Abstract
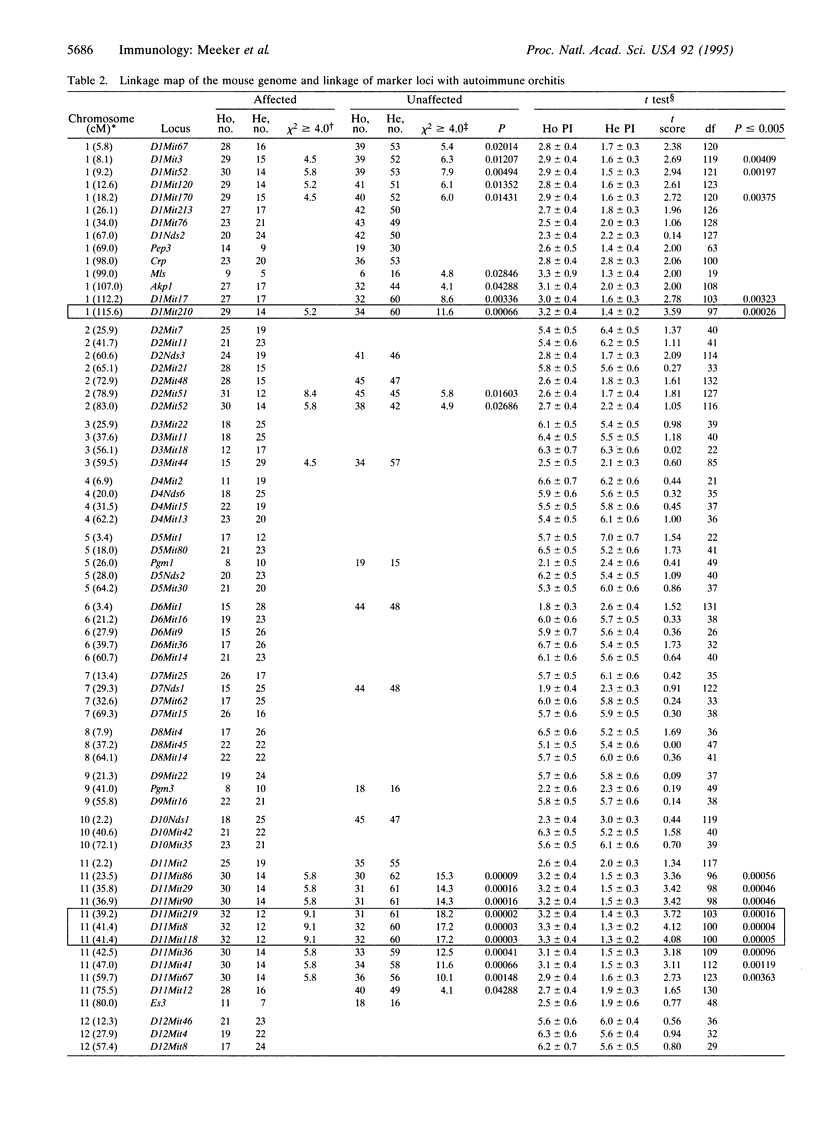
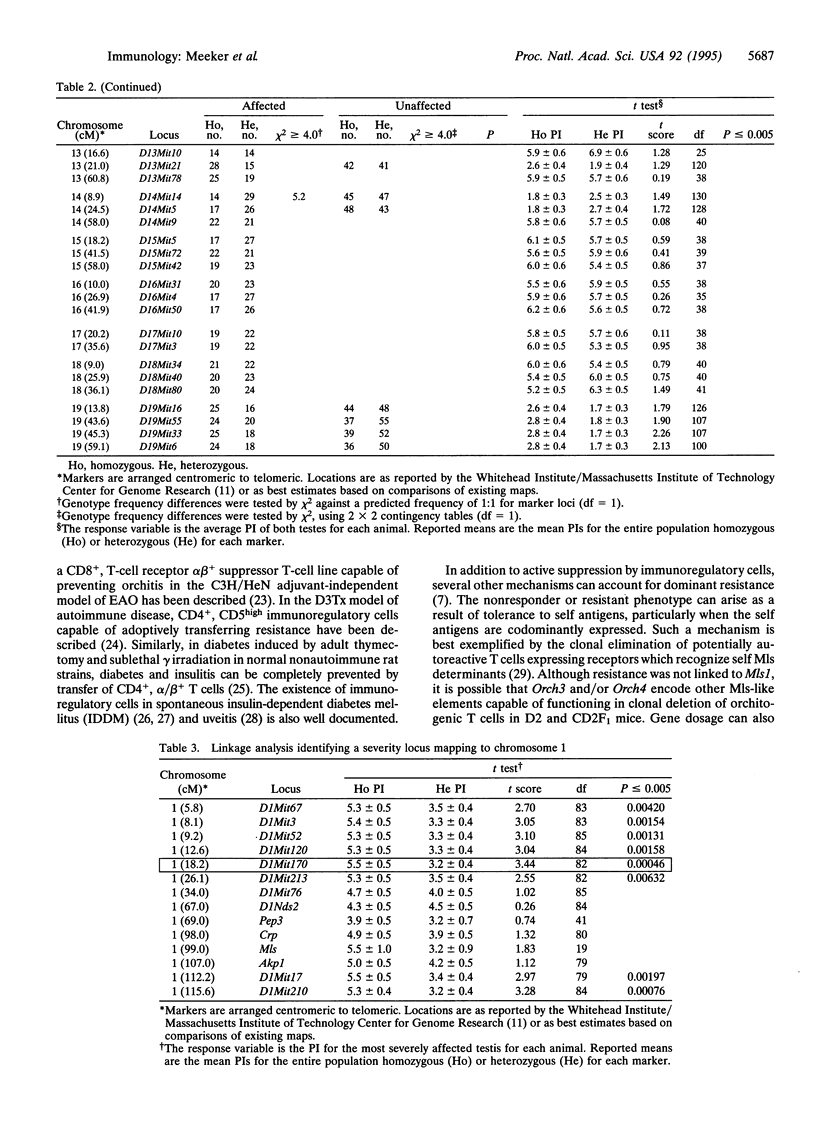
The existence of immunoregulatory genes conferring dominant resistance to autoimmunity is well documented. In an effort to better understand the nature and mechanisms of action of these genes, we utilized the murine model of autoimmune orchitis as a prototype. When the orchitis-resistant strain DBA/2J is crossed with the orchitis-susceptible strain BALB/cByJ, the F1 hybrid is completely resistant to the disease. By using reciprocal radiation bone marrow chimeras, the functional component mediating this resistance was mapped to the bone marrow-derived compartment. Resistance is not a function of either low-dose irradiation- or cyclophosphamide (20 mg/kg)-sensitive immunoregulatory cells, but can be adoptively transferred by primed splenocytes. Genome exclusion mapping identified three loci controlling the resistant phenotype. Orch3 maps to chromosome 11, whereas Orch4 and Orch5 map to the telomeric and centromeric regions of chromosome 1, respectively. All three genes are linked to a number of immunologically relevant candidate loci. Most significant, however, is the linkage of Orch3 to Idd4 and Orch5 to Idd5, two susceptibility genes which play a role in autoimmune insulin-dependent type 1 diabetes mellitus in the nonobese diabetic mouse.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beller D. I. Functional significance of the regulation of macrophage Ia expression. Eur J Immunol. 1984 Feb;14(2):138–143. doi: 10.1002/eji.1830140207. [DOI] [PubMed] [Google Scholar]
- Beraud E., Varriale S., Farnarier C., Bernard D. Suppressor cells in Lewis rats with experimental allergic encephalomyelitis: prevention of the disease and inhibition of lymphocyte proliferation by the suppressor cells or their products. Eur J Immunol. 1982 Nov;12(11):926–930. doi: 10.1002/eji.1830121106. [DOI] [PubMed] [Google Scholar]
- Blankenhorn E. P., Stranford S. A. Genetic factors in demyelinating diseases: genes that control demyelination due to experimental allergic encephalomyelitis (EAE) and Theiler's murine encephalitis virus. Reg Immunol. 1992 Sep-Oct;4(5):331–343. [PubMed] [Google Scholar]
- Bogen B. Dominant suppressive effect of the silent Eb alpha allele on an in vivo T helper cell response under Ed beta Ed alpha region-linked immune response gene control. Eur J Immunol. 1985 Oct;15(10):1033–1037. doi: 10.1002/eji.1830151014. [DOI] [PubMed] [Google Scholar]
- Boitard C., Yasunami R., Dardenne M., Bach J. F. T cell-mediated inhibition of the transfer of autoimmune diabetes in NOD mice. J Exp Med. 1989 May 1;169(5):1669–1680. doi: 10.1084/jem.169.5.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bureau J. F., Montagutelli X., Bihl F., Lefebvre S., Guénet J. L., Brahic M. Mapping loci influencing the persistence of Theiler's virus in the murine central nervous system. Nat Genet. 1993 Sep;5(1):87–91. doi: 10.1038/ng0993-87. [DOI] [PubMed] [Google Scholar]
- Chen Y., Kuchroo V. K., Inobe J., Hafler D. A., Weiner H. L. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science. 1994 Aug 26;265(5176):1237–1240. doi: 10.1126/science.7520605. [DOI] [PubMed] [Google Scholar]
- Cornall R. J., Prins J. B., Todd J. A., Pressey A., DeLarato N. H., Wicker L. S., Peterson L. B. Type 1 diabetes in mice is linked to the interleukin-1 receptor and Lsh/Ity/Bcg genes on chromosome 1. Nature. 1991 Sep 19;353(6341):262–265. doi: 10.1038/353262a0. [DOI] [PubMed] [Google Scholar]
- Davies J. L., Kawaguchi Y., Bennett S. T., Copeman J. B., Cordell H. J., Pritchard L. E., Reed P. W., Gough S. C., Jenkins S. C., Palmer S. M. A genome-wide search for human type 1 diabetes susceptibility genes. Nature. 1994 Sep 8;371(6493):130–136. doi: 10.1038/371130a0. [DOI] [PubMed] [Google Scholar]
- Dietrich W. F., Miller J. C., Steen R. G., Merchant M., Damron D., Nahf R., Gross A., Joyce D. C., Wessel M., Dredge R. D. A genetic map of the mouse with 4,006 simple sequence length polymorphisms. Nat Genet. 1994 Jun;7(2 Spec No):220–245. doi: 10.1038/ng0694supp-220. [DOI] [PubMed] [Google Scholar]
- Dietrich W., Katz H., Lincoln S. E., Shin H. S., Friedman J., Dracopoli N. C., Lander E. S. A genetic map of the mouse suitable for typing intraspecific crosses. Genetics. 1992 Jun;131(2):423–447. doi: 10.1093/genetics/131.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowell D., Mason D. Evidence that the T cell repertoire of normal rats contains cells with the potential to cause diabetes. Characterization of the CD4+ T cell subset that inhibits this autoimmune potential. J Exp Med. 1993 Mar 1;177(3):627–636. doi: 10.1084/jem.177.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowell D., Mason D. Evidence that the T cell repertoire of normal rats contains cells with the potential to cause diabetes. Characterization of the CD4+ T cell subset that inhibits this autoimmune potential. J Exp Med. 1993 Mar 1;177(3):627–636. doi: 10.1084/jem.177.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S., Palmer S. M., Rodrigues N. R., Cordell H. J., Hearne C. M., Cornall R. J., Prins J. B., McShane P., Lathrop G. M., Peterson L. B. Polygenic control of autoimmune diabetes in nonobese diabetic mice. Nat Genet. 1993 Aug;4(4):404–409. doi: 10.1038/ng0893-404. [DOI] [PubMed] [Google Scholar]
- Hara Y., Caspi R. R., Wiggert B., Chan C. C., Wilbanks G. A., Streilein J. W. Suppression of experimental autoimmune uveitis in mice by induction of anterior chamber-associated immune deviation with interphotoreceptor retinoid-binding protein. J Immunol. 1992 Mar 15;148(6):1685–1692. [PubMed] [Google Scholar]
- Kohno S., Munoz J. A., Williams T. M., Teuscher C., Bernard C. C., Tung K. S. Immunopathology of murine experimental allergic orchitis. J Immunol. 1983 Jun;130(6):2675–2682. [PubMed] [Google Scholar]
- Kono D. H., Burlingame R. W., Owens D. G., Kuramochi A., Balderas R. S., Balomenos D., Theofilopoulos A. N. Lupus susceptibility loci in New Zealand mice. Proc Natl Acad Sci U S A. 1994 Oct 11;91(21):10168–10172. doi: 10.1073/pnas.91.21.10168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lider O., Santos L. M., Lee C. S., Higgins P. J., Weiner H. L. Suppression of experimental autoimmune encephalomyelitis by oral administration of myelin basic protein. II. Suppression of disease and in vitro immune responses is mediated by antigen-specific CD8+ T lymphocytes. J Immunol. 1989 Feb 1;142(3):748–752. [PubMed] [Google Scholar]
- MacDonald H. R., Glasebrook A. L., Schneider R., Lees R. K., Pircher H., Pedrazzini T., Kanagawa O., Nicolas J. F., Howe R. C., Zinkernagel R. M. T-cell reactivity and tolerance to Mlsa-encoded antigens. Immunol Rev. 1989 Feb;107:89–108. doi: 10.1111/j.1600-065x.1989.tb00004.x. [DOI] [PubMed] [Google Scholar]
- Mahi-Brown C. A., Yule T. D., Tung K. S. Evidence for active immunological regulation in prevention of testicular autoimmune disease independent of the blood-testis barrier. Am J Reprod Immunol Microbiol. 1988 Apr;16(4):165–170. doi: 10.1111/j.1600-0897.1988.tb00190.x. [DOI] [PubMed] [Google Scholar]
- Miller A., Lider O., Roberts A. B., Sporn M. B., Weiner H. L. Suppressor T cells generated by oral tolerization to myelin basic protein suppress both in vitro and in vivo immune responses by the release of transforming growth factor beta after antigen-specific triggering. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):421–425. doi: 10.1073/pnas.89.1.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A., Lider O., Weiner H. L. Antigen-driven bystander suppression after oral administration of antigens. J Exp Med. 1991 Oct 1;174(4):791–798. doi: 10.1084/jem.174.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukasa A., Hiramine C., Hojo K. Generation and characterization of a continuous line of CD8+ suppressively regulatory T lymphocytes which down-regulates experimental autoimmune orchitis (EAO) in mice. Clin Exp Immunol. 1994 Apr;96(1):138–145. doi: 10.1111/j.1365-2249.1994.tb06243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira D. B., Mitchison N. A. Immune suppression genes. Clin Exp Immunol. 1989 Feb;75(2):167–177. [PMC free article] [PubMed] [Google Scholar]
- Person P. L., Snoek M., Demant P., Woodward S. R., Teuscher C. The immunogenetics of susceptibility and resistance to murine experimental allergic orchitis. Reg Immunol. 1992 Sep-Oct;4(5):284–297. [PubMed] [Google Scholar]
- Smith H., Lou Y. H., Lacy P., Tung K. S. Tolerance mechanism in experimental ovarian and gastric autoimmune diseases. J Immunol. 1992 Sep 15;149(6):2212–2218. [PubMed] [Google Scholar]
- Sudweeks J. D., Todd J. A., Blankenhorn E. P., Wardell B. B., Woodward S. R., Meeker N. D., Estes S. S., Teuscher C. Locus controlling Bordetella pertussis-induced histamine sensitization (Bphs), an autoimmune disease-susceptibility gene, maps distal to T-cell receptor beta-chain gene on mouse chromosome 6. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3700–3704. doi: 10.1073/pnas.90.8.3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teuscher C., Gasser D. L., Woodward S. R., Hickey W. F. Experimental allergic orchitis in mice. VI. Recombinations within the H-2S/H-2D interval define the map position of the H-2-associated locus controlling disease susceptibility. Immunogenetics. 1990;32(5):337–344. doi: 10.1007/BF00211648. [DOI] [PubMed] [Google Scholar]
- Teuscher C., Hickey W. F., Korngold R. Experimental allergic orchitis in mice. V. Resistance to actively induced disease in BALB/cJ substrain mice is mediated by CD4+ T cells. Immunogenetics. 1990;32(1):34–40. doi: 10.1007/BF01787326. [DOI] [PubMed] [Google Scholar]
- Teuscher C., Smith S. M., Goldberg E. H., Shearer G. M., Tung K. S. Experimental allergic orchitis in mice. I. Genetic control of susceptibility and resistance to induction of autoimmune orchitis. Immunogenetics. 1985;22(4):323–333. doi: 10.1007/BF00430916. [DOI] [PubMed] [Google Scholar]
- Todd J. A., Aitman T. J., Cornall R. J., Ghosh S., Hall J. R., Hearne C. M., Knight A. M., Love J. M., McAleer M. A., Prins J. B. Genetic analysis of autoimmune type 1 diabetes mellitus in mice. Nature. 1991 Jun 13;351(6327):542–547. doi: 10.1038/351542a0. [DOI] [PubMed] [Google Scholar]
- Varriale S., Béraud E., Barbaria J., Galibert R., Bernard D. Regulation of experimental autoimmune encephalomyelitis: inhibition of adoptive experimental autoimmune encephalomyelitis by 'recovery-associated suppressor cells'. J Neuroimmunol. 1994 Sep;53(2):123–131. doi: 10.1016/0165-5728(94)90022-1. [DOI] [PubMed] [Google Scholar]



