Abstract
The prevalence of heart failure (HF) increases with age. While clinical trials suggest that contemporary evidence-based HF therapies have reduced morbidity and mortality, these trials largely excluded the elderly. Questions remain regarding the clinical characteristics of elderly HF patients and the impact of contemporary therapies on their outcomes. This review presents the epidemiology of HF in the elderly and summarizes the data on the pathophysiology of the ageing heart. The clinical characteristics, treatment patterns, and outcomes of elderly HF patients are explored. Finally, the main gaps regarding HF therapies in the elderly and the opportunities for future trials are highlighted.
Keywords: Heart failure, Heart failure with preserved ejection fraction, Elderly, Outcomes, Therapy
Heart failure (HF) is a major and growing public health problem worldwide, with high morbidity, mortality, and cost.1 Despite recent improvements in the outcomes of patients with chronic HF through contemporary therapies,2 concerns exist as to whether the subjects included in major HF clinical trials were representative of real-world patients. In particular, the elderly are under-represented in clinical trials and may be at an increased risk for worse outcomes.
A consensus definition of elderly does not exist. Traditionally, 65 years have been considered the conventional threshold for older age, since this age cut-off has historically represented a common age for retirement in many cultures.3 However, increased life expectancy may make this age cut-off inappropriately low. Recent HF studies classified ‘elderly’ patients heterogeneously, as those older than 704 to 80 years,5–7 while patients older than 85 years were often classified as ‘very elderly’.8 This latter cut-off has been proposed as potentially a more appropriate threshold for old age.3
Regardless of the specific definition for elderly, it is clear that HF is primarily a condition of the older population in developed countries. Elderly HF patients demonstrate distinctive pathophysiological features, complex co-morbidity profiles, and unique issues of medication tolerance. Our understanding of proper patient management of the elderly is limited by their frequent referral to general practitioners (GPs) or geriatricians rather than cardiologists, as well as by their under-representation in major HF trials. In this review, we summarize the current data on HF in the elderly, focusing on the pathophysiology of the ageing heart, and the clinical characteristics and outcomes. The differential response to HF therapies in the elderly and the opportunities for future investigation are also highlighted.
Heart failure as a disease of the elderly
Available data on HF epidemiology show that HF increasingly represents a disease of the elderly. In epidemiological studies in the general population, the mean age at first diagnosis of HF increased over the years, now being 80 years old.9 The incidence of new HF events turns out to be much higher in elderly10 compared with younger subjects, and registry data show that the proportion of hospitalizations with a diagnosis of HF, as well as the prevalence of HF in the general population, is much higher in older patients.11–13
The epidemic of HF among the elderly may, in part, be explained by improved management of acute conditions and co-morbidities, with patients living longer and progressing to clinical HF. Longer exposure to risk factors and age-related changes may also make the elderly more prone to develop HF.14 However, although overall survival after HF onset has substantially improved with contemporary therapies,9 this benefit is less evident in older age groups.15,16 Advanced age remains a strong predictor for poor outcomes in patients with chronic17 or acute HF,11 and it is included in several prognostic models for mortality after hospitalization.18
Pathophysiology of the ageing heart
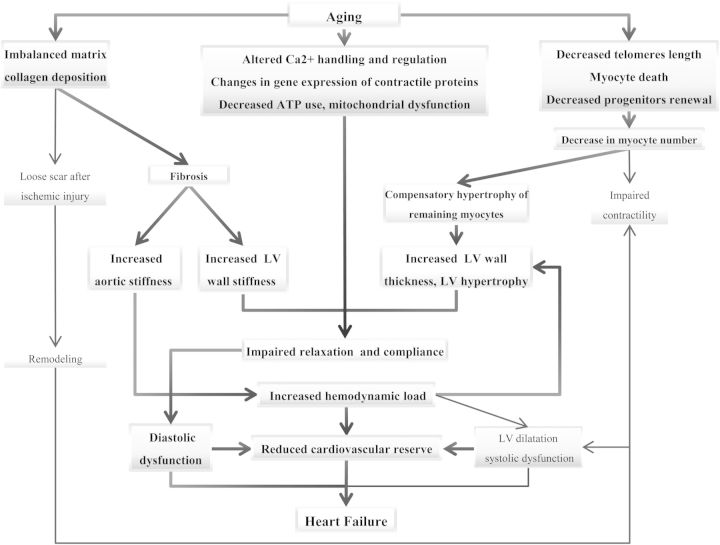
Several specific changes in cardiac structure and function are associated with cardiac ageing, and they may explain a number of pathophysiological and phenotypic features typical of the elderly. Among these, particularly important is the greater predisposition of the elderly to develop HF, particularly HF with preserved ejection fraction (HFpEF)4,12,19 (Figure 1).
Figure 1.
Suggested pathophysiological mechanisms predisposing to the development of diastolic dysfunction and heart failure in otherwise healthy ageing hearts.
With age, there is a decrease both in number and in function of myocytes, which occurs even in subjects without evidence of cardiovascular disease.20 The underlying mechanisms of such changes include enhanced necrosis and apoptosis,21 and a reduced regenerative capacity of cardiac progenitor cells. This prevents adequate reparation for myocyte loss either caused by ageing, or secondary to myocardial injury and ischaemia.21 The loss of functioning cardiomyocytes is compensated by the hypertrophy of the remaining cells.20
Alterations in the function of myocytes associated with age include impaired calcium metabolism and regulation, which reflects an alteration of processes of contraction and relaxation.22 In addition, contractile proteins change with age similarly to the alterations seen in hypertrophic hearts.23 Finally, ATP utilization is less efficient in the ageing heart. These abnormalities may provide the substrate for worsening cardiac function in the setting of exacerbating conditions, even in otherwise healthy hearts.22 Another potential mechanism associated with the higher risk of development of HF in advancing age is the shortening of telomeres, which has been suggested as a marker of biological and cellular ageing and associated with development of HF.21,24
Simultaneously with the reduced number, function, and compensatory hypertrophy of myocytes, the senescent myocardium is affected by an imbalance of extracellular matrix metabolism, with a subsequent detrimental increase in myocardial collagen content and development of fibrosis.25 Myocardial fibrosis is promoted by several mechanisms26 known to be up-regulated in HF at any age, and which are constitutively activated in the elderly. They include the up-regulation of the renin–angiotensin–aldosterone system,25 enhanced inflammatory activity,27 and oxidative stress.25
As a consequence, even otherwise healthy patients tend to show, as a function of age, an increased prevalence of LV hypertrophy and impaired relaxation.22 Moreover, age-associated changes affect the entire vascular system, causing arterial vascular wall fibrosis, thickening, and stiffening,28 and thus further increase cardiac afterload and worsen hypertrophy.25 These mechanisms may culminate in the development of clinically evident HFpEF. At first, symptoms related to HFpEF typically occur during exertion.26 Exercise capacity is primarily affected due to the lower heart rate increase during exercise shown by elderly patients, as well as a higher afterload and end-diastolic pressure due to vascular stiffness, and a stroke volume that is more preload dependent due to impaired relaxation.22
The above-mentioned mechanisms may also explain, in part, why the most common phenotype of HF in the elderly is HFpEF, while EF and contractility are usually initially preserved.22 Age-associated myocardial and vascular wall stiffness and the consequent increase in aortic impedance, as seen during peaks of systolic blood pressure, may lead to an increased end-diastolic pressure in a stiff ventricle,28 with resultant pulmonary oedema. Conditions which further impair ventricular filling, such as AF, may trigger HF decompensation more easily in the aged heart where cardiac reserve may be further reduced. Conversely, systolic HF is more typical of young patients, probably as a consequence of ischaemic heart disease.29 However, with time and additional insult, LV dilatation and dysfunction may occur as a final stage of HFpEF.
Clinical characteristics, assessment, and outcomes
Elderly patients hospitalized for acute HF are more likely to be female and to have higher EF and a higher prevalence of HFpEF5,6,8 compared with younger patients. They have an increased prevalence of co-morbidities, including AF, hypertension, cerebrovascular disease, anaemia, malignancy, and chronic kidney disease.5,6,8,19 Conversely, CAD and diabetes are less common in the very elderly.8,19 This may be explained by the longer survival of people not suffering from these diseases which, on the other hand, cause systolic dysfunction and HF at a younger age.29
In contrast to younger patients with systolic HF, elderly patients with acute decompensation of HF more often present with acute pulmonary oedema and hypertension, consistent with a significant vascular contribution to the underlying pathophysiology.30 Conversely, elderly outpatients may present with a gradual onset of symptoms and atypical findings, including loss of appetite and a decrease in body mass index,31 whereas traditional HF symptoms, such as shortness of breath, may be absent or difficult to interpret due to a lack of specificity.31,32 Several co-morbid conditions, such as osteoarthritis and problems with mobility,33 may act as confounders and prevent an early detection of HF either by the patient or by the physician.
This distinct clinical HF profile in the elderly may also influence processes of care in this population. Elderly HF outpatients are less likely to be referred to specialist care.34 Adherence to guidelines and HF management differ significantly between cardiologists and GPs; for instance, GPs are less likely to use echocardiography and natriuretic peptides for HF diagnosis,35 although these tools represent key diagnostic tests in the algorithm for the identification of HF,36 especially in patients where symptoms may be masked by co-morbidities such as lung disease.37 Likewise, elderly patients hospitalized with acute HF are less likely to be evaluated by a cardiologist during hospitalization,6 or to receive specific diagnostic procedures such as echocardiographic evaluation.5,6,38 At discharge, they may receive less counselling about HF management and follow-up.19 The above-mentioned differences may affect the patients' outcomes.38
With regard to prognosis and risk stratification, a variety of risk models have been developed to predict the mortality risk of general HF patients.18 However, less conclusive evidence is available for the elderly4,39 Several studies in elderly HF patients attempted to identify prognostic factors, but these studies differed significantly with regard to the definitions of ‘elderly’, study design, and duration of follow-up.4,6,8,31,39 This heterogeneity limits the ability to draw conclusions with respect to the risk profile of elderly compared with younger HF patients.
This lack of knowledge is important as available data show a different prognostic value of several variables in different age groups, hence highlighting the need for a specific evaluation for the elderly. For instance, while a lower EF is associated with higher mortality in the general HF population,40 prognostic models have actually shown that low EF may lose its prognostic importance in elderly compared with young HF patients.8,41 Therefore, the presence of HFpEF in the elderly should not be considered benign.8 The high prevalence of this condition among the elderly41 and the lack of effective treatment strategies highlight the significant burden of this disease.2 Conversely and possibly related to the pathophysiology of HFpEF, AF shows an increasing impact on prognosis with increasing age.8 Risk assessment in the elderly should also consider the importance of conditions not strictly related to cardiovascular disease, which reflect greater frailty and impaired functional status.5,31,39 The risk classification in the elderly with HF improves with the inclusion of the total number of co-morbidities,39 and conditions such as disability and dementia.33
Participation of elderly patients in clinical trials
Trials of HF have tended to exclude the elderly. More recently, several HF trials have focused specifically on the effect of therapies on the elderly, including the Irbesartan in Heart Failure with Preserved Systolic Function (I-PRESERVE)42 trial (mean age 73 years), the Study of the Effects of Nebivolol Intervention on Outcomes and Rehospitalisation in Seniors with Heart Failure (SENIORS)43 (mean age 76 years), the Cardiac Insufficiency Bisoprolol Study in Elderly (CIBIS-ELD) (mean age 73 years),44 the Evaluation of Losartan in the Elderly Study (ELITE, mean age 73 years),45 and the Perindopril in Elderly People with Chronic Heart Failure study (PEP-CHF, mean age 76 years).46
Nevertheless, many landmark HF trials upon which current treatment guidelines are based2 and recent HF trials47 mostly include younger patients. As a result, a gap still exists between the patients enrolled in clinical trials and those treated in daily clinical practice, and current evidence-based treatments have been investigated in a population which represents the minority of those patients likely ultimately to receive the therapies.
Several factors may explain the under-representation of the elderly in clinical trials. First, the fact that elderly patients are less likely to be referred to a cardiologist during hospitalization for acute HF5,38 and to receive specialist counselling for outpatient care34,35 may prevent their enrolment in trials and registries. Secondly, the inclusion criteria of randomized controlled trials have often explicitly excluded elderly patients; ∼ 30% of HF trials included specific age limitations.48 This issue is shared among trials on cardiovascular diseases in general.49 Moreover, additional enrolment criteria are often based on age-related conditions, including renal dysfunction, life expectancy, and cancer. Finally, most trials focused on HF with reduced EF.48 Therefore, elderly patients, who are more likely to have co-morbidities or HFpEF, are more likely to fail trial screening. Table 1 shows the age of study populations and key age-related study inclusion criteria that represent a potential age-related selection bias in several landmark trials which influenced current evidence-based guidelines and clinical practice.
Table 1.
Selected main practice-changing heart failure trials
| Trial | Year | Study treatmenta | No. of patients | Age (years)b | Key age-related inclusion criteria |
|---|---|---|---|---|---|
| SOLVD | 1991 | Enalapril | 2569 | 61 | Age <80; EF ≤ 35% |
| DIG (main trial) | 1997 | Digoxin | 6800 | 63 ± 11 | EF ≤45% |
| RALES | 1999 | Spironolactone | 1663 | 65 ± 12 | EF ≤35% |
| CIBIS II | 1999 | Bisoprolol | 2647 | 61 ± 11 | Age 18–80; EF ≤35% |
| ATLAS | 1999 | Low-dose vs. high-dose lisinopril | 3793 | 64 ± 10 | EF ≤30% |
| COPERNICUS | 2001 | Carvedilol | 2289 | 63 ± 12 | EF ≤25% |
| BEST | 2001 | Bucindolol | 2706 | 60 ± 12 | EF ≤35%. |
| EPHESUS | 2001 | Eplerenone | 6632 | 64 ± 11 | EF ≤40% |
| Val-HeFT | 2002 | Valsartan | 5010 | 62 ± 11 ACEi, 67 ± 10 no ACEi | EF ≤40% |
| MADIT II | 2002 | ICD | 1232 | 64 ± 10 | EF ≤30% |
| COMET | 2003 | Carvedilol vs. metoprolol | 3029 | 62 (11–4)c | EF ≤35% |
| CARE HF | 2005 | CRT vs. medical therapy alone | 813 | 66 (59–72) no CRT, 67 (60–73) CRTc | EF ≤35% |
| MADIT-CRT | 2009 | CRT-D vs. ICD | 1820 | 65 ± 11 | EF ≤30% |
| SHIFT | 2010 | Ivabradine | 6558 | 60 ± 11 | EF ≤35% |
| EMPHASIS | 2011 | Eplerenone | 2737 | 69 ± 8 | EF ≤35% |
Selected landmark heart failure trials, mean or median age of population enrolled, and key age-related inclusion criteria representing a potential age-related selection bias.
ACEi, ACE inhibitor; ATLAS, Assessment of Treatment with Lisinopril and Survival study; BEST, Beta-blocker Evaluation of Survival trial; CARE HF, Cardiac Resynchronization-Heart Failure; CIBIS-II, Cardiac Insufficiency Bisoprolol Trial II; COPERNICUS, Carvedilol Prospective Randomized Cumulative Survival Study; COMET, Carvedilol Or Metoprolol European Trial; CRT, cardiac resynchronization therapy; DIG, Digitalis Investigation Group; EMPHASIS, Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure; EPHESUS, Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study; ICD, implantable cardioverter defibrillator; MADIT, Multicenter Automatic Defibrillator Implantation Trial; MERIT-HF, Metoprolol Randomized Intervention Trial in Congestive Heart Failure; RALES, Randomized Aldactone Evaluation Study; SHIFT, Systolic Heart failure treatment with the If inhibitor ivabradine Trial; SOLVD, Studies Of Left Ventricular Dysfunction; Val-HeFT, Valsartan Heart Failure Trial.
aVersus placebo if not otherwise specified.
bMean ± SD, if not otherwise specified.
cMedian (interquartile range).
Therapy
As outlined in the previous sections, elderly patients with HF may differ from younger patients with respect to both the pathophysiology of their symptoms and their prognostic determinants. On the other hand, they also are under-represented in randomized controlled clinical trials. As a result, therapies recommended in current guidelines are based largely on studies in a younger population with a different clinical profile and have not been adequately tested in elderly patients.
Available data may suggest that the untoward effects of non-adherence to HF treatment,50 as well as the benefits of recommended HF medications, such as neurohormonal antagonists, may be similar in the elderly and in younger patients.5 However, conflicting data have been presented and they are mainly based on retrospective analyses.51–53 Data from recent trials focused on the elderly43−46 are still insufficient, and several gaps remain in evidence with regard to standard HF treatments as well as those of common cardiovascular co-morbidities such as AF.54,55
The lack of evidence-based therapies in the elderly represents a major issue since the management of medical therapy is a complex process for several reasons.
First, physiological age-related changes influence drug pharmacokinetics and dynamics. Ageing is often associated with a reduction in both lean body mass and total body water, and a relative increase in body fat; these changes result in a lower volume of distribution and higher plasma concentrations of hydrophilic drugs, while the plasma concentrations of lipophilic drugs tend to decrease.56 A reduction in metabolic capacity by the liver impairs first-pass metabolism, thus causing an enhanced activation of some drugs and a reduced activation of others; a decrease in renal or liver function also causes reduced clearance of several medications.56 Secondly, elderly patients more often have co-morbid conditions at higher risk for drug-related side effects, such as renal dysfunction,6,8 orthostatic hypotension, and bradycardia.22 Co-morbidities may also result in conflicting treatment recommendations,57 and polypharmacy frequently includes drugs which may themselves contribute to worsen HF (e.g. non-steroidal anti-inflammatory drugs) or increase the risk of drug–drug interactions (e.g. antidepressants and antiarrhymics, antibiotics and anticoagulants).56 Finally, social issues, including limited access to caregivers and specialists, cognitive impairment, and financial problems affect therapy adherence.58
The burden of disease management represents a challenge for the caregiver, also with regard to the choice of invasive strategies. Available subgroup analyses of implantable cardioverter defibrillator and CRT intervention trials do not allow definitive conclusions with regard to the risk–benefit ratio in this population with a shorter life expectancy.59–61 Likewise, the survival benefit of other procedures such as coronary artery bypass grafting or valve replacement is uncertain for elderly patients. The appropriateness of such interventions should be carefully evaluated on a case by case basis.
Older patients with end-stage HF may be ineligible for heart transplantation because of age and co-morbidities.62 Although extended listing criteria may be used, long-term survival is significantly lower in older compared with younger heart transplant recipients, due to the the higher risk of developing complications after transplant.63 These findings, together with the limited number of available organs for transplantation, have made the use of LV assist devices (LVADs) as destination therapy a potential option. However, advanced age has been identified as a risk factor for death in patients with LVAD therapy,64 and available data are still very limited in this regard.65
Limited life expectancy and advanced stage disease are closely connected in frail older patients and require an emphasis on continuity of care as well as palliative and end of life care.66 Disease management programmes may address a substantial need for the elderly, particularly given issues related to polypharmacy, complex co-morbidity profiles, and atypical symptom presentations. They may allow early detection of and intervention in disease progression or exacerbation, in order to counter the risk of therapy non-compliance and encourage adherence to lifestyle measures. Several trials investigating structured telephone support or non-invasive monitoring for chronic HF were conducted on patients with a mean age over 70 years,67,68 thus resembling a ‘real-world’ potential target population. Some recent meta-analyses seem to suggest a possible beneficial effect of these programmes with regard to lower rates of hospital admission.67,68 However, data are derived from heterogeneous studies and results remain inconsistent, without enough evidence to support a guideline recommendation.2
Unresolved issues and future directions
Elderly patients represent the majority of those with HF and have distinct features compared with younger patients commonly included in trials. The elderly HF phenotype is characterized by an increased prevalence of HFpEF, with a greater burden of cardiac and non-cardiac co-morbidities. While elderly patients represent the majority of the HF population and have a worse prognosis compared with the younger cohort, targeted treatment strategies have been insufficiently developed for them. Present knowledge is limited by the enrolment of patients with HF with reduced EF in most trials, with the exclusion of those with increased frailty.
Future trials may address these issues by taking into account appropriate inclusion criteria and a thorough assessment of elderly patients. Overall, specific enrolment goals should be included for the elderly (e.g. at least 30% of patients enrolled over 75 years). Furthermore, trials should pre-specify analyses of subpopulations such as the elderly where there may be a rationale for a differential effect. With regard to pathophysiology, new techniques to assess central blood pressure and aortic stiffness may be particularly important in elderly patients with HF, in order to provide insight into the exacerbating factors and potential therapeutic targets. Secondly, HF diagnosis and assessment in the elderly may require adjustment of trial metrics with respect of exercise capacity assessment and biomarker cut-offs.69 A diagnostic algorithm for HF in the elderly has been proposed37 and should be prospectively validated. Prognostic markers should specifically be studied in the elderly in order to allow personalized assessment and risk stratification. Likewise, non-cardiovascular conditions that are likely to influence outcomes should be taken into account in prognostic models. These models may also serve to estimate the prognostic benefit of additional medications or devices for individual patients or to facilitate decision-making concerning palliative care. Finally, implementation strategies for appropriate referral to a cardiologist or other specialty services should be empirically investigated in the elderly population. Cardiologists and HF specialists should also align with other disciplines to provide integrated, multispecialty management and follow-up. The risk–benefit ratio and cost-effectiveness of these strategies should be evaluated in prospective studies.
Conclusion
Heart failure in the elderly will continue to be an increasing health burden, as this population represents the majority of HF patients and demonstrates worse outcomes compared with younger patients. Sparse evidence exists for disease management in these patients, due to two related issues, i.e. under-representation in clinical trials and less frequent referral to specialist attention. Elderly patients with HF commonly have a complex profile characterized by multiple co-morbidities, polypharmacy, and social circumstances which may provide an additional clinical challenge, requiring a targeted and multidisciplinary approach. In order to improve the prognosis as well as resource allocation in the elderly HF population, we propose that distinct strategies for assessment, care, therapy, risk stratification, education and follow-up should be developed for this population.
Conflict of interest: none declared.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation 2012. 125 doi: 10.1161/CIR.0b013e31823ac046. e2–e220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA, Jaarsma T, Kober L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Ronnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A, Bax JJ, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck-Brentano C, Hasdai D, Hoes A, Kirchhof P, Knuuti J, Kolh P, McDonagh T, Moulin C, Popescu BA, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Vahanian A, Windecker S, McDonagh T, Sechtem U, Bonet LA, Avraamides P, Ben Lamin HA, Brignole M, Coca A, Cowburn P, Dargie H, Elliott P, Flachskampf FA, Guida GF, Hardman S, Iung B, Merkely B, Mueller C, Nanas JN, Nielsen OW, Orn S, Parissis JT, Ponikowski P. ESC guidelines for the diagnosis treatment of acute chronic heart failure 2012: the Task Force for the Diagnosis Treatment of Acute Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2012;14:803–869. doi: 10.1093/eurjhf/hfs105. [DOI] [PubMed] [Google Scholar]
- 3.Jugdutt BI. Aging and heart failure: changing demographics and implications for therapy in the elderly. Heart Fail Rev. 2010;15:401–405. doi: 10.1007/s10741-010-9164-8. [DOI] [PubMed] [Google Scholar]
- 4.Manzano L, Babalis D, Roughton M, Shibata M, Anker SD, Ghio S, van Veldhuisen DJ, Cohen-Solal A, Coats AJ, Poole-Wilson PP, Flather MD. Predictors of clinical outcomes in elderly patients with heart failure. Eur J Heart Fail. 2011;13:528–536. doi: 10.1093/eurjhf/hfr030. [DOI] [PubMed] [Google Scholar]
- 5.Komajda M, Hanon O, Hochadel M, Lopez-Sendon JL, Follath F, Ponikowski P, Harjola VP, Drexler H, Dickstein K, Tavazzi L, Nieminen M. Contemporary management of octogenarians hospitalized for heart failure in Europe: Euro Heart Failure Survey II. Eur Heart J. 2009;30:478–586. doi: 10.1093/eurheartj/ehn539. [DOI] [PubMed] [Google Scholar]
- 6.Barsheshet A, Shotan A, Cohen E, Garty M, Goldenberg I, Sandach A, Behar S, Zimlichman E, Lewis BS, Gottlieb S. Predictors of long-term (4-year) mortality in elderly and young patients with acute heart failure. Eur J Heart Fail. 2010;12:833–840. doi: 10.1093/eurjhf/hfq079. [DOI] [PubMed] [Google Scholar]
- 7.Hulsmann M, Berger R, Mortl D, Pacher R. Influence of age and in-patient care on prescription rate and long-term outcome in chronic heart failure: a data-based substudy of the EuroHeart Failure Survey. Eur J Heart Fail. 2005;7:657–661. doi: 10.1016/j.ejheart.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 8.Mogensen UM, Ersboll M, Andersen M, Andersson C, Hassager C, Torp-Pedersen C, Gustafsson F, Kober L. Clinical characteristics and major comorbidities in heart failure patients more than 85 years of age compared with younger age groups. Eur J Heart Fail. 2011;13:1216–1223. doi: 10.1093/eurjhf/hfr116. [DOI] [PubMed] [Google Scholar]
- 9.Levy D, Kenchaiah S, Larson MG, Benjamin EJ, Kupka MJ, Ho KK, Murabito JM, Vasan RS. Long-term trends in the incidence of and survival with heart failure. N Engl J Med. 2002;347:1397–1402. doi: 10.1056/NEJMoa020265. [DOI] [PubMed] [Google Scholar]
- 10.Bleumink GS, Knetsch AM, Sturkenboom MC, Straus SM, Hofman A, Deckers JW, Witteman JC, Stricker BH. Quantifying the heart failure epidemic: prevalence, incidence rate, lifetime risk and prognosis of heart failure. The Rotterdam Study. Eur Heart J. 2004;25:1614–1619. doi: 10.1016/j.ehj.2004.06.038. [DOI] [PubMed] [Google Scholar]
- 11.Abraham WT, Fonarow GC, Albert NM, Stough WG, Gheorghiade M, Greenberg BH, O'Connor CM, Sun JL, Yancy CW, Young JB. Predictors of in-hospital mortality in patients hospitalized for heart failure: insights from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF) J Am Coll Cardiol. 2008;52:347–356. doi: 10.1016/j.jacc.2008.04.028. [DOI] [PubMed] [Google Scholar]
- 12.Nieminen MS, Brutsaert D, Dickstein K, Drexler H, Follath F, Harjola VP, Hochadel M, Komajda M, Lassus J, Lopez-Sendon JL, Ponikowski P, Tavazzi L. EuroHeart Failure Survey II (EHFS II): a survey on hospitalized acute heart failure patients: description of population. Eur Heart J. 2006;27:2725–2736. doi: 10.1093/eurheartj/ehl193. [DOI] [PubMed] [Google Scholar]
- 13.Fang J, Mensah GA, Croft JB, Keenan NL. Heart failure-related hospitalization in the U.S., 1979 to 2004. J Am Coll Cardiol. 2008;52:428–434. doi: 10.1016/j.jacc.2008.03.061. [DOI] [PubMed] [Google Scholar]
- 14.Jugdutt BI. Prevention of heart failure in the elderly: when, where and how to begin? Heart Fail Rev. 2012;17:531–544. doi: 10.1007/s10741-012-9299-x. [DOI] [PubMed] [Google Scholar]
- 15.Roger VL, Weston SA, Redfield MM, Hellermann-Homan JP, Killian J, Yawn BP, Jacobsen SJ. Trends in heart failure incidence and survival in a community-based population. JAMA. 2004;292:344–350. doi: 10.1001/jama.292.3.344. [DOI] [PubMed] [Google Scholar]
- 16.Goldberg RJ, Glatfelter K, Burbank-Schmidt E, Farmer C, Spencer FA, Meyer T. Trends in mortality attributed to heart failure in Worcester, Massachusetts, 1992 to 2001. Am J Cardiol. 2005;95:1324–1328. doi: 10.1016/j.amjcard.2005.01.076. [DOI] [PubMed] [Google Scholar]
- 17.Pocock SJ, Wang D, Pfeffer MA, Yusuf S, McMurray JJ, Swedberg KB, Ostergren J, Michelson EL, Pieper KS, Granger CB. Predictors of mortality and morbidity in patients with chronic heart failure. Eur Heart J. 2006;27:65–75. doi: 10.1093/eurheartj/ehi555. [DOI] [PubMed] [Google Scholar]
- 18.Fonarow GC. Epidemiology and risk stratification in acute heart failure. Am Heart J. 2008;155:200–207. doi: 10.1016/j.ahj.2006.10.043. [DOI] [PubMed] [Google Scholar]
- 19.Forman DE, Cannon CP, Hernandez AF, Liang L, Yancy C, Fonarow GC. Influence of age on the management of heart failure: findings from Get With the Guidelines-Heart Failure (GWTG-HF) Am Heart J. 2009;157:1010–1017. doi: 10.1016/j.ahj.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 20.Olivetti G, Melissari M, Capasso JM, Anversa P. Cardiomyopathy of the aging human heart. Myocyte loss and reactive cellular hypertrophy. Circ Res. 1991;68:1560–1568. doi: 10.1161/01.res.68.6.1560. [DOI] [PubMed] [Google Scholar]
- 21.Wong LS, van der Harst P, de Boer RA, Huzen J, van Gilst WH, van Veldhuisen DJ. Aging, telomeres and heart failure. Heart Fail Rev. 2010;15:479–486. doi: 10.1007/s10741-010-9173-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part II: the aging heart in health: links to heart disease. Circulation. 2003;107:346–354. doi: 10.1161/01.cir.0000048893.62841.f7. [DOI] [PubMed] [Google Scholar]
- 23.Pandya K, Kim HS, Smithies O. Fibrosis, not cell size, delineates beta-myosin heavy chain reexpression during cardiac hypertrophy and normal aging in vivo. Proc Natl Acad Sci USA. 2006;103:16864–16869. doi: 10.1073/pnas.0607700103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Collerton J, Martin-Ruiz C, Kenny A, Barrass K, von Zglinicki T, Kirkwood T, Keavney B. Telomere length is associated with left ventricular function in the oldest old: the Newcastle 85+ study. Eur Heart J. 2007;28:172–176. doi: 10.1093/eurheartj/ehl437. [DOI] [PubMed] [Google Scholar]
- 25.Chen W, Frangogiannis NG. The role of inflammatory and fibrogenic pathways in heart failure associated with aging. Heart Fail Rev. 2010;15:415–422. doi: 10.1007/s10741-010-9161-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Udelson JE. Heart failure with preserved ejection fraction. Circulation. 2011;124 doi: 10.1161/CIRCULATIONAHA.111.071696. e540–e543. [DOI] [PubMed] [Google Scholar]
- 27.Cieslik KA, Taffet GE, Carlson S, Hermosillo J, Trial J, Entman ML. Immune-inflammatory dysregulation modulates the incidence of progressive fibrosis and diastolic stiffness in the aging heart. J Mol Cell Cardiol. 2011;50:248–256. doi: 10.1016/j.yjmcc.2010.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kawaguchi M, Hay I, Fetics B, Kass DA. Combined ventricular systolic and arterial stiffening in patients with heart failure and preserved ejection fraction: implications for systolic and diastolic reserve limitations. Circulation. 2003;107:714–720. doi: 10.1161/01.cir.0000048123.22359.a0. [DOI] [PubMed] [Google Scholar]
- 29.Jelani A, Jugdutt BI. STEMI and heart failure in the elderly: role of adverse remodeling. Heart Fail Rev. 2010;15:513–521. doi: 10.1007/s10741-010-9177-3. [DOI] [PubMed] [Google Scholar]
- 30.Metra M, Brutsaert D, Dei Cas L, Gheorghiade M. Acute heart failure: epidemiology, classification and pathophysiology. In: Tubaro MDN, Filippatos G, Goldstein P, Vranckx P, Zagher D, editors. The ESC Textbook of Intensive and Acute Cardiac Care. Oxford/New York: Oxford University Press; 2011. pp. 479–490. [Google Scholar]
- 31.Oudejans I, Mosterd A, Bloemen JA, Valk MJ, van Velzen E, Wielders JP, Zuithoff NP, Rutten FH, Hoes AW. Clinical evaluation of geriatric outpatients with suspected heart failure: value of symptoms, signs, and additional tests. Eur J Heart Fail. 2011;13:518–527. doi: 10.1093/eurjhf/hfr021. [DOI] [PubMed] [Google Scholar]
- 32.Ahmed A, Allman RM, Aronow WS, DeLong JF. Diagnosis of heart failure in older adults: predictive value of dyspnea at rest. Arch Gerontol Geriatr. 2004;38:297–307. doi: 10.1016/j.archger.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 33.Chaudhry SI, Wang Y, Gill TM, Krumholz HM. Geriatric conditions and subsequent mortality in older patients with heart failure. J Am Coll Cardiol. 2010;55:309–316. doi: 10.1016/j.jacc.2009.07.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johansson S, Wallander MA, Ruigómez A, García Rodríguez LA. Incidence of newly diagnosed heart failure in UK general practice. Eur J Heart Fail. 2001;3:225–231. doi: 10.1016/s1388-9842(00)00131-8. [DOI] [PubMed] [Google Scholar]
- 35.Rutten FH, Grobbee DE, Hoes AW. Differences between general practitioners and cardiologists in diagnosis and management of heart failure: a survey in every-day practice. Eur J Heart Fail. 2003;5:337–344. doi: 10.1016/s1388-9842(03)00050-3. [DOI] [PubMed] [Google Scholar]
- 36.Kelder JC, Cramer MJ, van Wijngaarden J, van Tooren R, Mosterd A, Moons KG, Lammers JW, Cowie MR, Grobbee DE, Hoes AW. The diagnostic value of physical examination and additional testing in primary care patients with suspected heart failure. Circulation. 2011;124:2865–2873. doi: 10.1161/CIRCULATIONAHA.111.019216. [DOI] [PubMed] [Google Scholar]
- 37.Manzano L, Escobar C, Cleland JG, Flather M. Diagnosis of elderly patients with heart failure. Eur J Heart Fail. 2012;14:1097–1103. doi: 10.1093/eurjhf/hfs109. [DOI] [PubMed] [Google Scholar]
- 38.Boom NK, Lee DS, Tu JV. Comparison of processes of care and clinical outcomes for patients newly hospitalized for heart failure attended by different physician specialists. Am Heart J. 2012;163:252–259. doi: 10.1016/j.ahj.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 39.Oudejans I, Mosterd A, Zuithoff NP, Hoes AW. Comorbidity drives mortality in newly diagnosed heart failure: a study among geriatric outpatients. J Card Fail. 2012;18:47–52. doi: 10.1016/j.cardfail.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 40.Meta-analysis Global Group in Chronic Heart Failure (MAGGIC) The survival of patients with heart failure with preserved or reduced left ventricular ejection fraction: an individual patient data meta-analysis. Eur Heart J. 2011;33:1750–1757. doi: 10.1093/eurheartj/ehr254. [DOI] [PubMed] [Google Scholar]
- 41.Satomura H, Wada H, Sakakura K, Kubo N, Ikeda N, Sugawara Y, Ako J, Momomura S. Congestive heart failure in the elderly: comparison between reduced ejection fraction and preserved ejection fraction. J Cardiol. 2012;59:215–219. doi: 10.1016/j.jjcc.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 42.Massie BM, Carson PE, McMurray JJ, Komajda M, McKelvie R, Zile MR, Anderson S, Donovan M, Iverson E, Staiger C, Ptaszynska A. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. 2008;359:2456–2467. doi: 10.1056/NEJMoa0805450. [DOI] [PubMed] [Google Scholar]
- 43.Flather MD, Shibata MC, Coats AJ, Van Veldhuisen DJ, Parkhomenko A, Borbola J, Cohen-Solal A, Dumitrascu D, Ferrari R, Lechat P, Soler-Soler J, Tavazzi L, Spinarova L, Toman J, Bohm M, Anker SD, Thompson SG, Poole-Wilson PA. Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS) Eur Heart J. 2005;26:215–225. doi: 10.1093/eurheartj/ehi115. [DOI] [PubMed] [Google Scholar]
- 44.Dungen HD, Apostolovic S, Inkrot S, Tahirovic E, Topper A, Mehrhof F, Prettin C, Putnikovic B, Neskovic AN, Krotin M, Sakac D, Lainscak M, Edelmann F, Wachter R, Rau T, Eschenhagen T, Doehner W, Anker SD, Waagstein F, Herrmann-Lingen C, Gelbrich G, Dietz R. Titration to target dose of bisoprolol vs. carvedilol in elderly patients with heart failure: the CIBIS-ELD trial. Eur J Heart Fail. 2011;13:670–680. doi: 10.1093/eurjhf/hfr020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pitt B, Segal R, Martinez FA, Meurers G, Cowley AJ, Thomas I, Deedwania PC, Ney DE, Snavely DB, Chang PI. Randomised trial of losartan versus captopril in patients over 65 with heart failure (Evaluation of Losartan in the Elderly Study, ELITE) Lancet. 1997;349:747–752. doi: 10.1016/s0140-6736(97)01187-2. [DOI] [PubMed] [Google Scholar]
- 46.Cleland JG, Tendera M, Adamus J, Freemantle N, Polonski L, Taylor J PEP-CHF Investigators. The perindopril in elderly people with chronic heart failure (PEP-CHF) study. Eur Heart J. 2006;27:2338–2345. doi: 10.1093/eurheartj/ehl250. [DOI] [PubMed] [Google Scholar]
- 47.Cherubini A, Oristrell J, Pla X, Ruggiero C, Ferretti R, Diestre G, Clarfield AM, Crome P, Hertogh C, Lesauskaite V, Prada GI, Szczerbinska K, Topinkova E, Sinclair-Cohen J, Edbrooke D, Mills GH. The persistent exclusion of older patients from ongoing clinical trials regarding heart failure. Arch Intern Med. 2011;171:550–556. doi: 10.1001/archinternmed.2011.31. [DOI] [PubMed] [Google Scholar]
- 48.Heiat A, Gross CP, Krumholz HM. Representation of the elderly, women, and minorities in heart failure clinical trials. Arch Intern Med. 2002;162:1682–1688. doi: 10.1001/archinte.162.15.1682. [DOI] [PubMed] [Google Scholar]
- 49.Forman DE, Rich MW, Alexander KP, Zieman S, Maurer MS, Najjar SS, Cleveland JC, Jr, Krumholz HM, Wenger NK. Cardiac care for older adults. Time for a new paradigm. J Am Coll Cardiol. 2011;57:1801–1810. doi: 10.1016/j.jacc.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Metra M, Dei Cas L, Massie BM. Treatment of heart failure in the elderly: never say it's too late. Eur Heart J. 2009;30:391–393. doi: 10.1093/eurheartj/ehp024. [DOI] [PubMed] [Google Scholar]
- 51.Flather MD, Yusuf S, Kober L, Pfeffer M, Hall A, Murray G, Torp-Pedersen C, Ball S, Pogue J, Moye L, Braunwald E. Long-term ACE-inhibitor therapy in patients with heart failure or left-ventricular dysfunction: a systematic overview of data from individual patients. ACE-Inhibitor Myocardial Infarction Collaborative Group. Lancet. 2000;355:1575–1581. doi: 10.1016/s0140-6736(00)02212-1. [DOI] [PubMed] [Google Scholar]
- 52.Cohen-Solal A, McMurray JJ, Swedberg K, Pfeffer MA, Puu M, Solomon SD, Michelson EL, Yusuf S, Granger CB. Benefits and safety of candesartan treatment in heart failure are independent of age: insights from the Candesartan in Heart failure–Assessment of Reduction in Mortality and morbidity programme. Eur Heart J. 2008;29:3022–3028. doi: 10.1093/eurheartj/ehn476. [DOI] [PubMed] [Google Scholar]
- 53.Dulin BR, Haas SJ, Abraham WT, Krum H. Do elderly systolic heart failure patients benefit from beta blockers to the same extent as the non-elderly? Meta-analysis of >12,000 patients in large-scale clinical trials. Am J Cardiol. 2005;95:896–898. doi: 10.1016/j.amjcard.2004.11.052. [DOI] [PubMed] [Google Scholar]
- 54.Kazemian P, Oudit G, Jugdutt BI. Atrial fibrillation and heart failure in the elderly. Heart Fail Rev. 2012;17:597–613. doi: 10.1007/s10741-011-9290-y. [DOI] [PubMed] [Google Scholar]
- 55.Marinigh R, Lip GY, Fiotti N, Giansante C, Lane DA. Age as a risk factor for stroke in atrial fibrillation patients implications for thromboprophylaxis: implications for thromboprophylaxis. J Am Coll Cardiol. 2010;56:827–837. doi: 10.1016/j.jacc.2010.05.028. [DOI] [PubMed] [Google Scholar]
- 56.Mangoni AA, Jackson SH. Age-related changes in pharmacokinetics and pharmacodynamics: basic principles and practical applications. Br J Clin Pharmacol. 2004;57:6–14. doi: 10.1046/j.1365-2125.2003.02007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Boyd CM, Darer J, Boult C, Fried LP, Boult L, Wu AW. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: implications for pay for performance. JAMA. 2005;294:716–724. doi: 10.1001/jama.294.6.716. [DOI] [PubMed] [Google Scholar]
- 58.van der Wal MH, Jaarsma T, van Veldhuisen DJ. Non-compliance in patients with heart failure; how can we manage it? Eur J Heart Fail. 2005;7:5–17. doi: 10.1016/j.ejheart.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 59.Tsai V, Goldstein MK, Hsia HH, Wang Y, Curtis J, Heidenreich PA. Influence of age on perioperative complications among patients undergoing implantable cardioverter-defibrillators for primary prevention in the United States. Circ Cardiovasc Qual Outcomes. 2011;4:549–556. doi: 10.1161/CIRCOUTCOMES.110.959205. [DOI] [PubMed] [Google Scholar]
- 60.Barsheshet A, Moss AJ, Huang DT, McNitt S, Zareba W, Goldenberg I. Applicability of a risk score for prediction of the long-term (8-year) benefit of the implantable cardioverter-defibrillator. J Am Coll Cardiol. 2012;59:2075–2079. doi: 10.1016/j.jacc.2012.02.036. [DOI] [PubMed] [Google Scholar]
- 61.Grimm W. Outcomes of elderly heart failure recipients of ICD and CRT. Int J Cardiol. 2008;125:154–160. doi: 10.1016/j.ijcard.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 62.Mehra MR, Kobashigawa J, Starling R, Russell S, Uber PA, Parameshwar J, Mohacsi P, Augustine S, Aaronson K, Barr M. Listing criteria for heart transplantation: International Society for Heart and Lung Transplantation guidelines for the care of cardiac transplant candidates–2006. J Heart Lung Transplant. 2006;25:1024–1042. doi: 10.1016/j.healun.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 63.Marelli D, Kobashigawa J, Hamilton MA, Moriguchi JD, Kermani R, Ardehali A, Patel J, Noguchi E, Beygui R, Laks H, Plunkett M, Shemin R, Esmailian F. Long-term outcomes of heart transplantation in older recipients. J Heart Lung Transplant. 2008;27:830–834. doi: 10.1016/j.healun.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 64.Kirklin JK, Naftel DC, Kormos RL, Stevenson LW, Pagani FD, Miller MA, Ulisney KL, Baldwin JT, Young JB. Third INTERMACS Annual Report: the evolution of destination therapy in the United States. J Heart Lung Transplant. 2011;30:115–123. doi: 10.1016/j.healun.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 65.Butler CR, Jugdutt BI. Mechanical circulatory support for elderly heart failure patients. Heart Fail Rev. 2012;17:663–669. doi: 10.1007/s10741-012-9298-y. [DOI] [PubMed] [Google Scholar]
- 66.Jaarsma T, Beattie JM, Ryder M, Rutten FH, McDonagh T, Mohacsi P, Murray SA, Grodzicki T, Bergh I, Metra M, Ekman I, Angermann C, Leventhal M, Pitsis A, Anker SD, Gavazzi A, Ponikowski P, Dickstein K, Delacretaz E, Blue L, Strasser F, McMurray J. Advanced Heart Failure Study Group of the HFA of the ESC. Palliative care in heart failure: a position statement from the palliative care workshop of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2009;11:433–443. doi: 10.1093/eurjhf/hfp041. [DOI] [PubMed] [Google Scholar]
- 67.Inglis SC, Clark RA, McAlister FA, Ball J, Lewinter C, Cullington D, Stewart S, Cleland JG. Structured telephone support or telemonitoring programmes for patients with chronic heart failure. Cochrane Database Syst Rev. 2010;8 doi: 10.1002/14651858.CD007228.pub2. CD007228. [DOI] [PubMed] [Google Scholar]
- 68.Whellan DJ, Hasselblad V, Peterson E, O'Connor CM, Schulman KA. Metaanalysis and review of heart failure disease management randomized controlled clinical trials. Am Heart J. 2005;149:722–729. doi: 10.1016/j.ahj.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 69.Blonde-Cynober F, Morineau G, Estrugo B, Fillie E, Aussel C, Vincent JP. Diagnostic and prognostic value of brain natriuretic peptide (BNP) concentrations in very elderly heart disease patients: specific geriatric cut-off and impacts of age, gender, renal dysfunction, and nutritional status. Arch Gerontol Geriatr. 2011;52:106–110. doi: 10.1016/j.archger.2010.02.010. [DOI] [PubMed] [Google Scholar]