Abstract
Biomineralization at pathological extraosseous sites (i.e. vasculature and soft tissues) is associated with increased morbidity and mortality. So-called ‘nanobacteria’ have been described as pathogenic agents causing many diseases including calcification. Initially, their appearance, and having a content consisting of nucleic acids plus proteins and properties of growing structures, suggested that they were living organisms. However, it could be demonstrated that the so-called nanobacteria were in fact mineralizing nanoparticles that contain mineral and non-mineral compounds, that these particles bind to charged molecules and that supersaturation enables in vitro growth of these nanoparticles. Recent data indicate that nanoparticles consisting of protein–mineral complexes can be seen both in vitro and in vivo as precursors of matrix calcification.
Keywords: biomineralization, nanobacteria, nanoparticles, vascular calcification
Introduction
In living vertebrates, biomineralization is a highly regulated cell-autonomous process, usually restricted to the skeleton and teeth. However, biomineralization may also occur as pathological extraosseous calcification in the vasculature or soft tissues, leading to an increased morbidity and/or mortality. Until approximately one decade ago, extraosseous calcification was mainly studied with regard to the chemical process of precipitation due to supersaturation of calcium and phosphate ions. Since it was discovered that tissues involved in pathological calcification also expressed genes initially discovered in bone metabolism, these putative osteogenic processes (active calcification) contrasted with the chemical precipitation of calcium salts (passive calcification). It is most likely that both processes contribute to extraosseous calcification [1, 2]. It is well-known that calcium, phosphate and mineralizable matrix-like collagen fibres are sufficient to induce tissue calcification in the absence of osteoblasts [3]. Dead cells and necrotic tissues form an excellent mineralizable matrix, and in this case, the process is called ‘dystrophic calcification’. One and a half decades ago, so-called ‘nanobacteria’ were described as pathogenic agents causing calcification. However, recent results demonstrate that this approach was merely a ‘red herring’, which put us on the wrong track.
The discovery of nanobacteria and evidence for their existence
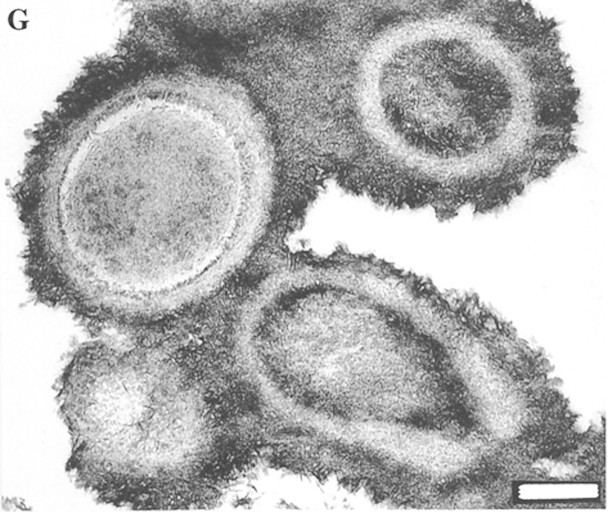
Around 15 years ago, nanoscopic life forms called nanobacteria entered the stage [4, 5] and eventually were described as the causative agent of many diseases including calcification. Initially, Folk [4] had studied rock specimens by electron microscopy and observed tiny structures with a cell-like appearance i.e. resembling cell walls and filamentous surface projections. These particles were only 10–200 nm in size and thus were much smaller than any other known bacteria. Therefore, Folk [4] called these tiny particles nanobacteria. A few years later, McKay et al. [5] observed similar tiny structures within a Martian meteorite, which caused considerable excitement, as these and other structures pointed to the possibility of extraterrestrial life. In 1998, Finnish researchers described similar structures (50–500 nm) with nucleic acids and proteins as potential pathogens in their cell culture (Figure 1) [6]. Interestingly, these small particles could change from small spherical bodies to films and clumps of mineralized material containing hydroxyapatite, the main mineral of bone. Further research revealed that similar structures existed in body fluids including blood and urine. These entities were deemed ‘infectious’ because ‘inoculating’ mineral-containing fluids with these entities caused a slow ‘reproduction’ of these entities. Ultimately, nanobacteria were considered the causative pathogens for many diseases from kidney stones to cancer [7, 8]. Polycystic kidney disease was assumed to be caused by these agents [9], and nanoparticles were associated with calcified blood vessels [10] as well. These particles seemed to contain DNA, proteins and synthesized RNA. These findings caused a major boom for nanobacteria, to the extent of receiving tabloid press coverage followed by the founding of highly promising start-up companies.
Fig. 1.
Transmission electron micrograph of so-called ‘nanobacteria’ after a 3-month culture period (bar = 200 nm). Photograph taken with permission from [6] (copyright 1998 National Academy of Sciences, USA).
Nanobacteria do not exist and simply represent nanoparticles
However, in the year 2000, Cisar et al. [11] explored the so-called nanobacteria and determined that phospholipids could bind and, thereby, facilitate the formation of calcium–phosphate crystals which resembled these nano-sized structures. Secondly, it was observed that the crystalline structures were shown to grow and replicate in vitro as if they were alive. Thirdly, it was demonstrated that the nucleic acid sequences thought to be a diagnostic marker of nanobacteria were in fact common sequences of nucleic acid frequently contaminating laboratories [11].
In addition, Martel and Young [12] performed a series of experiments on the origin of putative nanobacteria. Indeed, non-mineral compounds such as proteins interfered with the crystallization process. Surprisingly, calcium phosphate, together with non-mineral compounds, grew into nanoparticles that resembled the putative nanobacteria in structure and shape. Moreover, these nanoparticles showed a high-binding capacity to charged molecules such as ions, carbohydrates, lipids and nucleic acids. Depending on the ratio of mineral to non-mineral compounds, either crystallization to hydroxyapatite or to more complex forms took place [13, 14]. Raoult et al. [15] found that the main protein of the so-called nanobacteria was fetuin-A. Besides fetuin-A, other proteins such as albumin or apolipoproteins could also be identified [16]. Moreover, Young et al. [16] determined that polyclonal antisera raised against nanobacteria strongly cross-reacted with fetuin-A and albumin. Consequently, the term ‘nanobacteria’ was discarded and replaced with the term ‘nanoparticles’ [17].
Taken together, the putative living nanobacteria have been shown to be non-living nanoparticles containing both mineral and non-mineral compounds (Table 1) such as the calciprotein particles (CPPs) shown in Figure 2, which represent a possible multitude of calcifying nanoparticles in a very idealized form regarding both shape and composition. Nevertheless, the interaction of minerals with calcium-binding proteins suggests that these nanoparticles are part of the body’s defence mechanisms against unwanted calcification. Thus, mineral–protein complexes seem to be part of the normal mineral homeostasis. If the mineral supersaturation, and thus the balance of mineral versus mineral-binding proteins (i.e. calcification inhibitor proteins), is tilted towards the mineral component, crystallization can take place. In stages of disease i.e. kidney stones or vascular calcification, this imbalance, and consequentially calcification, can take place [21].
Table 1.
Nanoparticles as putative living organisms turned out to be a biochemical phenomenon
| Nanoparticles as putative living organisms | Nanoparticles as biochemical phenomenon | |
| Form and shape similar but much smaller than bacteria | → | Nanoparticles of mineral and non-mineral compounds |
| Content: nucleic acid and proteins | → | In vitro high binding to charged molecules |
| Growing structures | → | Saturated solution enables in vitro growth |
| Antibodies against nanobacteria | → | Antibodies against albumin and fetuin-A |
Fig. 2.
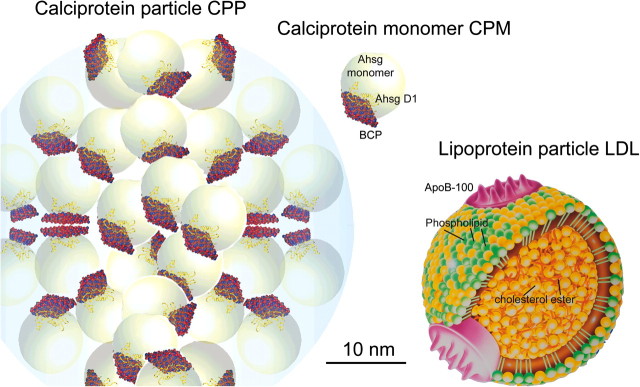
Illustration of a CPP, calciprotein monomer (modified after [18, 19]) and a low-density lipoprotein (LDL) particle. The LDL particle is ∼22 nm in diameter and contains many esterified cholesterol molecules in the hydrophobic core, cholesterol, phospholipids and a few apolipoprotein B-100 molecules in the hydrophilic coat (modified after [20]).
Nanoparticles and their role in extraosseous calcification
Crystal formation starts with nucleation and subsequently proceeds to growth (Figure 3). Nucleation starts with small mineral ion clusters forming in supersaturated solutions of constitutive mineral ions. Coordination of calcium ions by proteins and phospholipids may regulate these events [25]. A critical size is required for stable nuclei, otherwise re-dissolution may occur. In the presence of preformed nuclei, crystal growth proceeds at a fast pace even in the absence of supersaturation. Fetuin-A has been shown in vitro to inhibit mineralization on the level of crystal growth by the transient formation of soluble protein–mineral complexes containing fetuin-A, calcium and phosphate [22, 26]. These CCPs start out as nanoscopic (50–150 nm diameter) colloidal spheres. Initially, they are amorphous and soluble but become progressively more crystalline and insoluble in a time- and temperature-dependent fashion [22]. Amorphous mineral phases admixed with protein are now widely recognized as the earliest manifestations of biomineralization, both in the mollusc shell and in the vertebrate skeleton [27, 28]. The mineral part itself has also been shown to contain spherical mineral particles with nanocrystalline needles of ∼10 × 100 nm [29]. Self-assembly of nano-sized apatite particles seems to constitute a mechanism for the generation of larger biological mineral crystals [30]. Ultra high-resolution electron microscopy revealed that microcalcifications of 20–500 nm contained nanocrystals 2–10 nm in size [31]. Similar nanocrystals 20–25 nm in size were demonstrated in vascular calcifications [29, 32]. In addition, phosphate may induce calcification by enhancing nanocrystal formation [33]. These findings collectively suggest that early mineralization products are nanocrystalline and contain protein in the form of protein–mineral complexes. Furthermore protein–mineral complexes play an early and essential role in both physiological and pathological calcification. Not surprisingly, protein–mineral complexes have been described in experimental animal models of calcification [34] and, most recently, also in dialysis patients who are known to be at high risk of calcification [35]. Protein–mineral complexes/CPPs/calcifying nanoparticles should, however, not be confused with larger cell-derived and membrane-delineated vesicles including matrix vesicles or calcifying apoptotic vesicles that originate from actively mineralizing cells like osteoblasts or calcifying chondrocytes or from calcifying apoptotic or necrotic cells. Calcifying vesicles may nevertheless harbour protein–mineral complexes like the fetuin-A-rich vesicles associated with calcifying smooth muscle cells [36].
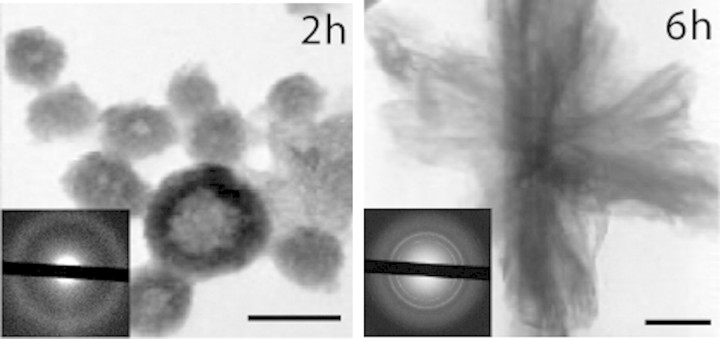
Fig. 3.
Electron microscopic picture of synthetic CCPs [22]. The CCPs initially have a diameter of 30–150 nm (2 h, 37°C) and are amorphous as shown by diffraction analysis. Nextly, the CPPs are transformed, dependent on temperature, mineral ion supersaturation and fetuin-A concentration, into larger and crystalline mineral particles [23]. These particles are still soluble until ∼24 h at 37°C. Similar particles have been detected in ascites of patients with sclerosing calcifying peritonitis [24]. Scale bars represents 100 nm. This research was originally published in [22] © the American Society for Biochemistry and Molecular Biology.
Taken together, nanoparticles consisting of protein–mineral complexes can be seen both in vitro and in vivo as precursors of matrix calcification. The interaction of mineral with mineral-binding proteins and low-molecular weight inhibitors thus constitute important facets of mineral transport and homeostasis. Fetuin-A, as well as other proteins, contained in soluble protein–mineral particles may be viewed as mineral chaperones [37] fulfilling a role in the stabilization, transport and recycling of water-insoluble mineral, similar to the role of lipoproteins in the metabolism of lipoprotein particles that contain water-insoluble lipids [20].
Acknowledgments
Conflict of interest statement. None declared.
References
- 1.Schinke T, McKee MD, Karsenty G. Extracellular matrix calcification: where is the action? Nat Genet. 1999;21:150–151. doi: 10.1038/5928. [DOI] [PubMed] [Google Scholar]
- 2.Jahnen-Dechent W, Heiss A, Schafer C, et al. Fetuin-a regulation of calcified matrix metabolism. Circ Res. 2011;108:1494–1509. doi: 10.1161/CIRCRESAHA.110.234260. [DOI] [PubMed] [Google Scholar]
- 3.Murshed M, Harmey D, Millan JL, et al. Unique coexpression in osteoblasts of broadly expressed genes accounts for the spatial restriction of ECM mineralization to bone. Genes Dev. 2005;19:1093–1104. doi: 10.1101/gad.1276205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Folk RL. SEM imaging of bacteria and nannobacteria in carbonate sediments and rocks. J Sediment Petrol. 1993;63:990–999. [Google Scholar]
- 5.McKay DS, Gibson EK, Jr, Thomas-Keprta KL, et al. Search for past life on Mars: possible relic biogenic activity in martian meteorite ALH84001. Science. 1996;273:924–930. doi: 10.1126/science.273.5277.924. [DOI] [PubMed] [Google Scholar]
- 6.Kajander EO, Ciftcioglu N. Nanobacteria: an alternative mechanism for pathogenic intra- and extracellular calcification and stone formation. Proc Natl Acad Sci U S A. 1998;95:8274–8279. doi: 10.1073/pnas.95.14.8274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ciftcioglu N, Bjorklund M, Kuorikoski K, et al. Nanobacteria: an infectious cause for kidney stone formation. Kidney Int. 1999;56:1893–1898. doi: 10.1046/j.1523-1755.1999.00755.x. [DOI] [PubMed] [Google Scholar]
- 8.Sedivy R, Battistutti WB. Nanobacteria promote crystallization of psammoma bodies in ovarian cancer. APMIS. 2003;111:951–954. doi: 10.1034/j.1600-0463.2003.1111006.x. [DOI] [PubMed] [Google Scholar]
- 9.Hjelle JT, Miller-Hjelle MA, Poxton IR, et al. Endotoxin and nanobacteria in polycystic kidney disease. Kidney Int. 2000;57:2360–2374. doi: 10.1046/j.1523-1755.2000.00096.x. [DOI] [PubMed] [Google Scholar]
- 10.Miller VM, Rodgers G, Charlesworth JA, et al. Evidence of nanobacterial-like structures in calcified human arteries and cardiac valves. Am J Physiol Heart Circ Physiol. 2004;287:H1115–H1124. doi: 10.1152/ajpheart.00075.2004. [DOI] [PubMed] [Google Scholar]
- 11.Cisar JO, Xu DQ, Thompson J, et al. An alternative interpretation of nanobacteria-induced biomineralization. Proc Natl Acad Sci U S A. 2000;97:11511–11515. doi: 10.1073/pnas.97.21.11511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martel J, Young JD. Purported nanobacteria in human blood as calcium carbonate nanoparticles. Proc Natl Acad Sci U S A. 2008;105:5549–5554. doi: 10.1073/pnas.0711744105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Young JD, Martel J, Young D, et al. Characterization of granulations of calcium and apatite in serum as pleomorphic mineralo-protein complexes and as precursors of putative nanobacteria. PLoS One. 2009;4:e5421. doi: 10.1371/journal.pone.0005421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu CY, Martel J, Young D, et al. Fetuin-A/albumin-mineral complexes resembling serum calcium granules and putative nanobacteria: demonstration of a dual inhibition-seeding concept. PLoS One. 2009;4:e8058. doi: 10.1371/journal.pone.0008058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raoult D, Drancourt M, Azza S, et al. Nanobacteria are mineralo fetuin complexes. PLoS Pathog. 2008;4:e41. doi: 10.1371/journal.ppat.0040041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Young JD, Martel J, Young L, et al. Putative nanobacteria represent physiological remnants and culture by-products of normal calcium homeostasis. PLoS One. 2009;4:e4417. doi: 10.1371/journal.pone.0004417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ciftcioglu N, McKay DS. Pathological calcification and replicating calcifying-nanoparticles: general approach and correlation. Pediatr Res. 2010;67:490–499. doi: 10.1203/PDR.0b013e3181d476ce. [DOI] [PubMed] [Google Scholar]
- 18.Schlieper G, Westenfeld R, Brandenburg V, et al. Inhibitors of calcification in blood and urine. Semin Dial. 2007;20:113–121. doi: 10.1111/j.1525-139X.2007.00257.x. [DOI] [PubMed] [Google Scholar]
- 19.Genge BR, Wu LN, Wuthier RE. In vitro modeling of matrix vesicle nucleation: synergistic stimulation of mineral formation by annexin A5 and phosphatidylserine. J Biol Chem. 2007;282:26035–26045. doi: 10.1074/jbc.M701057200. [DOI] [PubMed] [Google Scholar]
- 20.Price PA, Nguyen TM, Williamson MK. Biochemical characterization of the serum fetuin-mineral complex. J Biol Chem. 2003;278:22153–22160. doi: 10.1074/jbc.M300739200. [DOI] [PubMed] [Google Scholar]
- 21.Heiss A, DuChesne A, Denecke B, et al. Structural basis of calcification inhibition by alpha 2-HS glycoprotein/fetuin-A. Formation of colloidal calciprotein particles. J Biol Chem. 2003;278:13333–13341. doi: 10.1074/jbc.M210868200. [DOI] [PubMed] [Google Scholar]
- 22.Aizenberg J, Weiner S, Addadi L. Coexistence of amorphous and crystalline calcium carbonate in skeletal tissues. Connect Tissue Res. 2003;44(Suppl 1):20–25. [PubMed] [Google Scholar]
- 23.Mahamid J, Aichmayer B, Shimoni E, et al. Mapping amorphous calcium phosphate transformation into crystalline mineral from the cell to the bone in zebrafish fin rays. Proc Natl Acad Sci U S A. 2010;107:6316–6321. doi: 10.1073/pnas.0914218107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sandin K, Kloo L, Nevsten P, et al. Formation of carbonated apatite particles from a supersaturated inorganic blood serum model. J Mater Sci Mater Med. 2009;20:1677–1687. doi: 10.1007/s10856-009-3735-z. [DOI] [PubMed] [Google Scholar]
- 25.Robinson C. Self-oriented assembly of nano-apatite particles: a subunit mechanism for building biological mineral crystals. J Dent Res. 2007;86:677–679. doi: 10.1177/154405910708600801. [DOI] [PubMed] [Google Scholar]
- 26.Schlieper G, Aretz A, Verberckmoes SC, et al. Ultrastructural analysis of vascular calcifications in uremia. J Am Soc Nephrol. 2010;21:689–696. doi: 10.1681/ASN.2009080829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Becker A, Epple M, Muller KM, et al. A comparative study of clinically well-characterized human atherosclerotic plaques with histological, chemical, and ultrastructural methods. J Inorg Biochem. 2004;98:2032–2038. doi: 10.1016/j.jinorgbio.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 28.Neven E, De Schutter TM, De Broe ME, et al. Cell biological and physicochemical aspects of arterial calcification. Kidney Int. 2011;79:1166–1177. doi: 10.1038/ki.2011.59. [DOI] [PubMed] [Google Scholar]
- 29.Price PA, Williamson MK, Nguyen TM, et al. Serum levels of the fetuin-mineral complex correlate with artery calcification in the rat. J Biol Chem. 2004;279:1594–1600. doi: 10.1074/jbc.M305199200. [DOI] [PubMed] [Google Scholar]
- 30.Hamano T, Matsui I, Mikami S, et al. Fetuin-mineral complex reflects extraosseous calcification stress in CKD. J Am Soc Nephrol. 2010;21:1998–2007. doi: 10.1681/ASN.2009090944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reynolds JL, Skepper JN, McNair R, et al. Multifunctional roles for serum protein fetuin-a in inhibition of human vascular smooth muscle cell calcification. J Am Soc Nephrol. 2005;16:2920–2930. doi: 10.1681/ASN.2004100895. [DOI] [PubMed] [Google Scholar]
- 32.Jahnen-Dechent W, Schafer C, Ketteler M, et al. Mineral chaperones: a role for fetuin-A and osteopontin in the inhibition and regression of pathologic calcification. J Mol Med. 2008;86:379–389. doi: 10.1007/s00109-007-0294-y. [DOI] [PubMed] [Google Scholar]
- 33.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 34.Jahnen-Dechent W. Lotś wifeś problem revisted: how we prevent pathological calcification. In: Baeuerlein E, editor. Biomineralization Progress in Biology, Molecular Biology and Application. Weinheim: Wiley-VCH; 2004. pp. 245–267. [Google Scholar]
- 35.Heiss A, Pipich V, Jahnen-Dechent W, et al. Fetuin-A is a mineral carrier protein: small angle neutron scattering provides new insight on fetuin-a controlled calcification inhibition. Biophys J. 2010;99:3986–3995. doi: 10.1016/j.bpj.2010.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wald J, Wiese S, Eckert T, et al. Structure and stability of calcium phosphate - fetuin-A colloids probed by time-resolved dynamic light scattering. Soft Matter. 2011;7:2869–2874. [Google Scholar]
- 37.Olde Loohuis KM, Jahnen-Dechent W, van DW. The case: milky ascites is not always chylous. Kidney Int. 2010;77:77–78. doi: 10.1038/ki.2009.407. [DOI] [PubMed] [Google Scholar]