HDL is further esterified to cholesteryl esters by lecithin-cholesterol acyltransferase (LCAT) leading to the formation of mature HDL particles. HDL particles deliver cholesterol to the liver via SRB1 receptor, which is also regulated by several miRNAs including miR-185, miR-223, miR-96, and miR-185. This figure was performed using the Servier Medical Art illustration resources (http://www.servier.com).
The efflux of cholesterol and HDL formation through the ABCA1 pathway remains predominant. This finding was brought to light when the mutation in Tangier disease, a condition characterized by low plasma HDL levels, was attributed to mutations in the ABCA1 gene.9–11 However, other proteins also play a role in HDL metabolism, including ABCG1 and the scavenger receptor class B type 1 (SRB1), which is involved in the maturation of HDL particles. Studies in vitro have shown that ABCA1 and ABCG1 synergistically mediate cholesterol efflux to HDL.12 These models propose that the lipidated ApoA1 formed after the ApoA1/ABCA1 interaction serves as an acceptor for cholesterol that is effluxed from cells in an ABCG1-dependent process.12 The importance of ABCG1 in regulating cholesterol efflux is well established; however, the mechanism of ABCG1 action is controversial. In contrast to ABCA1, it has been recently reported that ABCG1 is localized in endocytic vesicles where facilitate the distribution of specific intracellular sterols away from the endoplasmatic reticulum.13 Absence of ABCA1 and ABCG1 in mice results in massive accumulation of macrophage foam cells in various tissues such as in the spleen, heart, thymus, liver, and lung.14,15 However, there are conflicting data regarding the role of both transporters during the progression of atherosclerosis. While transplantation of bone marrow from Abca1−/− mice into Ldlr−/− or ApoE−/− recipients caused an increase in atherosclerosis,16 deficiency of ABCG1 in bone marrow cells resulted in either a modest increase, or decrease of atherosclerosis.17,18 Despite the well-established role of ABCA1 in HDL formation, its effect on atherogenesis is less clear. Even though the increase in the incidence of atherosclerosis has been reported in people affected with Tangier disease, not all the subjects develop atherosclerosis. Moreover, mice lacking ABCA1 in the liver develop similar atherosclerotic lesions than Ldlr−/− mice.19 Interestingly, absence of ABCA1 in the liver markedly diminish plasma HDL-C levels (less than 50%) but cause also a marked reduction in circulating VLDL and LDL.
In addition to ABCA1/ABCG1-mediated cholesterol efflux, excess intracellular cholesterol can also be eliminated by aqueous diffusion. This process consists of desorption of free cholesterol molecules from the plasma cell membrane into the surrounding aqueous phase. Collision of desorbed cholesterol molecules with HDL particles diffusing in the extracellular aqueous space leads to their rapid uptake into the lipoprotein acceptor.
SRB1 mediates bidirectional flux of cholesterol between cells and HDL, modulating changes in the composition and structure of HDL particles.20,21 SRB1 facilitates the delivery of cholesterol to steroidogenic tissues and liver. Mouse studies have revealed the importance of this receptor in controlling lipoprotein metabolism and the progression of atherosclerosis.22,23 Remarkably, absence of SRB1 results in a dramatic increase of atherosclerosis at a very young age. Srb1−/−ApoE−/− mice develop atherosclerotic plaques in the coronary arteries, a rare phenotype observed in mouse models of atherosclerosis.22
HDL particles are constantly remodelled by a number of plasma enzymes and proteins. The lecithin-cholesterol acyltransferase (LCAT), a liver synthesized glycoprotein, mediates the transfer of fatty acids from phospholipids to free cholesterol present in the pre β-HDL to form cholesteryl ester.24,25 Glomset proposed that esterification of cholesterol would drive the net efflux from cells because esterification would prevent the back-exchange of cholesterol from HDL to cells.4,26 The cholesteryl ester generated by LCAT in HDL could be transferred to other lipoproteins, including very-low density lipoproteins (VLDL). This process is catalysed by the cholesteryl ester transfer protein (CETP), an enzyme that facilitates the transport of cholesteryl esters and triglycerides between the lipoproteins.27,28 This neutral lipid transfer process results in a net gain of triglycerides and net loss of cholesteryl ester in HDL.28 In addition to CETP, human plasma also contains a second lipid transfer protein, designated phospholipid transfer protein (PLTP), which mediates transfer of phospholipids from ApoB-containing lipoproteins to HDL. Moreover, two members of the triglyceride lipase family, the hepatic lipase (HL) and the endothelial lipase (EL), also participate in HDL metabolism. HL is a glycoprotein synthesized primarily by the liver and has both triglyceride lipase and phospholipase and hydrolyzes HDL, generating smaller subspecies, including pre β-HDL.29 EL acts primarily as a phospholipase and hydrolyzes HDL phospholipids and is primarily located in vascular endothelial cells.30
HDL particles deliver cholesterol and cholesterol esters to the liver and steroidogenic tissues through the scavenger receptor SRB1 (Figure 1). Within the liver, a portion of cholesterol is enzymatically converted in bile salt molecules. This process is unique to the liver because only hepatocytes express high levels of the enzyme cholesterol 7a-hydroxilase (CYP7A1), which initiates and rate-limits the multi-step conversion process.31 Cholesterol and bile acid molecules have different physical properties. While cholesterol is insoluble in water, bile acid salts are biological amphiphiles and highly soluble allowing the transport of cholesterol in the digestive system by forming micelles. Biliary lipids are secreted across the apical (canalicular) membrane of hepatocytes by three different transmembrane transporters: ABCB11 (aka BESP), ABCG5/ABCG8 (an obligate heterodimer that facilitates cholesterol efflux), and ABCB4 (aka MDR3; which pumps phospholipids).32 Another transporter, ATP8B1 maintains the asymmetry of phospholipids to promote the required lipid packing of the canalicular membrane for resistance to hydrophobic bile salts and canalicular membrane transport.33,34 All these transporters play an essential role in the removal of cholesterol from the peripheral tissues towards the liver for excretion.35,36 This process represents the last step of RCT, a protective mechanism against the development of atherosclerosis.
2. Transcriptional regulation of ABCA1 and ABCG1 expression
As mentioned earlier, ABCA1 regulates cellular cholesterol efflux and HDL biogenesis. Importantly, ABCA1 mRNA and protein half-life is very short (1–2 h), suggesting that de novo transcription and translation are critical for controlling its expression in response to environmental changes, such as cholesterol loading. ABCA1 and ABCG1 are oxysterol-regulated genes and both are transcriptionally regulated by the liver X receptors (LXRs).12,37,38 These nuclear hormone receptors [(LXRα (NR1H3) and LXRβ (NR1H2)] form heterodimers with the retinoic-X-receptor (RXR)39 leading to the transcriptional activation of both ABC transporters. Genetic deletion of LXRα and LXRβ results in a massive accumulation of cholesterol in peripheral tissues, suggesting the critical role of both transcription factors in controlling cholesterol removal from cells.40,41 Moreover, absence of LXRα and LXRβ markedly increase the progression of atherosclerosis in mice.40 In addition to the transcriptional regulation of ABCA1 and ABCG1 by LXRs, several groups have recently identified that the expression of both transporters are significantly post-transcriptionally regulated by microRNAs (miRNAs)42–44 (Table 1).
Table 1.
miRNA regulation of HDL metabolism
| MiRNA | Target genes | Cell type /tissue | Ref. |
|---|---|---|---|
| miR-33a/b | ABCA1, ABCG1, NPC1, CPT1A, SIRT6, AMPK, HADHB, CROT, CYP7A1, ABCB11, ATP8B1, NSF, SRC3, PCK1, G6PC, IRS2, RIP140, NFYC, SREBP1 | Macrophages (primary mouse peritoneal macs, THP-1), endothelial cells (EAhy926), hepatic cells (HepG2, HEPA, Fu5aH, Hep3B), and mouse liver | 45–52 |
| miR-33* | NPC1, RIP140, SRC3, NFYC, IRS2, CROT | Hepatic cells (Huh-7), macrophages (THP-1) | 53 |
| miR-758 | ABCA1 | Macrophages (primary mouse peritoneal macs, J774, THP-1), hepatic cells (HepG2, Huh-7, HEPA), and neuroglyomal cells (H4) | 54 |
| miR-26 | ABCA1, ARL7 | Macrophages (Raw264.7 and THP-1) | 55 |
| miR-145 | ABCA1 | Hepatic cells (HepG2) and pancreatic β cells (MIN6 and primary mouse β cells) | 56 |
| miR-106b | ABCA1 | Mouse primary hippocampal neurons (DIV14) and mouse neuroblastoma cells (Neuro2a) | 57 |
| miR-10b | ABCA1, ABCG1 | Macrophages (primary mouse peritoneal macs, J774,THP-1) | 58 |
| miR-144 | ABCA1 | Macrophages (mouse peritoneal macrophages, J774, THP-1), hepatic cells (HepG2, Huh-7, Hepa), endothelial cells (EAhy926), mouse liver, human hepatic cells (Hep3B), and mouse primary hepatocytes | 59–61 |
| miR-27 | ABCA1 | Macrophages (THP-1) | 62 |
| miR-206 | LXRα | Macrophages (THP-1) | 63 |
| miR-613 | LXRα | Hepatic cells (HepG2) | 64 |
| miR-96 | SRB1 | Hepatic cells (HepG2) | 65 |
| miR-223 | SRB1 | Carried on HDL | 65 |
| miR-185 | SRB1 | Hepatic cells (HepG2) | 65 |
| miR-125a | SRB1 | Hepatic cells (Hepa), steroidogenic cell lines (MLTC-1, granulosa cells) | 66 |
| miR-455 | SRB1 | Hepatic cells (Hepa), steroidogenic cell lines (MLTC-1, granulosa cells) | 66 |
3. microRNAs and lipid metabolism
MicroRNAs (miRNAs) are small (18–25 nucleotides), evolutionarily conserved, non-coding RNAs that have an important function in gene regulation, acting predominantly at the post-transcriptional level.67,68 Since miRNAs have been described in Caenorhabditis elegans, hundreds of miRNAs have been identified in animals, plants, and viruses.67,68 They have been shown to participate in almost every cellular process investigated, including cholesterol homeostasis and lipoprotein metabolism.42–44,67,68 Mature miRNA products are generated from precursors called pri-miRNAs, which are composed of hundreds or thousands of nucleotides through sequential processing by the ribonuclease DROSHA.69,70 This ultimately produces a nuclear hairpin precursor called the pre-miRNA, which is then exported to the cytoplasm where it is processed by DICER to produce the mature miRNA.69,70 miRNAs typically control the expression of their target genes by imperfect base pairing to the 3′-untranslated region (3′UTR) of mRNAs. miRNAs are preferentially incorporated into the RISC complex where they associate with Argonaute proteins directing the binding of the RISC complex to the 3′UTR of their target mRNAs. This association produces mRNA repression either by transcript destabilization, translational inhibition, or both.69,70 One miRNA often regulates multiple genes that are involved in a specific signalling cascade or cellular mechanism, thus making miRNAs potent biological regulators. In the past few years, several groups have demonstrated the important role of miRNAs including miR-33, miR-122, and miR-30c in controlling lipoprotein metabolism.45–47,71–73 In the following sections of this review article, we will discuss the most recent findings regarding the importance of miRNAs in regulating HDL metabolism.
4. miR-33 regulation of HDL metabolism and atherogenesis
The miR-33 family consists of two intronic miRNAs, miR-33a and miR-33b, which are encoded within the introns of the sterol regulatory element-binding proteins (SREBP) 2 and 1 genes, respectively.45–48 The SREBPs are a family of membrane-bound transcription factors that regulate cellular lipid synthesis and clearance of pro-atherogenic lipoproteins.74,75 SREBP2 regulates the expression of genes involved in the cholesterol biosynthetic pathway as well as the low-density lipoprotein receptor (LDLR). The function of SREBP1 is more complex because the same gene encodes two different SREBP isoforms (SREBP1c and SREBP1a). SREBP1c increases the expression of genes that regulate fatty acid synthesis and SREBP1a regulates genes that control cholesterol metabolism and fatty acid synthesis. SREBP1c is the predominant SREBP1 isoform in adult liver and it is activated in response to insulin. The fact that miR-33a and miR-33b are co-transcribed with their respective host genes suggests that miR-33a/b regulate related physiological processes controlled by SREBP2 and SREBP1.45–48 Indeed, it has been demonstrated that miR-33a and miR-33b help boost cellular cholesterol and fatty acid levels during times of need. Under conditions that stimulate SREBP transcription, miR-33a/b are co-expressed with their host genes and reciprocally regulate genes involved in cellular cholesterol efflux/HDL biogenesis (ABCA1 and ABCG1) and fatty acid degradation (CPT1A, CROT, HADHB, AMPK1A).45–48 These findings illustrate an elegant genetic regulatory mechanism by which miR-33a/b and their host genes cooperate to tightly regulate intracellular cholesterol and fatty acid levels. In addition to the genes mentioned above, Baldan and colleagues49 have reported that miR-33 also regulates the expression of a number of bile acid transporters, including ABCB11 and ATP8B1 that control bile secretion. Moreover, a recent study also identified CYP7A1 as a miR-33 target gene.50 Altogether, these findings support the hypothesis that miR-33 controls whole-body cholesterol homeostasis by affecting HDL biogenesis (via ABCA1), cellular cholesterol efflux from peripheral tissues (via ABCA1 and ABCG1) and bile acid synthesis (via CYP7A1) and secretion (via ATP8B1 and ABCB11).
Given that miR-33 levels markedly regulate ABCA1 expression, several groups assessed the efficacy of anti-miR-33 therapy for increasing circulating HDL-C. Three independent studies demonstrated that silencing of miR-33 in mice using modified anti-sense oligonucleotides, or viral delivery of hairpin inhibitors, increased hepatic ABCA1 expression and plasma HDL-C levels by 25–35%.45–47 In addition to the elevated circulating HDL-C levels observed in mice treated with anti-miR-33 oligonucleotides, antagonism of miR-33 in vivo also enhanced RCT.47,49 The effect of miR-33 inhibitors on plasma HDL-C levels was later confirmed in miR-33 deficient mice, which showed a significant increase in hepatic ABCA1 expression and a 25% increase in serum HDL-C compared with wild-type mice.51 Most importantly, anti-miR-33 therapy also resulted in increased plasma HDL-C levels in non-human primates.76,77
A number of observational studies, including the Framingham Heart Study, have shown a strong inverse correlation of plasma HDL-C levels with coronary heart disease. To demonstrate whether anti-miR-33 therapy reduces the progression and enhances the regression of atherosclerosis in atherosclerosis-prone mouse models, several groups inhibited miR-33 using chemically modified antisense oligonucleotides.78–80 The first study reported that a 4-week treatment with 2′-fluoro/methoxylethyl (2′F/MOE) anti-miR-33 oligonucleotides of Ldlr null mice fed previously a western diet (WD) for 14 weeks increased circulating HDL-C and enhanced the regression of atherosclerosis.79 The results of the atherosclerosis progression studies, however, are somehow conflicting. While Baldan's group showed that prolonged anti-miR-33 therapy failed to raise plasma HDL-C and did not prevent the progression of atherosclerosis,78 our group demonstrated that antagonism of miR-33 reduced atherogenesis despite the fact that HDL-C levels were not affected.80 The different outcomes observed in the last two studies might be explained by different cholesterol content in the western diets (0.3 and 1.25%), oligonucleotide chemical modifications, and length of treatment. Indeed, while we demonstrated that the (2′F/MOE) anti-miR-33 oligonucleotides enhance ABCA1 expression in the artery wall, Baldan's study did not assess the efficacy of anti-miR-33 therapy in increasing ABCA1 expression in atherosclerotic plaques. Finally, the fact that miR-33 null mice have significant protection against the progression of atherosclerosis strongly suggests that inhibiting miR-33 in vivo might be useful for treating atherosclerotic vascular disease.81
5. miR-33 regulation of fatty acid and glucose metabolism
Besides the main role of miR-33 in regulating cholesterol metabolism, miR-33 also contributes to the regulation of additional metabolic pathways such as fatty acid metabolism and insulin signalling.48,52 miR-33 regulates the expression of carnitine O-octanyl transferase (CROT), carnitine palmitoyltransferase 1A (CPT1A), and hydroxyacyl-coenzyme A dehydrogenase-3-ketoacyl-coenzyme A thiolase-enoyl-coenzyme A hydratase (trifunctional protein) β-subunit (HADHB), thereby controlling fatty acid β-oxidation.48,52 CROT and CPT1A regulate the transport of fatty acids to the mitochondria for their degradation and HADHB is required for the last steps of the mitochondria β-oxidation pathway. Inhibition of miR-33 in human hepatic cells increases the degradation of fatty acids, suggesting that anti-miR-33 therapy may be useful for treating hepatic steatosis by increasing the degradation rate of fatty acids in the liver.48,52
miR-33 also regulates the post-transcriptional expression of the AMPK-activated protein kinase (AMPK), sirtuin 6 (SIRT6), and insulin receptor substrate 2 (IRS2), thus controlling fatty acid and glucose metabolism.48 This observation suggests that anti-miR-33 therapy could increase insulin sensitivity. In addition to the regulation of insulin signalling in human hepatic cells, it has recently been shown that miR-33 also modulates the expression of ABC transporters and insulin secretion in human and mouse pancreatic islets. Of note, inhibition of miR-33 in pancreatic islets increases ABCA1 expression and enhances insulin secretion while overexpression of miR-33 has the opposite effects.82 Altogether, these findings indicate that antagonism of miR-33 might increase plasma HDL-C and insulin sensitivity and reduce hepatic lipid accumulation and plasma triglyceride levels. However, it has been recently shown that miR-33-deficient mice develop obesity, hepatic steatosis, and insulin resistance.83 Mechanistically, Horie et al.83 found that miR-33 inhibits SREBP1 expression, thereby increasing fatty acid synthesis. Moreover, we have also reported that miR-33 regulates gluconeogenesis, suggesting that derepression of miR-33 might increase hepatic glucose production.84 Together, these observations indicate that miR-33 regulates multiple metabolic processes and that further experiments are warranted to fully understand the molecular mechanism by which miR-33 controls lipid and glucose metabolism.
Although most of the studies have focused on the role of miR-33a-5p (guide strand), we have also recently discovered that the guide strand (miR-33-3p; aka miR-33*) accumulates in a number of tissues and targets similar genes as miR-33.53 These findings suggest that both strands of the miR-33 locus may work together to control cellular lipid metabolism. Collectively, these studies have illuminated the key role of miR-33 in regulating lipid and glucose metabolism and how targeting miR-33 might be a useful therapy for treating cardiometabolic disorders.
6. Other miRNAs that regulate ABCA1 expression and HDL metabolism
miR-33 was the first miRNA described to regulate hepatic ABCA1 expression and plasma HDL-C levels in vivo. However, in the last years, it has become clear that ABCA1 expression is highly regulated at the post-transcriptional level by multiple miRNAs, including miR-758, miR-26, miR-106b, miR-27, miR-145, miR-10b, and miR-144.53–58,85,86 These miRNAs can regulate ABCA1 expression and function in a variety of cell types such as macrophages, neurons, pancreatic β-cells, enterocytes, and hepatocytes. The relative importance of these miRNAs in controlling ABCA1 expression will most likely be dictated by their relative abundance and the expression of other miRNA targets in specific cells or tissues. Additionally, the expression of these miRNAs and mRNA targets can also be regulated by physiological stimuli that alter miRNA expression levels. Importantly, several reports have demonstrated that cellular lipid metabolism influences miRNA expression, representing positive or negative feedback models that contribute to the complex regulation of ABCA1 expression. This is the case of miR-758, an intergenic miRNA that, similarly to miR-33, is downregulated after cholesterol loading in macrophages and in the liver of mice fed a high-fat diet.54 Additionally, two reports have recently demonstrated the role of miR-144 in regulating cholesterol metabolism.59,60 miR-144 is synthesized as a polycistronic transcript together with miR-451. In vertebrates, this conserved miRNA cluster plays an important role in eritropoiesis and cancer and was first described to bypass the classic Dicer processing step during miRNA biogenesis.87–89 We identified miR-144 using an unbiased genome-wide screen of miRNAs modulated by LXR ligands in combination with bioinformatic tools for miRNA target predictions. We found that miR-144 directly targets ABCA1 and its overexpression markedly reduces ABCA1 protein levels in human and mouse macrophages and hepatic cell lines. Importantly, our in vivo results indicated that delivery of miR-144 mimics to mice inhibits hepatic ABCA1 expression levels and reduces circulating HDL-C.86 More importantly, inhibition of endogenous miR-144 levels using anti-miR-144 conjugated particles in mice increases hepatic ABCA1 expression and raises plasma HDL levels. In a second report, de Aguiar Vallim et al.60 identified miR-144 using a genome-wide screening aimed at identifying miRNAs regulated by farnesoid X receptor (FXR), a nuclear receptor that controls hepatic sterol and bile acid levels. Similar to our findings, gain- and loss-of-function experiments also showed that changes in hepatic miR-144 levels influence hepatic ABCA1 expression and circulating HDL-C. These results suggest a novel model by which miR-144 contributes to the FXR effect by inhibiting hepatic ABCA1 and promoting the redirection of hepatic cholesterol to biliary excretion.60 The role of miR-144 in regulating plasma HDL levels has also recently been confirmed in a mouse model of atherosclerosis.61 Overexpression of miR-144 accelerates the progression of atherosclerosis by impairing RCT and promoting pro-inflammatory cytokine production in ApoE−/− mice.
A recent study has shown that the intestinal microbiota can regulate RCT by modulating the expression of miR-10b expression. The authors found that protocatechuic acid (PCA), a metabolite produced by the gut microbiota from cyaniding-3 to O-β-glucoside (Cy-3-G) inhibits ABCA1 and ABCG1 expression. Importantly, PCA accelerates macrophage cholesterol efflux and Cy-3-G consumption promotes RCT and regresses atherosclerosis in ApoE−/− mice. miR-145 also regulates ABCA1 expression in HepG2 cells and in murine pancreatic islets. Overexpression of miR-145 inhibits cholesterol efflux in HepG2 cells and causes cholesterol accumulation in pancreatic islets resulting in a marked decrease in glucose-stimulated insulin secretion. Finally, miR-26 and miR-27 inhibit ABCA1 expression and cholesterol efflux in mouse and human macrophage cell lines, respectively.55,56,62
miRNAs also regulate the expression of LXR, thereby controlling the transcriptional activation of ABCA1. LXR is directly targeted by miR-1, miR-206, miR-613, and miR-155.63,64,90 miR-1, miR-206, and miR-613 suppress lipogenesis by inhibiting LXRα and its target genes including SREBP1, acetyl-CoA carboxylase (ACC), and fatty acid synthase (FAS).63,64,90 miR-155 also inhibits LXR expression and its absence confers protection against hepatic steatosis in mice.90 LXR activation also regulates the expression of miRNAs that control the expression levels of some LXR-induced genes such as ABCA1 and the ADP-ribosylation factor-like-7 (ARL7).55 Interestingly, miR-26 expression is inhibited in cells treated with LXR agonists, suggesting that the downregulation of miR-26 might cooperate with the LXR transcriptional activation to increase ABCA1 expression.55
RXRα is also regulated by miRNAs including miR-128-2.91 This miRNA targets RXRα, thus inhibiting LXR-induced ABCA1 expression. Interestingly, miR-128-2 increases SREBP2 expression and decreases SREBP1, ABCA1, ABCG1, and RXRα expression. Overall, these data suggest the existence of a complex miRNA network operating under different physiological conditions in a number of different cell types and tissues to orchestrate post-transcriptional regulation of lipid metabolism (Figure 1).
7. Post-transcriptional regulation of ABCA1 by HuR
Although the importance of miRNAs in the regulation of ABCA1 and ABCG1 expression has been demonstrated in a number of cells and tissues, recent findings suggest that miRNA action might occur in conjunction with RNA-binding proteins (RBPs).92–94 RBPs bind to AU-rich elements (AREs) in the 3′UTR of genes, thereby modulating their expression by increasing or decreasing translation and/or mRNA stability. Around 20 RBPs, including the well-known members of the ELAV family (HuR, HuB, HuC, and HuD), have been identified.95 Interestingly, we have recently found that HuR binds to the 3′UTR of ABCA1 and increases its expression by enhancing protein translation.96 A number of studies have shown that RBP compete or cooperate with miRNAs to control gene expression.97,98 As such, the miRNA-mediated regulation of ABCA1 expression may be influenced by HuR binding to the ARE motifs in the 3′UTR (Figure 2). Further studies would be important to determine whether HuR might compete or cooperate with miRNAs in the regulation of ABCA1 expression.
Figure 2.
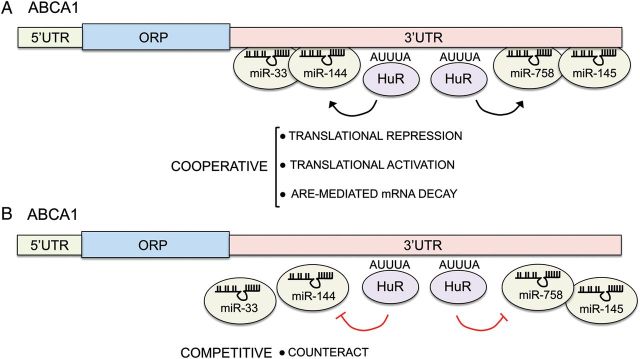
Interplay between miRNAs and HuR in the post-transcriptional regulation of ABCA1 expression. HuR regulates ABCA1 expression at post-transcriptional level. HuR might cooperate with miRNAs to enhance translational repression or activation and ARE-mediated mRNA decay (A) or compete by counteracting the miRNA binding to the 3′UTR (B).
8. miRNA regulation of SRB1 expression
SRB1 regulates the cholesterol transport from HDL to the liver for excretion.35,36 Absence of SRB1 impairs RCT and causes massive atherosclerosis in mice.22 Indeed, SRB1 null mice develop coronary atherosclerosis, a rare phenotype observed in few mouse models of atherosclerosis.22,99,100 SRB1 expression is regulated by different transcription factors including LXR, SREBP, liver receptor homologue 1 (LRH-1), and peroxisome proliferator-activated receptors (PPAR).101 Moreover, SRB1 expression is controlled at the post-transcriptional level by alternative splicing and its interaction with PDX domain containing 1 (PDZK1), a scaffolding protein that regulates SRB1 cellular localization and function.102,103 In addition to this mechanism of regulation, SRB1 expression is also controlled by miRNAs. In this regard, a number of recent studies have uncovered several miRNAs, including miR-455, miR-125a, miR-185, miR-96, and miR-223 that bind directly to the 3′UTR of SRB1 and suppress its expression.65,66 Overexpression of miR-455, miR-125a, miR-185, miR-96, and miR-223 reduces SRB1 protein levels and HDL-C uptake. Conversely, antagonism of these miRNAs enhances SRB1 expression and increases HDL-C uptake. Interestingly, the levels of miR-96 and miR-185 inversely correlate with the increased expression of SRB1 in the livers of ApoE knockout mice fed a high-fat diet.65 Overall, these observations suggest that SRB1 expression is regulated at the post-transcriptional level by alternative splicing, protein localization, and miRNAs.
9. HDL transports endogenous miRNAs and delivers them to recipient cells
In addition to the classic view of HDL as sterol transporter that facilitates the movement if sterols from peripheral cells to the liver for its reutilization or excretion, it is now recognized that HDL function is much more complex in terms of differential lipids and proteins that it transports. Surprisingly, it has been recently reported that HDL can transport miRNAs and deliver them to the receiving cells influencing their gene expression.104 Among of them, miR-223 is one of the most abundant miRNAs (10000 copies/μg of HDL). Notably, HDL-derived miR-223 is transferred to human hepatic cells (Huh7) via SRB1 receptor leading to a significant reduction of miR-223 target genes expression.
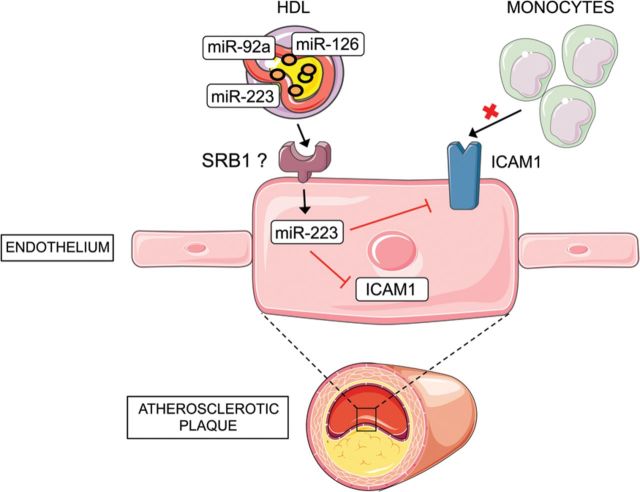
Similarly, HDL-derived miR-223 is also transferred to endothelial cells and inhibits the intracellular adhesion molecule 1 (ICAM-1), thereby reducing monocyte adhesion and inflammation105 (Figure 3). This finding might explain in part the well-known anti-inflammatory effects of HDL. However, additional studies are necessary to address some important questions including the mechanism of miRNA loading in HDL particles and the receptors that mediate the transfer of HDL-derived miRNAs and the recipient cells within the atherosclerotic plaques (endothelial cells, macrophages, and smooth muscle cells).
Figure 3.
HDL-derived miR-223 is transferred to endothelial cells and reduces inflammation. HDL transports miRNAs including miR-92a, miR-126, and miR-223. miR-223 can be transferred to endothelial cells (ECs). The receptor that facilitates the transfer in ECs is unknown but previous studies demonstrate that SRB1 regulates the transport in human hepatic cells (Huh7). miR-223 inhibit ICAM-1 expression in ECs thereby reducing monocyte adhesion and inflammation.
10. Conclusions
miRNAs have emerged as critical regulators of almost all biological processes including lipoprotein metabolism. Work over the last years has demonstrated that miRNAs play an important role in regulating HDL metabolism (Figure 1). A number of genes associated with HDL biogenesis, cellular cholesterol efflux, and biliary secretion are post-trancriptionally regulated by miRNAs. Most of the studies have identified miRNAs that regulate ABCA1 and SRB1 expression (Table 1); however, it is not known whether or not the expression of other key players that control HDL metabolism, such as CETP and LCAT, are modulated by miRNAs. This possibility is unlikely as both genes have a very short 3′UTR (less than 200 nt) and no conserved miRNA-binding sites within their 3′UTR across species.
Antagonizing the expression of some miRNAs, including miR-33, has shown to markedly increase circulating HDL-C in mice and non-human primates. Moreover, anti-miR-33 therapy reduces the progression and enhances the regression of atherosclerosis in mice. These findings suggest that antagonizing a set of miRNAs in the liver to increase ABCA1 and SRB1 expression might enhance RCT. Most of the therapies aimed to increase plasma HDL-C levels, such as CETP inhibitors, fail to protect against coronary artery disease. Moreover, recent results from Mendelian randomization studies also fail to demonstrate an association between circulating HDL-C levels and cardiovascular risk.106 Even thought, these studies argue about the benefit of HDL-C to protect against myocardial infarction, it is clear that HDL-C levels are not necessarily reflective of the broad antiatherogenic properties of HDL particles, including RCT. Indeed, Rader and colleagues107 have shown that the cholesterol efflux capacity from macrophages, a metric of HDL function, has a strong inverse association with both carotid intima-media thickness and the likelihood of angiographic coronary artery disease, independently of HDL-C. It may be possible that anti-miR-33 therapies, which influence HDL metabolism by controlling HDL biogenesis, cellular cholesterol efflux, and bile excretion might be useful for treating dyslipidaemias and cardiovascular related disorders.
Conflict of interest: none declared.
Funding
C.F.-H. laboratory is supported by the National Institutes of Health (R01HL107953 and R01HL106063) and The Leducq Foundation.
References
- 1.Chen HW, Heiniger HJ, Kandutsch AA. Stimulation of sterol and DNA synthesis in leukemic blood cells by low concentrations of phytohemagglutinin. Exp Cell Res. 1977;109:253–262. doi: 10.1016/0014-4827(77)90004-0. [DOI] [PubMed] [Google Scholar]
- 2.Fernandez C, Lobo Md Mdel V, Gomez-Coronado D, Lasuncion MA. Cholesterol is essential for mitosis progression and its deficiency induces polyploid cell formation. Exp Cell Res. 2004;300:109–120. doi: 10.1016/j.yexcr.2004.06.029. [DOI] [PubMed] [Google Scholar]
- 3.Ikonen E. Mechanisms for cellular cholesterol transport: defects and human disease. Physiol Rev. 2006;86:1237–1261. doi: 10.1152/physrev.00022.2005. [DOI] [PubMed] [Google Scholar]
- 4.Glomset JA, Norum KR. The metabolic role of lecithin: cholesterol acyltransferase: perspectives form pathology. Adv Lipid Res. 1973;11:1–65. [PubMed] [Google Scholar]
- 5.Eisenberg S. High density lipoprotein metabolism. J Lipid Res. 1984;25:1017–1058. [PubMed] [Google Scholar]
- 6.Duong PT, Collins HL, Nickel M, Lund-Katz S, Rothblat GH, Phillips MC. Characterization of nascent HDL particles and microparticles formed by ABCA1-mediated efflux of cellular lipids to apoA-I. J Lipid Res. 2006;47:832–843. doi: 10.1194/jlr.M500531-JLR200. [DOI] [PubMed] [Google Scholar]
- 7.Karlsson H, Leanderson P, Tagesson C, Lindahl M. Lipoproteomics II: mapping of proteins in high-density lipoprotein using two-dimensional gel electrophoresis and mass spectrometry. Proteomics. 2005;5:1431–1445. doi: 10.1002/pmic.200401010. [DOI] [PubMed] [Google Scholar]
- 8.Vedhachalam C, Duong PT, Nickel M, Nguyen D, Dhanasekaran P, Saito H, Rothblat GH, Lund-Katz S, Phillips MC. Mechanism of ATP-binding cassette transporter A1-mediated cellular lipid efflux to apolipoprotein A-I and formation of high density lipoprotein particles. J Biol Chem. 2007;282:25123–25130. doi: 10.1074/jbc.M704590200. [DOI] [PubMed] [Google Scholar]
- 9.Bodzioch M, Orso E, Klucken J, Langmann T, Bottcher A, Diederich W, Drobnik W, Barlage S, Buchler C, Porsch-Ozcurumez M, Kaminski WE, Hahmann HW, Oette K, Rothe G, Aslanidis C, Lackner KJ, Schmitz G. The gene encoding ATP-binding cassette transporter 1 is mutated in Tangier disease. Nat Genet. 1999;22:347–351. doi: 10.1038/11914. [DOI] [PubMed] [Google Scholar]
- 10.Brooks-Wilson A, Marcil M, Clee SM, Zhang LH, Roomp K, van Dam M, Yu L, Brewer C, Collins JA, Molhuizen HO, Loubser O, Ouelette BF, Fichter K, Ashbourne-Excoffon KJ, Sensen CW, Scherer S, Mott S, Denis M, Martindale D, Frohlich J, Morgan K, Koop B, Pimstone S, Kastelein JJ, Genest J, Jr, Hayden MR. Mutations in ABC1 in Tangier disease and familial high-density lipoprotein deficiency. Nat Genet. 1999;22:336–345. doi: 10.1038/11905. [DOI] [PubMed] [Google Scholar]
- 11.Rust S, Rosier M, Funke H, Real J, Amoura Z, Piette JC, Deleuze JF, Brewer HB, Duverger N, Denefle P, Assmann G. Tangier disease is caused by mutations in the gene encoding ATP-binding cassette transporter 1. Nat Genet. 1999;22:352–355. doi: 10.1038/11921. [DOI] [PubMed] [Google Scholar]
- 12.Gelissen IC, Harris M, Rye KA, Quinn C, Brown AJ, Kockx M, Cartland S, Packianathan M, Kritharides L, Jessup W. ABCA1 and ABCG1 synergize to mediate cholesterol export to apoA-I. Arterioscler Thromb Vasc Biol. 2006;26:534–540. doi: 10.1161/01.ATV.0000200082.58536.e1. [DOI] [PubMed] [Google Scholar]
- 13.Tarling EJ, Edwards PA. ATP binding cassette transporter G1 (ABCG1) is an intracellular sterol transporter. Proc Natl Acad Sci USA. 2011;108:19719–19724. doi: 10.1073/pnas.1113021108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baldan A, Tarr P, Vales CS, Frank J, Shimotake TK, Hawgood S, Edwards PA. Deletion of the transmembrane transporter ABCG1 results in progressive pulmonary lipidosis. J Biol Chem. 2006;281:29401–29410. doi: 10.1074/jbc.M606597200. [DOI] [PubMed] [Google Scholar]
- 15.Westerterp M, Murphy AJ, Wang M, Pagler TA, Vengrenyuk Y, Kappus MS, Gorman DJ, Nagareddy PR, Zhu X, Abramowicz S, Parks JS, Welch C, Fisher EA, Wang N, Yvan-Charvet L, Tall AR. Deficiency of ATP-binding cassette transporters A1 and G1 in macrophages increases inflammation and accelerates atherosclerosis in mice. Circ Res. 2013;112:1456–1465. doi: 10.1161/CIRCRESAHA.113.301086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aiello RJ, Brees D, Bourassa PA, Royer L, Lindsey S, Coskran T, Haghpassand M, Francone OL. Increased atherosclerosis in hyperlipidemic mice with inactivation of ABCA1 in macrophages. Arterioscler Thromb Vasc Biol. 2002;22:630–637. doi: 10.1161/01.atv.0000014804.35824.da. [DOI] [PubMed] [Google Scholar]
- 17.Out R, Hoekstra M, Hildebrand RB, Kruit JK, Meurs I, Li Z, Kuipers F, Van Berkel TJ, Van Eck M. Macrophage ABCG1 deletion disrupts lipid homeostasis in alveolar macrophages and moderately influences atherosclerotic lesion development in LDL receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2006;26:2295–2300. doi: 10.1161/01.ATV.0000237629.29842.4c. [DOI] [PubMed] [Google Scholar]
- 18.Ranalletta M, Wang N, Han S, Yvan-Charvet L, Welch C, Tall AR. Decreased atherosclerosis in low-density lipoprotein receptor knockout mice transplanted with Abcg1-/- bone marrow. Arterioscler Thromb Vasc Biol. 2006;26:2308–2315. doi: 10.1161/01.ATV.0000242275.92915.43. [DOI] [PubMed] [Google Scholar]
- 19.Bi X, Zhu X, Duong M, Boudyguina EY, Wilson MD, Gebre AK, Parks JS. Liver ABCA1 deletion in LDLrKO mice does not impair macrophage reverse cholesterol transport or exacerbate atherogenesis. Arterioscler Thromb Vasc Biol. 2013;33:2288–2296. doi: 10.1161/ATVBAHA.112.301110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ji Y, Jian B, Wang N, Sun Y, Moya ML, Phillips MC, Rothblat GH, Swaney JB, Tall AR. Scavenger receptor BI promotes high density lipoprotein-mediated cellular cholesterol efflux. J Biol Chem. 1997;272:20982–20985. doi: 10.1074/jbc.272.34.20982. [DOI] [PubMed] [Google Scholar]
- 21.Jian B, de la Llera-Moya M, Ji Y, Wang N, Phillips MC, Swaney JB, Tall AR, Rothblat GH. Scavenger receptor class B type I as a mediator of cellular cholesterol efflux to lipoproteins and phospholipid acceptors. J Biol Chem. 1998;273:5599–5606. doi: 10.1074/jbc.273.10.5599. [DOI] [PubMed] [Google Scholar]
- 22.Braun A, Trigatti BL, Post MJ, Sato K, Simons M, Edelberg JM, Rosenberg RD, Schrenzel M, Krieger M. Loss of SR-BI expression leads to the early onset of occlusive atherosclerotic coronary artery disease, spontaneous myocardial infarctions, severe cardiac dysfunction, and premature death in apolipoprotein E-deficient mice. Circ Res. 2002;90:270–276. doi: 10.1161/hh0302.104462. [DOI] [PubMed] [Google Scholar]
- 23.Rigotti A, Trigatti BL, Penman M, Rayburn H, Herz J, Krieger M. A targeted mutation in the murine gene encoding the high density lipoprotein (HDL) receptor scavenger receptor class B type I reveals its key role in HDL metabolism. Proc Natl Acad Sci USA. 1997;94:12610–12615. doi: 10.1073/pnas.94.23.12610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fielding CJ, Shore VG, Fielding PE. A protein cofactor of lecithin:cholesterol acyltransferase. Biochem Biophys Res Commun. 1972;46:1493–1498. doi: 10.1016/0006-291x(72)90776-0. [DOI] [PubMed] [Google Scholar]
- 25.Jonas A. Lecithin-cholesterol acyltransferase in the metabolism of high-density lipoproteins. Biochim Biophys Acta. 1991;1084:205–220. doi: 10.1016/0005-2760(91)90062-m. [DOI] [PubMed] [Google Scholar]
- 26.Glomset JA. The plasma lecithins:cholesterol acyltransferase reaction. J Lipid Res. 1968;9:155–167. [PubMed] [Google Scholar]
- 27.Fielding CJ, Fielding PE. Molecular physiology of reverse cholesterol transport. J Lipid Res. 1995;36:211–228. [PubMed] [Google Scholar]
- 28.Tall AR. Plasma cholesteryl ester transfer protein. J Lipid Res. 1993;34:1255–1274. [PubMed] [Google Scholar]
- 29.Connelly PW. The role of hepatic lipase in lipoprotein metabolism. Clin Chim Acta. 1999;286:243–255. doi: 10.1016/s0009-8981(99)00105-9. [DOI] [PubMed] [Google Scholar]
- 30.Shimizu M, Kanazawa K, Hirata K, Ishida T, Hiraoka E, Matsuda Y, Iwai C, Miyamoto Y, Hashimoto M, Kajiya T, Akita H, Yokoyama M. Endothelial lipase gene polymorphism is associated with acute myocardial infarction, independently of high-density lipoprotein-cholesterol levels. Circ J. 2007;71:842–846. doi: 10.1253/circj.71.842. [DOI] [PubMed] [Google Scholar]
- 31.de Aguiar Vallim TQ, Tarling EJ, Edwards PA. Pleiotropic roles of bile acids in metabolism. Cell Metab. 2013;17:657–669. doi: 10.1016/j.cmet.2013.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Esteller A. Physiology of bile secretion. World J Gastroenterol. 2008;14:5641–5649. doi: 10.3748/wjg.14.5641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paulusma CC, Folmer DE, Ho-Mok KS, de Waart DR, Hilarius PM, Verhoeven AJ, Oude Elferink RP. ATP8B1 requires an accessory protein for endoplasmic reticulum exit and plasma membrane lipid flippase activity. Hepatology. 2008;47:268–278. doi: 10.1002/hep.21950. [DOI] [PubMed] [Google Scholar]
- 34.Paulusma CC, Groen A, Kunne C, Ho-Mok KS, Spijkerboer AL, Rudi de Waart D, Hoek FJ, Vreeling H, Hoeben KA, van Marle J, Pawlikowska L, Bull LN, Hofmann AF, Knisely AS, Oude Elferink RP. Atp8b1 deficiency in mice reduces resistance of the canalicular membrane to hydrophobic bile salts and impairs bile salt transport. Hepatology. 2006;44:195–204. doi: 10.1002/hep.21212. [DOI] [PubMed] [Google Scholar]
- 35.Acton S, Rigotti A, Landschulz KT, Xu S, Hobbs HH, Krieger M. Identification of scavenger receptor SR-BI as a high density lipoprotein receptor. Science. 1996;271:518–520. doi: 10.1126/science.271.5248.518. [DOI] [PubMed] [Google Scholar]
- 36.Kozarsky KF, Donahee MH, Rigotti A, Iqbal SN, Edelman ER, Krieger M. Overexpression of the HDL receptor SR-BI alters plasma HDL and bile cholesterol levels. Nature. 1997;387:414–417. doi: 10.1038/387414a0. [DOI] [PubMed] [Google Scholar]
- 37.Tontonoz P. Transcriptional and posttranscriptional control of cholesterol homeostasis by liver X receptors. Cold Spring Harb Symp Quant Biol. 2011;76:129–137. doi: 10.1101/sqb.2011.76.010702. [DOI] [PubMed] [Google Scholar]
- 38.Venkateswaran A, Laffitte BA, Joseph SB, Mak PA, Wilpitz DC, Edwards PA, Tontonoz P. Control of cellular cholesterol efflux by the nuclear oxysterol receptor LXR alpha. Proc Natl Acad Sci USA. 2000;97:12097–12102. doi: 10.1073/pnas.200367697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Willy PJ, Umesono K, Ong ES, Evans RM, Heyman RA, Mangelsdorf DJ. LXR, a nuclear receptor that defines a distinct retinoid response pathway. Genes Dev. 1995;9:1033–1045. doi: 10.1101/gad.9.9.1033. [DOI] [PubMed] [Google Scholar]
- 40.Bradley MN, Hong C, Chen M, Joseph SB, Wilpitz DC, Wang X, Lusis AJ, Collins A, Hseuh WA, Collins JL, Tangirala RK, Tontonoz P. Ligand activation of LXR beta reverses atherosclerosis and cellular cholesterol overload in mice lacking LXR alpha and apoE. J Clin Invest. 2007;117:2337–2346. doi: 10.1172/JCI31909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peet DJ, Turley SD, Ma W, Janowski BA, Lobaccaro JM, Hammer RE, Mangelsdorf DJ. Cholesterol and bile acid metabolism are impaired in mice lacking the nuclear oxysterol receptor LXR alpha. Cell. 1998;93:693–704. doi: 10.1016/s0092-8674(00)81432-4. [DOI] [PubMed] [Google Scholar]
- 42.Fernandez-Hernando C, Ramirez CM, Goedeke L, Suarez Y. MicroRNAs in metabolic disease. Arterioscler Thromb Vasc Biol. 2013;33:178–185. doi: 10.1161/ATVBAHA.112.300144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fernandez-Hernando C, Suarez Y, Rayner KJ, Moore KJ. MicroRNAs in lipid metabolism. Curr Opin Lipidol. 2011;22:86–92. doi: 10.1097/MOL.0b013e3283428d9d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moore KJ, Rayner KJ, Suarez Y, Fernandez-Hernando C. microRNAs and cholesterol metabolism. Trends Endocrinol Metab. 2010;21:699–706. doi: 10.1016/j.tem.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marquart TJ, Allen RM, Ory DS, Baldan A. miR-33 links SREBP-2 induction to repression of sterol transporters. Proc Natl Acad Sci USA. 2010;107:12228–12232. doi: 10.1073/pnas.1005191107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Najafi-Shoushtari SH, Kristo F, Li Y, Shioda T, Cohen DE, Gerszten RE, Naar AM. MicroRNA-33 and the SREBP host genes cooperate to control cholesterol homeostasis. Science. 2010;328:1566–1569. doi: 10.1126/science.1189123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rayner KJ, Suarez Y, Davalos A, Parathath S, Fitzgerald ML, Tamehiro N, Fisher EA, Moore KJ, Fernandez-Hernando C. MiR-33 contributes to the regulation of cholesterol homeostasis. Science. 2010;328:1570–1573. doi: 10.1126/science.1189862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Davalos A, Goedeke L, Smibert P, Ramirez CM, Warrier NP, Andreo U, Cirera-Salinas D, Rayner K, Suresh U, Pastor-Pareja JC, Esplugues E, Fisher EA, Penalva LO, Moore KJ, Suarez Y, Lai EC, Fernandez-Hernando C. miR-33a/b contribute to the regulation of fatty acid metabolism and insulin signaling. Proc Natl Acad Sci USA. 2011;108:9232–9237. doi: 10.1073/pnas.1102281108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Allen RM, Marquart TJ, Albert CJ, Suchy FJ, Wang DQ, Ananthanarayanan M, Ford DA, Baldan A. miR-33 controls the expression of biliary transporters, and mediates statin- and diet-induced hepatotoxicity. EMBO Mol Med. 2012;4:882–895. doi: 10.1002/emmm.201201228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li T, Francl JM, Boehme S, Chiang JY. Regulation of cholesterol and bile acid homeostasis by the cholesterol 7alpha-hydroxylase/steroid response element-binding protein 2/microRNA-33a axis in mice. Hepatology. 2013;58:1111–1121. doi: 10.1002/hep.26427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Horie T, Ono K, Horiguchi M, Nishi H, Nakamura T, Nagao K, Kinoshita M, Kuwabara Y, Marusawa H, Iwanaga Y, Hasegawa K, Yokode M, Kimura T, Kita T. MicroRNA-33 encoded by an intron of sterol regulatory element-binding protein 2 (Srebp2) regulates HDL in vivo. Proc Natl Acad Sci USA. 2010;107:17321–17326. doi: 10.1073/pnas.1008499107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gerin I, Clerbaux LA, Haumont O, Lanthier N, Das AK, Burant CF, Leclercq IA, MacDougald OA, Bommer GT. Expression of miR-33 from an SREBP2 intron inhibits cholesterol export and fatty acid oxidation. J Biol Chem. 2010;285:33652–33661. doi: 10.1074/jbc.M110.152090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Goedeke L, Vales-Lara FM, Fenstermaker M, Cirera-Salinas D, Chamorro-Jorganes A, Ramirez CM, Mattison JA, de Cabo R, Suarez Y, Fernandez-Hernando C. A regulatory role for microRNA 33* in controlling lipid metabolism gene expression. Mol Cell Biol. 2013;33:2339–2352. doi: 10.1128/MCB.01714-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ramirez CM, Davalos A, Goedeke L, Salerno AG, Warrier N, Cirera-Salinas D, Suarez Y, Fernandez-Hernando C. MicroRNA-758 regulates cholesterol efflux through posttranscriptional repression of ATP-binding cassette transporter A1. Arterioscler Thromb Vasc Biol. 2011;31:2707–2714. doi: 10.1161/ATVBAHA.111.232066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sun D, Zhang J, Xie J, Wei W, Chen M, Zhao X. MiR-26 controls LXR-dependent cholesterol efflux by targeting ABCA1 and ARL7. FEBS Lett. 2012;586:1472–1479. doi: 10.1016/j.febslet.2012.03.068. [DOI] [PubMed] [Google Scholar]
- 56.Kang MH, Zhang LH, Wijesekara N, de Haan W, Butland S, Bhattacharjee A, Hayden MR. Regulation of ABCA1 protein expression and function in hepatic and pancreatic islet cells by miR-145. Arterioscler Thromb Vasc Biol. 2013;33:2724–2732. doi: 10.1161/ATVBAHA.113.302004. [DOI] [PubMed] [Google Scholar]
- 57.Kim J, Yoon H, Ramirez CM, Lee SM, Hoe HS, Fernandez-Hernando C. MiR-106b impairs cholesterol efflux and increases Abeta levels by repressing ABCA1 expression. Exp Neurol. 2012;235:476–483. doi: 10.1016/j.expneurol.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang D, Xia M, Yan X, Li D, Wang L, Xu Y, Jin T, Ling W. Gut microbiota metabolism of anthocyanin promotes reverse cholesterol transport in mice via repressing miRNA-10b. Circ Res. 2012;111:967–981. doi: 10.1161/CIRCRESAHA.112.266502. [DOI] [PubMed] [Google Scholar]
- 59.Ramirez CM, Rotllan N, Vlassov AV, Davalos A, Li M, Goedeke L, Aranda JF, Cirera-Salinas D, Araldi E, Salerno A, Wanschel A, Zavadil J, Castrillo A, Kim J, Suarez Y, Fernandez-Hernando C. Control of cholesterol metabolism and plasma high-density lipoprotein levels by microRNA-144. Circ Res. 2013;112:1592–1601. doi: 10.1161/CIRCRESAHA.112.300626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.de Aguiar Vallim TQ, Tarling EJ, Kim T, Civelek M, Baldan A, Esau C, Edwards PA. MicroRNA-144 regulates hepatic ATP binding cassette transporter A1 and plasma high-density lipoprotein after activation of the nuclear receptor farnesoid X receptor. Circ Res. 2013;112:1602–1612. doi: 10.1161/CIRCRESAHA.112.300648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hu YW, Hu YR, Zhao JY, Li SF, Ma X, Wu SG, Lu JB, Qiu YR, Sha YH, Wang YC, Gao JJ, Zheng L, Wang Q. An Agomir of miR-144–3p accelerates plaque formation through impairing reverse cholesterol transport and promoting pro-inflammatory cytokine production. PLoS ONE. 2014;9:e94997. doi: 10.1371/journal.pone.0094997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang M, Wu JF, Chen WJ, Tang SL, Mo ZC, Tang YY, Li Y, Wang JL, Liu XY, Peng J, Chen K, He PP, Lv YC, Ouyang XP, Yao F, Tang DP, Cayabyab FS, Zhang DW, Zheng XL, Tian GP, Tang CK. MicroRNA-27a/b regulates cellular cholesterol efflux, influx and esterification/hydrolysis in THP-1 macrophages. Atherosclerosis. 2014;234:54–64. doi: 10.1016/j.atherosclerosis.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 63.Zhong D, Huang G, Zhang Y, Zeng Y, Xu Z, Zhao Y, He X, He F. MicroRNA-1 and microRNA-206 suppress LXRalpha-induced lipogenesis in hepatocytes. Cell Signal. 2013;25:1429–1437. doi: 10.1016/j.cellsig.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 64.Ou Z, Wada T, Gramignoli R, Li S, Strom SC, Huang M, Xie W. MicroRNA hsa-miR-613 targets the human LXRalpha gene and mediates a feedback loop of LXRalpha autoregulation. Mol Endocrinol. 2011;25:584–596. doi: 10.1210/me.2010-0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang L, Jia XJ, Jiang HJ, Du Y, Yang F, Si SY, Hong B. MicroRNAs 185, 96, and 223 repress selective high-density lipoprotein cholesterol uptake through posttranscriptional inhibition. Mol Cell Biol. 2013;33:1956–1964. doi: 10.1128/MCB.01580-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hu Z, Shen WJ, Kraemer FB, Azhar S. MicroRNAs 125a and 455 repress lipoprotein-supported steroidogenesis by targeting scavenger receptor class B type I in steroidogenic cells. Mol Cell Biol. 2012;32:5035–5045. doi: 10.1128/MCB.01002-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 68.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 69.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Filipowicz W, Bhattacharyya SN, Sonenberg N. Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nat Rev Genet. 2008;9:102–114. doi: 10.1038/nrg2290. [DOI] [PubMed] [Google Scholar]
- 71.Elmen J, Lindow M, Silahtaroglu A, Bak M, Christensen M, Lind-Thomsen A, Hedtjarn M, Hansen JB, Hansen HF, Straarup EM, McCullagh K, Kearney P, Kauppinen S. Antagonism of microRNA-122 in mice by systemically administered LNA-antimiR leads to up-regulation of a large set of predicted target mRNAs in the liver. Nucleic Acids Res. 2008;36:1153–1162. doi: 10.1093/nar/gkm1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Esau C, Davis S, Murray SF, Yu XX, Pandey SK, Pear M, Watts L, Booten SL, Graham M, McKay R, Subramaniam A, Propp S, Lollo BA, Freier S, Bennett CF, Bhanot S, Monia BP. miR-122 regulation of lipid metabolism revealed by in vivo antisense targeting. Cell Metab. 2006;3:87–98. doi: 10.1016/j.cmet.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 73.Soh J, Iqbal J, Queiroz J, Fernandez-Hernando C, Hussain MM. MicroRNA-30c reduces hyperlipidemia and atherosclerosis in mice by decreasing lipid synthesis and lipoprotein secretion. Nat Med. 2013;19:892–900. doi: 10.1038/nm.3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest. 2002;109:1125–1131. doi: 10.1172/JCI15593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yokoyama C, Wang X, Briggs MR, Admon A, Wu J, Hua X, Goldstein JL, Brown MS. SREBP-1, a basic-helix-loop-helix-leucine zipper protein that controls transcription of the low density lipoprotein receptor gene. Cell. 1993;75:187–197. [PubMed] [Google Scholar]
- 76.Rayner KJ, Esau CC, Hussain FN, McDaniel AL, Marshall SM, van Gils JM, Ray TD, Sheedy FJ, Goedeke L, Liu X, Khatsenko OG, Kaimal V, Lees CJ, Fernandez-Hernando C, Fisher EA, Temel RE, Moore KJ. Inhibition of miR-33a/b in non-human primates raises plasma HDL and lowers VLDL triglycerides. Nature. 2011;478:404–407. doi: 10.1038/nature10486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rottiers V, Obad S, Petri A, McGarrah R, Lindholm MW, Black JC, Sinha S, Goody RJ, Lawrence MS, deLemos AS, Hansen HF, Whittaker S, Henry S, Brookes R, Najafi-Shoushtari SH, Chung RT, Whetstine JR, Gerszten RE, Kauppinen S, Naar AM. Pharmacological inhibition of a microRNA family in nonhuman primates by a seed-targeting 8-mer antimiR. Sci Transl Med. 2013;5:212. doi: 10.1126/scitranslmed.3006840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Marquart TJ, Wu J, Lusis AJ, Baldan A. Anti-miR-33 therapy does not alter the progression of atherosclerosis in low-density lipoprotein receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2013;33:455–458. doi: 10.1161/ATVBAHA.112.300639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rayner KJ, Sheedy FJ, Esau CC, Hussain FN, Temel RE, Parathath S, van Gils JM, Rayner AJ, Chang AN, Suarez Y, Fernandez-Hernando C, Fisher EA, Moore KJ. Antagonism of miR-33 in mice promotes reverse cholesterol transport and regression of atherosclerosis. J Clin Invest. 2011;121:2921–2931. doi: 10.1172/JCI57275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rotllan N, Ramirez CM, Aryal B, Esau CC, Fernandez-Hernando C. Therapeutic silencing of microRNA-33 inhibits the progression of atherosclerosis in Ldlr-/- mice--brief report. Arterioscler Thromb Vasc Biol. 2013;33:1973–1977. doi: 10.1161/ATVBAHA.113.301732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Horie T, Baba O, Kuwabara Y, Chujo Y, Watanabe S, Kinoshita M, Horiguchi M, Nakamura T, Chonabayashi K, Hishizawa M, Hasegawa K, Kume N, Yokode M, Kita T, Kimura T, Ono K. MicroRNA-33 deficiency reduces the progression of atherosclerotic plaque in ApoE-/- mice. J Am Heart Assoc. 2012;1:e003376. doi: 10.1161/JAHA.112.003376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wijesekara N, Zhang LH, Kang MH, Abraham T, Bhattacharjee A, Warnock GL, Verchere CB, Hayden MR. miR-33a modulates ABCA1 expression, cholesterol accumulation, and insulin secretion in pancreatic islets. Diabetes. 2012;61:653–658. doi: 10.2337/db11-0944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Horie T, Nishino T, Baba O, Kuwabara Y, Nakao T, Nishiga M, Usami S, Izuhara M, Sowa N, Yahagi N, Shimano H, Matsumura S, Inoue K, Marusawa H, Nakamura T, Hasegawa K, Kume N, Yokode M, Kita T, Kimura T, Ono K. MicroRNA-33 regulates sterol regulatory element-binding protein 1 expression in mice. Nat Commun. 2013;4:2883. doi: 10.1038/ncomms3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ramirez CM, Goedeke L, Rotllan N, Yoon JH, Cirera-Salinas D, Mattison JA, Suarez Y, de Cabo R, Gorospe M, Fernandez-Hernando C. MicroRNA 33 regulates glucose metabolism. Mol Cell Biol. 2013;33:2891–2902. doi: 10.1128/MCB.00016-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.de Aguiar Vallim T, Tarling E, Kim T, Civelek M, Baldan A, Esau C, Edwards P. MicroRNA-144 regulates hepatic ABCA1 and plasma HDL following activation of the nuclear receptor FXR. Circ Res. 2013;112:1602–1612. doi: 10.1161/CIRCRESAHA.112.300648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ramirez CM, Rotllan N, Vlassov AV, Davalos A, Li M, Goedeke L, Aranda JF, Cirera-Salinas D, Araldi E, Salerno A, Wanschel AC, Zavadil J, Castrillo A, Jungsu K, Suarez Y, Fernandez-Hernando C. Control of cholesterol metabolism and plasma HDL levels by miRNA-144. Circ Res. 2013;112:1592–1601. doi: 10.1161/CIRCRESAHA.112.300626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cheloufi S, Dos Santos CO, Chong MM, Hannon GJ. A dicer-independent miRNA biogenesis pathway that requires Ago catalysis. Nature. 2010;465:584–589. doi: 10.1038/nature09092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cifuentes D, Xue H, Taylor DW, Patnode H, Mishima Y, Cheloufi S, Ma E, Mane S, Hannon GJ, Lawson ND, Wolfe SA, Giraldez AJ. A novel miRNA processing pathway independent of Dicer requires Argonaute2 catalytic activity. Science. 2010;328:1694–1698. doi: 10.1126/science.1190809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yang JS, Maurin T, Robine N, Rasmussen KD, Jeffrey KL, Chandwani R, Papapetrou EP, Sadelain M, O'Carroll D, Lai EC. Conserved vertebrate mir-451 provides a platform for Dicer-independent, Ago2-mediated microRNA biogenesis. Proc Natl Acad Sci USA. 2010;107:15163–15168. doi: 10.1073/pnas.1006432107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Miller AM, Gilchrist DS, Nijjar J, Araldi E, Ramirez CM, Lavery CA, Fernandez-Hernando C, McInnes IB, Kurowska-Stolarska M. MiR-155 has a protective role in the development of non-alcoholic hepatosteatosis in mice. PLoS ONE. 2013;8:e72324. doi: 10.1371/journal.pone.0072324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Adlakha YK, Khanna S, Singh R, Singh VP, Agrawal A, Saini N. Pro-apoptotic miRNA-128–2 modulates ABCA1, ABCG1 and RXRalpha expression and cholesterol homeostasis. Cell Death Dis. 2013;4:e780. doi: 10.1038/cddis.2013.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ciafre SA, Galardi S. microRNAs and RNA-binding proteins: a complex network of interactions and reciprocal regulations in cancer. RNA Biol. 2013;10:935–942. doi: 10.4161/rna.24641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ho JJ, Marsden PA. Competition and collaboration between RNA-binding proteins and microRNAs. RNA. 2014;5:69–86. doi: 10.1002/wrna.1197. [DOI] [PubMed] [Google Scholar]
- 94.Srikantan S, Tominaga K, Gorospe M. Functional interplay between RNA-binding protein HuR and microRNAs. Curr Protein Pept Sci. 2012;13:372–379. doi: 10.2174/138920312801619394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Srikantan S, Gorospe M. HuR function in disease. Front Biosci. 2012;17:189–205. doi: 10.2741/3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ramirez CM, Lin CS, Abdelmohsen K, Goedeke L, Yoon JH, Madrigal-Matute J, Martin-Ventura JL, Vo DT, Uren PJ, Penalva LO, Gorospe M, Fernandez-Hernando C. RNA-binding protein HuR regulates expression of ABCA1. J Lipid Res. 2014;55:1066–1076. doi: 10.1194/jlr.M044925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bhattacharyya SN, Habermacher R, Martine U, Closs EI, Filipowicz W. Relief of microRNA-mediated translational repression in human cells subjected to stress. Cell. 2006;125:1111–1124. doi: 10.1016/j.cell.2006.04.031. [DOI] [PubMed] [Google Scholar]
- 98.Eiring AM, Harb JG, Neviani P, Garton C, Oaks JJ, Spizzo R, Liu S, Schwind S, Santhanam R, Hickey CJ, Becker H, Chandler JC, Andino R, Cortes J, Hokland P, Huettner CS, Bhatia R, Roy DC, Liebhaber SA, Caligiuri MA, Marcucci G, Garzon R, Croce CM, Calin GA, Perrotti D. miR-328 functions as an RNA decoy to modulate hnRNP E2 regulation of mRNA translation in leukemic blasts. Cell. 2010;140:652–665. doi: 10.1016/j.cell.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fernandez-Hernando C, Ackah E, Yu J, Suarez Y, Murata T, Iwakiri Y, Prendergast J, Miao RQ, Birnbaum MJ, Sessa WC. Loss of Akt1 leads to severe atherosclerosis and occlusive coronary artery disease. Cell Metab. 2007;6:446–457. doi: 10.1016/j.cmet.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kuhlencordt PJ, Gyurko R, Han F, Scherrer-Crosbie M, Aretz TH, Hajjar R, Picard MH, Huang PL. Accelerated atherosclerosis, aortic aneurysm formation, and ischemic heart disease in apolipoprotein E/endothelial nitric oxide synthase double-knockout mice. Circulation. 2001;104:448–454. doi: 10.1161/hc2901.091399. [DOI] [PubMed] [Google Scholar]
- 101.Rigotti A, Miettinen HE, Krieger M. The role of the high-density lipoprotein receptor SR-BI in the lipid metabolism of endocrine and other tissues. Endocr Rev. 2003;24:357–387. doi: 10.1210/er.2001-0037. [DOI] [PubMed] [Google Scholar]
- 102.Kocher O, Krieger M. Role of the adaptor protein PDZK1 in controlling the HDL receptor SR-BI. Curr Opin Lipidol. 2009;20:236–241. doi: 10.1097/MOL.0b013e32832aee82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kocher O, Yesilaltay A, Cirovic C, Pal R, Rigotti A, Krieger M. Targeted disruption of the PDZK1 gene in mice causes tissue-specific depletion of the high density lipoprotein receptor scavenger receptor class B type I and altered lipoprotein metabolism. J Biol Chem. 2003;278:52820–52825. doi: 10.1074/jbc.M310482200. [DOI] [PubMed] [Google Scholar]
- 104.Vickers KC, Palmisano BT, Shoucri BM, Shamburek RD, Remaley AT. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat Cell Biol. 2011;13:423–433. doi: 10.1038/ncb2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tabet F, Vickers KC, Cuesta Torres LF, Wiese CB, Shoucri BM, Lambert G, Catherinet C, Prado-Lourenco L, Levin MG, Thacker S, Sethupathy P, Barter PJ, Remaley AT, Rye KA. HDL-transferred microRNA-223 regulates ICAM-1 expression in endothelial cells. Nat Commun. 2014;5:3292. doi: 10.1038/ncomms4292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Voight BF, Peloso GM, Orho-Melander M, Frikke-Schmidt R, Barbalic M, Jensen MK, Hindy G, Holm H, Ding EL, Johnson T, Schunkert H, Samani NJ, Clarke R, Hopewell JC, Thompson JF, Li M, Thorleifsson G, Newton-Cheh C, Musunuru K, Pirruccello JP, Saleheen D, Chen L, Stewart A, Schillert A, Thorsteinsdottir U, Thorgeirsson G, Anand S, Engert JC, Morgan T, Spertus J, Stoll M, Berger K, Martinelli N, Girelli D, McKeown PP, Patterson CC, Epstein SE, Devaney J, Burnett MS, Mooser V, Ripatti S, Surakka I, Nieminen MS, Sinisalo J, Lokki ML, Perola M, Havulinna A, de Faire U, Gigante B, Ingelsson E, Zeller T, Wild P, de Bakker PI, Klungel OH, Maitland-van der Zee AH, Peters BJ, de Boer A, Grobbee DE, Kamphuisen PW, Deneer VH, Elbers CC, Onland-Moret NC, Hofker MH, Wijmenga C, Verschuren WM, Boer JM, van der Schouw YT, Rasheed A, Frossard P, Demissie S, Willer C, Do R, Ordovas JM, Abecasis GR, Boehnke M, Mohlke KL, Daly MJ, Guiducci C, Burtt NP, Surti A, Gonzalez E, Purcell S, Gabriel S, Marrugat J, Peden J, Erdmann J, Diemert P, Willenborg C, Konig IR, Fischer M, Hengstenberg C, Ziegler A, Buysschaert I, Lambrechts D, Van de Werf F, Fox KA, El Mokhtari NE, Rubin D, Schrezenmeir J, Schreiber S, Schafer A, Danesh J, Blankenberg S, Roberts R, McPherson R, Watkins H, Hall AS, Overvad K, Rimm E, Boerwinkle E, Tybjaerg-Hansen A, Cupples LA, Reilly MP, Melander O, Mannucci PM, Ardissino D, Siscovick D, Elosua R, Stefansson K, O'Donnell CJ, Salomaa V, Rader DJ, Peltonen L, Schwartz SM, Altshuler D, Kathiresan S. Plasma HDL cholesterol and risk of myocardial infarction: a mendelian randomisation study. Lancet. 2012;380:572–580. doi: 10.1016/S0140-6736(12)60312-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Khera AV, Cuchel M, de la Llera-Moya M, Rodrigues A, Burke MF, Jafri K, French BC, Phillips JA, Mucksavage ML, Wilensky RL, Mohler ER, Rothblat GH, Rader DJ. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Eng J Med. 2011;364:127–135. doi: 10.1056/NEJMoa1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]