Abstract
Recent advances in tumor biology have made remarkable achievements in the development of therapy for metastatic castrate-resistant prostate cancer. These advances reflect a growing appreciation for the role of the tumor microenvironment in promoting prostate cancer progression. Prostate cancer is no longer viewed predominantly as a disease of abnormally proliferating epithelial cells but rather as a disease of complex interactions between prostate cancer epithelial cells (epithelial compartment) and the surrounding tissues (stromal compartment) in which they reside. For example, prostate cancers frequently metastasize to bone, an organ that contains a microenvironment rich in extracellular matrix proteins and stromal cells including hematopoietic cells, osteoblasts, osteoclasts fibroblasts, endothelial cells, adipocytes, immune cells, and mesenchymal stem cells. Multiple signaling pathways provide crosstalk between the epithelial and the stromal compartments to enhance tumor growth, including androgen receptor signaling, tyrosine kinase receptor signaling, and immune surveillance. The rationale to disrupt this “two-compartment” crosstalk has led to the development of drugs that target tumor stromal elements in addition to the cancer epithelial cell.
Prostate cancer remains the most common noncutaneous malignancy among men in the United States. In 2010, it is estimated that 220 000 men were newly diagnosed with prostate cancer and 32 050 men died of the disease (1). Prostate cancer is a biologically heterogeneous disease that produces variable clinical outcomes. Since the advent of prostate-specific antigen (PSA) testing, most patients diagnosed with prostate cancer have disease confined to the prostate gland (organ-confined disease) (2,3). For some men, prostate cancer follows a relatively indolent clinical course that does not require immediate treatment or in some cancer cases, any treatment at all (4,5). In contrast, up to 75% of newly diagnosed patients present with potentially aggressive prostate cancers that warrant treatment (6). For these patients with clinically significant disease, tumor progression occurs in a well-recognized anatomical pattern (7). Tumors that are initially organ confined can spread to locoregional lymph nodes but more commonly disseminate hematogenously to distant organs with a striking predilection for the skeleton (8). Prostate cancer that progresses despite castrate levels of serum testosterone is defined as “castrate resistant” (9).
Over the past decade, insights into the biological basis of prostate cancer development and progression have influenced our approach to treating patients with advanced disease. Although research efforts have historically focused on the prostate cancer epithelial cell to identify genetic alterations associated with malignant transformation, there is growing evidence that the host tissue microenvironment is critical for the progression from localized disease to distant metastases (10–13). For example, prostate cancer epithelial cells preferentially metastasize to bone. This is a multistep nonrandom process that involves 1) dissemination of cancer cells into the vascular system, 2) adhesion of cancer cells to the skeletal microvasculature, 3) extravasation of cancer cells into bone marrow, and 4) survival and proliferation of prostate cancer cells within the bone microenvironment. The normal bone microenvironment is composed of multiple types of stromal cells including hematopoietic cells, fibroblasts, endothelial cells, adipocytes, macrophages, osteoblasts, osteoclasts, and mesenchymal stem cells. In addition, the bone marrow microenvironment contains a soluble extracellular matrix rich in growth factors and cytokines (14).
The “Two-Compartment” Model
According to the “seed and soil” hypothesis, the bone microenvironment provides “fertile soil” for prostate cancer epithelial cells to “seed” (15). Once “seeded,” the ability of prostate cancer cells to “germinate” into tumors depends on bidirectional interactions between prostate cancer epithelial cells (the “epithelial compartment”) and the bone microenvironment (the “stromal compartment”). In contrast to most other solid tumor malignancies, prostate cancer bone metastases are typically “bone forming” rather than “bone destructive.” These lesions are produced when autocrine and paracrine feedback loops created between the prostate cancer epithelial cell and the bone microenvironment usurp normal bone homeostasis maintained by osteoblasts, osteoclasts, endothelial cells, and other bone stromal elements. These events lead to the formation of abnormal unstructured bone, termed “woven” bone, which is susceptible to the development of pain and/or fracture (16). Thus, the “lethal phenotype” of metastatic castrate-resistant prostate cancer (mCRPC) does not depend solely on the presence of cancer epithelial cells in the bone per se but also on the host stromal response to this presence. The interaction between the epithelial and stromal compartments defines a “vicious cycle” of prostate cancer progression in the bone (17).
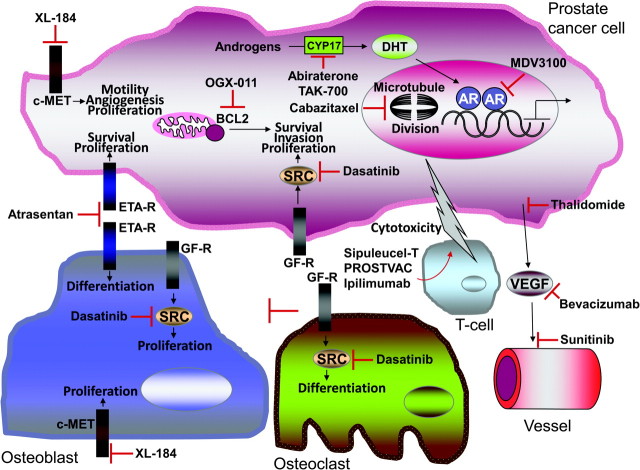
Elucidating the bidirectional interactions between the cancer cell and host bone microenvironment is now an important area of prostate cancer research (18). An increasing number of epithelial–stromal interacting pathways have been shown to contribute to the development, progression, and bone tropism of prostate cancer. A guiding principle derived from this effort has been the discovery that growth signaling pathways involved in normal prostate gland development and bone homeostasis frequently become dysregulated in prostate cancer (18). These pathways present novel targets for small-molecule therapeutics. The knowledge gained from this two-compartment model has led to novel treatment strategies that target the bone microenvironment in addition to the epithelial cell (13) (Figure 1).
Figure 1.
The two-compartment model in bone for novel therapeutics in metastatic castrate-resistant prostate cancer. The “epithelial compartment” contains the prostate cancer epithelial cell (top). The “stromal compartment” contains multiple different cell types including osteoblasts, osteoclasts, T-cells, and endothelial cells (bottom). Multiple autocrine and paracrine signaling pathways that contribute to prostate cancer progression are depicted. The different novel therapeutics that target these pathways are also shown. Please refer to the body of the text for additional details. AR = androgen receptor; ETA-R = endothelin type A receptor; VEGF = vascular endothelial growth factor; DHT = dihydrotestosterone; GF-R = growth factor receptor.
This review focuses on novel therapies being developed for the treatment of patients with mCRPC. Although the precise mechanisms whereby these therapeutic agents elicit an antitumor response are complex and every agent likely affects both compartments to some extent, we conceptually divide therapies into one of three different categories based on which compartment(s) is principally targeted: 1) epithelial targeting therapies, 2) stromal targeting therapies, and 3) epithelial–stromal targeting therapies. Specific biomarkers permit quantization and localization of therapy-induced effects within each compartment. For example, PSA levels reflect modulation of cancer epithelial cells, bone-specific alkaline phosphatase (BAP) levels reflect modulation of osteoblast activity, and urinary N-telopeptide (uNTx) levels reflect modulation of osteoclast activity (19).
Targeted agents are categorized based on which compartment they principally target and provide a conceptual framework that links understanding the underlying biology of cancer progression in the bone to candidate rational drug combinations. Nonetheless, an important caveat to this framework is that the mechanism of action for individual therapies often comes from molecular–pathologic evidence derived from preclinical (in vitro and in vivo) models of prostate cancer rather than directly from human tumors. Because preclinical models that use well-established prostate cancer cell lines do not recapitulate the heterogeneity of either the genetics or the epithelial–stromal interactions present in human tumors, data from preclinical models have historically correlated poorly with data from human patients. More recently, however, our group has sought to overcome this limitation by establishing human prostate cancer xenografts derived from biopsies of human tumors (20,21). These xenografts, implanted directly into severe combined immunodeficiency mice, preserve the genetics and epithelial–stromal interactions of the original tumors and are invaluable for elucidating mechanisms for both prostate cancer progression and therapy development.
Epithelial Targeting Agents
To date, the best known epithelial targeting agents remain conventional cytotoxic chemotherapy agents. Chemotherapy produces an antitumor effect primarily through apoptosis of prostate cancer epithelial cells. Docetaxel-based chemotherapy, approved by the Food and Drug Administration (FDA) in 2004, substantially palliates cancer-associated symptoms and modestly prolongs survival in patients with mCRPC (22,23). Correspondingly, a prevailing hypothesis in the medical oncology field is that the inability of chemotherapy to cure mCRPC is attributable to intrinsic defects in epithelial cell apoptosis such as B-cell CLL/lymphoma 2 (BCL2) overexpression and/or phosphatase and tensin homolog (PTEN) loss (24,25). These findings support the strategy of developing more potent chemotherapy agents and/or novel agents that overcome resistance mechanisms to existing chemotherapies (Table 1).
Table 1.
Ongoing phase II and phase III trials of novel therapies in mCRPC*
| Agent | Clinicaltrials.gov ID | Randomization | Primary endpoint | Patient population | Trial status (planned accrual) |
| Epithelial | |||||
| Cabazitaxel | NCT00417079 | Cabazitaxel + prednisone vs mitoxantrone + prednisone | OS | Post-docetaxel | Completed (755 patients) |
| OGX-011 (Custirsen) | NCT01188187 | Docetaxel + prednisone + custirsen vs docetaxel + prednisone | OS | Chemo naive | Recruiting (800 patients) |
| NCT01083615 | Custirsen vs placebo | Pain | Post-docetaxel | Recruiting (292 patients) | |
| Stromal | |||||
| Atrasentan | NCT00134056 | Docetaxel + prednisone + atrasentan vs Docetaxel + prednisone | OS and PFS | Chemo naive | Ongoing, not recruiting (930 patients) |
| NCT00036556 | Atrasentan vs placebo | TTP | CRPC, nonmetastatic | Completed (941 patients) | |
| NCT00036543 | Atrasentan vs placebo | TTP | Chemo naive | Completed (1000 patients) | |
| Antiangiogenesis | |||||
| Bevacizumab | NCT00110214 | Docetaxel + prednisone + bevacizumab vs docetaxel + prednisone | OS | Chemo naive | Ongoing, not recruiting (1020 patients) |
| Thalidomide (phase II) | NCT00004635 | Thalidomide vs placebo | TTPF | Androgen dependent | Completed (101 patients) |
| NCT00089609 | Docetaxel + thalidomide + bevacizumab | TTPF immunologic changes | Chemo naive | Ongoing, not recruiting (73 patients) | |
| Sunitinib | NCT00676650 | Sunitinib + prednisone vs prednisone | OS | Post-docetaxel | Expected 873 patients, prematurely discontinued |
| Antiandrogens | |||||
| Abiraterone acetate | NCT00887198 | AA + prednisone vs prednisone | OS and PFS | Chemo naive | Ongoing, not recruiting (1000 patients) |
| NCT00638690 | AA + prednisone vs prednisone | OS | Post-docetaxel | Ongoing, not recruiting (1158 patients) | |
| TAK-700 (phase I/II) (phase III) | NCT00569153 | TAK-700 | RR | Chemo naive | Ongoing, not recruiting (123 patients) |
| NCT01193244 | TAK-700 + prednisone vs prednisone | OS and PFS | Chemo naive | Recruiting (1454 patients) | |
| NCT01193257 | TAK-700 + prednisone vs prednisone | OS and PFS | Post-docetaxel | Recruiting (1083 patients) | |
| MDV3100 | NCT00974311 | MDV3100 vs placebo | OS | Post-docetaxel | Ongoing, not recruiting |
| NCT01212991 | MDV3100 vs placebo | OS and PFS | Chemo naive | Recruiting (1680 patients) | |
| Targeted | |||||
| Dasatinib | NCT00744497 | Docetaxel + prednisone + dasatinib vs docetaxel + prednisone | OS | Chemo naive | Recruiting (1500 patients) |
| Dovitinib (TKI258) (Phase II) | NCT00831792 | Dovitinib | OS and TTPF | CRPC | Ongoing, not recruiting (40 patients) |
| XL-184 (Cabozantinib) (phase II) | NCT00940225 | XL-184 | RR | Solid tumors | Recruiting (1300 patients) |
| Immunotherapy | |||||
| Provenge (Sipuleucel-T) | NCT00779402 | Provenge vs placebo | TTPF | Hormone-sensitive PSA increase | Ongoing, not recruiting (159 patients) |
| NCT01133704 | Provenge vs placebo | TTP | Chemo naive | Completed (98 patients) | |
| NCT00065442 | Provenge vs Placebo | OS | Chemo naive | Completed (512 patients) | |
| NCT00005947 | Provenge vs placebo | TTP | Chemo naive | Completed (127 patients) | |
| PROSTVAC-F/TRICOM | NCT01322490 | PROSTVAC-F/TRICOM ± GM-CSF vs placebo | OS | Chemo naive | Not recruiting (1200 patients) |
| Ipilimumab | NCT00861614 | Ipilimumab vs placebo | OS | Post-docetaxel | Recruiting (800 patients) |
| NCT01057810 | Ipilimumab vs placebo | OS | Chemo naive | Recruiting (600 patients) | |
AA = Abiraterone acetate; CRPC = castrate-resistant prostate cancer; GM-CSF = granulocyte-macrophage colony-stimulating factor; OS = overall survival; PFS = progression-free survival; RR = response rate; PSA = prostate-specific antigen; TTP = time to disease progression; TTPF = time to PSA failure.
Until recently, this strategy has been relatively unsuccessful. With regard to cytotoxic strategies, several non–taxane chemotherapy combinations are active in mCRPC, but none has passed the threshold of response to warrant comparison to docetaxel in a phase III frontline clinical trial with survival as a primary endpoint (26). With regard to drugs that overcome resistance mechanisms, the experience with oblimersen, a BCL2-specific antisense oligonucleotide, was disappointing because it did not enhance the efficacy of docetaxel and was associated with toxicity (27). More recently, however, the development of two novel agents, cabazitaxel and clusterin, suggest that improvements in targeting the epithelial compartment are still possible.
Cabazitaxel
Cabazitaxel is a semisynthetic member of the taxane family of cytotoxic agents and was developed building on the knowledge of sensitivity of prostate cancer to microtubular poisons (28). Like other taxanes, cabazitaxel stabilizes tubulin to induce cell cycle arrest and inhibit cell proliferation. In contrast to other taxanes such as docetaxel, cabazitaxel is less affected by the multidrug resistance P-glycoprotein efflux pump and overcomes docetaxel resistance in in vitro and in vivo preclinical models (28).
Cabazitaxel was recently compared with the topoisomerase type II inhibitor mitoxantrone in a randomized phase III trial in patients with mCRPC previously treated with docetaxel (cabazitaxel plus prednisone compared with mitoxantrone plus prednisone in hormone-refractory metastatic prostate cancer [TROPIC] trial) (29). Seven hundred and fifty-five men were randomly assigned to either mitoxantrone (12 mg/m2 every 21 days) or cabazitaxel (25 mg/m2 every 21 days) for a maximum of 10 cycles, and overall survival was the primary endpoint. Both groups received 10 mg of prednisone daily. After a median follow-up of more than a year, the risk for mortality was statistically significantly decreased in the cabazitaxel group (hazard ratio [HR] = 0.70, P < .001). This translated to a median overall survival of 15.1 months in the cabazitaxel group vs 12.7 months in the mitoxantrone group. Cabazitaxel also demonstrated statistically significant PSA responses (>50% decrease in PSA serum levels) and decrease in size of measurable lesions (by Response Evaluation Criteria in Solid Tumors [RECIST] criteria). The principal side effect of cabazitaxel was neutropenia, with statistically significantly more episodes of febrile neutropenia in the cabazitaxel group vs the mitoxantrone group (7.5% vs 1.3%, respectively). Based on the findings of the TROPIC trial, cabazitaxel was approved by the FDA in June 2010 for use in mCRPC patients after treatment with docetaxel.
Despite the positive result of the TROPIC trial, some important considerations remain about the optimal use of cabazitaxel. For example, although all of the patients enrolled in the TROPIC trial had evidence for disease progression following previous treatment with docetaxel, at least 25% of the patients did not have truly “docetaxel-refractory” disease (defined as disease that progresses during therapy or within 30 days of the last docetaxel dose) (24). It has therefore been argued that a substantial proportion of patients in the TROPIC trial would likely have responded to retreatment with docetaxel (30). In addition, the choice of mitoxantrone as a control group was arguably not ideal, given its very modest activity and the existence of other chemotherapy combinations with established activity in mCRPC (eg, cyclophosphamide combined with vincristine and decadron) (26,31). In light of these considerations, it remains debatable whether cabazitaxel should be accepted as the “de facto” best second-line chemotherapy choice for patients who truly have docetaxel-refractory disease. Nonetheless, the potential lack of cross-resistance between docetaxel and cabazitaxel has renewed interest in understanding how microtubule biology contributes to castrate-resistant disease progression and how best to exploit tubulins as a therapy target.
Clusterin
In response to external stress stimuli such as radiation and chemotherapy, epithelial cancer cells have developed multiple adaptive responses that are cytoprotective (32). One of these is mediated by clusterin, a chaperone protein involved in cell proliferation and survival (33). In prostate cancer cell lines, inhibition of clusterin was associated with greater susceptibility to cytotoxic agents and radiation (34,35). Therapeutic approaches to target clusterin are based on antisense oligonucleotides, the most promising of which is custirsen (also known as OGX-011) (36). Custirsen is a 2′-methoxyethyl-modified phosphorothioate antisense oligonucleotide that is complementary to the clusterin mRNA. Custersin has been shown to decrease clusterin expression in both in vitro and in vivo preclinical models. In a recent randomized phase II trial, 82 patients with mCRPC were randomly assigned to docetaxel and prednisone with or without OGX-011 (640 mg administered intravenously weekly) (37). All patients could receive up to 10 cycles of therapy, and the primary endpoint was the proportion of patients with a PSA decline of greater than 50% from baseline. The percentage of patients who achieved a greater than 50% decline in PSA was similar between the two groups (58% vs 54% respectively). Despite this, OGX-011 was associated with an improved overall survival (23.8 months in patients receiving docetaxel and prednisone plus OGX-011 vs 16.9 months in patients receiving docetaxel and prednisone alone). Based on these data, a randomized phase III trial is currently comparing docetaxel and prednisone with docetaxel and prednisone plus custirsen in 800 patients with mCRPC in the frontline setting, with overall survival as the primary endpoint (ClinicalTrials.gov identifier: NCT01188187).
Stromal Targeting Agents
Stromal targeting agents specifically inhibit the ability of the tumor microenvironment from contributing to disease progression (13). As such, these agents generally target molecular pathways that affect the ability of stromal cells (eg, endothelial cells, osteoclasts, and osteoblasts) to support and enhance cancer cell growth rather than directly targeting the epithelial cell per se. Studies to date have suggested that stromal targeting agents are only modestly effective when used as monotherapy in patients with mCRPC, despite evidence of therapy-induced target effects on the tumor microenvironment. For example, although zoledronic acid (an osteoclast inhibitor), imatinib (a multitarget tyrosine kinase inhibitor of platelet-derived growth factor [PDGF], C-Kit, and ABL1), and atrasentan (a selective endothelin A receptor antagonist that inhibits osteoblast proliferation) have all been shown to modulate the bone microenvironment, none has demonstrated any beneficial impact on disease progression or overall survival (38–40). Thus, the optimal use of stromal-targeting agents appears to be in combination with epithelial-targeting agents (such as chemotherapy). Nonetheless, single-agent trials have provided “proof of principal” that candidate stromal-targeting drugs can modulate the tumor microenvironment and permit development of the most specific biomarkers for the pathway being targeted (eg, levels of soluble vascular endothelial growth factor [VEGF] in patients receiving antiangiogenic therapy).
Atrasentan
In the bone microenvironment, both osteoblasts and osteoclasts express cell surface endothelin type A (ETA) receptors at high density. In response to ligand binding of endothelin-1 to the ETA receptor, osteoblasts become activated and stimulated to proliferate, whereas osteoclasts are inhibited (41). The net effect of endothelin-1/ETA signaling on the bone microenvironment is stimulation of new bone growth. Signaling through the ETA receptor induces osteoblastic metastases in mouse models (42,43).
Atrasentan (pyrrolidine-3-carboxylic acid) is a highly selective and potent ETA receptor antagonist that potently inhibits the osteoblast-dependent formation of new bone induced by metastatic cancer cells in a variety of preclinical model systems. Recent phase II and phase III trials have evaluated the role of atrasentan monotherapy in mCRPC (40,44). Although these trials established the ability of atrasentan to modulate the bone microenvironment (eg, by changes in bone-specific markers), there was no measurable clinical benefit. However, there is continued interest in the ability of atrasentan to enhance the response to docetaxel. A randomized phase III trial is currently comparing docetaxel and prednisone plus atrasentan to docetaxel and prednisone in patients with stage IV prostate cancer and bone metastases as first-line therapy (ClinicalTrials.gov identifier: NCT00134056). The primary endpoint is overall survival.
Denosumab
Although prostate cancer bone metastases are osteoblastic, the development of these lesions involves an osteolytic response mediated by osteoclasts. Interactions between receptor activator of nuclear factor of κB ligand (receptor activated nuclear factor-κB ligand [RANKL]) and its receptor (RANK) are critical in regulating both osteoclastogenesis and bone remodeling involved in the formation of prostate cancer bone metastases. Prostate cancer epithelial cells in bone metastases overexpress RANKL compared with cancer cells in primary tumors. Denosumab is a human monoclonal antibody against RANKL. In a recent randomized double-blind study, denosumab was superior to zoledronic acid in preventing skeletal-related events in patients with mCRPC (45). The median time to the first skeletal-related event on study was 20.1 vs 17.1 months in patients receiving denosumab vs zoledronic acid, respectively. Among all skeletal-related events, the onset of radiation to the bone was the event that was most delayed by denosumab. Interestingly, denosumab was more potent than zoledronic acid in reducing both uNTx and bone-specific alkaline phosphatase levels. Despite these results, there was no difference in overall survival between the two groups. Future studies will test whether denosumab can enhance the survival benefit of chemotherapy for patients with mCRPC, realizing that the role of denosumab, based on its unique biology, might be more complex than being a mere alternative to bisphosphonates, which are widely used to treat bone metastases in solid tumors and multiple myeloma.
Antiangiogenic Agents
Blocking angiogenesis to inhibit tumor growth is an archetypal stromal-targeting strategy that has proven to be successful in treating a variety of different metastatic tumor types, including kidney, colon, and lung cancers (46). As monotherapy, the principal antitumor mechanism of antiangiogenic agents (antiangiogenics) is through inhibition of endothelial cell function, an event that leads to a reduction in tumor blood flow, tumor hypoxia, and cell death. Furthermore, antiangiogenics can cooperate with the antitumoral effects of cytotoxic chemotherapies, although an alternative mechanism has been proposed whereby antiangiogenics selectively “prune” structurally defective neovessels, leading to increased blood flow and enhanced delivery of chemotherapy to the tumor (47,48). In the case of prostate cancer skeletal metastases, it is well known that the bone microenvironment is highly vascular with an abundant sinusoid microvasculature (14). Several preclinical studies have established the utility of blocking angiogenesis to inhibit prostate cancer bone metastases.
One of the first antiangiogenic agents studied in patients with mCRPC was thalidomide (49). Although its exact mechanism of action is incompletely understood, preclinical models suggest that thalidomide inhibits secretion of proangiogenic cytokines from both the epithelial and stromal compartments (50). In phase II studies, thalidomide produced only modest reductions in PSA when used as monotherapy but was considerably more potent when combined with docetaxel (49,51,52). For example, in a randomized phase II study, the addition of thalidomide to docetaxel led to proportionally more patients with a 50% or more reduction in PSA and a trend toward improvement in overall survival compared with docetaxel alone (28.9 vs 14.7 months, respectively; P = 0.11) (53). Although interest in thalidomide persists, fewer studies have been done with this interesting agent because of its relative toxicity (thromboembolic events), the fact that its exact mechanism for angiogenesis inhibition remains unknown, and the emergence of more potent and specific angiogenesis inhibitors (eg, bevacizumab and sunitinib, discussed below). In contrast, lenalidomide, a thalidomide analog with improved tolerability, is now being actively investigated in patients with mCRPC (54). A randomized placebo-controlled phase III trial is currently investigating the effect of the addition of lenalidomide to docetaxel in patients with CRPC on overall survival (ClinicalTrials.gov identifier: NCT00988208). In another single-group phase II trial, for metastatic prostate cancer, patients with mCRPC are receiving a combination of lenalidomide, bevacizumab, and prednisone to assess the safety and efficacy of this combination. This trial is expected to finish accrual in the middle of year 2012 (ClinicalTrials.gov identifier: NCT00942578).
Despite the compelling rationale to apply antiangiogenic therapies to metastatic prostate cancer, two recent negative phase III studies have raised important questions about this approach. The first study, Cancer and Leukemia Group B (CALGB) 90401, tested the ability of bevacizumab, a monoclonal antibody that binds to VEGFA to enhance the survival benefit of docetaxel in the frontline setting for patients with mCRPC (55). Although there was an improvement in progression-free survival, there was no difference in overall survival between patients with mCRPC who received docetaxel plus bevacizumab vs docetaxel alone (55). In addition, treatment-related adverse events were higher in the bevacizumab group. The second study, Pfizer’s Sun 1120 (ClinicalTrials.gov identifier: NCT00676650), tested the ability of sunitinib, a multi-tyrosine inhibitor with high specific activity against receptors for PDGF and VEGF, to enhance the ability of prednisone to prolong survival in patients with mCRPC previously treated with docetaxel-based chemotherapy. The trial was stopped following an interim analysis showing that the addition of sunitinib to prednisone was unlikely to affect overall survival. Although one might conclude from these studies that antiangiogenic therapies are ineffective in mCRPC, we believe these negative data highlight an important biologic principle in prostate cancer angiogenesis that should inform the design of future trials. Specifically, the bone marrow microenvironment contains multiple proangiogenic factors in addition to VEGF including PDGF, basic fibroblast growth factor (bFGF), interleukin 8, and other soluble cytokines. This multiplicity of angiogenic pathways creates “redundancy” and the potential for “tumor escape” from antiangiogenic therapies and suggests that blocking multiple pathways simultaneously, rather than VEGF alone, may be necessary to effectively block angiogenesis in mCRPC. In support of this, our experience with clinical trials suggests that blocking PDGF and VEGF simultaneously (with sunitinib) is more potent in eliciting PSA responses in patients with mCRPC than blocking either VEGF alone (with bevacizumab) or PDGF alone (with imatinib) (56). Reflecting these data, studies are currently underway using tyrosine kinase inhibitors that target multiple angiogenic pathways (eg, TKI258, which potently blocks VEGF, PDGF, and bFGF) (ClinicalTrials.gov identifier: NCT00831792), or alternatively, combine agents that block angiogenesis through different mechanisms (eg, combining bevacizumab plus lenalidomide). In addition, in a recent phase I/II study combining sunitinib and docetaxel for the treatment of mCRPC in the frontline setting, patients demonstrated reductions in both PSA levels and tumor burden that were more substantial than a historical cohort of patients receiving docetaxel alone (57).
The observation that both bevacizumab and sunitinib have shown prolongation of progression-free survival without differences in overall survival also raises the possibility that sustained suppression of angiogenesis is required to affect overall survival. Traditionally, phase III clinical trials with overall survival as the primary endpoint are designed such that patients receive experimental therapy until there is objective evidence of disease progression. At that point, the experimental therapy is stopped, and patients are eligible for additional therapies while being followed for survival. The rationale is that it would be futile to continue an experimental therapy that is not stopping tumor growth. In testing novel antiangiogenics, however, traditional phase III trial designs have two potentially important limitations. First, an experimental antiangiogenic therapy that no longer stops disease progression by standard criteria (eg, using changes in PSA levels and/or RECIST) may still sufficiently slow the growth rate of the tumor such that patients would ultimately experience a prolongation in survival had they remained on the drug. Second, enhanced tumor growth following cessation of antiangiogenic therapy has been described, a “rebound” phenomenon that could influence overall survival (58). To address these limitations, it may be necessary to continue antiangiogenic therapy beyond standard definitions of disease progression to observe a beneficial impact on overall survival.
Epithelial–Stromal Targeting Agents
Many agents demonstrate evidence for modulating both the epithelial and stromal compartments. For example, drugs that target androgen receptor (AR) signaling fall into this category. The AR is ubiquitously expressed on both prostate cancer epithelial cells and stromal cells within the tumor microenvironment (59). In addition to directly stimulating epithelial cell proliferation, AR signaling also promotes tumor growth through its activity on stromal cells (60). Thus, agents that block AR signaling modulate both the epithelial and stromal compartments in a therapeutically favorable manner. This is evidenced in patients by reductions in serum PSA (a biomarker reflecting modulation of the epithelial compartment) and bone-specific alkaline phosphatase (a biomarker reflecting modulation osteoblast activity within the stromal compartment).
Novel Agents That Interfere With Androgen Signaling
There is now clear evidence that even with castrate levels of serum testosterone, prostate cancer bone metastases continue to rely on androgen signaling for growth (61,62). Potential mechanisms accounting for this include intratumoral amplification of the AR, mutations of the AR, changes in levels of AR cofactors, increased expression of enzymes involved in androgen synthesis, and enhanced intracellular conversion of adrenal androgens to testosterone and dihydrotestosterone within the tumor microenvironment, and ligand-independent activation of the AR (63). Reflecting these processes, there is a gradual shift during prostate cancer progression from endocrine sources of androgens (ie, from the testes and adrenal glands) to paracrine, autocrine, and intracrine sources within the tumor microenvironment. Although all these events can occur in the setting of a low serum testosterone, tumors may still respond to agents that block AR signaling within the tumor microenvironment.
Abiraterone is a small-molecule inhibitor of 17 alpha-monooxygenase (17 alpha-hydroxylase and C17,20-lyase, referred to as the CYP17 complex), a member of the cytochrome P450 family that catalyzes the 17 alpha-hydroxylation of intermediates of steroid biosynthesis involved in testosterone synthesis (64). Administration of this agent in mice and humans suppresses testosterone production by both the testes and the adrenals to castrate range levels. In the biogenesis of testosterone, 17 alpha-hydroxylase is required to convert pregnenolone to 17-OH-pregnenolone, which is further downstream converted by C17,20-lyase to dehydroepiandrostenedione, a precursor of testosterone. Inhibition of the CYP17 complex thus leads to accumulation of upstream mineral corticoids (eg, corticosterone) and reduction of downstream steroids including testosterone and estradiol (65). In phase I and II studies, abiraterone treatment consistently suppressed testosterone levels and led to statistically significant reductions in PSA level, regression of radiological lesions, and improvement in symptoms (65,66). Adverse events were sequelae of secondary mineralocorticoid excess and included hypokalemia, hypertension, peripheral edema, and headaches. These side effects were well managed with a mineralocorticoid receptor antagonist.
Data from phase II studies with abiraterone acetate suggested that abiraterone was active in patients with mCRPC regardless of whether or not they had previously received docetaxel treatment (67,68). These observations led to two randomized placebo-controlled phase III trials testing the ability of abiraterone to improve survival in patients with mCRPC. The first trial, COU-AA-301, compared abiraterone plus prednisone with placebo plus prednisone in patients with mCRPC who had previously received docetaxel-based chemotherapy (69). Results from this study demonstrated an improvement in overall survival for patients receiving abiraterone (14.8 vs 10.9 months, HR = 0.646; P < .001) and led to FDA approval of this agent (69). The second trial, COU-AA-302 (NCT00887198), is comparing abiraterone and prednisone with placebo and prednisone in patients with mCRPC who are chemotherapy naive. COU-AA-302 has completed accrual, and results are pending.
The successful development of abiraterone supports the hypothesis that castrate-resistant prostate cancers utilize autocrine and paracrine sources of testosterone for continued growth. This biologic feature of mCRPC was arguably underappreciated in past decades because of the fact that ketoconazole, the best-known CYP17 inhibitor before the discovery of abiraterone, is considerably less potent and less clinically active than abiraterone. Reflecting these differences between abiraterone and ketoconazole, abiraterone still demonstrates antitumor activity in patients with mCRPC who progress on ketoconazole.
Additional CYP17 inhibitors are currently under development. TAK-700 is a selective, nonsteroidal potent CYP17 inhibitor (Millennium Pharmaceuticals, Cambridge, MA). Compared with abiraterone, TAK-700 more potently and specifically inhibits 17, 20-lyase enzymatic activity than 17-hydroxylase activity. This may make TAK-700 safer and more tolerable than abiraterone (because TAK-700 is less likely to suppress cortisol and produce a compensatory rise in adrenocorticotropic hormone (ACTH), leading to a physiological state of mineral corticoid excess). In a recent phase I study of 15 patients with mCRPC who received TAK-700 (≥300 mg for three or more cycles), 12 (80%) patients showed 50% or greater reduction and four (27%) patients showed 90% or greater reduction in PSA level (70). Two ongoing phase III trials with overall survival as primary endpoint are randomly assigning patients with mCRPC to TAK-700 or placebo in the chemotherapy-naive (ClinicalTrials.gov Identifier: NCT01193244) and post-docetaxel settings (ClinicalTrials.gov Identifier: NCT01193257), respectively.
MDV3100 is a novel small-molecule AR antagonist that overcomes resistance to conventional antiandrogens mediated by increased expression (71). Like other antiandrogens such as bicalutamide, MDV3100 inhibits AR function by blocking AR ligand binding, nuclear translocation, and DNA binding (72). In contrast to bicalutamide, however, MDV3100 does not possess agonist activity when AR is overexpressed (71). The results of a phase I/II study in patients with mCRPC have recently been published (73). In this study, the dose of MDV3100 was escalated in cohorts of three to six patients, starting at 30 mg daily to a maximal dose of 600 mg daily. A total of 140 patients with progressive mCRPC were enrolled, with approximately 54% having previously received chemotherapy. A dose of 240 mg/d was determined to be the maximum tolerated dose, with the most common toxicity being grade 3–4 fatigue (11% of all patients). A total of 78 (56%) of 140 patients showed a 50% or greater reduction in PSA level, 13 (22%) of 59 patients with measurable disease had a partial response, and 61 (56%) of 109 patients with bone disease experienced stable disease. For all patients, the median time to progression was 47 weeks. Based on these encouraging results, two placebo-controlled phase III trials are currently ongoing to evaluate the effect of MDV3100 on overall survival in patients with mCRPC. The first trial evaluates the impact of MDV3100 vs placebo on overall survival in patients with mCRPC who have previously received docetaxel-based chemotherapy (ClinicalTrials.gov Identifier: NCT00974311). This trial has finished accrual, and results are pending. The second phase III trial evaluates the impact of MDV3100 vs placebo on overall survival in patients with mCRPC who are chemotherapy naive (ClinicalTrials.gov Identifier: NCT01212991). This trial is actively accruing, and results are pending.
Targeted Agents
Dasatinib is an oral tyrosine kinase inhibitor that targets BCR-ABL and SRC family kinases, EPH receptor A2 (EPHA2), c-KIT, and PDGF receptor beta polypeptide (PDGFRB) (74). Dasatinib was recently evaluated as a single agent in chemotherapy-naive patients with mCRPC in a phase II trial (75). PSA doubling time improved in 29 (80.1%) of 36 patients, and one patient showed a greater than 50% reduction in PSA level. In 27 patients who had bone scans, one patient had improvement and 16 others had stable disease at 12 weeks. In 15 patients evaluable by RECIST, 10 (67%) had stable disease. There was also a decrease in serum markers of bone turnover (including BAP) in 21 (57%) of 37 patients. The drug was well tolerated with few side effects.
Although these data suggested only modest clinical activity for dasatinib monotherapy, the ability of dasatinib to modulate both the epithelial and stromal compartments prompted us to combine it with docetaxel in a phase I/II study of patients with mCRPC (76). Additional rationale for this combination came from preclinical data suggesting that dasatinib may enhance the antitumoral effect of docetaxel in orthotopic (intratibial) models of prostate cancer (77). In the clinical trial, the combination of dasatinib and docetaxel therapy was generally well tolerated. Durable PSA declines of 50% or greater occurred in 26 (57%) of 46 of patients, and in 30 patients with measurable disease, 18 (60%) had a partial response. Of 46 patients, 14 (30%) had disappearance of a lesion on bone scan. Correlative studies revealed a decline in bone turnover marker uNTx in patients who responded, and pharmacokinetic studies demonstrated an association between peak dasatinib levels with a decrease in serum interleukin 8, providing mechanistic insight into the action of dasatinib and docetaxel (78,79). In addition, several patients who responded to the combination therapy and were subsequently maintained on dasatinib monotherapy (after docetaxel discontinuation) have experienced prolonged periods of disease stabilization (76). Given this favorable activity, a randomized double-blind phase III trial comparing docetaxel plus dasatinib vs docetaxel plus placebo in castration-resistant prostate cancer is currently ongoing and has recently finished accrual (ClinicalTrials.gov Identifier: NCT00744497).
XL-184 (Also Known as Cabozantinib)
Signaling through the hepatocyte growth factor (HGF) and its receptor, met proto-oncogene (c-MET), is aberrantly activated in mCRPC and promotes tumor growth through dual stimulatory effects on both prostate cancer epithelial cells and tumor stromal elements (eg, osteoblasts) (80,81). In preclinical studies, androgen ablation is associated with increased expression of c-MET, suggesting a role for c-MET in mediating resistance to antitumor effects induced by androgen ablation (82). In support of this, small-molecule inhibitors of c-MET are more potent at inhibiting castration-resistant than androgen-dependent orthotopic models of prostate cancer (83).
XL-184 is an orally bioavailable novel tyrosine kinase inhibitor of c-MET and VEGF receptor 2 (VEGFR2) (84). Its ability to simultaneously inhibit c-MET and VEGFR2 is what distinguishes XL-184 from other well-known VEGFR2 inhibitors such as sunitinib (85). XL-184 is currently being tested in a randomized discontinuation study in adult patients with advanced malignancies (ClinicalTrials.gov Identifier: NCT00940225). In this trial design, all patients are initially treated with open-label XL-184 for 12 weeks. Subjects who have responded during this “lead-in” continue on XL-184. Patients with stable disease are randomized in a double-blind fashion to either XL-184 or placebo. Subjects who progress discontinue XL-184. In a recent abstract, 100 patients with mCRPC were evaluable (86). Forty-seven percent had visceral disease, 88% had nodal disease, 78% had bone metastasis, and 47% were previously treated with docetaxel-based chemotherapy. Tumor shrinkage occurred in 84% of patients, and 86% of patients had complete or partial resolution of lesions on bone scan as early as week 6. In patients receiving narcotics for bone pain, 64% had improvements in pain and 45% decreased or halted narcotics during the study. Effects on osteoclasts and osteoblasts were also observed, with 55% of patients experiencing a 50% or greater reduction in uNTx and 56% of patients experiencing a 50% or greater reduction in serum alkaline phosphatase. The most common grade 3–4 toxic effects were fatigue (11%), hypertension (7%), and hand–foot syndrome (5%). Statistically significant responses were seen in both chemotherapy-naive and chemotherapy-treated groups. Given these exceptional results, a phase II nonrandomized expansion cohort of XL-184 is currently underway in patients with mCRPC who have previously received docetaxel-based therapy (ClinicalTrials.gov Identifier: NCT00940225).
Immunotherapy
It is well established that epithelial tumors generate a host immune response within the tumor microenvironment (87). However, this immune response is largely ineffective in eradicating the tumor because the tumor establishes mechanisms for “immune evasion” (88). These mechanisms include weak antigenicity of the tumor (ie, tumor-associated antigens are recognized as “self” rather than “foreign”), development of immunoresistance by the tumor, and inadequate immune T-cell effect or function within the tumor microenvironment. In addition, tumor cells stimulate immune cells to produce inflammatory cytokines that promote tumor proliferation, invasion, and angiogenesis. Thus, inflammation within the tumor microenvironment contributes to prostate cancer progression. These observations have prompted numerous efforts to modulate the immune response into an effective antitumor therapy. Immunotherapy represents an epithelial–stromal targeting therapy because it stimulates the immune system to target the tumor (rather than directly targeting the tumor per se).
Sipuleucel-T (also known as Provenge) is a cellular immunotherapy produced by incubating the patient’s peripheral blood mononuclear cells ex vivo with a recombinant fusion protein consisting of prostatic acid phosphatase (PAP), an antigen expressed predominantly on prostate cancer epithelial cells, and the immunostimulatory cytokine granulocyte-macrophage colony-stimulating factor (GM-CSF) (89). This procedure is intended to enhance the activity of the patients’ autologous antigen-presenting cells to elicit a cytotoxic T-lymphocyte response against PAP when reinfused back into the patient. Three phase III trials have evaluated the efficacy of sipuleucel-T in advanced prostate cancer. Two trials, D9901 and D9902A, with a total of 127 patients, were reported together (90,91). Men with asymptomatic mCRPC received either three infusions of sipuleucel-T (n = 82 patients) or placebo (n = 45 patients) every 2 weeks. Cross-over was allowed at the time of progression because frozen cells from all patients were available. Although the time to progression was similar in both groups (11.7 vs 10.0 weeks), there was a statistically significant difference in median overall survival in favor of sipuleucel-T (25.0 vs 21.4 months, P = .01). These findings were confirmed in the IMPACT trial, which was a larger randomized, double-blind placebo-controlled phase III trial of three doses of sipuleucel-T (n = 341 patients) or placebo (n = 171 patients) (92). Again, a statistically significant survival advantage was demonstrated in men who received sipuleucel-T (HR = 0.775, P = .032). The most commonly reported adverse events were chills, headaches, pyrexia, and flu-like symptoms; most of which were reported as grade 1 or 2 toxicity and subsided within 1–2 days. Based on the survival advantage data, sipuleucel-T was approved in April 2010 by the FDA for treatment of men with asymptomatic mCRPC.
Although the successful development of sipuleucel-T represents a remarkable achievement in the field of immunotherapy, many questions remain regarding its precise mechanism(s) of action. For example, the survival benefit observed with sipuleucel-T is not accompanied by favorable effects on PSA, tumor regression, time to progression, or quality of life. As a possible explanation for this discrepancy, it has been suggested that immunotherapy permits continued tumor growth but at a substantially slower kinetic rate, which results in a prolongation in survival (93,94). Furthermore, the immune response to sipuleucel-T is generally not observed until several months after initiation of therapy, at which point most patients have progressed. Thus, if adequate numbers of memory cells are generated at the time of vaccine administration, disease progression may actually “boost” the antitumoral immune response in a delayed manner to affect survival (89).
Other immune therapy approaches have focused on regulating costimulatory molecules to boost the T-effector cell response to mCRPC. For example, the PROSTVAC-F/TRICOM vaccine consists of three principal components: 1) a vaccinia virus expressing the entire PSA transgene used for the first immunization, 2) a PSA fowlpox vector expressing the entire PSA transgene used for subsequent boost doses (to minimize the development of neutralizing antibody responses), and 3) a viral vector encoding three major costimulatory molecules (B-lymphocyte activation antigen B7-1 [B7.1], intercellular adhesion molecule 1 [ICAM-1], and lymphocyte function-associated antigen 3 [LFA-3], termed TRICOM) which have a crucial role for lymphocyte activation during antigen presentation by antigen-presenting cells. In initial phase I and single-group phase II studies, safety and immune response profiles were established (95–97). The effects on clinical outcomes (progression-free and overall survival) in patients with mCRPC were subsequently reported in a randomized phase II trial (98). Patients with mCRPC and minimal symptoms were randomly assigned in a 2:1 fashion to receive either PROSTVAC-F/TRICOM (priming followed by six boosters plus GM-CSF) or placebo. A total of 122 patients were enrolled. Similar to the observations with sipuleucel-T, the progression-free survival was similar in both study groups, but there was a statistically significant overall survival advantage at 3 years in favor of the PROSTVAC-VF group (30% vs 17%; P = .0061) (98). Based on an 8.5-month improvement in median overall survival observed in this trial, a randomized double-blind phase III trial has been designed which will compare the effect of PROSTVAC-F/TRICOM with or without GM-CSF vs placebo on overall survival in men with minimally symptomatic mCRPC. This study is planned to start accrual in August 2011 and will enroll 1200 patients (ClinicalTrials.gov Identifier: NCT01322490). Three ongoing phase II trials are currently evaluating PROSTVAC-F/TRICOM in non-mCRPC as well as in combination with chemo- and radioimmunotherapy (ClinicalTrials.gov Identifiers: NCT00450463, NCT01145508, and NCT00450619).
Ipilimumab is a monoclonal antibody that blocks the activity of T-cell inhibitory receptor cytotoxic T lymphocyte-associated 4 (CTLA4). CTLA4 is expressed on the surface of Helper T cells and transmits an inhibitory (antiproliferative) signal to in response to “self-antigens.” Thus, ipilimumab is a potent immunotherapy strategy that works by inhibiting immune tolerance to tumors. In a landmark study, ipilimumab was recently shown to improve survival in malignant melanoma (99). In mCRPC, ipilimumab was given in a pilot trial at 3 mg/kg as a single dose to 14 patients (100). It was found to be safe, and two patients experienced a PSA decline of more than 50%. In a subsequent phase I trial of patients with mCRPC treated with ipilimumab plus GM-CSF, 50% of the patients (three of six patients) treated in the highest dose cohort had a PSA response, and one demonstrated a partial response in visceral metastases (101). In a phase II trial, the safety and efficacy of ipilimumab with or without one single dose of docetaxel was evaluated in mCRPC (46 patients) (102). In each treatment group (23 patients in each group), three patients had a PSA response (total six of 46 patients showed PSA response). Five serious adverse events reported in three patients were considered to be possible immune breakthrough events associated with drug exposure and consistent with an immune-based mechanism of action. Based on these findings, there are currently two ongoing phase III trials in mCRPC. These trials are comparing the overall survival in patients with mCRPC treated with ipilimumab or placebo in the pre- and post-docetaxel settings, respectively (ClinicalTrials.gov Identifier: NCT00861614 and NCT01057810).
Conclusions
Advances in our understanding of the biology of mCRPC have led to the development of many new promising therapeutic agents to treat this disease. These advances reflect a general paradigm shift away from the traditional approach of targeting predominantly the cancer epithelial cell toward a strategy that also targets the tumor microenvironment. The fruits of this approach are evidenced by the FDA approval of three novel agents that have each been shown to prolong life in patients with mCRPC (cabazitaxel, abiraterone, and provenge). Despite these remarkable achievements, mCRPC remains an incurable disease. Further research is needed to identify which patients will benefit most from individual therapies and which combinations of therapies will be most effective.
Funding
There was no funding for this study.
Footnotes
The authors are solely responsible for writing the article and the decision to submit the article for publication.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Catalona WJ, Smith DS, Ratliff TL, Basler JW. Detection of organ-confined prostate cancer is increased through prostate-specific antigen-based screening. JAMA. 1993;270:948–954. [PubMed] [Google Scholar]
- 3.Shao YH, Albertsen PC, Roberts CB, et al. Risk profiles and treatment patterns among men diagnosed as having prostate cancer and a prostate-specific antigen level below 4.0 ng/ml. Arch Intern Med. 2010;170:1256–1261. doi: 10.1001/archinternmed.2010.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johansson JE, Andren O, Andersson SO, et al. Natural history of early, localized prostate cancer. JAMA. 2004;291:2713–2719. doi: 10.1001/jama.291.22.2713. [DOI] [PubMed] [Google Scholar]
- 5.Lu-Yao GL, Albertsen PC, Moore DF, et al. Outcomes of localized prostate cancer following conservative management. JAMA. 2009;302:1202–1209. doi: 10.1001/jama.2009.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ploussard G, Epstein JI, Montironi R, et al. The contemporary concept of significant versus insignificant prostate cancer. Eur Urol. doi: 10.1016/j.eururo.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 7.Saitoh H, Hida M, Shimbo T, Nakamura K, Yamagata J, Satoh T. Metastatic patterns of prostatic cancer. Correlation between sites and number of organs involved. Cancer. 1984;54:3078–3084. doi: 10.1002/1097-0142(19841215)54:12<3078::aid-cncr2820541245>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 8.Logothetis CJ, Lin SH. Osteoblasts in prostate cancer metastasis to bone. Nat Rev Cancer. 2005;5:21–28. doi: 10.1038/nrc1528. [DOI] [PubMed] [Google Scholar]
- 9.Scher HI, Halabi S, Tannock I, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26:1148–1159. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chung LW, Baseman A, Assikis V, Zhau HE. Molecular insights into prostate cancer progression: the missing link of tumor microenvironment. J Urol. 2005;173:10–20. doi: 10.1097/01.ju.0000141582.15218.10. [DOI] [PubMed] [Google Scholar]
- 11.Cher ML, Towler DA, Rafii S, et al. Cancer interaction with the bone microenvironment: a workshop of the National Institutes of Health Tumor Microenvironment Study Section. Am J Pathol. 2006;168:1405–1412. doi: 10.2353/ajpath.2006.050874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morrissey C, Vessella RL. The role of tumor microenvironment in prostate cancer bone metastasis. J Cell Biochem. 2007;101:873–886. doi: 10.1002/jcb.21214. [DOI] [PubMed] [Google Scholar]
- 13.Karlou M, Tzelepi V, Efstathiou E. Therapeutic targeting of the prostate cancer microenvironment. Nat Rev Urol. 2010;7:494–509. doi: 10.1038/nrurol.2010.134. [DOI] [PubMed] [Google Scholar]
- 14.Bussard KM, Gay CV, Mastro AM. The bone microenvironment in metastasis; what is special about bone? Cancer Metastasis Rev. 2008;27:41–55. doi: 10.1007/s10555-007-9109-4. [DOI] [PubMed] [Google Scholar]
- 15.Paget S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev. 1989;8:98–101. [PubMed] [Google Scholar]
- 16.Roudier MP, True LD, Higano CS, et al. Phenotypic heterogeneity of end-stage prostate carcinoma metastatic to bone. Hum Pathol. 2003;34:646–653. doi: 10.1016/s0046-8177(03)00190-4. [DOI] [PubMed] [Google Scholar]
- 17.Loberg RD, Logothetis CJ, Keller ET, Pienta KJ. Pathogenesis and treatment of prostate cancer bone metastases: targeting the lethal phenotype. J Clin Oncol. 2005;23:8232–8241. doi: 10.1200/JCO.2005.03.0841. [DOI] [PubMed] [Google Scholar]
- 18.Efstathiou E, Logothetis CJ. A new therapy paradigm for prostate cancer founded on clinical observations. Clin Cancer Res. 2010;16:1100–1107. doi: 10.1158/1078-0432.CCR-09-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cook RJ, Coleman R, Brown J, et al. Markers of bone metabolism and survival in men with hormone-refractory metastatic prostate cancer. Clin Cancer Res. 2006;12:3361–3367. doi: 10.1158/1078-0432.CCR-06-0269. [DOI] [PubMed] [Google Scholar]
- 20.Li ZG, Mathew P, Yang J, et al. Androgen receptor-negative human prostate cancer cells induce osteogenesis in mice through FGF9-mediated mechanisms. J Clin Invest. 2008;118:2697–2710. doi: 10.1172/JCI33093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aparicio A, Tzelepi V, Araujo JC, et al. Neuroendocrine prostate cancer xenografts with large-cell and small-cell features derived from a single patient’s tumor: morphological, immunohistochemical, and gene expression profiles. Prostate. 2010;71:846–856. doi: 10.1002/pros.21301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tannock IF, de WR, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 23.Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–1520. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 24.Mathew P, Dipaola R. Taxane refractory prostate cancer. J Urol. 2007;178:S36–S41. doi: 10.1016/j.juro.2007.04.032. [DOI] [PubMed] [Google Scholar]
- 25.Galsky MD, Vogelzang NJ. Docetaxel-based combination therapy for castration-resistant prostate cancer. Ann Oncol. 2010;21:2135–2144. doi: 10.1093/annonc/mdq050. [DOI] [PubMed] [Google Scholar]
- 26.Thall PF, Logothetis C, Pagliaro LC, et al. Adaptive therapy for androgen-independent prostate cancer: a randomized selection trial of four regimens. J Natl Cancer Inst. 2007;99:1613–1622. doi: 10.1093/jnci/djm189. [DOI] [PubMed] [Google Scholar]
- 27.Sternberg CN, Dumez H, Van PH, et al. Docetaxel plus oblimersen sodium (Bcl-2 antisense oligonucleotide): an EORTC multicenter, randomized phase II study in patients with castration-resistant prostate cancer. Ann Oncol. 2009;20:1264–1269. doi: 10.1093/annonc/mdn784. [DOI] [PubMed] [Google Scholar]
- 28.Mita AC, Denis LJ, Rowinsky EK, et al. Phase I and pharmacokinetic study of XRP6258 (RPR 116258A), a novel taxane, administered as a 1-hour infusion every 3 weeks in patients with advanced solid tumors. Clin Cancer Res. 2009;15:723–730. doi: 10.1158/1078-0432.CCR-08-0596. [DOI] [PubMed] [Google Scholar]
- 29.de Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376:1147–1154. doi: 10.1016/S0140-6736(10)61389-X. [DOI] [PubMed] [Google Scholar]
- 30.Beer TM, Garzotto M, Henner WD, Eilers KM, Wersinger EM. Multiple cycles of intermittent chemotherapy in metastatic androgen-independent prostate cancer. Br J Cancer. 2004;91:1425–1427. doi: 10.1038/sj.bjc.6602198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berthold DR, Sternberg CN, Tannock IF. Management of advanced prostate cancer after first-line chemotherapy. J Clin Oncol. 2005;23:8247–8252. doi: 10.1200/JCO.2005.03.1435. [DOI] [PubMed] [Google Scholar]
- 32.Tiligada E, Miligkos V, Delitheos A. Cross-talk between cellular stress, cell cycle and anticancer agents: mechanistic aspects. Curr Med Chem Anticancer Agents. 2002;2:553–566. doi: 10.2174/1568011023353976. [DOI] [PubMed] [Google Scholar]
- 33.Zoubeidi A, Chi K, Gleave M. Targeting the cytoprotective chaperone, clusterin, for treatment of advanced cancer. Clin Cancer Res. 2010;16:1088–1093. doi: 10.1158/1078-0432.CCR-09-2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gleave ME, Miyake H, Zellweger T, et al. Use of antisense oligonucleotides targeting the antiapoptotic gene, clusterin/testosterone-repressed prostate message 2, to enhance androgen sensitivity and chemosensitivity in prostate cancer. Urology. 2001;58:39–49. doi: 10.1016/s0090-4295(01)01241-9. [DOI] [PubMed] [Google Scholar]
- 35.Zellweger T, Chi K, Miyake H, et al. Enhanced radiation sensitivity in prostate cancer by inhibition of the cell survival protein clusterin. Clin Cancer Res. 2002;8:3276–3284. [PubMed] [Google Scholar]
- 36.Chi KN, Zoubeidi A, Gleave ME. Custirsen (OGX-011): a second-generation antisense inhibitor of clusterin for the treatment of cancer. Expert Opin Investig Drugs. 2008;17:1955–1962. doi: 10.1517/13543780802528609. [DOI] [PubMed] [Google Scholar]
- 37.Chi KN, Hotte SJ, Yu EY, et al. Randomized phase II study of docetaxel and prednisone with or without OGX-011 in patients with metastatic castration-resistant prostate cancer. J Clin Oncol. 2010;28:4247–4254. doi: 10.1200/JCO.2009.26.8771. [DOI] [PubMed] [Google Scholar]
- 38.Saad F, Gleason DM, Murray R, et al. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst. 2002;94:1458–1468. doi: 10.1093/jnci/94.19.1458. [DOI] [PubMed] [Google Scholar]
- 39.Mathew P, Thall PF, Bucana CD, et al. Platelet-derived growth factor receptor inhibition and chemotherapy for castration-resistant prostate cancer with bone metastases. Clin Cancer Res. 2007;13:5816–5824. doi: 10.1158/1078-0432.CCR-07-1269. [DOI] [PubMed] [Google Scholar]
- 40.Carducci MA, Saad F, Abrahamsson PA, et al. A phase 3 randomized controlled trial of the efficacy and safety of atrasentan in men with metastatic hormone-refractory prostate cancer. Cancer. 2007;110:1959–1966. doi: 10.1002/cncr.22996. [DOI] [PubMed] [Google Scholar]
- 41.Chiao JW, Moonga BS, Yang YM, et al. Endothelin-1 from prostate cancer cells is enhanced by bone contact which blocks osteoclastic bone resorption. Br J Cancer. 2000;83:360–365. doi: 10.1054/bjoc.2000.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nelson JB, Nguyen SH, Wu-Wong JR, et al. New bone formation in an osteoblastic tumor model is increased by endothelin-1 overexpression and decreased by endothelin A receptor blockade. Urology. 1999;53:1063–1069. doi: 10.1016/s0090-4295(98)00658-x. [DOI] [PubMed] [Google Scholar]
- 43.Yin JJ, Mohammad KS, Kakonen SM, et al. A causal role for endothelin-1 in the pathogenesis of osteoblastic bone metastases. Proc Natl Acad Sci U S A. 2003;100:10954–10959. doi: 10.1073/pnas.1830978100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Michaelson MD, Kaufman DS, Kantoff P, Oh WK, Smith MR. Randomized phase II study of atrasentan alone or in combination with zoledronic acid in men with metastatic prostate cancer. Cancer. 2006;107:530–535. doi: 10.1002/cncr.22043. [DOI] [PubMed] [Google Scholar]
- 45.Fizazi K, Carducci M, Smith M, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet. 2011;377:813–822. doi: 10.1016/S0140-6736(10)62344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kerbel R, Folkman J. Clinical translation of angiogenesis inhibitors. Nat Rev Cancer. 2002;2:727–739. doi: 10.1038/nrc905. [DOI] [PubMed] [Google Scholar]
- 47.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 48.Folkman J. Angiogenesis: an organizing principle for drug discovery? Nat Rev Drug Discov. 2007;6:273–286. doi: 10.1038/nrd2115. [DOI] [PubMed] [Google Scholar]
- 49.Figg WD, Dahut W, Duray P, et al. A randomized phase II trial of thalidomide, an angiogenesis inhibitor, in patients with androgen-independent prostate cancer. Clin Cancer Res. 2001;7:1888–1893. [PubMed] [Google Scholar]
- 50.Madan RA, Dahut WL. Angiogenesis inhibition in the treatment of prostate cancer. Anticancer Agents Med Chem. 2009;9:1070–1078. doi: 10.2174/187152009789735035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Figg WD, Arlen P, Gulley J, et al. A randomized phase II trial of docetaxel (taxotere) plus thalidomide in androgen-independent prostate cancer. Semin Oncol. 2001;28:62–66. doi: 10.1016/s0093-7754(01)90157-5. [DOI] [PubMed] [Google Scholar]
- 52.Drake MJ, Robson W, Mehta P, Schofield I, Neal DE, Leung HY. An open-label phase II study of low-dose thalidomide in androgen-independent prostate cancer. Br J Cancer. 2003;88:822–827. doi: 10.1038/sj.bjc.6600817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dahut WL, Gulley JL, Arlen PM, et al. Randomized phase II trial of docetaxel plus thalidomide in androgen-independent prostate cancer. J Clin Oncol. 2004;22:2532–2539. doi: 10.1200/JCO.2004.05.074. [DOI] [PubMed] [Google Scholar]
- 54.Dahut WL, ragon-Ching JB, Woo S, et al. Phase I study of oral lenalidomide in patients with refractory metastatic cancer. J Clin Pharmacol. 2009;49:650–660. doi: 10.1177/0091270009335001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kelly WK, Halabi MA, Crducci MA, et al. A randomized, double-blind, placebo-controlled phase III trial comparing docetaxel, prednisone, and placebo with docetaxel, prednisone, and bevacizumab in men with metastatic castration-resistant prostate cancer (mCRPC): survival results of CALGB 90401. J Clin Oncol. 2010;28(suppl):18s. doi: 10.1200/JCO.2011.39.4767. Abstract LBA4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zurita AJ, Shore N, Krall JM, et al. Distinct patterns of PSA modulation by single-agent sunitinib before combination with docetaxel and prednisone in patients with metastatic castrate-resistant prostate cancer (CRPCa) J Clin Oncol. 2007;25(suppl):18S. Abstract 5134. 2007 ASCO Annual Meeting Proceedings Part I. [Google Scholar]
- 57.Zurita AJ, Liu J, Hutson TE, et al. Sunitinib in combination with docetaxel and prednisone in patients (pts) with metastatic hormone-refractory prostate cancer (mHRPC) J Clin Oncol. 2009;27(suppl):15s. Abstract 5166. [Google Scholar]
- 58.Ebos JM, Kerbel RS. Antiangiogenic therapy: impact on invasion, disease progression, and metastasis. Nat Rev Clin Oncol. 2011;8:210–221. doi: 10.1038/nrclinonc.2011.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mantalaris A, Panoskaltsis N, Sakai Y, et al. Localization of androgen receptor expression in human bone marrow. J Pathol. 2001;193:361–366. doi: 10.1002/1096-9896(0000)9999:9999<::AID-PATH803>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 60.Niu Y, Chang TM, Yeh S, Ma WL, Wang YZ, Chang C. Differential androgen receptor signals in different cells explain why androgen-deprivation therapy of prostate cancer fails. Oncogene. 2010;29:3593–3604. doi: 10.1038/onc.2010.121. [DOI] [PubMed] [Google Scholar]
- 61.Scher HI, Sawyers CL. Biology of progressive, castration-resistant prostate cancer: directed therapies targeting the androgen-receptor signaling axis. J Clin Oncol. 2005;23:8253–8261. doi: 10.1200/JCO.2005.03.4777. [DOI] [PubMed] [Google Scholar]
- 62.Montgomery RB, Mostaghel EA, Vessella R, et al. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res. 2008;68:4447–4454. doi: 10.1158/0008-5472.CAN-08-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mostaghel EA, Montgomery B, Nelson PS. Castration-resistant prostate cancer: targeting androgen metabolic pathways in recurrent disease. Urol Oncol. 2009;27:251–257. doi: 10.1016/j.urolonc.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Reid AH, Attard G, Barrie E, de Bono JS. CYP17 inhibition as a hormonal strategy for prostate cancer. Nat Clin Pract Urol. 2008;5:610–620. doi: 10.1038/ncpuro1237. [DOI] [PubMed] [Google Scholar]
- 65.Attard G, Reid AH, Yap TA, et al. Phase I clinical trial of a selective inhibitor of CYP17, abiraterone acetate, confirms that castration-resistant prostate cancer commonly remains hormone driven. J Clin Oncol. 2008;26:4563–4571. doi: 10.1200/JCO.2007.15.9749. [DOI] [PubMed] [Google Scholar]
- 66.Attard G, Reid AH, A’Hern R, et al. Selective inhibition of CYP17 with abiraterone acetate is highly active in the treatment of castration-resistant prostate cancer. J Clin Oncol. 2009;27:3742–3748. doi: 10.1200/JCO.2008.20.0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Danila DC, Morris MJ, de Bono JS, et al. Phase II multicenter study of abiraterone acetate plus prednisone therapy in patients with docetaxel-treated castration-resistant prostate cancer. J Clin Oncol. 2010;28:1496–1501. doi: 10.1200/JCO.2009.25.9259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Reid AH, Attard G, Danila DC, et al. Significant and sustained antitumor activity in post-docetaxel, castration-resistant prostate cancer with the CYP17 inhibitor abiraterone acetate. J Clin Oncol. 2010;28:1489–1495. doi: 10.1200/JCO.2009.24.6819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dreicer R, Agus DB, MacVicar GR, Wang H, Stadler WM. Safety, pharmacokinetics, and efficacy of TAK-700 in metastatic castration-resistant prostrate cancer: a phase I/II, open-label study. J Clin Oncol. 2010;28(suppl):15s. Abstract 3084. [Google Scholar]
- 71.Tran C, Ouk S, Clegg NJ, et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science. 2009;324:787–790. doi: 10.1126/science.1168175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Attard G, Swennenhuis JF, Olmos D, et al. Characterization of ERG, AR and PTEN gene status in circulating tumor cells from patients with castration-resistant prostate cancer. Cancer Res. 2009;69:2912–2918. doi: 10.1158/0008-5472.CAN-08-3667. [DOI] [PubMed] [Google Scholar]
- 73.Scher HI, Beer TM, Higano CS, et al. Antitumour activity of MDV3100 in castration-resistant prostate cancer: a phase 1-2 study. Lancet. 2010;375:1437–1446. doi: 10.1016/S0140-6736(10)60172-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shah NP, Tran C, Lee FY, Chen P, Norris D, Sawyers CL. Overriding imatinib resistance with a novel ABL kinase inhibitor. Science. 2004;305:399–401. doi: 10.1126/science.1099480. [DOI] [PubMed] [Google Scholar]
- 75.Yu EY, Wilding G, Posadas E, et al. Phase II study of dasatinib in patients with metastatic castration-resistant prostate cancer. Clin Cancer Res. 2009;15:7421–7428. doi: 10.1158/1078-0432.CCR-09-1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Araujo JC, Mathew P, Armstrong AJ, et al. Dasatinib and docetaxel combination treatment for patients with castration-resistant progressive prostate cancer: a phase I/II study (CA180086) Cancer. 2011 In press. [Google Scholar]
- 77.Koreckij T, Nguyen H, Brown LG, Yu EY, Vessella RL, Corey E. Dasatinib inhibits the growth of prostate cancer in bone and provides additional protection from osteolysis. Br J Cancer. 2009;101:263–268. doi: 10.1038/sj.bjc.6605178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dayyani F, Gallick GE, Thompson E, Trudel GC, Logothetis CJ, Araujo JC. Correlation of dasatinib (DAS) peak levels with interleukin-8 (IL-8) and monocyte chemotactic protein-1 (MCP-1) levels in patients with castration-resistant progressive prostate cancer (CRPC) J Clin Oncol. 2010;28:15s. [Google Scholar]
- 79.Dayyani F, Gallick GE, Trudel GC, Logothetis CJ, Araujo JC. Decline in bone markers and response to combination therapy with dasatinib and docetaxel in patients with castration-resistant progressive prostate cancer (CRPC) 2010 In: ASCO 2010 Genitourinary Cancers Symposium. San Francisco, CA. ;abstract 136. [Google Scholar]
- 80.Birchmeier C, Birchmeier W, Gherardi E, Vande Woude GF. Met, metastasis, motility and more. Nat Rev Mol Cell Biol. 2003;4:915–925. doi: 10.1038/nrm1261. [DOI] [PubMed] [Google Scholar]
- 81.Knudsen BS, Edlund M. Prostate cancer and the met hepatocyte growth factor receptor. Adv Cancer Res. 2004;91:31–67. doi: 10.1016/S0065-230X(04)91002-0. [DOI] [PubMed] [Google Scholar]
- 82.Verras M, Lee J, Xue H, Li TH, Wang Y, Sun Z. The androgen receptor negatively regulates the expression of c-Met: implications for a novel mechanism of prostate cancer progression. Cancer Res. 2007;67:967–975. doi: 10.1158/0008-5472.CAN-06-3552. [DOI] [PubMed] [Google Scholar]
- 83.Tu WH, Zhu C, Clark C, Christensen JG, Sun Z. Efficacy of c-Met inhibitor for advanced prostate cancer. BMC Cancer. 2010;10:556. doi: 10.1186/1471-2407-10-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.You WK, Sennino B, Williamson CW, et al. VEGF and c-Met blockade amplify angiogenesis inhibition in pancreatic islet cancer. Cancer Res. 2011;71:4758–4768. doi: 10.1158/0008-5472.CAN-10-2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Verbeek HH, Alves MM, de Groot JW, et al. The effects of four different tyrosine kinase inhibitors on medullary and papillary thyroid cancer cells. J Clin Endocrinol Metab. 2011;96:E991–E995. doi: 10.1210/jc.2010-2381. [DOI] [PubMed] [Google Scholar]
- 86.Hussain M, Smith A, Sweeney C, et al. Phase II study of XL184 in a cohort of patients with castration-resistant prostate cancer (CRPC) and measurable soft tissue disease. J Clin Oncol. 2011;29(suppl 7) Abstract 127. [Google Scholar]
- 87.Whiteside TL. The tumor microenvironment and its role in promoting tumor growth. Oncogene. 2008;27:5904–5912. doi: 10.1038/onc.2008.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Whiteside TL, Mandapathil M, Szczepanski M, Szajnik M. Mechanisms of tumor escape from the immune system: adenosine-producing Treg, exosomes and tumor-associated TLRs. Bull Cancer. 2011;98:E25–E31. doi: 10.1684/bdc.2010.1294. [DOI] [PubMed] [Google Scholar]
- 89.So-Rosillo R, Small EJ. Sipuleucel-T (APC8015) for prostate cancer. Expert Rev Anticancer Ther. 2006;6:1163–1167. doi: 10.1586/14737140.6.9.1163. [DOI] [PubMed] [Google Scholar]
- 90.Small EJ, Schellhammer PF, Higano CS, et al. Placebo-controlled phase III trial of immunologic therapy with sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J Clin Oncol. 2006;24:3089–3094. doi: 10.1200/JCO.2005.04.5252. [DOI] [PubMed] [Google Scholar]
- 91.Higano CS, Schellhammer PF, Small EJ, et al. Integrated data from 2 randomized, double-blind, placebo-controlled, phase 3 trials of active cellular immunotherapy with sipuleucel-T in advanced prostate cancer. Cancer. 2009;115:3670–3679. doi: 10.1002/cncr.24429. [DOI] [PubMed] [Google Scholar]
- 92.Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 93.Stein WD, Gulley JL, Schlom J, et al. Tumor regression and growth rates determined in five intramural NCI prostate cancer trials: the growth rate constant as an indicator of therapeutic efficacy. Clin Cancer Res. 2011;17:907–917. doi: 10.1158/1078-0432.CCR-10-1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Madan RA, Gulley JL. The current and emerging role of immunotherapy in prostate cancer. Clin Genitourin Cancer. 2010;8:10–16. doi: 10.3816/CGC.2010.n.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dipaola RS, Plante M, Kaufman H, et al. A phase I trial of pox PSA vaccines (PROSTVAC-VF) with B7-1, ICAM-1, and LFA-3 co-stimulatory molecules (TRICOM) in patients with prostate cancer. J Transl Med. 2006;4:1. doi: 10.1186/1479-5876-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Arlen PM, Skarupa L, Pazdur M, et al. Clinical safety of a viral vector based prostate cancer vaccine strategy. J Urol. 2007;178:1515–1520. doi: 10.1016/j.juro.2007.05.117. [DOI] [PubMed] [Google Scholar]
- 97.Gulley JL, Arlen PM, Madan RA, et al. Immunologic and prognostic factors associated with overall survival employing a poxviral-based PSA vaccine in metastatic castrate-resistant prostate cancer. Cancer Immunol Immunother. 2010;59:663–674. doi: 10.1007/s00262-009-0782-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kantoff PW, Schuetz TJ, Blumenstein BA, et al. Overall survival analysis of a phase II randomized controlled trial of a Poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J Clin Oncol. 2010;28:1099–1105. doi: 10.1200/JCO.2009.25.0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Small EJ, Tchekmedyian NS, Rini BI, Fong L, Lowy I, Allison JP. A pilot trial of CTLA-4 blockade with human anti-CTLA-4 in patients with hormone-refractory prostate cancer. Clin Cancer Res. 2007;13:1810–1815. doi: 10.1158/1078-0432.CCR-06-2318. [DOI] [PubMed] [Google Scholar]
- 101.Fong L, Kwek SS, O’Brien S, et al. Potentiating endogenous antitumor immunity to prostate cancer through combination immunotherapy with CTLA4 blockade and GM-CSF. Cancer Res. 2009;69:609–615. doi: 10.1158/0008-5472.CAN-08-3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Small E, Higano C, Tchekmedyian NS, et al. Randomized phase II study comparing 4 monthly doses of ipilimumab (MDX-010) as a single agent or in combination with a single dose of docetaxel in patients with hormone-refractory prostate cancer. In: ASCO 2006 Annual Meeting, Atlanta, GA; 2006; abstract 4609 [Google Scholar]