Background: Hypotonicity stimulates transepithelial ion and water flux in the Drosophila renal tubule through unknown mechanisms.
Results: The with-no-lysine (WNK) and Ste20-related proline/alanine-rich (SPAK)/oxidative stress response (OSR1) kinase cascade regulates transepithelial K+ flux through the fly sodium-potassium-2-chloride cotransporter (NKCC).
Conclusion: The kinase/NKCC pathway is required for the tubule's response to hypotonicity.
Significance: This provides a molecular mechanism by which flies osmoregulate.
Keywords: Epithelium, Ion-sensitive Electrode, Na-K-Cl Cotransporter (NKCC), Potassium Transport, Renal Physiology, Serine/Threonine Protein Kinase, Fray, Malpighian Tubule, SLC12 Cotransporters, Epithelial Ion Transport
Abstract
The ability to osmoregulate is fundamental to life. Adult Drosophila melanogaster maintain hemolymph osmolarity within a narrow range. Osmolarity modulates transepithelial ion and water flux in the Malpighian (renal) tubules of the fly, which are in direct contact with hemolymph in vivo, but the mechanisms causing increased transepithelial flux in response to hypotonicity are unknown. Fly renal tubules secrete a KCl-rich fluid. We have previously demonstrated a requirement for Ncc69, the fly sodium-potassium-2-chloride cotransporter (NKCC), in tubule K+ secretion. Mammalian NKCCs are regulated by a kinase cascade consisting of the with-no-lysine (WNK) and Ste20-related proline/alanine-rich (SPAK)/oxidative stress response (OSR1) kinases. Here, we show that decreasing Drosophila WNK activity causes a reduction in K+ flux. Similarly, knocking down the SPAK/OSR1 homolog fray also decreases K+ flux. We demonstrate that a hierarchical WNK-Fray signaling cascade regulates K+ flux through Ncc69, because (i) a constitutively active Fray mutant rescues the wnk knockdown phenotype, (ii) Fray directly phosphorylates Ncc69 in vitro, and (iii) the effect of wnk and fray knockdown is abolished in Ncc69 mutants. The stimulatory effect of hypotonicity on K+ flux is absent in wnk, fray, or Ncc69 mutant tubules, suggesting that the Drosophila WNK-SPAK/OSR1-NKCC cascade is an essential molecular pathway for osmoregulation, through its effect on transepithelial ion flux and fluid generation by the renal tubule.
Introduction
The ability to osmoregulate is a fundamental property of living organisms across kingdoms (1). Single cell organisms as well as individual cells within multicellular organisms regulate internal osmolarity and, consequently, cell volume. Multicellular organisms will often maintain the osmolarity of extracellular fluids within a narrow range in order to avoid perturbations in cell volume and intracellular osmolarity, which may have deleterious effects on cell function.
Adult Drosophila melanogaster maintain extracellular osmolarity within a narrow range despite environmental stressors (2), but how osmoregulation is achieved is poorly understood. Insect iono- and osmoregulation is achieved through epithelial ion transport across the midgut, Malpighian (renal) tubules, and hindgut/rectum (3, 4). Because the renal tubules are in direct contact with hemolymph (the blood compartment of the insect), they can directly sense changes in extracellular osmolarity. In adult Drosophila, fluid secretion rates in renal tubules vary inversely with osmolarity: decreased fluid secretion at higher osmolarity and increased secretion at lower osmolarity (5). Increased osmolarity inhibits tubule sensitivity to the diuretic effects of tyramine (5), but the mechanism by which hypotonicity stimulates fluid secretion is unknown.
Fluid secretion in the main segment of the adult fly tubule is driven by secretion of a potassium chloride-rich fluid. This occurs through the parallel transepithelial secretion of cations, primarily K+, through the principal cell and Cl− secretion through the stellate cell (6–9). We have previously shown that the fly sodium-potassium-2-chloride cotransporter (NKCC),2 Ncc69, mediates ∼30% of principal cell transepithelial K+ transport (10).
The solute-linked carrier 12 (SLC12) family of sodium-coupled cotransporters (the sodium-coupled cotransporter (NCC) and NKCC1 and NKCC2) play important roles in mammalian renal physiology. NCC and NKCC2 mediate sodium chloride reabsorption in the distal convoluted tubule and thick ascending limb, respectively (11), whereas NKCC1 plays a role in transepithelial ion transport in the intercalated cell of the collecting duct (12–14). In addition, the activity of these transporters also influences the renal handling of water, divalent cations, and acid-base homeostasis. Regulation of these transporters is therefore key in allowing the kidney to respond to variations in dietary solute and water intake, and pathogenic dysregulation leads to abnormalities in serum electrolytes, osmolarity, and blood pressure in humans and in mouse models (11).
The WNK-SPAK/OSR1 kinase cascade is a key regulator of NCC and NKCC transport activity. Mutations in WNK1 and WNK4 result in human disease, pseudohypoaldosteronism type II (also known as familial hyperkalemia and hypertension or Gordon's syndrome), characterized by high serum potassium levels and high blood pressure (15). A number of in vitro, cultured cell, and Xenopus oocyte studies support a model in which WNK kinases phosphorylate the related SPAK and OSR1 kinases on two sites, thereby activating SPAK/OSR1 (16–18). SPAK and OSR1 then phosphorylate the N termini of NCC, NKCC1, and NKCC2 on conserved serines and threonines, increasing transporter activity (18–26), although some studies suggest that WNK4 negatively regulates NCC (27–32), at least under certain conditions. In vivo data are also consistent with positive regulatory roles of this kinase cascade in the regulation of NKCC2 in thick ascending limb and NCC in the distal convoluted tubule (33–39). However, although multiple studies have examined the effects of the WNK-SPAK/OSR1 kinase cascade on transporter phosphorylation in vivo, the effects on transporter activity have almost exclusively been examined in heterologous systems, such as Xenopus oocytes or HEK293 cells. However, the ion transport physiology of individual cells versus transporting epithelia may be different. Only one study, examining a dominant-negative isoform of WNK1, has directly demonstrated a role for WNK regulation of NKCC in renal tubule transepithelial ion flux (40).
Our goal was to examine roles for the WNK-SPAK/OSR1-NKCC pathway in the Drosophila renal tubule, a physiologically accessible transporting epithelium that also offers ease of genetic manipulation. Here, we demonstrate that Drosophila WNK and the SPAK/OSR1 homolog Fray regulate transepithelial K+ flux in the main segment of the fly renal tubule through their actions on Ncc69. Activation of the WNK-SPAK/OSR1-NKCC pathway in hypotonic conditions results in increased K+ flux and fluid secretion by the Malpighian tubules. Because the tubules generate an isosmotic fluid that is subsequently modified (similar to the glomerular filtrate), increased fluid generation by the tubules is the first step in clearance of a water load and the homeostatic maintenance of extracellular osmolarity.
EXPERIMENTAL PROCEDURES
Chemicals and Reagents
All chemicals and reagents were from Sigma or Fisher unless otherwise specified.
Fly Stocks and Genetics
The following D. melanogaster strains were used: wBerlin (wild-type), obtained from Dr. Adrian Rothenfluh (University of Texas Southwestern Medical Center, Dallas, TX); w;Ncc69r2, obtained from Dr. William Leiserson (Yale University, New Haven, CT) (41); w;c42-GAL4, expressing GAL4 in the principal cells of the main and lower segments as well as bar-shaped cells in the initial and transitional segments (42), obtained from Dr. Julian Dow (University of Glasgow, Glasgow, UK) and outcrossed for five generations to wBerlin. w;UAS-WNKRNAi, an RNAi line targeting Drosophila wnk, was obtained from the Vienna Drosophila Resource Center (Vienna, Austria; line 106928) (43) and was outcrossed for five generations to wBerlin; upon outcrossing, two eye colors became apparent, one with a “gradient” eye in heterozygotes and one with a heterozygous orange eye. The gradient eye stock was used for all experiments. All transgenic fly lines generated (described below) were outcrossed for five generations to wBerlin.
Flies were reared at 28 °C unless otherwise noted on cornmeal/yeast/molasses food prepared in a central kitchen at University of Texas Southwestern Medical Center. Female flies were collected within 48 h of eclosion and kept on standard food for 3–5 days before tubule dissection.
Cloning and Plasmid Construction
Primer sequences are listed in Table 1.
TABLE 1.
Primers
| Name | Sequence (5′–3′) |
|---|---|
| W5837D | CACCATGGTACTACCTCCTGCACCACGCAACCTCAAAGG |
| W5024D | TGACTCTCGCACGCGTTCAATCC |
| W6483U | TGCTCGTGTGTGCAGAAAGTGTGC |
| W6777D | CACCATGGATAGCAAAATAGAACCTGCAGATGC |
| W9433U | GACTGCCTGTCGGCGAAATGACCTGG |
| W9291D | CACCGGCTATTCCGTCACCGGCTATTCCCATGC |
| W15754-2U | TGTGTTTGCGGAGCTAGTGGACGTCGGATTCGATGAGG |
| W13711D | TCGCACATCCATCGACAACAGC |
| W15754U | TGTGTTTGCGGAGCTAGTGGACGT |
| N16285D | CACCATGTCGGACACAATCTCTTTCGAGTTGG |
| N26955U | CGAGTAGAAGGTCAGCACACTCGTCTG |
| Ncc69N204F | CACCATGTCGGACACAATCTCTTTCGAG |
| Ncc69N204R | CTAGCCCGACTCCGGGTCCTGAT |
| F1D | CACCATGACCTCCATACCCGCCAATCTGTCTAGC |
| F1656U | GTCCGTGATGGAGATCTGCGCATATCC |
| F606D | AGTGCGCCACGAGTTTGTGGGCACACC |
| F628U | TGCCCACAAACTCGTGGCGCACTTTCTGC |
| F548D | TCGCTGCCTTCGGTGTGAGTGC |
| F569U | GCACTCACACCGAAGGCAGCG |
| W7655D | GTTGACTGGTGTTCCAGTTGCCTGGTGC |
| W8322U | CCAAGGCGCCGATTTTAACACTACCAG |
| W8307D | TGGTAGTGTTAAAATCGGCGCCTTGG |
| W9433U | GACTGCCTGTCGGCGAAATGACCTGG |
| FRS2 | ctagcagtGGATTTATATAGTACGTAAAGtagttatattcaagcataCTTTACGTACTATATAAATCCgcg |
| FRR2 | aattcgcGGATTTATATAGTACGTAAAGtatgcttgaatataactaCTTTACGTACTATATAAATCCactg |
| Rp422D | CGCACCAAGCACTTCATC |
| Rp597U | TACTGTCCCTTGAAGCGG |
| W13320D | CCAGCAATGTCCTCCCTAA |
| W13736U | AGTTGTAGACGGAGGAGCCT |
wnk Cloning
RNA was isolated from whole wBerlin adult flies using TRIzol reagent (Invitrogen). cDNA was prepared using SuperScript II reverse transcriptase (Invitrogen) and an oligo(dT)20 primer. Initial attempts to obtain a full-length wnk clone based on the annotated sequence were unsuccessful. Therefore, PCR was performed to clone three segments of the open reading frame separately, which were then ligated together. All amplifications were performed with high-fidelity Pfx polymerase (Invitrogen). Initial attempts to clone the 5′-end, using primers W5837D and W9433U, revealed a misannotated splice site. A more extensive analysis of the cDNA was therefore undertaken. PCR was performed with primers W5024D and W6483U, revealing an alternatively spliced exon within the 5′-UTR as well as redefining the splicing events in the 5′-UTR and suggesting a “new” start codon. Based on these findings, the 5′-end was amplified with primers W6777D and W9433U. The second segment was amplified with W9291D and W15754-2U. The third segment was amplified with primers W13711D and W15754U. The three segments were individually cloned into pENTR (Invitrogen) using the Topo reaction (Invitrogen). pENTR containing segments two and three was digested with PciI and BglI (New England Biolabs, Ipswich, MA) and ligated together using T4 DNA ligase (New England Biolabs, Ipswich, MA). The resultant product, as well as pENTR containing segment one, was then digested with ApaLI (New England Biolabs, Ipswich, MA) and ligated together to yield the final full-length wnk cDNA in pENTR. The cDNA was sequenced in its entirety, and the sequence has been deposited in GenBankTM.
Generation of WNKD420A
In order to mutate Asp-420 to alanine, base pairs 1258:1260 of the wnk open reading frame were mutated from GAC to GCC. The top strand mutation was introduced by performing PCR with primers W7655D and W8332U. The bottom strand mutation was introduced by performing PCR with primers W8307D and W9433U. Top and bottom strands were then “zippered” together by performing PCR with primers W7655D and W9433U. This fragment was then cloned into pENTR using the TOPO reaction (Invitrogen). All PCR was performed with Pfx polymerase (Invitrogen), and the resulting mutant cDNA sequence was confirmed by sequencing. pENTR plasmids containing full-length wild-type wnk and the mutant wnk fragment were cut with SpeI and BstBI (New England Biolabs, Ipswich, MA). The mutant wnk fragment was ligated into the full-length wnk backbone using T4 DNA ligase (New England Biolabs, Ipswich, MA).
Ncc69 Cloning
Clone GH27027 was obtained from the Drosophila Genomics Resource Center (Bloomington, IN). The open reading frame was amplified by PCR using Pfx polymerase (Invitrogen) and primers N16285D and N26955U, cloned into pENTR using the TOPO reaction (Invitrogen), and sequenced in its entirety. To generate the GST-tagged N terminus of Ncc69 (amino acids 1–204) for use in the in vitro kinase assay, primers Ncc69N204F and Ncc69N204R were used with Pfx polymerase (Invitrogen), and the amplified fragment was cloned into pENTR using the TOPO reaction (Invitrogen). The Ncc69 fragment was then recombined into pDEST15 using the clonase reaction (Invitrogen), and the sequence was confirmed.
fray Cloning
A full-length cDNA encoding fray (RE53265) was obtained from the Drosophila Genomics Resource Center (Bloomington, IN). The full-length open reading frame was obtained by PCR with Pfx polymerase (Invitrogen) and primers F1D and F1656U and cloned into pENTR using TOPO (Invitrogen). In order to introduce the T206E mutation, base pairs 616:618 were mutated from ACC to GAG. The top strand mutation was introduced by using primers F1D and F628U, and the bottom strand mutation was introduced by using primers F606D and F1656U. Top and bottom strands were then “zippered” together by performing PCR with primers F1D and F1656U, and the resulting PCR product was cloned into pENTR using the TOPO reaction (Invitrogen). All PCR was performed with Pfx polymerase (Invitrogen), and the resulting cDNA sequence was confirmed by sequencing. A similar strategy was used to introduce the D185A kinase-dead mutation into wild type or frayT206E, using primers F548D and F569U. To generate GST-tagged Fray for use in the in vitro kinase assay, wild-type and mutant fray were recombined into pDEST15 using the clonase reaction (Invitrogen).
Expression of GST-tagged Fusion Proteins
pDEST15 constructs were transformed into BL21(DE3) Escherichia coli. Protein expression and purification were undertaken as described in the GST Gene Fusion System Handbook of GE Healthcare. Briefly, cells were grown at 37 °C in 2× YT medium to an A600 of 1–2 OD. Isopropyl-d-galactosidase was added to a final concentration of 0.1 mm to induce protein expression, and the cells were cultured for another 16 h at 20 °C. Cells were harvested by centrifugation and lysed by sonication in PBS with SIGMAFASTTM protease inhibitor. GST-tagged proteins were purified from the lysates using GSTrapTM FF (GE Healthcare) and eluted from the column in 50 mm Tris-HCl, pH 8.0, with 10 mm glutathione. After gel electrophoresis, Coomassie Blue-stained proteins were compared with a BSA standard to determine approximate protein concentrations.
Drosophila Transgenics
Generation of UAS-frayT206E and UAS-WNKD420A Transgenic Flies
A clonase reaction (Invitrogen) was performed to recombine the relevant open reading frame from pENTR into the pUASg.attB vector, obtained from Johannes Bischof and Konrad Basler (Zurich, Switzerland) (44). Plasmid DNA was prepared using the Qiafilter plasmid midi kit (Qiagen, Valencia, CA). DNA was injected into y1M{vas-int.Dm}ZH-2A w*; M{3xP3-RFP.attP′}ZH-22A (44) embryos by Rainbow Transgenic Flies, Inc. (Camarillo, CA). Transformants were outcrossed for five generations to wBerlin. The presence of the correct insert was confirmed by genomic DNA PCR and sequencing.
Generation of UAS-frayRNAi
Primers targeting the 3′-UTR of fray were designed using the DSIR program, siRNA 21 nucleotides. The top hit was chosen, and BLASTn was performed against the Drosophila genome, which showed 16-bp off-target hits to heterochromatin/U only and additional 14-bp off-target hits. The FRS2 and FRR2 primers were designed based on the TRiP protocol (45). These were then annealed and ligated into pWALIUM20 provided by the TRiP project (Harvard University, Boston, MA) after EcoRI/NheI digestion (New England Biolabs, Ipswich, MA). DNA was injected into y1 w67c23; P{CaryP}attP2 (Bloomington stock no. 8622) by Rainbow Transgenic Flies, Inc. The presence of the insert was confirmed by genomic DNA PCR and sequencing.
Kinase Assay
The in vitro kinase assay was performed as described (46) with the following modifications. Approximately 2.5 μg of GST-Fray and 20 μg of GST-Ncc69(1–204) were incubated in 50 μl of kinase buffer containing 10 mm HEPES (pH 7.4), 1 mm DTT, 5 mm MgCl2, 10 μm ATP, 10 μCi of [γ-32P]ATP (3000 Ci/mmol, PerkinElmer Life Sciences), protease inhibitor (cOmplete ULTRA tablets, EDTA-free, Roche Applied Science), and PhosSTOP (Roche Applied Science). After incubation for 2 h at 30 °C, phosphorylated substrates were subjected to SDS-PAGE. 32P emission was detected by a PhosphorImager, model STORM860 (GE Healthcare).
RT-PCR
Measuring wnk Transcript Levels
Three to four sets of tubules, n = 40 tubules/genotype (w;UAS-WNKRNAi/+;c42-GAL4/+ versus w;UAS-WNKRNAi/+ and w;UAS-WNKRNAi/+;Ncc69r2/Ncc69r2 versus w;UAS-WNKRNAi/+;c42-GAL4 Ncc69r2/Ncc69r2), were compared. Tubules were dissected in Drosophila saline and transferred to 600 μl of ZR RNA buffer from the Zymo Quick-RNA microprep kit (Zymo Research, Irvine, CA). Tubules were homogenized by 10–20 passes with a 27-gauge needle. RNA was isolated according to the Zymo protocol and eluted in 6 μl of DNase/RNase-free H2O. DNase treatment was performed using DNase I (Ambion/Invitrogen). cDNA was prepared using an oligo(dT)20 primer and SuperScript II reverse transcriptase (Invitrogen). Quantitative PCR was performed using iTaq SYBR Green Supermix with Rox (Bio-Rad) on a Bio-Rad iCycler. Primers used for the rp49 control were Rp422D and Rp597U. Primers used for wnk were W13320D and W13736U. The PCR cycle was 95 °C for 3 min, 95 °C for 30 s, 58 °C for 30 s, 72 °C for 40 s (repeated 40 times) and then 55 °C for 1 min. Primer efficiency (E) was calculated for both primer sets by generating a standard curve of whole-fly cDNA (see “wnk cloning” section) diluted 1:10, 1:100, 1:1000, and 1:10,000 for rp49 and 1:10, 1:100, and 1:1000 for wnk. Relative wnk transcript in control versus experimental samples was then calculated according to the method of Pfaffl (47) (i.e. ratio = (Ewnk)Δ-CP-wnk(control sample)/(Erp49)Δ-CP-rp49(control sample).
Measuring fray Transcript Levels
Four sets of tubules, n = 50 tubules/genotype (w;c42-GAL4/+ versus yw/w;c42-GAL4/UAS-frayRNAi), were compared. RNA was prepared as above. 150 ng of total RNA was used for reverse transcription using SuperScript II reverse transcriptase (Invitrogen) and random hexamer primers according to the manufacturer's instructions. Quantitative PCR was performed using the CFX Connect real-time PCR detecting system (Bio-Rad) with the iTaqTM Universal Probes Supermix (Bio-Rad). The TaqMan primer/probe sets for fray (Dm02361684_s1) and endogenous control RpL32 (Dm02151827_g1) were ordered from Invitrogen. Results were analyzed with Bio-Rad CFX Manager.
Ramsay Assay and Ion-specific Electrodes
The Ramsay assay was set up as described previously (10, 48). Malpighian tubules were dissected from adult females under Drosophila saline consisting of 117.5 mm NaCl, 20 mm KCl, 2 mm CaCl2, 8.5 mm MgCl2, 10.2 mm NaHCO3, 4.3 mm NaH2PO4, 15 mm HEPES, and 20 mm glucose, pH 7.0. Anterior tubules are more easily dissected and were used ∼90% of the time. Tubule pairs were transferred to a 10–20-μl bathing droplet consisting of standard bathing medium, a 1:1 mixture of Drosophila saline and Schneider's medium (Invitrogen), under mineral oil (Fisher). The composition of Schneider's medium is 3.33 mm glycine, 2.3 mm l-arginine, 3.01 mm l-aspartic acid, 0.496 mm l-cysteine, 0.417 mm l-cystine, 5.44 mm l-glutamic acid, 12.33 mm l-glutamine, 2.58 mm l-histidine, 1.15 mm l-isoleucine, 1.15 mm l-leucine, 9.02 mm l-lysine hydrochloride, 5.37 mm l-methionine, 0.909 mm l-phenylalanine, 14.78 mm l-proline, 2.38 mm l-serine, 2.94 mm l-threonine, 0.49 mm l-tryptophan, 2.76 mm l-tyrosine, 2.56 mm l-valine, 5.62 mm β-alanine, 5.41 mm CaCl2, 15.06 mm MgSO4, 21.33 mm KCl, 3.31 mm KH2PO4, 4.76 mm NaHCO3, 36.21 mm NaCl, 4.94 mm Na2HPO4, 1.37 mm α-ketoglutaric acid, 11.11 mm d-glucose, 0.862 mm fumaric acid, 0.746 mm malic acid, 0.847 mm succinic acid, 5.85 mm trehalose, and 2000 mg/liter yeast olate. Osmolality of 250-μl undiluted samples of standard bathing medium was measured in triplicate using an Advanced Instruments Osmometer, model 303 (Norwood, MA), according to the manufacturer's instructions. Hypotonic saline was obtained by adding 90 μl of H2O to 300 μl of standard bathing medium, and osmolality was measured in triplicate. Hypertonic saline was obtained by adding sucrose to the standard bathing medium to a final concentration of 0.1 m.
One tubule of the dissected pair remained in the droplet, whereas the other tubule of the pair was wrapped around a Minutien pin (Fine Science Tools, Foster City, CA) as an anchor. The secreted fluid droplet was examined at ∼2 h, and its diameter was measured using an ocular micrometer in a dissecting stereomicroscope (Nikon, Melville, NY) at ×50 magnification. The volume of the droplet was calculated assuming spherical geometry (4/3 πr3). The secretion rate was calculated for each tubule by dividing volume/time.
Ion-specific and reference electrodes were prepared according to the method of Maddrell et al. (49) as described previously (10). Unfilamented borosilicate glass capillaries with an outside diameter of 1.2 mm (Harvard Apparatus, Holliston, MA) were washed for 5 min with nitric acid, rinsed 3–5 times with deionized water, and dried on a hot plate set to 200 °C for a minimum of 20 min. Pipettes were pulled to a tip size of 1–2 μm using a vertical pipette puller (Narishige, East Meadow, NY). They were then dried for at least 10 min on the hot plate and lightly silanized by application of a drop of dichlorodimethylsilane inside a 15-cm Pyrex dish, which was inverted over the pipettes on the hot plate for a minimum of 20 min. Silanized pipettes were stored over silica gel (Fisher) until use. For measuring K+ flux, backfill solution of 0.5 m KCl was added to the pipette, and a small amount of potassium ionophore I mixture B was aspirated into the tip of the pipette by application of negative pressure. The reference electrode was prepared from filamented borosilicate glass capillaries with an outside diameter of 1.2 mm (Harvard Apparatus), pulled in a manner similar to the ion-specific electrode. The tip and shank were filled with 1 m sodium acetate, and the electrode was backfilled with 3 m KCl. Selectivity of the potassium ion-specific electrode for K+ compared with Na+ is greater than 103.9 (50).
K+ activity was measured as described previously (10). Calibration drops consisting of 15, 75, 150, and 200 mm KCl were measured by immersing the reference and ion-specific electrodes into the fluid drop and recording the potential using a Digidata 1200 amplifier (Axon Instruments, Union City, CA) and a FD223a dual-channel electrometer (World Precision Instruments, Sarasota, FL). The ion-specific electrode was calibrated before and after each set of experimental measurements. Slope/decile change in K+ concentration was calculated using the Nernst equation for the difference between 15 and 150 mm, 75 and 150 mm, and 150 and 200 mm, and the average slope was then calculated. K+ activity in the experimental fluid was measured, and concentration was calculated according to Equation 1,
where [K+]e is the potassium concentration of the experimental drop, [K+]c is the potassium concentration of the calibration drop, V is the change in potential (mV) between the experimental drop and the calibration drop, and s is the slope (mV) for a 10-fold change in K+ concentration, determined by measurements from the calibration drops. [K+]c was determined by the mean of the two 200 mm calibration drops (pre- and postexperiment). The K+ flux of each tubule was calculated by multiplying K+ concentration by the secretion rate for each tubule.
Statistics
Results comprising two groups were compared using an unpaired two-tailed t test. For results with three or more groups, one-way analysis of variance (ANOVA) was used. Bonferroni's test or Newman-Keuls test was used for post hoc testing of one-way ANOVA results. Two-tailed Fisher's exact test was used for categorical variables. Significance level was set at p < 0.05. All statistical analyses were performed using GraphPad Prism, version 5.0 or 6.0 (GraphPad Software, La Jolla, CA).
RESULTS
We have previously shown that the Drosophila NKCC, Ncc69, is required in the cation-conducting principal cell of the fly renal tubule for normal transepithelial K+ flux (10). Here we examined whether WNK-SPAK/OSR1 signaling regulates this NKCC. Drosophila wnk is an essential gene. We therefore used the GAL4-UAS system (51) to knock down wnk in the tubule principal cell using the GAL4 driver c42-GAL4 (42), allowing analysis of fluid secretion and K+ flux in the tubule of the adult fly using the Ramsay assay (48). Because the initial segment of the tubule is not fluid-secreting, and the lower segment of the tubule lies outside the bathing droplet in our experiments, this assay measures main segment fluid secretion and ion flux (48). The effect of wnk knockdown on fluid secretion rate, K+ concentration, and K+ flux is shown in Table 2, and the effect on K+ flux is illustrated in Fig. 1. wnk knockdown decreased K+ flux by about 30% (Fig. 1A), similar to the decrease in K+ flux seen in tubules homozygous for a null mutation in Ncc69 (10).
TABLE 2.
Secretion rate, [K+], and K+ flux
All values shown are mean ± S.E. “Slope” is the mean slope per 10-fold difference in [K+] of the electrodes used for each experiment. Note that K+ flux was calculated separately for each tubule analyzed, and therefore the value of mean secretion rate × mean [K+] may differ slightly from measured K+ flux, due to rounding.
| Genotype | Secretion rate | [K+] | K+ flux | n | Slope |
|---|---|---|---|---|---|
| nl/min/tubule | mm | pmol/min/tubule | |||
| Principal cell wnk knockdown | |||||
| w;c42-GAL4/+ | 0.39 ± 0.030 | 169 ± 6.1 | 71 ± 5.5 | 38 | 52.5 |
| w;UAS-WNKRNAi/+;c42-GAL4/+ | 0.34 ± 0.030 | 141 ± 6.6 | 49 ± 5.0 | 42 | |
| w;UAS-WNKRNAi/+ | 0.49 ± 0.025 | 162 ± 4.8 | 80 ± 4.6 | 38 | |
| Kinase-dead WNK in principal cells | |||||
| w;c42-GAL4/+ | 0.47 ± 0.030 | 147 ± 6.2 | 69 ± 5.6 | 30 | 52.1 |
| w;UAS-WNKD420A/+;c42-GAL4/+ | 0.35 ± 0.025 | 144 ± 7.5 | 50 ± 4.4 | 31 | |
| w;UAS-WNKD420A/+ | 0.48 ± 0.031 | 141 ± 5.0 | 68 ± 4.4 | 30 | |
| Principal cell fray knockdown | |||||
| w;c42-GAL4/+ | 0.52 ± 0.031 | 144 ± 7.1 | 77 ± 6.3 | 46 | 51.7 |
| yw/w;UAS-frayRNAi/c42-GAL4 | 0.35 ± 0.030 | 112 ± 6.7 | 41 ± 4.8 | 41 | |
| yw/w;UAS-frayRNAi/+ | 0.48 ± 0.035 | 142 ± 4.1 | 68 ± 5.5 | 46 | |
| Principal cell constitutively active Fray expression | |||||
| w;c42-GAL4/+ | 0.33 ± 0.034 | 174 ± 11.1 | 57 ± 6.2 | 38 | 53.8 |
| w;UAS-frayT206E/+;c42-GAL4/+ | 0.38 ± 0.044 | 170 ± 5.8 | 68 ± 8.1 | 38 | |
| w;UAS-frayT206E/+ | 0.33 ± 0.028 | 170 ± 7.2 | 55 ± 5.4 | 38 | |
| Rescue of wnk knockdown with constitutively active Fray | |||||
| w;UAS-WNKRNAi UAS-frayT206E/+ | 0.51 ± 0.068 | 182 ± 9.6 | 89 ± 9.1 | 11 | 51.1 |
| w;UAS-WNKRNAi/+;c42-GAL4/+ | 0.38 ± 0.057 | 161 ± 12.2 | 60 ± 7.6 | 14 | |
| w;UAS-WNKRNAi UAS-frayT206E/+;c42-GAL4/+ | 0.53 ± 0.045 | 178 ± 7.6 | 94 ± 8.7 | 12 | |
| wnk knockdown in Ncc69r2 mutant tubules | |||||
| w;c42-GAL4 Ncc69r2/Ncc69r2 | 0.50 ± 0.033 | 127 ± 5.3 | 65 ± 4.7 | 29 | 52.4 |
| w;UAS-WNKRNAi/+;c42-GAL4 Ncc69r2/Ncc69r2 | 0.43 ± 0.035 | 145 ± 6.6 | 62 ± 4.8 | 26 | |
| w;UAS-WNKRNAi/+;Ncc69r2/Ncc69r2 | 0.47 ± 0.030 | 143 ± 5.2 | 66 ± 4.3 | 28 | |
| fray knockdown in Ncc69r2 mutant tubules | |||||
| yw/w;c42-GAL4 Ncc69r2/Ncc69r2 | 0.54 ± 0.030 | 129 ± 3.4 | 70 ± 4.7 | 32 | 57.4 |
| yw/w;UAS-frayRNAi Ncc69r2/c42-GAL4 Ncc69r2 | 0.50 ± 0.028 | 127 ± 3.9 | 64 ± 4.4 | 31 | |
| Effect of hypertonic batha | |||||
| w;c42-GAL4/+ | 0.41 ± 0.053 | 121 ± 8.3 | 54 ± 9.1 | 27/22 | 54.0 |
| w;c42-GAL4/+, hypertonic | 0.15 ± 0.023 | 146 ± 10.4 | 26 ± 5.8 | 27/18 | |
| yw/w;UAS-frayRNAi/c42-GAL4 | 0.31 ± 0.045 | 107 ± 8.5 | 34 ± 5.8 | 29/22 | |
| yw/w;UAS-frayRNAi/c42-GAL4, hypertonic | 0.11 ± 0.022 | 158 ± 14.7 | 12 ± 3.8 | 24/14 | |
| Effect of hypotonic bath | |||||
| w;c42-GAL4/+ | 0.53 ± 0.056 | 132 ± 3.6 | 74 ± 8.4 | 37 | 52.2 |
| w;c42-GAL4/+, hypotonic | 0.91 ± 0.080 | 108 ± 3.0 | 99 ± 9.1 | 38 | |
| w;UAS-WNKRNAi/+;c42-GAL4/+ | 0.41 ± 0.035 | 124 ± 5.1 | 51 ± 5.4 | 34 | |
| w; UAS-WNKRNAi/+;c42-GAL4/+, hypotonic | 0.57 ± 0.067 | 97 ± 3.6 | 59 ± 7.8 | 37 | |
| yw/w;UAS-frayRNAi/ c42-GAL4 | 0.38 ± 0.043 | 94 ± 6.3 | 39 ± 4.8 | 32 | |
| yw/w;UAS-frayRNAi/ c42-GAL4, hypotonic | 0.39 ± 0.068 | 82 ± 6.7 | 40 ± 8.6 | 34 | |
| wBerlin | 0.72 ± 0.057 | 138 ± 6.6 | 103 ± 8.5 | 22 | 53.6 |
| wBerlin, hypotonic | 1.45 ± 0.092 | 114 ± 3.1 | 164 ± 10.5 | 21 | |
| w;Ncc69r2/Ncc69r2 | 0.62 ± 0.055 | 132 ± 4.7 | 80 ± 6.4 | 20 | |
| w;Ncc69r2/Ncc69r2, hypotonic | 0.80 ± 0.064 | 100 ± 3.8 | 80 ± 5.2 | 21 | |
a In this experiment, [K+] was measured in a subset of tubules; n for secretion is followed by n for [K+] and K+ flux.
FIGURE 1.
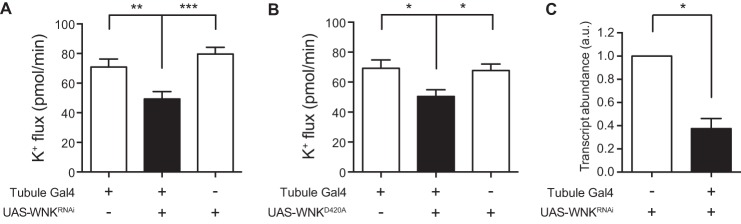
Decreasing WNK activity in the principal cell decreases transepithelial tubule K+ flux. The GAL4-UAS system, using the principal cell GAL4 driver c42-GAL4, was used to knock down wnk in the principal cells of the tubule or to express a dominant-negative WNK isoform. Fluid secretion rates were measured in isolated tubules using the Ramsay assay, and K+ concentration was determined using ion-specific electrodes, allowing calculation of K+ flux. A, K+ flux (pmol/min/tubule) was measured in control tubules (w;c42-GAL4/+ and w;UAS-WNKRNAi/+) versus wnk principal cell knockdown tubules (w;UAS-WNKRNAi/+;c42-GAL4/+). n = 38–42 tubules/genotype. There was no significant difference in K+ flux between the two control genotypes. K+ flux was decreased in the wnk knockdown tubules compared with both controls. B, K+ flux (pmol/min/tubule) was measured in control tubules (w;c42-GAL4/+ and w;UAS-WNKD420A/+) versus tubules expressing dominant-negative, kinase-dead WNK in the principal cells (w;UAS-WNKD420A/+;c42-GAL4/+). n = 30–31 tubules/genotype. There was no significant difference in K+ flux between the two control genotypes. K+ flux was decreased in the tubules expressing kinase-dead WNK compared with both controls. C, wnk transcript levels were measured by quantitative RT-PCR in control tubules (w;UAS-WNKRNAi/+) versus wnk knockdown tubules (w;UAS-WNKRNAi/+;c42-GAL4/+), n = 3 sets of tubules, 40 tubules/genotype in each set. All data are presented as the mean ± S.E. (error bars) *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Mutating Asp-420 in WNK to Ala abolishes kinase activity (46). Overexpression of WNKD420A phenocopies wnk loss of function in flies (46), indicating that this kinase-dead variant has dominant-negative properties. We therefore expressed WNKD420A in the principal cell of the tubule and found that this decreased K+ flux to a similar extent as seen with wnk knockdown (Fig. 1B). Together, these data indicate that WNK positively regulates K+ flux in the Drosophila renal tubule.
To confirm that wnk transcripts were indeed decreased in the knockdown tubules, we performed quantitative RT-PCR. Compared with control tubules, wnk transcript levels in knockdown tubules were decreased by 63% (Fig. 1C). This probably underestimates the degree of wnk knockdown in the principal cells because c42-GAL4 is expressed in only a subset of the tubule cells examined.
Mammalian WNKs activate the Sterile 20 kinases SPAK and OSR1 by phosphorylating them (16–18). Similarly, Sato and Shibuya (46) and Serysheva et al. (52) have shown that Drosophila WNK phosphorylates the fly SPAK/OSR1 homolog Fray. In addition, Sato and Shibuya (46) demonstrated that Fray phosphorylates the N terminus of mammalian NCC. Because four of the N-terminal serines and threonines phosphorylated by SPAK/OSR1 in mammalian NCC and NKCC1/2 are conserved in Drosophila Ncc69 (41), we hypothesized that Fray also phosphorylates Ncc69. To demonstrate this, we performed in vitro kinase assays. We observed autophosphorylation of wild-type Fray, as has previously been observed for bacterially expressed SPAK and OSR1 (25, 53), but wild-type Fray did not phosphorylate the N terminus of Ncc69 (Fig. 2). Similar to previous studies with bacterially purified SPAK and OSR1 (25, 53), after bacterial expression and purification of full-length Fray, we observed both full-length and truncated Fray isoforms, both of which were autophosphorylated. Interestingly, we observed less autophosphorylation of wild-type Fray in the presence of Ncc69, suggesting that in the absence of activation by WNK, Fray may be non-productively binding Ncc69. Mutation of the T-loop threonine of SPAK and OSR1, a target of WNK phosphorylation that is required for SPAK/OSR1 activation, to a phospho-mimicking glutamic acid results in constitutive SPAK/OSR1 activity in vitro (26) and in vivo in C. elegans (54). We therefore introduced a similar mutation into Fray, T206E. FrayT206E phosphorylated the N terminus of Ncc69 in the absence of WNK (Fig. 2), indicating that this mutation renders Fray constitutively active and that Ncc69 is a target of Fray phosphorylation. No kinase activity, either autophosphorylation or Ncc69 phosphorylation, was seen when a mutation predicted to render the kinase inactive, D185A, was introduced into wild-type or constitutively active Fray (Fig. 2).
FIGURE 2.
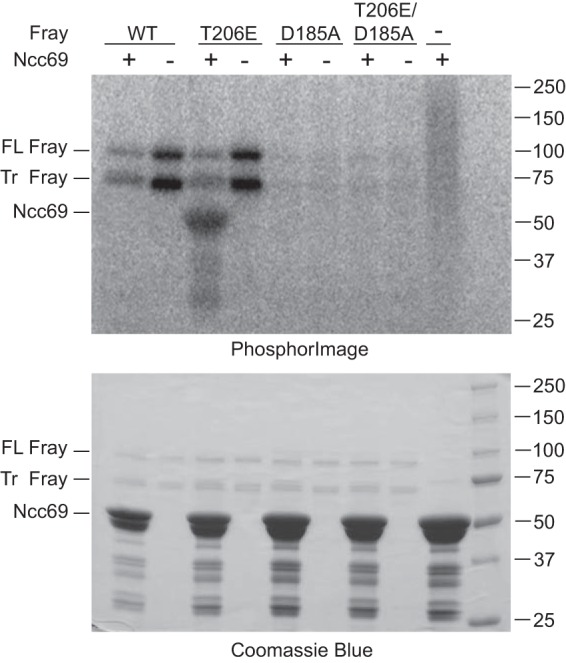
Fray phosphorylates the N terminus of Ncc69. In vitro kinase assays were performed with ∼2.5 μg of full-length GST-tagged Fray: wild-type (WT), constitutively active (T206E), kinase-dead (D185A), or constitutively active/kinase-dead (D185A/T206E), in the presence or absence of ∼20 μg GST-tagged N terminus of Ncc69 (amino acids 1–204), purified from bacteria. Bacterial expression of full-length Fray yielded both a full-length (FL) and a truncated (Tr) form, both of which autophosphorylated, whereas the kinase-dead mutants did not. Ncc69 was phosphorylated only by constitutively active FrayT206E. The top panel shows the PhosphorImager image of the Coomassie-stained gel below; this is one representative example of four experiments performed.
Like wnk, fray is an essential gene in the fly (55). We therefore knocked down fray in the principal cell of the tubule using the GAL4-UAS system. Again, we observed a decrease in K+ flux in the fray knockdown tubules (Table 2 and Fig. 3A), similar to the phenotype seen with wnk knockdown. Quantitative RT-PCR showed a 51% decrease in whole-tubule fray transcript levels (Fig. 3B).
FIGURE 3.
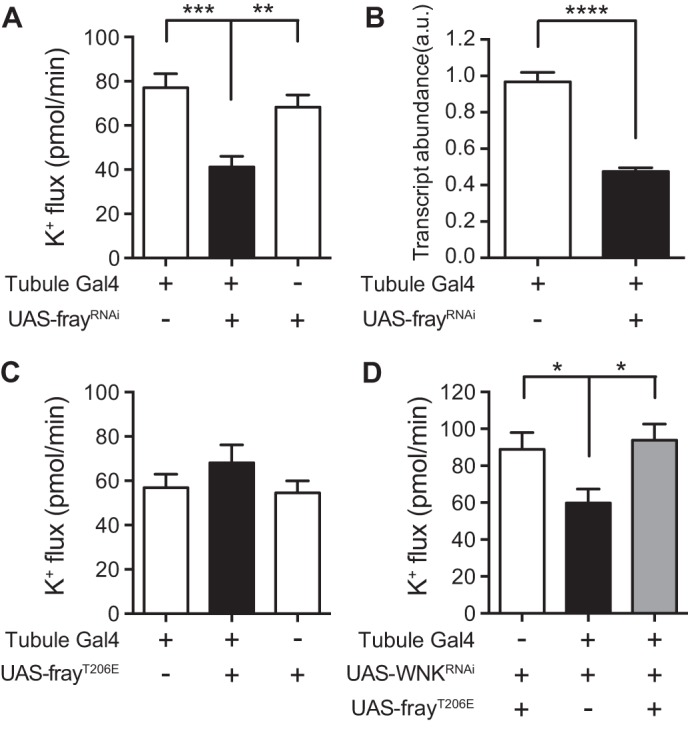
Fray positively regulates K+ flux downstream of WNK. A, K+ flux (pmol/min/tubule) was measured in control tubules (w;c42-GAL4/+ and yw/w;UAS-frayRNAi/+) versus fray principal cell knockdown tubules (yw/w;c42-GAL4/UAS-frayRNAi). n = 41–46 tubules/genotype. There was no significant difference in K+ flux between the two control genotypes. K+ flux was decreased in the fray knockdown tubules compared with both controls. B, fray transcript levels were measured by quantitative RT-PCR in control tubules (w;c42-GAL4/+) versus fray knockdown tubules (yw/w;c42-GAL4/UAS-frayRNAi), n = 4 sets of tubules, 50 tubules/genotype in each set. C, K+ flux (pmol/min/tubule) was measured in control tubules (w;c42-GAL4/+ and w;UAS-frayT206E/+) versus tubules expressing constitutively active FrayT206E in the principal cell (w;UAS-frayT206E/+;c42-GAL4/+). n = 38 tubules/genotype. Expression of constitutively active Fray in a wild-type background had no effect on K+ flux (one-way ANOVA, p = 0.3079). D, K+ flux (pmol/min/tubule) was measured in control tubules (w;UAS-WNKRNAi UAS-frayT206E/+), wnk principal cell knockdown tubules (w;UAS-WNKRNAi/+;c42-GAL4/+), and wnk principal cell knockdown tubules expressing constitutively active FrayT206E in the principal cells (w;UAS-WNKRNAi UAS-frayT206E/+;c42-GAL4/+). n = 11–14 tubules/genotype. wnk knockdown decreased K+ flux, and simultaneous expression of constitutively active Fray rescued this phenotype. There was no significant difference in K+ flux between the control and rescue tubules. All data are presented as the mean ± S.E. (error bars). *, p < 0.05; **, p < 0.01; ***, p < 0.001; ****, p < 0.0001.
Expression of the constitutively active FrayT206E isoform in the principal cell of the tubule did not increase K+ flux (Fig. 3C), suggesting that increasing Fray activity is not sufficient to increase ion flux. A possible explanation for this is that mouse protein-25/calcium-binding protein 39 (Mo25/Cab39) is limiting in wild-type tubules. Mo25/Cab39 has been shown to dramatically increase the in vitro kinase activity of SPAK/OSR1, including the SPAK and OSR1 constitutively active mutants analogous to FrayT206E (56), perhaps by facilitating domain swapping of SPAK/OSR1 dimers (57), and Fray and Mo25 also act together in Drosophila neuroblast asymmetric cell division (58). Nevertheless, expression of FrayT206E was sufficient to rescue the decreased K+ flux seen in wnk knockdown tubules back to control levels (Fig. 3D). This indicates that Fray acts downstream of WNK in the positive regulation of K+ flux and that WNK probably acts by phosphorylation of Fray on the T-loop threonine Thr-206.
Because Fray is acting downstream of WNK and Fray phosphorylates Ncc69, we predicted that the WNK-Fray kinase cascade is regulating K+ flux in the tubule through the regulation of the Ncc69 NKCC. To test this, we examined the effect of wnk knockdown in tubules homozygous for a null mutation in Ncc69, w;Ncc69r2/Ncc69r2 (10, 41). Unlike in the wild-type background, wnk knockdown in the Ncc69r2/Ncc69r2 mutant background had no effect on K+ flux (Table 2 and Fig. 4A), indicating that Ncc69 is downstream of WNK signaling. To verify that wnk transcripts were decreased in the Ncc69 mutant background, we performed quantitative RT-PCR. wnk transcript levels were decreased 48% in knockdown tubules compared with control (Fig. 4B), and the decrease in wnk transcript levels was not significantly different in the wild-type and Ncc69r2/Ncc69r2 mutant backgrounds (p = 0.5413). Similarly, knocking down fray in the principal cell of the tubule in the Ncc69r2/Ncc69r2 mutant background also had no effect on K+ flux (Fig. 4C), indicating that Ncc69 is also downstream of Fray signaling.
FIGURE 4.

WNK and Fray regulate principal cell K+ flux through NKCC. A, K+ flux (pmol/min/tubule) was measured in tubules with a homozygous null mutation for the NKCC Ncc69, either with principal cell wnk knockdown (w;UAS-WNKRNAi/+;c42-GAL4 Ncc69r2/Ncc69r2) or without (w;c42-GAL4 Ncc69r2/Ncc69r2 and w;UAS-WNKRNAi/+;Ncc69r2/Ncc69r2). n = 26–29 tubules/genotype. wnk knockdown had no effect on K+ flux in the Ncc69 null mutant background. One-way ANOVA, p = 0.7758. B, wnk transcript levels were measured by quantitative RT-PCR in control tubules (w;UAS-WNKRNAi/+;Ncc69r2/Ncc69r2) versus wnk knockdown tubules (w; UAS-WNKRNAi/+;c42-GAL4 Ncc69r2/Ncc69r2), n = 4 sets of tubules, 40 tubules/genotype in each set. wnk transcripts were decreased in the knockdown tubules in the Ncc69 null mutant background. C, K+ flux (pmol/min/tubule) was measured in tubules with a homozygous null mutation for the NKCC Ncc69, either with principal cell fray knockdown (yw/w;UAS-frayRNAi Ncc69r2/c42-GAL4 Ncc69r2) or without (w;c42-GAL4 Ncc69r2/Ncc69r2). n = 31–32 tubules/genotype. fray knockdown had no effect on K+ flux in the Ncc69 null mutant background. Two-tailed unpaired t test, p = 0.2898. All data are presented as the mean ± S.E. (error bars) *, p < 0.05.
Stimulation of the WNK-SPAK/OSR1 kinase cascade has been observed in cultured mammalian cells in both hypertonic and hypotonic conditions (17, 23, 24, 53, 59–61). Whether changes in tonicity can stimulate transepithelial renal tubule ion transport by activation of this pathway has not been examined. We therefore examined the effects of altering tonicity of the tubule bathing medium in our assay. It has previously been shown that fluid secretion rates are decreased in hypertonic bathing medium (5). Similarly, we found that increasing bathing medium tonicity by the addition of 0.1 m sucrose resulted in decreased fluid secretion and K+ flux in control tubules (Table 2 and Fig. 5). The inhibitory effect of hypertonicity was also observed in fray knockdown tubules (Fig. 5). We also noted that a high proportion of both control and fray knockdown tubules failed to secrete fluid at all, again consistent with an inhibitory effect of hypertonicity on secretion; 29 of 56 (52%) w;c42-GAL4/+ tubules bathed in hypertonic medium failed to secrete, and 25 of 49 (51%) yw/w;UAS-frayRNAi/c42-GAL4 tubules failed to (p = 1.000, Fisher's exact test).
FIGURE 5.
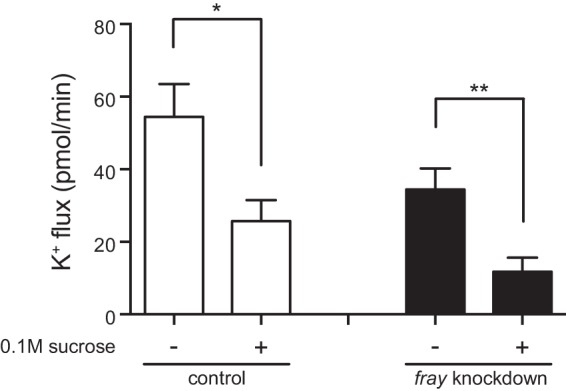
Hypertonicity decreases K+ flux in control and fray knockdown tubules. K+ flux (pmol/min/tubule) was measured in control (w;c42-GAL4/+) and principal cell fray knockdown (yw/w;UAS-frayRNAi/c42-GAL4) tubules in standard bathing medium or hypertonic bathing medium (sucrose added to a final concentration of 0.1 m). n = 14–22 tubules/genotype/condition. K+ flux was decreased in hypertonic conditions in both control and fray knockdown tubules. *, p < 0.05; **, p < 0.01. Error bars, S.E.
Fluid secretion rates increase when tonicity of bathing medium is decreased (5). We replicated this result in w;c42-GAL4/+ control tubules and showed that K+ flux is also increased (Table 2 and Fig. 6A). However, the increase in K+ flux was not observed in wnk or fray knockdown tubules (Fig. 6A). Similarly, hypotonic bathing medium stimulated K+ flux in a second control strain, wBerlin, but not in w;Ncc69r2/Ncc69r2 mutant tubules lacking the NKCC transporter (Fig. 6B). Thus, whereas Ncc69 mediates ∼20–30% of K+ flux under baseline conditions, under hypotonic conditions, Ncc69 mediates ∼50% of K+ flux (Table 2 and Fig. 6B) (10). Similarly, the WNK- and Fray-sensitive components of K+ flux are increased under hypotonic conditions. These results indicate that hypotonic bathing medium stimulates K+ flux by activating the WNK/Fray/Ncc69 pathway.
FIGURE 6.
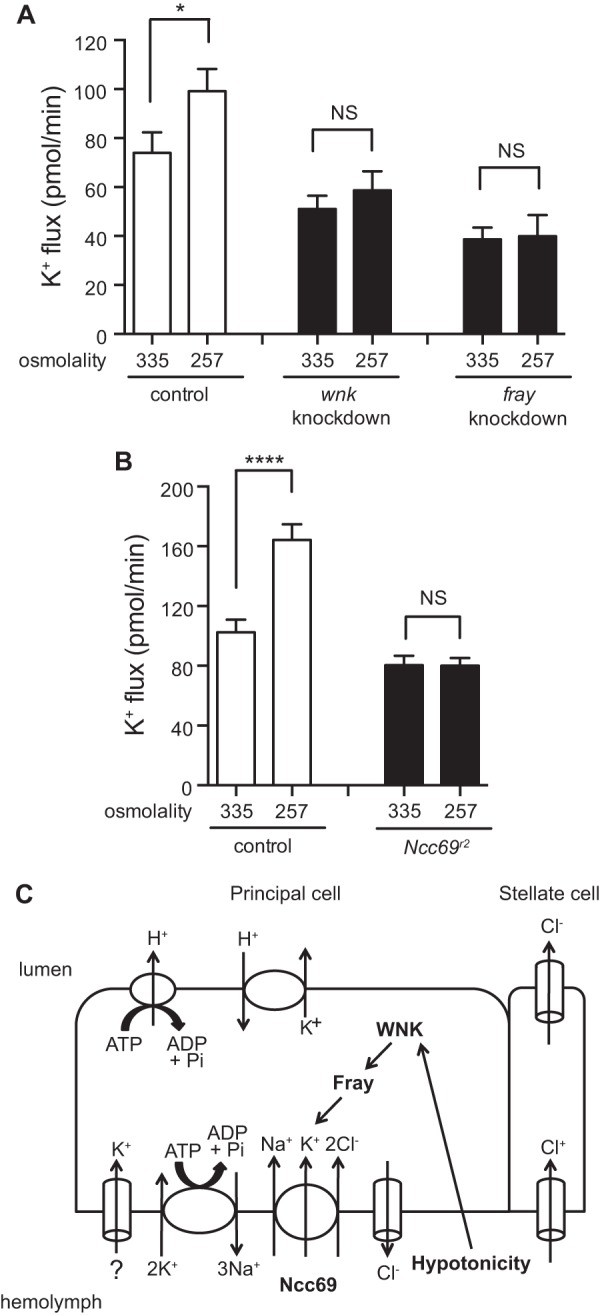
Hypotonicity stimulates K+ flux by activating the WNK/Fray/NKCC pathway. A, K+ flux (pmol/min/tubule) was measured in control (w;c42-GAL4/+), principal cell wnk knockdown (w;UAS-WNKRNAi/+;c42-GAL4/+), and principal cell fray knockdown (yw/w;UAS-frayRNAi/c42-GAL4) tubules in standard bathing medium (measured osmolality = 335 mosm/kg) or hypotonic bathing medium (standard bathing medium diluted with water to a measured osmolality of 257 mosm/kg). n = 32–38 tubules/genotype/condition. K+ flux was stimulated in control tubules under hypotonic conditions but was not stimulated in wnk or fray knockdown tubules. B, K+ flux (pmol/min/tubule) was measured in control (wBerlin) and Ncc69 homozygous null mutant (w;Ncc69r2/Ncc69r2) tubules in standard bathing medium (osmolality = 335 mosm/kg) or hypotonic bathing medium (osmolality = 257 mosm/kg). Flies in this experiment were reared at room temperature, ∼23 °C. n = 20–22 tubules/genotype/condition. K+ flux was stimulated in control tubules under hypotonic conditions, but was not stimulated in Ncc69 null tubules. All data are presented as the mean ± S.E. (error bars). *, p < 0.05; ****, p < 0.0001. C, model for NKCC regulation. Our previous data are consistent with a role for Ncc69 in K+ uptake from the hemolymph into the principal cell; additional K+ uptake pathways are indicated with question marks. K+ is then secreted across the apical membrane in exchange for H+, which is extruded into the lumen by the apical vacuolar H+-ATPase. The data presented here are consistent with a positive regulatory role for the WNK-SPAK/OSR1 (Fray) kinase cascade in NKCC (Ncc69) regulation. Hypotonicity stimulates K+ flux by activating the WNK-SPAK/OSR1-NKCC pathway.
DISCUSSION
Lytle and Forbush (62) demonstrated in isolated shark rectal gland, a transporting epithelium, that both hypotonic and hypertonic conditions activate NKCC, and multiple studies have shown that in isolated cells, WNK-SPAK/OSR1 signaling is activated in hypertonic and hypotonic media (17, 23, 24, 53, 59–61). Why would extracellular hypertonicity and hypotonicity, which cause opposite effects on cell volume (shrinkage and swelling, respectively), both activate NKCC activity? Activation of the WNK-SPAK/OSR1-NKCC pathway under hypertonic conditions increases intracellular Cl−; the accompanying inward flow of osmotically obliged water restores cell volume after hypertonic shrinkage. What, then, is the purpose of hypotonic activation of the WNK-SPAK/OSR1-NKCC pathway? A clue to this comes from the same study of Lytle and Forbush (62), in which transepithelial tubule secretion only occurred under hypotonic and not under hypertonic conditions despite NKCC activation in both situations. Under hypertonic conditions, NKCC activation in the absence of net transepithelial transport would result in restoration of tubule epithelial cell volume. However, under isotonic or hypotonic conditions, NKCC activation on the basolateral membrane of this secreting epithelium would result in cell swelling if it were not coupled to apical ion efflux; thus, under hypotonic conditions, both basolateral NKCC and apical transport mechanisms are stimulated, resulting in net fluid secretion. Thus, it is likely that activation of NKCC by hypertonicity primarily defends cell volume (i.e. regulatory volume increase after cell shrinkage), whereas activation by hypotonicity is functionally designed to stimulate transepithelial solute and fluid fluxes. Here, we have directly proven for the first time that in a transporting epithelium, hypotonicity does result in increased transepithelial ion flux through the activation of the WNK-SPAK/OSR1-NKCC pathway.
The Malpighian tubules of insects are renal epithelia that play an essential role in iono- and osmoregulation (3, 4). Because the fly tubules are bathed in hemolymph, they can directly sense changes in its osmolarity. Indeed, in isolated tubules, changes in bathing solution osmolarity directly regulate fluid secretion and ion flux (5, 63). Here, we demonstrate an inverse relationship between tubule bathing osmolarity and K+ flux in the Drosophila renal tubule. We previously demonstrated a role for the fly NKCC, Ncc69, in transepithelial K+ flux (10). We therefore examined whether Ncc69 is required for the increased K+ flux seen in hypotonic conditions. We demonstrate that both Ncc69 and its kinase regulators, WNK and Fray, are required for the response to hypotonicity. Our results are consistent with a model in which WNK activates the SPAK/OSR1 homolog Fray, which then phosphorylates the N terminus of Ncc69 to result in transporter activation, K+ uptake into the principal cell, and eventual K+ secretion across the apical membrane and into the urine (Fig. 6C). Hypotonicity activates the WNK-Fray-Ncc69 pathway because no stimulation of K+ flux is seen in tubules with decreased wnk, fray, or Ncc69. Increased KCl secretion drives an increase in osmotically obliged water flux, presumably through aquaporins expressed in the tubule (64), resulting in increased fluid secretion.
In contrast, as in the shark rectal gland, hypertonicity does not result in increased fluid secretion or transepithelial ion flux. In fact, K+ flux and fluid secretion decrease, and this occurs independently of the WNK-SPAK/OSR1-NKCC pathway. Blumenthal (5, 65) has previously demonstrated that tyramine is a positive regulator of transepithelial fluid secretion, by increasing chloride flux through the stellate cells. Increased osmolarity decreases tubule sensitivity to tyramine, resulting in decreased fluid secretion (5, 65). Our results suggest that this process occurs independently of WNK-SPAK/OSR1 signaling.
How is activation of the WNK-SPAK/OSR1 pathway occurring in tubules exposed to hypotonicity? The typical cellular response to hypotonicity is cell swelling, followed by a decrease in cellular volume (regulatory volume decrease), typically achieved by extrusion of KCl (66). Swelling followed by regulatory volume decrease in renal epithelial cells of the proximal tubule and cortical collecting duct was first demonstrated in the 1970s, and it was proposed to occur through loss of KCl (67). More recently, intracellular chloride concentrations were directly measured during cell swelling and regulatory volume decrease in the A6 Xenopus renal epithelial cell line. An immediate decrease in intracellular chloride occurred at the time of cell swelling due to dilution, followed by an ongoing decrease in intracellular chloride as cell volume decreased toward normal, suggesting that extrusion of intracellular chloride was serving as a mechanism of regulatory volume decrease (68). Similarly, measurement of intracellular chloride in the renal tubules of another insect, the grasshopper-like New Zealand alpine weta, demonstrated a decrease in intracellular chloride when the tubules were bathed in hypotonic saline as well as an increase in fluid secretion rate and K+ flux (63), as seen in our study of Drosophila tubules.
WNKs have been proposed to function as chloride sensors (69, 70), and very recently, the structural basis for chloride inhibition of WNK autophosphorylation and activation has been described (71). The sequence of the chloride-binding site of mammalian WNK1 is perfectly conserved in Drosophila WNK. Thus, a decrease in intracellular chloride occurring during cell swelling and regulatory volume decrease could stimulate WNK through relief of chloride inhibition, followed by Fray and Ncc69 activation, stimulating an increase in transepithelial ion and water flux. Indeed, intracellular Cl− concentration in the fly tubule is 30 mm in isotonic conditions (72), slightly higher than the apparent median inhibitory concentration of 20 mm for Cl− inhibition of mammalian WNK1 autophosphorylation (71). This suggests that tubule WNK-Fray signaling is poised to respond to changes in intracellular Cl−.
A similar mechanism probably explains the activation of sodium-coupled chloride cotransporters expressed in Xenopus oocytes. For example, Ponce-Coria et al. (73) observed activation of NKCC2 by WNK3-SPAK signaling by either bathing Xenopus oocytes in low Cl− hypotonic medium or by decreasing intracellular Cl− via coexpression of the potassium-chloride cotransporter, KCC2. Similarly, WNK3 powerfully stimulates both NCC and NKCC1 in Xenopus oocytes even under hypotonic conditions, in which cotransporter activity is otherwise suppressed (70, 74, 75).
Hypotonic cell swelling is thought to activate a volume-regulated anion channel (also called IClswell). Recently, LRRC8A has been identified as a key component of this channel in vertebrate cells (76, 77), whereas two groups have shown that in Drosophila S2 cells, Bestrophin-1 encodes IClswell (78–80). Bestrophin-1 is robustly expressed in adult fly tubule (FlyAtlas database) (81), suggesting a possible mechanism for lowering of intracellular Cl− in response to hypotonicity, with subsequent activation of the WNK-Fray signaling cascade.
In the adult fly, fluid secretion by the main segment is followed by reabsorption of ∼30% of the fluid in the lower segment (82). In addition, although it has not been studied in adult Drosophila, in other adult insects, including Dipterans, the hindgut and rectum are major sites of water and ion reabsorption regulating the final osmolarity of the excreta (3, 83, 84). Similarly, in Drosophila larvae, the anal papillae play a role in osmoregulation (85). Thus, following a hypotonic meal that results in decreased hemolymph osmolarity, water clearance could be achieved by first increasing the rate of fluid secretion by the main segment, as observed in our study; preferential reabsorption of ions over water in more “downstream” segments, such as the lower segment of the tubule, hindgut, and rectum, would then allow excretion of a water load without excess ion loss.
In summary, we have shown that the WNK-SPAK/OSR1 kinase cascade regulates the fly NKCC to modulate transepithelial ion flux in the Drosophila renal tubule. We provide the first direct demonstration that this pathway is required for the increased transepithelial ion flux that occurs upon hypotonic stimulation. The resulting increase in urine generation by the Malpighian tubule provides a means for clearance of a water load in order to maintain homeostasis of hemolymph osmolarity.
Acknowledgments
We thank Julian Dow, Billy Leiserson, and Adrian Rothenfluh for generous gifts of fly strains. Fly strains were also obtained from the Vienna Drosophila Resource Center. The Ncc69 and fray cDNAs were obtained from the Drosophila Genomics Resource Center. Johannes Bischof and Konrad Basler generously provided the pUASg.attB plasmid. We thank the TRiP project at Harvard Medical School for providing the pWALIUM20 plasmid vector. Adrian Rothenfluh participated in many thoughtful discussions and provided comments on the manuscript.
This work was supported, in whole or in part, by National Institutes of Health, NIDDK, Grants DK091316 (to A. R. R.), DK007257 (institutional T32), DK59530 (to C. L. H.), and DK079328 (University of Texas Southwestern O'Brien Center P30). This work was also supported by the American Society of Nephrology Gottschalk Award (to A. R. R.). The Drosophila Genomics Resource Center is supported by National Institutes of Health Grant 2P40OD010949-10A1, and the TRiP project at Harvard Medical School is supported by National Institutes of Health, NIGMS, Grant R01-GM084947.
- NKCC
- sodium-potassium-2-chloride cotransporter
- WNK
- with-no-lysine kinase
- SPAK
- Ste20-related proline/alanine-rich kinase
- OSR1
- oxidative stress response kinase
- SLC
- solute linked carrier
- NCC
- sodium-coupled chloride cotransporter
- ANOVA
- analysis of variance.
REFERENCES
- 1. Chamberlin M. E., Strange K. (1989) Anisosmotic cell volume regulation: a comparative view. Am. J. Physiol. 257, C159–C173 [DOI] [PubMed] [Google Scholar]
- 2. Albers M. A., Bradley T. J. (2004) Osmotic regulation in adult Drosophila melanogaster during dehydration and rehydration. J. Exp. Biol. 207, 2313–2321 [DOI] [PubMed] [Google Scholar]
- 3. Phillips J. (1981) Comparative physiology of insect renal function. Am. J. Physiol. 241, R241–R257 [DOI] [PubMed] [Google Scholar]
- 4. Larsen E. H., Deaton L. E., Onken H., O'Donnell M., Grosell M., Dantzler W. H., Weihrauch D. (2014) Osmoregulation and excretion. Compr. Physiol. 4, 405–573 [DOI] [PubMed] [Google Scholar]
- 5. Blumenthal E. M. (2005) Modulation of tyramine signaling by osmolality in an insect secretory epithelium. Am. J. Physiol. Cell Physiol. 289, C1261–C1267 [DOI] [PubMed] [Google Scholar]
- 6. O'Donnell M. J., Rheault M. R., Davies S. A., Rosay P., Harvey B. J., Maddrell S. H., Kaiser K., Dow J. A. (1998) Hormonally controlled chloride movement across Drosophila tubules is via ion channels in stellate cells. Am. J. Physiol. 274, R1039–R1049 [DOI] [PubMed] [Google Scholar]
- 7. Linton S. M., O'Donnell M. J. (1999) Contributions of K+:Cl− cotransport and Na+/K+-ATPase to basolateral ion transport in malpighian tubules of Drosophila melanogaster. J. Exp. Biol. 202, 1561–1570 [DOI] [PubMed] [Google Scholar]
- 8. Rheault M. R., O'Donnell M. J. (2001) Analysis of epithelial K+ transport in Malpighian tubules of Drosophila melanogaster: evidence for spatial and temporal heterogeneity. J. Exp. Biol. 204, 2289–2299 [DOI] [PubMed] [Google Scholar]
- 9. O'Donnell M. J., Dow J. A., Huesmann G. R., Tublitz N. J., Maddrell S. H. (1996) Separate control of anion and cation transport in malpighian tubules of Drosophila melanogaster. J. Exp. Biol. 199, 1163–1175 [DOI] [PubMed] [Google Scholar]
- 10. Rodan A. R., Baum M., Huang C. L. (2012) The Drosophila NKCC Ncc69 is required for normal renal tubule function. Am. J. Physiol. Cell Physiol. 303, C883–C894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gamba G. (2005) Molecular physiology and pathophysiology of electroneutral cation-chloride cotransporters. Physiol. Rev. 85, 423–493 [DOI] [PubMed] [Google Scholar]
- 12. Wall S. M., Fischer M. P., Mehta P., Hassell K. A., Park S. J. (2001) Contribution of the Na+-K+-2Cl− cotransporter NKCC1 to Cl− secretion in rat OMCD. Am. J. Physiol. Renal Physiol. 280, F913–F921 [DOI] [PubMed] [Google Scholar]
- 13. Pech V., Thumova M., Kim Y. H., Agazatian D., Hummler E., Rossier B. C., Weinstein A. M., Nanami M., Wall S. M. (2012) ENaC inhibition stimulates Cl- secretion in the mouse cortical collecting duct through an NKCC1-dependent mechanism. Am. J. Physiol. Renal Physiol. 303, F45–F55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu W., Schreck C., Coleman R. A., Wade J. B., Hernandez Y., Zavilowitz B., Warth R., Kleyman T. R., Satlin L. M. (2011) Role of NKCC in BK channel-mediated net K+ secretion in the CCD. Am. J. Physiol. Renal Physiol. 301, F1088–F1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wilson F. H., Disse-Nicodème S., Choate K. A., Ishikawa K., Nelson-Williams C., Desitter I., Gunel M., Milford D. V., Lipkin G. W., Achard J. M., Feely M. P., Dussol B., Berland Y., Unwin R. J., Mayan H., Simon D. B., Farfel Z., Jeunemaitre X., Lifton R. P. (2001) Human hypertension caused by mutations in WNK kinases. Science 293, 1107–1112 [DOI] [PubMed] [Google Scholar]
- 16. Vitari A. C., Deak M., Morrice N. A., Alessi D. R. (2005) The WNK1 and WNK4 protein kinases that are mutated in Gordon's hypertension syndrome phosphorylate and activate SPAK and OSR1 protein kinases. Biochem. J. 391, 17–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Moriguchi T., Urushiyama S., Hisamoto N., Iemura S., Uchida S., Natsume T., Matsumoto K., Shibuya H. (2005) WNK1 regulates phosphorylation of cation-chloride-coupled cotransporters via the STE20-related kinases, SPAK and OSR1. J. Biol. Chem. 280, 42685–42693 [DOI] [PubMed] [Google Scholar]
- 18. Anselmo A. N., Earnest S., Chen W., Juang Y. C., Kim S. C., Zhao Y., Cobb M. H. (2006) WNK1 and OSR1 regulate the Na+,K+,2Cl-cotransporter in HeLa cells. Proc. Natl. Acad. Sci. U.S.A. 103, 10883–10888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Richardson C., Sakamoto K., de los Heros P., Deak M., Campbell D. G., Prescott A. R., Alessi D. R. (2011) Regulation of the NKCC2 ion cotransporter by SPAK-OSR1-dependent and -independent pathways. J. Cell Sci. 124, 789–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rinehart J., Kahle K. T., de Los Heros P., Vazquez N., Meade P., Wilson F. H., Hebert S. C., Gimenez I., Gamba G., Lifton R. P. (2005) WNK3 kinase is a positive regulator of NKCC2 and NCC, renal cation-Cl− cotransporters required for normal blood pressure homeostasis. Proc. Natl. Acad. Sci. U.S.A. 102, 16777–16782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gagnon K. B., England R., Delpire E. (2007) A single binding motif is required for SPAK activation of the Na-K-2Cl cotransporter. Cell Physiol. Biochem. 20, 131–142 [DOI] [PubMed] [Google Scholar]
- 22. Gagnon K. B., England R., Delpire E. (2006) Volume sensitivity of cation-Cl− cotransporters is modulated by the interaction of two kinases: Ste20-related proline-alanine-rich kinase and WNK4. Am. J. Physiol. Cell Physiol. 290, C134–C142 [DOI] [PubMed] [Google Scholar]
- 23. Dowd B. F., Forbush B. (2003) PASK (proline-alanine-rich STE20-related kinase), a regulatory kinase of the Na-K-Cl cotransporter (NKCC1). J. Biol. Chem. 278, 27347–27353 [DOI] [PubMed] [Google Scholar]
- 24. Richardson C., Rafiqi F. H., Karlsson H. K., Moleleki N., Vandewalle A., Campbell D. G., Morrice N. A., Alessi D. R. (2008) Activation of the thiazide-sensitive Na+-Cl− cotransporter by the WNK-regulated kinases SPAK and OSR1. J. Cell Sci. 121, 675–684 [DOI] [PubMed] [Google Scholar]
- 25. Gagnon K. B., England R., Delpire E. (2006) Characterization of SPAK and OSR1, regulatory kinases of the Na-K-2Cl cotransporter. Mol. Cell Biol. 26, 689–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vitari A. C., Thastrup J., Rafiqi F. H., Deak M., Morrice N. A., Karlsson H. K., Alessi D. R. (2006) Functional interactions of the SPAK/OSR1 kinases with their upstream activator WNK1 and downstream substrate NKCC1. Biochem. J. 397, 223–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yang C. L., Angell J., Mitchell R., Ellison D. H. (2003) WNK kinases regulate thiazide-sensitive Na-Cl cotransport. J. Clin. Invest. 111, 1039–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cai H., Cebotaru V., Wang Y. H., Zhang X. M., Cebotaru L., Guggino S. E., Guggino W. B. (2006) WNK4 kinase regulates surface expression of the human sodium chloride cotransporter in mammalian cells. Kidney Int. 69, 2162–2170 [DOI] [PubMed] [Google Scholar]
- 29. Zhou B., Zhuang J., Gu D., Wang H., Cebotaru L., Guggino W. B., Cai H. (2010) WNK4 enhances the degradation of NCC through a sortilin-mediated lysosomal pathway. J. Am. Soc. Nephrol. 21, 82–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Subramanya A. R., Liu J., Ellison D. H., Wade J. B., Welling P. A. (2009) WNK4 diverts the thiazide-sensitive NaCl cotransporter to the lysosome and stimulates AP-3 interaction. J. Biol. Chem. 284, 18471–18480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wilson F. H., Kahle K. T., Sabath E., Lalioti M. D., Rapson A. K., Hoover R. S., Hebert S. C., Gamba G., Lifton R. P. (2003) Molecular pathogenesis of inherited hypertension with hyperkalemia: the Na-Cl cotransporter is inhibited by wild-type but not mutant WNK4. Proc. Natl. Acad. Sci. U.S.A. 100, 680–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mu S., Shimosawa T., Ogura S., Wang H., Uetake Y., Kawakami-Mori F., Marumo T., Yatomi Y., Geller D. S., Tanaka H., Fujita T. (2011) Epigenetic modulation of the renal β-adrenergic-WNK4 pathway in salt-sensitive hypertension. Nat. Med. 17, 573–580 [DOI] [PubMed] [Google Scholar]
- 33. Castañeda-Bueno M., Cervantes-Pérez L. G., Vázquez N., Uribe N., Kantesaria S., Morla L., Bobadilla N. A., Doucet A., Alessi D. R., Gamba G. (2012) Activation of the renal Na+:Cl− cotransporter by angiotensin II is a WNK4-dependent process. Proc. Natl. Acad. Sci. U.S.A. 109, 7929–7934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ohta A., Rai T., Yui N., Chiga M., Yang S. S., Lin S. H., Sohara E., Sasaki S., Uchida S. (2009) Targeted disruption of the Wnk4 gene decreases phosphorylation of Na-Cl cotransporter, increases Na excretion and lowers blood pressure. Hum. Mol. Genet. 18, 3978–3986 [DOI] [PubMed] [Google Scholar]
- 35. Rafiqi F. H., Zuber A. M., Glover M., Richardson C., Fleming S., Jovanović S., Jovanović A., O'Shaughnessy K. M., Alessi D. R. (2010) Role of the WNK-activated SPAK kinase in regulating blood pressure. EMBO Mol. Med. 2, 63–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yang S. S., Lo Y. F., Wu C. C., Lin S. W., Yeh C. J., Chu P., Sytwu H. K., Uchida S., Sasaki S., Lin S. H. (2010) SPAK-knockout mice manifest Gitelman syndrome and impaired vasoconstriction. J. Am. Soc. Nephrol. 21, 1868–1877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Grimm P. R., Taneja T. K., Liu J., Coleman R., Chen Y. Y., Delpire E., Wade J. B., Welling P. A. (2012) SPAK isoforms and OSR1 regulate sodium-chloride co-transporters in a nephron-specific manner. J. Biol. Chem. 287, 37673–37690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lin S. H., Yu I. S., Jiang S. T., Lin S. W., Chu P., Chen A., Sytwu H. K., Sohara E., Uchida S., Sasaki S., Yang S. S. (2011) Impaired phosphorylation of Na+-K+-2Cl− cotransporter by oxidative stress-responsive kinase-1 deficiency manifests hypotension and Bartter-like syndrome. Proc. Natl. Acad. Sci. U.S.A. 108, 17538–17543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Takahashi D., Mori T., Nomura N., Khan M. Z., Araki Y., Zeniya M., Sohara E., Rai T., Sasaki S., Uchida S. (2014) WNK4 is the major WNK positively regulating NCC in the mouse kidney. Biosci. Rep. 34, e00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cheng C. J., Truong T., Baum M., Huang C. L. (2012) Kidney-specific WNK1 inhibits sodium reabsorption in the cortical thick ascending limb. Am. J. Physiol. Renal Physiol. 303, F667–F673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Leiserson W. M., Forbush B., Keshishian H. (2011) Drosophila glia use a conserved cotransporter mechanism to regulate extracellular volume. Glia 59, 320–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rosay P., Davies S. A., Yu Y., Sozen M. A., Kaiser K., Dow J. A. (1997) Cell-type specific calcium signalling in a Drosophila epithelium. J. Cell Sci. 110, 1683–1692 [DOI] [PubMed] [Google Scholar]
- 43. Dietzl G., Chen D., Schnorrer F., Su K. C., Barinova Y., Fellner M., Gasser B., Kinsey K., Oppel S., Scheiblauer S., Couto A., Marra V., Keleman K., Dickson B. J. (2007) A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature 448, 151–156 [DOI] [PubMed] [Google Scholar]
- 44. Bischof J., Maeda R. K., Hediger M., Karch F., Basler K. (2007) An optimized transgenesis system for Drosophila using germ-line-specific phiC31 integrases. Proc. Natl. Acad. Sci. U.S.A. 104, 3312–3317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ni J. Q., Zhou R., Czech B., Liu L. P., Holderbaum L., Yang-Zhou D., Shim H. S., Tao R., Handler D., Karpowicz P., Binari R., Booker M., Brennecke J., Perkins L. A., Hannon G. J., Perrimon N. (2011) A genome-scale shRNA resource for transgenic RNAi in Drosophila. Nat. Methods 8, 405–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sato A., Shibuya H. (2013) WNK signaling is involved in neural development via Lhx8/Awh expression. PLoS One 8, e55301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pfaffl M. W. (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29, e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Dow J. A., Maddrell S. H., Görtz A., Skaer N. J., Brogan S., Kaiser K. (1994) The malpighian tubules of Drosophila melanogaster: a novel phenotype for studies of fluid secretion and its control. J. Exp. Biol. 197, 421–428 [DOI] [PubMed] [Google Scholar]
- 49. Maddrell S. H., O'Donnell M. J., Caffrey R. (1993) The regulation of haemolymph potassium activity during initiation and maintenance of diuresis in fed Rhodnius prolixus. J. Exp. Biol. 177, 273–285 [DOI] [PubMed] [Google Scholar]
- 50. Messerli M. A., Kurtz I., Smith P. J. (2008) Characterization of optimized Na+ and Cl− liquid membranes for use with extracellular, self-referencing microelectrodes. Anal. Bioanal. Chem. 390, 1355–1359 [DOI] [PubMed] [Google Scholar]
- 51. Brand A. H., Perrimon N. (1993) Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118, 401–415 [DOI] [PubMed] [Google Scholar]
- 52. Serysheva E., Berhane H., Grumolato L., Demir K., Balmer S., Bodak M., Boutros M., Aaronson S., Mlodzik M., Jenny A. (2013) Wnk kinases are positive regulators of canonical Wnt/β-catenin signalling. EMBO Rep. 14, 718–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chen W., Yazicioglu M., Cobb M. H. (2004) Characterization of OSR1, a member of the mammalian Ste20p/germinal center kinase subfamily. J. Biol. Chem. 279, 11129–11136 [DOI] [PubMed] [Google Scholar]
- 54. Hisamoto N., Moriguchi T., Urushiyama S., Mitani S., Shibuya H., Matsumoto K. (2008) Caenorhabditis elegans WNK-STE20 pathway regulates tube formation by modulating ClC channel activity. EMBO Rep. 9, 70–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Leiserson W. M., Harkins E. W., Keshishian H. (2000) Fray, a Drosophila serine/threonine kinase homologous to mammalian PASK, is required for axonal ensheathment. Neuron 28, 793–806 [DOI] [PubMed] [Google Scholar]
- 56. Filippi B. M., de los Heros P., Mehellou Y., Navratilova I., Gourlay R., Deak M., Plater L., Toth R., Zeqiraj E., Alessi D. R. (2011) MO25 is a master regulator of SPAK/OSR1 and MST3/MST4/YSK1 protein kinases. EMBO J. 30, 1730–1741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ponce-Coria J., Gagnon K. B., Delpire E. (2012) Calcium-binding protein 39 facilitates molecular interaction between Ste20p proline alanine-rich kinase and oxidative stress response 1 monomers. Am. J. Physiol. Cell Physiol. 303, C1198–C1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Yamamoto Y., Izumi Y., Matsuzaki F. (2008) The GC kinase Fray and Mo25 regulate Drosophila asymmetric divisions. Biochem. Biophys. Res. Commun. 366, 212–218 [DOI] [PubMed] [Google Scholar]
- 59. Naito S., Ohta A., Sohara E., Ohta E., Rai T., Sasaki S., Uchida S. (2011) Regulation of WNK1 kinase by extracellular potassium. Clin. Exp. Nephrol. 15, 195–202 [DOI] [PubMed] [Google Scholar]
- 60. Lenertz L. Y., Lee B. H., Min X., Xu B. E., Wedin K., Earnest S., Goldsmith E. J., Cobb M. H. (2005) Properties of WNK1 and implications for other family members. J. Biol. Chem. 280, 26653–26658 [DOI] [PubMed] [Google Scholar]
- 61. Zagórska A., Pozo-Guisado E., Boudeau J., Vitari A. C., Rafiqi F. H., Thastrup J., Deak M., Campbell D. G., Morrice N. A., Prescott A. R., Alessi D. R. (2007) Regulation of activity and localization of the WNK1 protein kinase by hyperosmotic stress. J. Cell Biol. 176, 89–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lytle C., Forbush B., 3rd. (1992) Na-K-Cl cotransport in the shark rectal gland. II. Regulation in isolated tubules. Am. J. Physiol. 262, C1009–C1017 [DOI] [PubMed] [Google Scholar]
- 63. Leader J. P., Neufeld D. S. (1997) Electrochemical characteristics of ion secretion in malpighian tubules of the New Zealand alpine weta (Hemideina maori). J. Insect. Physiol. 44, 39–48 [DOI] [PubMed] [Google Scholar]
- 64. Spring J. H., Robichaux S. R., Hamlin J. A. (2009) The role of aquaporins in excretion in insects. J. Exp. Biol. 212, 358–362 [DOI] [PubMed] [Google Scholar]
- 65. Blumenthal E. M. (2003) Regulation of chloride permeability by endogenously produced tyramine in the Drosophila Malpighian tubule. Am. J. Physiol. Cell Physiol. 284, C718–C728 [DOI] [PubMed] [Google Scholar]
- 66. Hoffmann E. K., Lambert I. H., Pedersen S. F. (2009) Physiology of cell volume regulation in vertebrates. Physiol. Rev. 89, 193–277 [DOI] [PubMed] [Google Scholar]
- 67. Dellasega M., Grantham J. J. (1973) Regulation of renal tubule cell volume in hypotonic media. Am. J. Physiol. 224, 1288–1294 [DOI] [PubMed] [Google Scholar]
- 68. Miyazaki H., Shiozaki A., Niisato N., Marunaka Y. (2007) Physiological significance of hypotonicity-induced regulatory volume decrease: reduction in intracellular Cl− concentration acting as an intracellular signaling. Am. J. Physiol. Renal Physiol. 292, F1411–F1417 [DOI] [PubMed] [Google Scholar]
- 69. Kahle K. T., Rinehart J., Ring A., Gimenez I., Gamba G., Hebert S. C., Lifton R. P. (2006) WNK protein kinases modulate cellular Cl− flux by altering the phosphorylation state of the Na-K-Cl and K-Cl cotransporters. Physiology 21, 326–335 [DOI] [PubMed] [Google Scholar]
- 70. Pacheco-Alvarez D., Gamba G. (2011) WNK3 is a putative chloride-sensing kinase. Cell Physiol. Biochem. 28, 1123–1134 [DOI] [PubMed] [Google Scholar]
- 71. Piala A. T., Moon T. M., Akella R., He H., Cobb M. H., Goldsmith E. J. (2014) Chloride sensing by WNK1 involves inhibition of autophosphorylation. Science Signal. 7, ra41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ianowski J. P., O'Donnell M. J. (2004) Basolateral ion transport mechanisms during fluid secretion by Drosophila Malpighian tubules: Na+ recycling, Na+:K+:2Cl− cotransport and Cl− conductance. J. Exp. Biol. 207, 2599–2609 [DOI] [PubMed] [Google Scholar]
- 73. Ponce-Coria J., San-Cristobal P., Kahle K. T., Vazquez N., Pacheco-Alvarez D., de Los Heros P., Juárez P., Muñoz E., Michel G., Bobadilla N. A., Gimenez I., Lifton R. P., Hebert S. C., Gamba G. (2008) Regulation of NKCC2 by a chloride-sensing mechanism involving the WNK3 and SPAK kinases. Proc. Natl. Acad. Sci. U.S.A. 105, 8458–8463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kahle K. T., Rinehart J., de Los Heros P., Louvi A., Meade P., Vazquez N., Hebert S. C., Gamba G., Gimenez I., Lifton R. P. (2005) WNK3 modulates transport of Cl− in and out of cells: implications for control of cell volume and neuronal excitability. Proc. Natl. Acad. Sci. U.S.A. 102, 16783–16788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Pacheco-Alvarez D., Vázquez N., Castañeda-Bueno M., de-Los-Heros P., Cortes-González C., Moreno E., Meade P., Bobadilla N. A., Gamba G. (2012) WNK3-SPAK interaction is required for the modulation of NCC and other members of the SLC12 family. Cell Physiol. Biochem. 29, 291–302 [DOI] [PubMed] [Google Scholar]
- 76. Qiu Z., Dubin A. E., Mathur J., Tu B., Reddy K., Miraglia L. J., Reinhardt J., Orth A. P., Patapoutian A. (2014) SWELL1, a plasma membrane protein, is an essential component of volume-regulated anion channel. Cell 157, 447–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Voss F. K., Ullrich F., Münch J., Lazarow K., Lutter D., Mah N., Andrade-Navarro M. A., von Kries J. P., Stauber T., Jentsch T. J. (2014) Identification of LRRC8 heteromers as an essential component of the volume-regulated anion channel VRAC. Science 344, 634–638 [DOI] [PubMed] [Google Scholar]
- 78. Chien L. T., Hartzell H. C. (2008) Rescue of volume-regulated anion current by bestrophin mutants with altered charge selectivity. J. Gen. Physiol. 132, 537–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Chien L. T., Hartzell H. C. (2007) Drosophila bestrophin-1 chloride current is dually regulated by calcium and cell volume. J. Gen. Physiol. 130, 513–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Stotz S. C., Clapham D. E. (2012) Anion-sensitive fluorophore identifies the Drosophila swell-activated chloride channel in a genome-wide RNA interference screen. PLoS One 7, e46865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Chintapalli V. R., Wang J., Dow J. A. (2007) Using FlyAtlas to identify better Drosophila melanogaster models of human disease. Nat. Genet. 39, 715–720 [DOI] [PubMed] [Google Scholar]
- 82. O'Donnell M. J., Maddrell S. H. (1995) Fluid reabsorption and ion transport by the lower Malpighian tubules of adult female Drosophila. J. Exp. Biol. 198, 1647–1653 [DOI] [PubMed] [Google Scholar]
- 83. Gupta B. L., Wall B. J., Oschman J. L., Hall T. A. (1980) Direct microprobe evidence of local concentration gradients and recycling of electrolytes during fluid absorption in the rectal papillae of Calliphora. J. Exp. Biol. 88, 21–47 [Google Scholar]
- 84. Bradley T. J. (1987) Physiology of osmoregulation in mosquitoes. Annu. Rev. Entomol. 32, 439–462 [DOI] [PubMed] [Google Scholar]
- 85. te Velde J. H., Molthoff C. F. M., Scharloo W. (1988) The function of anal papillae in salt adaptation of Drosophila melanogaster larvae. J. Evol. Biol. 2, 139–153 [Google Scholar]


