Abstract
d-Serine, an N-methyl d-aspartate receptor coagonist, and its regulatory enzymes, d-amino acid oxidase (DAO; degradation) and serine racemase (SR; synthesis), have been implicated in crucial roles of the developing central nervous system, yet the functional position that they play in regulating the availability of d-serine throughout development of the mammalian retina is not well-known. Using capillary electrophoresis and a sensitive method of enantiomeric amino acid separation, we were able to determine total levels of d-serine at specific ages during postnatal development of the mouse retina in two different strains of mice, one of which contained a loss-of-function point mutation for DAO while the other was a SR knockout line. Each mouse line was tested against conspecific wild type (WT) mice for each genetic strain. The universal trend in all WT and transgenic mice was a large amount of total retinal d-serine at postnatal age 2 (P2), followed by a dramatic decrease as the mice matured into adulthood (P70–80). SR knockout mice retinas had 41% less d-serine than WT retinas at P2, and 10 times less as an adult. DAO mutant mice retinas had significantly elevated levels of d-serine when compared to WT retinas at P2 (217%), P4 (223%), P8 (194%), and adulthood (227%).
Keywords: d-Serine, d-amino acid oxidase, serine racemase, retinal development, NMDA receptor, capillary electrophoresis
N-Methyl d-aspartate receptors (NMDARs) are unique among the major ionotropic glutamate receptors because they require an endogenous coagonist, in addition to glutamate, for ion channel gating. This coagonist was initially identified as glycine;1 but additional studies have determined that other amino acids, including d-serine, can substitute for, or even predominate over, glycine.2 Hashimoto et al. developed an HPLC technique that separated amino acid enantiomers and discovered the presence of d-serine with significantly high levels in areas of the brain that are also rich in NMDA receptors.3,4 The discovery of serine racemase (SR), the enzyme that converts l-serine to d-serine, established d-serine as a viable NMDAR coagonist5,6 rather than a substance that varied according to extraneous sources, such as dietary intake. d-Serine, NMDARs, and the d-serine regulatory enzymes, d-amino acid oxidase (DAO) and SR, have been implicated in multiple aspects of nervous system development, including regulation of dendritic morphology in pyramidal neurons,7 cell migration in the cerebellar cortex,8,9 and shaping synaptogenesis and neuronal circuitry.10d-Serine was more recently identified in the retina,11 where it, rather than glycine, is the principal endogenous coagonist of NMDARs on retinal ganglion cells (RGCs).12,13 Importantly, the coagonist sites of NMDA receptors in ganglion cells remain subsaturated, positioning the sensitivity of NMDA receptors to be further modulated by coagonist introduction, as evidenced by exogenously applied d-serine enhancing light-evoked NMDA receptor currents in RGCs.14 There is also evidence that d-serine can inhibit α-amino-3-hydroxy-5-methyl-4-isoxazole-proprionic acid (AMPA) and kainate receptors in the retina15 as well as regulate cerebellar long-term depression through the ionotropic glutamate receptor, delta-2 (GluD2),16 which is present in the mammalian retina.17
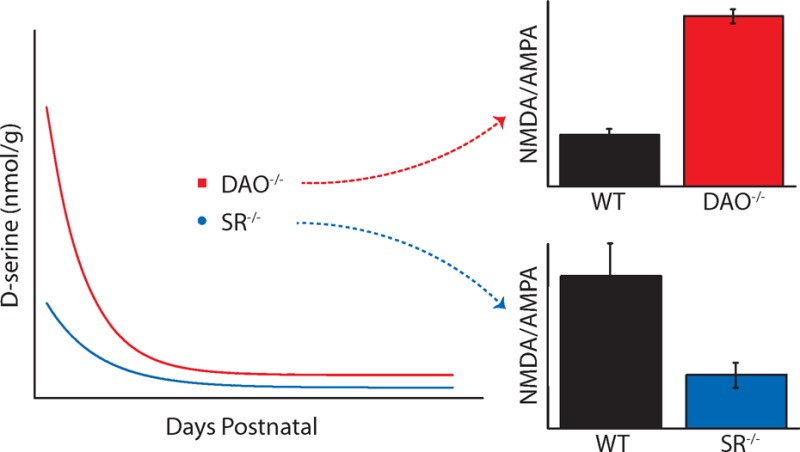
The enzymes SR and DAO are believed to be central for the enzymatic regulation of d-serine, and disabling the function of either enzyme typically increases or decreases d-serine levels depending on the role of the enzyme. SR is present in the mammalian brain and can catalyze the racemization of d-serine from its enantiomer, l-serine.5 Mice lacking SR (SR–/–) have significantly reduced levels of d-serine in multiple brain regions,18 including the adult retina, in which the NMDAR to AMPA receptor ratio of light-evoked currents is notably diminished.13 DAO, on the other hand, catalyzes the degradation of d-amino acids into keto acids. Mice transfected with DAO loss-of-function gene mutations (DAO–/–)19 have elevated levels of d-serine in the cerebellum and medulla oblongata, where wild-type DAO activity is particularly high.20 We have reported a similar d-serine elevation in the DAO–/– retina with a concomitant increase in NMDAR contributions to light-evoked responses of RGCs when compared to WT controls.21 Elevated levels of endogenous d-serine in DAO–/– mice also leave RGC NMDAR coagonist sites fully occupied and less available to coagonist modulation, in contrast to wild-type mice of either strain or to SR–/– mice.21 In addition, the GlyT1 transporter plays an important role in setting local concentrations of glycine to d-serine ratios. Heterozygote mice deficient in the GlyT1 transporter show saturation of the coagonist site of retinal ganglion cells.22
The present study was undertaken to explore the postnatal development of d-serine in mice with deficiencies in SR and DAO; it was stimulated by results which suggest that d-serine plays an important role in determining the NMDA to AMPA receptor ratio in adult mice.13,21 We used capillary electrophoresis (CE) to measure the levels of d-serine in the mouse retina at discrete ages throughout postnatal development. Our results show high postnatal d-serine in the retinas of all wild-type and transgenic strains followed by a decline into adulthood. We found that d-serine levels were increased in DAO–/– mice and decreased in SR–/– mice when compared to their age-matched wild-type counterparts for specific developmental periods.
Results and Discussion
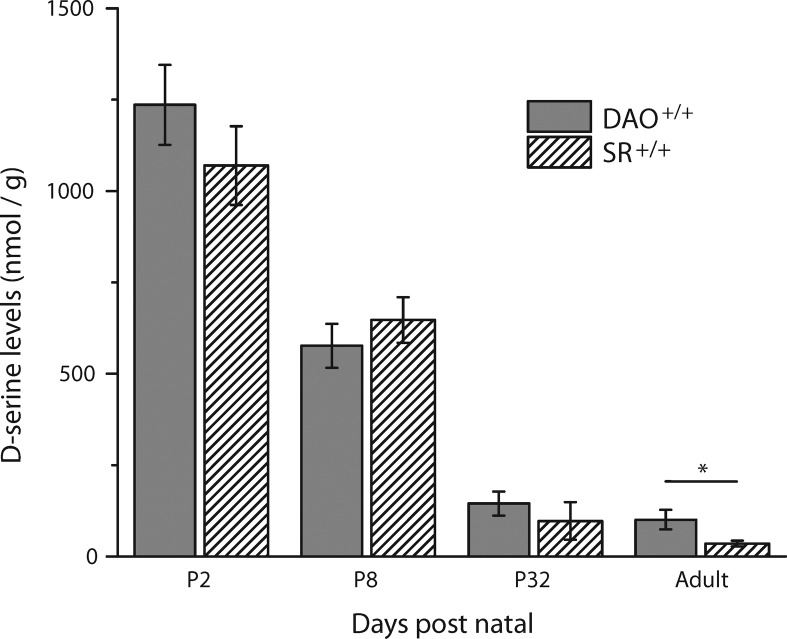
Figure 1 illustrates a comparison of total d-serine between the WT retinas for the C57 background strain of the SR–/– mice (SR+/+) and the ddY background strain of the DAO–/– mice (DAO+/+). In both strains, we found a significant elevation in early postnatal d-serine levels, which taper off during the course of development. This dramatic developmental decrease in SR+/+d-serine has been similarly reported in the mouse cerebellum24,25 and previously in the retina based on immunostaining techniques.23 The observed early high concentration of d-serine theoretically positions it to influence the expression and physiological characteristics of NMDARs during a critical developmental period in the first week of life. Although both strains demonstrated the same pattern of retinal d-serine changes over time, adult DAO+/+ levels (101 ± 26.7 nmol/g, N = 6, ddY background) were about 3 times larger than those in adult SR+/+ mice (35.1 ± 8.15 nmol/g, N = 7, C57 background). The differing adult d-serine levels in the two WT mice are most likely strain differences, as NMDA receptor activity is known to be strain dependent.26,27
Figure 1.
Comparison between d-serine levels in DAO+/+ and SR+/+ homogenized retinas. The only significant difference was between the adult animals of both strains (* indicates p < 0.05, two-tailed Student’s t test; SR+/+ P2 N = 7, P8 N = 6, P32 N = 6, adult N = 6; DAO+/+ P2 through adult N = 6 each).
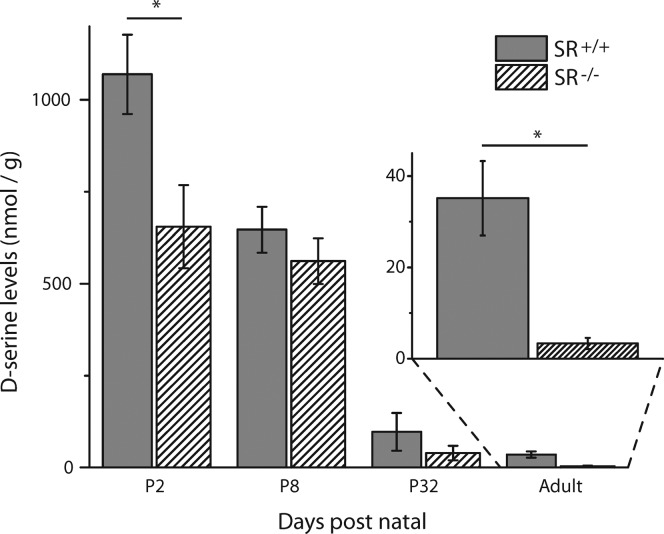
Figure 2 illustrates the time course of changes in ambient d-serine levels comparing P2, P8, P32, and adult SR+/+/SR–/– retinas (magnified in the inset). Significant differences of total retinal d-serine levels were observed between SR+/+ and SR–/– mice at P2 (SR+/+, 1110 ± 98.6 nmol/g, N = 7; SR–/–, 655 ± 113 nmol/g, N = 6) and in adulthood (SR+/+, 35.1 ± 8.15 nmol/g, N = 7; SR–/–, 3.34 ± 1.23 nmol/g, N = 6), with the transgenic mice expressing less d-serine than controls. Figure 4C illustrates the prominent difference between adult SR+/+ and SR–/– electropherograms. At P8 and P32, d-serine levels did not differ significantly between SR+/+ and SR–/– retinas, but the mean levels in SR–/– mice trended lower. Low levels of ambient d-serine during a period when glutamatergic receptor expression levels are being set could easily have diminished the demand for NMDAR development relative to d-serine insensitive AMPARs (at the levels reported, but see ref (15)), which ultimately dominate retinal ganglion cell activity in SR–/– adults.13 Although diminished d-serine has not been shown to directly impact NMDAR protein expression in the retina, we have previously found alterations to adult SR+/+ retinal glutamatergic activity that is consistent with this hypothesis,21 and these mice do show an increased expression of GluN1 in the cerebellum when compared to SR+/+ mice.28
Figure 2.
d-Serine levels in SR+/+ and SR–/– retinal homogenates. At postnatal day 2 (P2), the SR–/– retinal homogenates had 41% less d-serine than the SR+/+ retina. The disparity between strains was increased to a 10-fold difference in adulthood. From P2 through adulthood (P70–80), d-serine levels steadily declined in both SR+/+ and SR–/– retinas, for a 32-fold difference and 196-fold difference, respectively. There was no significant difference between strains at P8 or P32 (* indicates p < 0.01, one-tailed Student’s t test; SR+/+ P2 N = 7, P8 N = 6, P32 N = 6, adult N = 6; SR–/– P2 N = 6, P8 N = 6, P32 N = 7, adult N = 6).
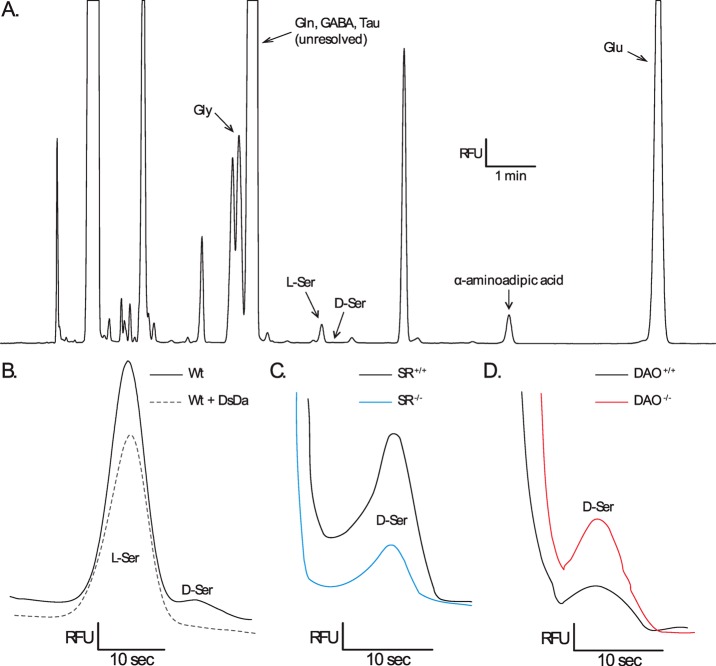
Figure 4.
Identification of capillary electrophoretic peaks from homogenized mouse retinas. (A) Electropherogram (relative fluorescent units (RFU) vs time) of an adult SR+/+ mouse retina showing the relative peak locations of NBD-F derivatized glycine (Gly), glutamine (Gln), γ-aminobutyric acid (GABA), taurine (Tau), l-serine (l-Ser), d-serine (d-Ser), α-aminoadipic acid (internal standard), and glutamate (Glu). (B) Normalized electropherograms of l-serine and d-serine in an adult SR+/+ vs the addition of d-serine deaminase (DsDa), which completely abolished the d-serine peak. (C) Normalized electropherograms of d-serine in an adult SR+/+ sample vs an adult SR–/– sample. (D) Normalized electropherograms of d-serine in an adult DAO+/+ sample vs an adult DAO–/–sample.
Surprisingly, although SR is believed to be the primary source of endogenous d-serine, the loss of this enzyme did not entirely eliminate the presence of retinal d-serine. Early P2 d-serine was still 196-fold higher in the SR–/– adult retina, compared to a 32-fold difference observed between the same points in the wild-type control. Furthermore, there were no differences in l-serine, the substrate for SR-based racemization to d-serine, between SR+/+ and SR–/– mice at any postnatal age, although l-serine levels were elevated at P2 (44 700 ± 1910 nmol/g, N = 10) and decreased into adulthood (4000 ± 382 nmol/g, N = 14). One alternate source of d-serine might be as a dietary component of the mouse feed.29 However, we tested a group of SR–/– animals, retained on a d-serine free diet from birth, and found no change in the adult retinal d-serine concentration (data not shown). Gut bacteria has also been suggested to supplement systemic d-serine, although published results have been mixed and highlight tissue-specific variability.29−31 A concentrated introduction through nutrient enriched maternal milk could account for initial peaks in d-serine and l-serine concentrations in SR–/– mice while the timing of progressive weaning is coincident with the noted period of decline. Finally, it is entirely possible that unidentified catalytic pathways exist for the production of d-serine, independent of SR.32−34 Although an alternative source is yet to be established, local transport systems could easily adjust d-serine in a tissue specific manner, including ASC-type transporters in the retina.35 This might also explain why SR–/– exhibited wild-type levels of d-serine at the whole eyeball level.36
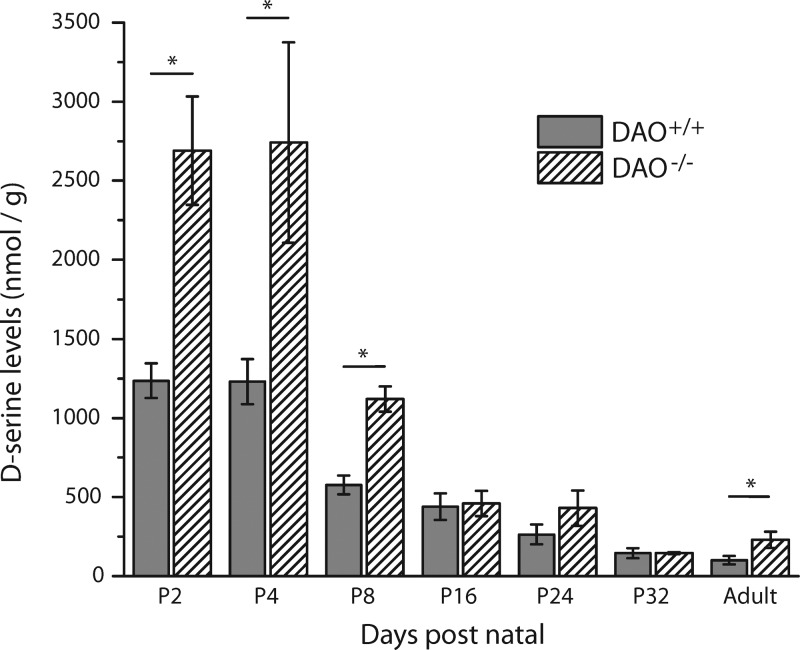
Figure 3 illustrates that d-serine levels were significantly higher in adult DAO–/– mice (229 ± 51.2 nmol/g, N = 7) in comparison to adult DAO+/+ mice (101 ± 26.7 nmol/g, N = 6). The visual distinction between adult DAO+/+ and DAO–/– electropherograms is depicted in Figure 4D. In addition, the DAO–/– mice displayed an approximate 2-fold increase in retinal d-serine at P2 (DAO+/+, 1240 ± 110 nmol/g, N = 6; DAO–/–, 2690 ± 342 nmol/g, N = 7), P4 (DAO+/+, 1230 ± 143 nmol/g, N = 6; DAO–/–, 2740 ± 633 nmol/g, N = 7), and P8 (DAO+/+, 577 ± 60.1 nmol/g, N = 6; DAO–/–, 1120 ± 80.2 nmol/g, N = 8). This prolonged period of elevated d-serine in DAO–/– mice could have encouraged higher levels of NMDAR development, which along with enhanced coagonist availability would contribute to the higher NMDAR/AMPAR ratios seen in light-evoked RGC responses and GluN2A/B to GluA2 expression of the adult DAO–/– retina.21 It remains possible, however, that elevated coagonist availability in adulthood could alone account for the receptor expression and activity changes we have observed. Similar to the retinas of SR–/– mice, the transgene effect had no significant impact on d-serine levels beyond P8, suggesting that the biological demand for manipulable d-serine regulation during retinal development is focused on the first few days of postnatal life.
Figure 3.
d-Serine levels in DAO+/+ and DAO–/– homogenized retinas. At postnatal day 2 (P2), the DAO–/– retinal homogenates had 2.2 times more d-serine than their WT control. The difference between strains was significant at all ages, apart from P16–32. Adult retinal homogenates indicated a 2.3-fold difference. From P2 through adulthood, d-serine steadily declined by a 12-fold difference in both DAO+/+ and DAO–/– retinas (* indicates p < 0.05, one-tailed Student’s t test; DAO+/+ P2 through adult N = 6 each; DAO–/– P2 N = 7, P4 N = 7, P8 N = 8, P16 N = 6, P24 N = 6, P32 N = 7, adult N = 7).
Despite the absence of d-serine’s primary catabolic pathway, DAO–/– retinas demonstrated high P2 d-serine, declining 12-fold by adulthood. This could be explained by the local transport systems or the maternal milk hypotheses described above, the latter of which could also account for the elevated P2 (45 300 ± 1640 nmol/g, N = 13) and adulthood decline (6500 ± 348 nmol/g, N = 13) of l-serine in the DAO–/– mice. d-Serine does have an alternate degradation pathway through an SR-catalyzed α,β-elimination reaction,37 but this mechanism could not account for declines in the developing SR–/– retinas. It remains possible that a pathway for d-serine elimination, independent of DAO or SR, is yet to be identified.
Although we have argued for a critical developmental period in which early postnatal d-serine shapes glutamatergic circuitry in the adult retina, our constitutional mouse models show significant changes in d-serine in both early life and adulthood. Therefore, it could be argued that the altered receptor activity ratios we have previously observed can be solely attributed to the synaptic availability of d-serine in the adult knockouts. Several pieces of circumstantial evidence, however, weigh in favor of a significant developmental period influence. While we have proposed that high coagonist availability promotes NMDAR activity and low availability diminishes it, similar conditions in the adult animal would not necessarily encourage the same result. Adult application of d-serine has been shown to promote the internalization of membrane NMDARs,38 while chronic receptor antagonism, which could be viewed as a period of diminished NMDAR activity, has been well documented to increase NMDAR expression and function.39,40 In addition, d-serine has been identified as a critical regulator of postnatal development in other CNS regions,41,42 specifically influencing NMDAR participation in synaptic activity with prolonged effects.10,43 Finally, two studies suggest the sufficiency of neonatal d-serine changes to permanently impact on adult glutamatergic function. Mice with a 3-PGDH deficiency lack both l-serine and d-serine throughout their lives, but in one investigation maternal d-serine supplementation alone was able to completely reverse the otherwise abnormal neurological phenotype.10,44 In another study, an SRR inhibitor was administered to rats from P7 to P9, which led to adult behavioral deficits despite a normal amino acid profile in multiple brain regions. Deficits in this latter model were consistent with schizophrenia, similar to our SR–/– mouse, and could be rescued by preadulthood treatment with d-serine.45 Nevertheless, a conclusive argument about the relative significance of postnatal and adult d-serine concentrations will require a detailed study of NMDAR activity and expression patterns in the retina at key points between birth and adulthood.
Conclusion
We have found that the retinal levels of d-serine are tightly controlled during the first week of postnatal life and vary according to deficiencies in either DAO or SR, and that the changes over the course of development are well-positioned to regulate the expression of NMDARs. During this period, diminished d-serine in SR–/– or elevated d-serine in the DAO–/– could conceivably contribute to the altered NMDAR/AMPAR ratios observed in the adult retina of these mice. The fundamentals of d-serine function, in the retina and elsewhere, remain a fruitful topic of study, both as a part of normal physiological activity and as a mechanistic contributor to various disease pathologies. In particular, alternative sources of d-serine regulation outside of SR and DAO should be examined with a thorough application of the newest research tools, such as cell-type specific transgenic mice and antibodies with confirmed staining specificity,46,47 both of which will be invaluable in confirming past work and resolving fundamental questions. In addition to synaptic shaping, the source of d-serine release, neuronal vs glial, continues to be vigorously debated. We note that the cortical and hippocampal regions where neuronal-dominant d-serine release has been identified47−49 also possess high SR and low DAO activity.25,47,50 By contrast in the retina and cerebellum, where astrocytic d-serine release is demonstrably critical,12,24,41,51−53 the relative enzyme contributions seem reversed,16,54 raising the intriguing hypothesis that the tissue-specific balance of regulatory enzyme activity might influence the predominant site of d-serine release. Recent discoveries have also pointed toward a glycine vs d-serine differentiation in the modulation of excitotoxic LTD-inducing extrasynaptic NMDARs vs neuroprotective LTP-promoting synaptic NMDARs.55,56 It is at least plausible that changes in d-serine availability, fueled by SR and DAO regulation, could have downstream effects on cell excitability, viability, and plasticity. For example, SR–/– mice appear to compensate for diminished d-serine by expanding the role of glycine as a synaptic coagonist,56 but this less-powerful NMDAR activator could easily exacerbate the hypoglutamatergia believed to underlie the schizophrenic phenotype of these mice.57 The results of the present study add to this growing body of work to highlight a critical but incompletely characterized role for d-serine in the multisystem development of glutamatergic circuitry in the retina and beyond.
Methods
Subjects
SR–/– mice and their wild-type (SR+/+) controls were generated on a C57/BL6 background strain18 and obtained from Joe Coyle of Harvard University. DAO–/– mice and their wild-type (DAO+/+) controls were generated on a ddY background strain.19 All mice were group housed in a conventional facility on a 12:12 light cycle with ad libitum access to food and water. Both males and females were used indiscriminately for all experiments at ages between postnatal day 2 (P2) and adult (>P70), with a group number of N = 6–8 per reported age category. All procedures were performed in accordance with protocols approved by the Institutional Animal Care and Use Committee at the University of Minnesota.
Retina Isolation and Preparation
Mice were euthanized by an overdose of pentobarbital (0.1 mL of 50 mg/mL concentration, I.P.) or by decapitation for neonatal mice (<P16) followed by bilateral pneumothorax to ensure death. The eyes were enucleated and placed in mammalian bicarbonate Ringer solution containing 111 mM NaCl, 3 mM KCl, 2 mM CaCl2, 1 mM Mg2SO4, 32 mM NaHCO3, 0.5 mM NaH2PO4, and 15 mM dextrose, bubbled at room temperature with 95% O2 and 5% CO2 until use, final pH 7.4. Retinas from both eyes of each animal were surgically isolated before being processed for capillary electrophoresis.
We pooled the retinas from each animal into ice cold 0.6 M perchloric acid (PCA, 115 μL) and homogenized them with a sonicator probe to precipitate proteins. An internal standard, 300 μM α-aminoadipic acid (5 μL), was added to the homogenate, which was then neutralized with 2 M KOH (35 μL). After the sample was pelleted in a tabletop centrifuge at 4 °C, the supernatant was spun again through a Spin-X centrifuge tube filter (Corning Inc., 0.22 μm cellulose acetate). Next, 120 μL of the remaining supernatant was combined with a 1 M borate and 0.15 M KOH solution (20 μL) and then frozen at −20 °C until CE analysis. The pellet was resuspended in 2 M NaOH (120 μL) and then quantified for protein concentration using a bicinchoninic acid assay (BCA; Pierce, Rockford, IL).
Capillary Electrophoresis
We used CE to determine d-serine concentrations in processed tissue homogenates as previously described35 due to its high sensitivity. Each sample was reacted at 60 °C for 15 min with 4-fluoro-7-nitrobenz-2-oxa-1,3-daizole (NBD-F, Molecular Probes, Eugene, OR) dissolved in acetonitrile (1 mg/100 μL) to fluorescently label primary and secondary amines.58 Separation was performed at 18 kV (80 μA) on a commercial CE instrument (Beckman-Coulter MDQ, Fullerton, CA) using a 50 μm inner diameter, 40 cm length capillary with laser-induced fluorescence (LIF) detection. The separation buffer consisted of 165 mM borate pH 10.00–10.15 (dependent on separation quality) and 34 mM (2-hydroxypropyl)-β-cyclodextrin (Sigma-Aldrich, St Louis, MO). Electropherograms (fluorescence vs time) were digitally recorded with 32 Karat software (Beckman-Coulter) from fluorescent signals detected with a photomultiplier tube (Figure 4A). Amino acid levels were quantified by integrating electropherogram peaks, normalizing the amino acid peaks to an internal standard, and then comparing them to known standards. Standards were prepared in triplicate ranging from 5 nM to 1.3 μM for d-serine and 50 nM to 13 μM for l-serine. The limit of detection (LOD) for our assay was 3.4 nM d-serine per sample before protein normalization and was calculated with our standard curve data and regression statistics (the LOD was equal to the y-intercept plus three times the standard deviation of the regression). The calculated LOD applied to both d-serine and l-serine, as peaks were identical for equal concentrations of the isomers. Only two samples, both of which were SR–/– adults, fell below the limit of detection, and their values were reported as zero. d-Serine presence was confirmed with peak depletion by degrading samples with d-serine deaminase (20 min at room temperature), which has been shown previously to efficiently and completely degrade d-serine under these conditions14 (Figure 4B).
Statistical Analysis
Each CE data point (N) was derived from a homogenization of two pooled retinas from a single animal. The measured values within each age category do not violate normality assumptions as revealed from Shapiro-Wilk tests. However, the strains DAO+/+/DAO–/– and SR+/+/SR–/– are quite dissimilar from one another and produced considerable fluctuations in their group variances. We refrained from using the standard one-way analysis of variance (ANOVA) method, and instead used independent 2-sample Student’s t tests for comparison at specified time points because we were only interested in comparing these strains between +/+ and −/– mice at specific ages and not across the spectrum of different time points (i.e., we avoided comparing P2 with P4, etc.). Specifically, Student’s two-tailed t test was used to calculate the significance between wild type strains for each age category in Figure 1, and Student’s one-tailed t test was used to calculate significance between strains for each age category in Figures 2 and 3. All data is expressed as mean ± SE (nmol/g), and significance was defined as p < 0.05.
Acknowledgments
We thank Manny Esguerra and Eric Gustafson for advice and guidance. We also thank Michael Bowser for CE optimization advice and Derek Miller for editorial assistance.
Glossary
Abbreviations
- NBD-F
4-fluoro-7-nitrobenz-2-oxa-1,3-daizole
- BCA
bicinchoninic acid assay
- SR+/+
C57/BL6 wild-type mice
- CE
capillary electrophoresis
- DAO
d-amino acid oxidase
- DAO–/–
d-amino acid oxidase mutant mice
- DAO+/+
ddY wild-type mice
- d-Ser
d-serine
- DsDa
d-serine deaminase
- Glu
glutamate
- Gln
glutamine
- Gly
glycine
- l-Ser
l-serine
- NMDA
N-methyl d-aspartate
- NMDAR
N-methyl d-aspartate receptors
- RFU
relative fluorescent units
- RGC
retinal ganglion cell
- SR
serine racemase
- SR–/–
serine racemase knockout mice
- Tau
taurine
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazole-proprionic acid
- GABA
γ-aminobutyric acid
Author Contributions
G.E.R., A.D.L., C.W.M., and R.F.M. designed research; G.E.R. and A.D.L. performed research; G.E.R. and D.B. analyzed data; and G.E.R., A.D.L., C.W.M., D.B., and R.F.M. wrote the paper.
National Eye Institute, Grant No. RO1-EY03014 to R.F.M.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
References
- Johnson J. W.; Ascher P. (1987) Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature 325, 529–531. [DOI] [PubMed] [Google Scholar]
- Kleckner N. W.; Dingledine R. (1988) Requirement for glycine in activation of NMDA-receptors expressed in Xenopus oocytes. Science 241, 835–837. [DOI] [PubMed] [Google Scholar]
- Hashimoto A.; Nishikawa T.; Hayashi T.; Fujii N.; Harada K.; Oka T.; Takahashi K. (1992) The presence of free d-serine in rat brain. FEBS Lett. 296, 33–36. [DOI] [PubMed] [Google Scholar]
- Hashimoto A.; Nishikawa T.; Oka T.; Takahashi K. (1993) Endogenous d-serine in rat brain: N-methyl-d-aspartate receptor-related distribution and aging. J. Neurochem. 60, 783–786. [DOI] [PubMed] [Google Scholar]
- Wolosker H.; Sheth K. N.; Takahashi M.; Mothet J. P.; Brady R. O. Jr.; Ferris C. D.; Snyder S. H. (1999) Purification of serine racemase: biosynthesis of the neuromodulator d-serine. Proc. Natl. Acad. Sci. U.S.A. 96, 721–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolosker H.; Blackshaw S.; Snyder S. H. (1999) Serine racemase: A glial enzyme synthesizing d-serine to regulate glutamate-N-methyl-d-aspartate neurotransmission. Proc. Natl. Acad. Sci. U.S.A. 96, 13409–13414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVito L. M.; Balu D. T.; Kanter B. R.; Lykken C.; Basu A. C.; Coyle J. T.; Eichenbaum H. (2011) Serine racemase deletion disrupts memory for order and alters cortical dendritic morphology. Genes, Brain Behav. 10, 210–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komuro H.; Rakic P. (1992) Selective role of N-type calcium channels in neuronal migration. Science 257, 806–809. [DOI] [PubMed] [Google Scholar]
- Komuro H.; Rakic P. (1993) Modulation of neuronal migration by NMDA receptors. Science 260, 95–97. [DOI] [PubMed] [Google Scholar]
- Fuchs S. A.; Roeleveld M. W.; Klomp L. W.; Berger R.; de Koning T. J. (2012) d-Serine influences synaptogenesis in a P19 cell model. JIMD Rep. 6, 47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien K. B.; Esguerra M.; Klug C. T.; Miller R. F.; Bowser M. T. (2003) A high-throughput on-line microdialysis-capillary assay for d-serine. Electrophoresis 24, 1227–1235. [DOI] [PubMed] [Google Scholar]
- Stevens E. R.; Esguerra M.; Kim P. M.; Newman E. A.; Snyder S. H.; Zahs K. R.; Miller R. F. (2003) d-Serine and serine racemase are present in the vertebrate retina and contribute to the physiological activation of NMDA receptors. Proc. Natl. Acad. Sci. U.S.A. 100, 6789–6794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan S. J.; Esguerra M.; Wickham R. J.; Romero G. E.; Coyle J. T.; Miller R. F. (2011) Serine racemase deletion abolishes light-evoked NMDA receptor currents in retinal ganglion cells. J. Physiol. 589, 5997–6006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson E. C.; Stevens E. R.; Wolosker H.; Miller R. F. (2007) Endogenous d-serine contributes to NMDA-receptor-mediated light-evoked responses in the vertebrate retina. J. Neurophysiol. 98, 122–130. [DOI] [PubMed] [Google Scholar]
- Daniels B. A.; Wood L.; Tremblay F.; Baldridge W. H. (2012) Functional evidence for d-serine inhibition of non-N-methyl-d-aspartate ionotropic glutamate receptors in retinal neurons. Eur. J. Neurosci. 35, 56–65. [DOI] [PubMed] [Google Scholar]
- Kakegawa W.; Miyoshi Y.; Hamase K.; Matsuda S.; Matsuda K.; Kohda K.; Emi K.; Motohashi J.; Konno R.; Zaitsu K.; Yuzaki M. (2011) d-Serine regulates cerebellar LTD and motor coordination through the delta2 glutamate receptor. Nat. Neurosci. 14, 603–611. [DOI] [PubMed] [Google Scholar]
- Grunert U.; Haverkamp S.; Fletcher E. L.; Wassle H. (2002) Synaptic distribution of ionotropic glutamate receptors in the inner plexiform layer of the primate retina. J. Comp. Neurol. 447, 138–151. [DOI] [PubMed] [Google Scholar]
- Basu A. C.; Tsai G. E.; Ma C. L.; Ehmsen J. T.; Mustafa A. K.; Han L.; Jiang Z. I.; Benneyworth M. A.; Froimowitz M. P.; Lange N.; Snyder S. H.; Bergeron R.; Coyle J. T. (2009) Targeted disruption of serine racemase affects glutamatergic neurotransmission and behavior. Mol. Psychiatry 14, 719–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konno R.; Yasumura Y. (1983) Mouse mutant deficient in d-amino acid oxidase activity. Genetics 103, 277–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamase K.; Konno R.; Morikawa A.; Zaitsu K. (2005) Sensitive determination of d-amino acids in mammals and the effect of d-amino-acid oxidase activity on their amounts. Biol. Pharm. Bull. 28, 1578–1584. [DOI] [PubMed] [Google Scholar]
- Gustafson E. C.; Morgans C. W.; Tekmen M.; Sullivan S. J.; Esguerra M.; Konno R.; Miller R. F. (2013) Retinal NMDA receptor function and expression are altered in a mouse lacking d-amino acid oxidase. J. Neurophysiol. 110, 2718–2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed B. T.; Sullivan S. J.; Tsai G.; Coyle J. T.; Esguerra M.; Miller R. F. (2009) The glycine transporter GlyT1 controls N-methyl-d-aspartic acid receptor coagonist occupancy in the mouse retina. Eur. J. Neurosci. 30, 2308–2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dun Y.; Duplantier J.; Roon P.; Martin P. M.; Ganapathy V.; Smith S. B. (2008) Serine racemase expression and d-serine content are developmentally regulated in neuronal ganglion cells of the retina. J. Neurochem. 104, 970–978. [DOI] [PubMed] [Google Scholar]
- Schell M. J.; Brady R. O. Jr.; Molliver M. E.; Snyder S. H. (1997) d-serine as a neuromodulator: regional and developmental localizations in rat brain glia resemble NMDA receptors. J. Neurosci. 17, 1604–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto A.; Oka T.; Nishikawa T. (1995) Anatomical distribution and postnatal changes in endogenous free d-aspartate and d-serine in rat brain and periphery. Eur. J. Neurosci. 7, 1657–1663. [DOI] [PubMed] [Google Scholar]
- Deutsch S. I.; Rosse R. B.; Paul S. M.; Riggs R. L.; Mastropaolo J. (1997) Inbred mouse strains differ in sensitivity to “popping” behavior elicited by MK-801. Pharmacol., Biochem. Behav. 57, 315–317. [DOI] [PubMed] [Google Scholar]
- Deutsch S. I.; Mastropaolo J.; Powell D. G.; Rosse R. B.; Bachus S. E. (1998) Inbred mouse strains differ in their sensitivity to an antiseizure effect of MK-801. Clin. Neuropharmacol. 21, 255–257. [PubMed] [Google Scholar]
- Mustafa A. K.; Ahmad A. S.; Zeynalov E.; Gazi S. K.; Sikka G.; Ehmsen J. T.; Barrow R. K.; Coyle J. T.; Snyder S. H.; Dore S. (2010) Serine racemase deletion protects against cerebral ischemia and excitotoxicity. J. Neurosci. 30, 1413–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asakura S.; Konno R. (1997) Origin of o-serine present in urine of mutant mice lacking d-amino-acid oxidase activity. Amino Acids 12, 213–223. [Google Scholar]
- Konno R.; Hamase K.; Maruyama R.; Zaitsu K. (2010) Mutant mice and rats lacking d-amino acid oxidase. Chem. Biodiversity 7, 1450–1458. [DOI] [PubMed] [Google Scholar]
- Savignac H. M.; Corona G.; Mills H.; Chen L.; Spencer J. P.; Tzortzis G.; Burnet P. W. (2013) Prebiotic feeding elevates central brain derived neurotrophic factor, N-methyl-d-aspartate receptor subunits and d-serine. Neurochem. Int. 63, 756–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwama H.; Takahashi K.; Kure S.; Hayashi F.; Narisawa K.; Tada K.; Mizoguchi M.; Takashima S.; Tomita U.; Nishikawa T. (1997) Depletion of cerebral d-serine in non-ketotic hyperglycinemia: possible involvement of glycine cleavage system in control of endogenous d-serine. Biochem. Biophys. Res. Commun. 231, 793–796. [DOI] [PubMed] [Google Scholar]
- Wood P. L.; Hawkinson J. E.; Goodnough D. B. (1996) Formation of d-serine from l-phosphoserine in brain synaptosomes. J. Neurochem. 67, 1485–1490. [DOI] [PubMed] [Google Scholar]
- Wolosker H.; Mori H. (2012) Serine racemase: an unconventional enzyme for an unconventional transmitter. Amino Acids 43, 1895–1904. [DOI] [PubMed] [Google Scholar]
- O’Brien K. B.; Miller R. F.; Bowser M. T. (2005) d-Serine uptake by isolated retinas is consistent with ASCT-mediated transport. Neurosci. Lett. 385, 58–63. [DOI] [PubMed] [Google Scholar]
- Horio M.; Kohno M.; Fujita Y.; Ishima T.; Inoue R.; Mori H.; Hashimoto K. (2011) Levels of d-serine in the brain and peripheral organs of serine racemase (Srr) knock-out mice. Neurochem. Int. 59, 853–859. [DOI] [PubMed] [Google Scholar]
- Baumgart F.; Rodriguez-Crespo I. (2008) d-Amino acids in the brain: the biochemistry of brain serine racemase. FEBS J. 275, 3538–3545. [DOI] [PubMed] [Google Scholar]
- Nong Y.; Huang Y. Q.; Ju W.; Kalia L. V.; Ahmadian G.; Wang Y. T.; Salter M. W. (2003) Glycine binding primes NMDA receptor internalization. Nature 422, 302–307. [DOI] [PubMed] [Google Scholar]
- Follesa P.; Ticku M. K. (1996) NMDA receptor upregulation: molecular studies in cultured mouse cortical neurons after chronic antagonist exposure. J. Neurosci. 16, 2172–2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy J. (2008) Alcohol related changes in regulation of NMDA receptor functions. Curr. Neuropharmacol. 6, 39–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P. M.; Aizawa H.; Kim P. S.; Huang A. S.; Wickramasinghe S. R.; Kashani A. H.; Barrow R. K.; Huganir R. L.; Ghosh A.; Snyder S. H. (2005) Serine racemase: Activation by glutamate neurotransmission via glutamate receptor interacting protein and mediation of neuronal migration. Proc. Natl. Acad. Sci. U.S.A. 102, 2105–2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puyal J.; Martineau M.; Mothet J. P.; Nicolas M. T.; Raymond J. (2006) Changes in d-serine levels and localization during postnatal development of the rat vestibular nuclei. J. Comp. Neurol. 497, 610–621. [DOI] [PubMed] [Google Scholar]
- Oliver M. W.; Larson J.; Lynch G. (1990) Activation of the glycine site associated with the NMDA receptor is required for induction of LTP in neonatal hippocampus. Int. J. Dev. Neurosci. 8, 417–424. [DOI] [PubMed] [Google Scholar]
- de Koning T. J.; Klomp L. W. J.; van Oppen A. C. C.; Beemer F. A.; Dorland L.; van den Berg I. E. T.; Berger R. (2004) Prenatal and early postnatal treatment in 3-phosphoglycerate-dehydrogenase deficiency. Lancet 364, 2221–2222. [DOI] [PubMed] [Google Scholar]
- Hagiwara H.; Iyo M.; Hashimoto K. (2013) Neonatal disruption of serine racemase causes schizophrenia-like behavioral abnormalities in adulthood: Clinical rescue by d-serine. PLoS One 8, e62438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miya K.; Inoue R.; Takata Y.; Abe M.; Natsume R.; Sakimura K.; Hongou K.; Miyawaki T.; Mori H. (2008) Serine racemase is predominantly localized in neurons in mouse brain. J. Comp. Neurol. 510, 641–654. [DOI] [PubMed] [Google Scholar]
- Wolosker H.; Radzishevsky I. (2013) The serine shuttle between glia and neurons: Implications for neurotransmission and neurodegeneration. Biochem. Soc. Trans. 41, 1546–1550. [DOI] [PubMed] [Google Scholar]
- Kartvelishvily E.; Shleper M.; Balan L.; Dumin E.; Wolosker H. (2006) Neuron-derived d-serine release provides a novel means to activate N-methyl-d-aspartate receptors. J. Biol. Chem. 281, 14151–14162. [DOI] [PubMed] [Google Scholar]
- Takayasu N.; Yoshikawa M.; Watanabe M.; Tsukamoto H.; Suzuki T.; Kobayashi H.; Noda S. (2008) The serine racemase mRNA is expressed in both neurons and glial cells of the rat retina. Arch. Histol. Cytol. 71, 123–129. [DOI] [PubMed] [Google Scholar]
- Benneyworth M. A.; Li Y.; Basu A. C.; Bolshakov V. Y.; Coyle J. T. (2012) Cell selective conditional null mutations of serine racemase demonstrate a predominate localization in cortical glutamatergic neurons. Cell Mol. Neurobiol. 32, 613–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schell M. J.; Molliver M. E.; Snyder S. H. (1995) d-serine, an endogenous synaptic modulator: localization to astrocytes and glutamate-stimulated release. Proc. Natl. Acad. Sci. U.S.A. 92, 3948–3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mothet J. P.; Pollegioni L.; Ouanounou G.; Martineau M.; Fossier P.; Baux G. (2005) Glutamate receptor activation triggers a calcium-dependent and SNARE protein-dependent release of the gliotransmitter d-serine. Proc. Natl. Acad. Sci. U.S.A. 102, 5606–5611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan S. J.; Miller R. F. (2010) AMPA receptor mediated d-serine release from retinal glial cells. J. Neurochem. 115, 1681–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi Y.; Konno R.; Sasabe J.; Ueno K.; Tojo Y.; Mita M.; Aiso S.; Hamase K. (2012) Alteration of intrinsic amounts of d-serine in the mice lacking serine racemase and d-amino acid oxidase. Amino Acids 43, 1919–1931. [DOI] [PubMed] [Google Scholar]
- Papouin T.; Ladepeche L.; Ruel J.; Sacchi S.; Labasque M.; Hanini M.; Groc L.; Pollegioni L.; Mothet J. P.; Oliet S. H. (2012) Synaptic and extrasynaptic NMDA receptors are gated by different endogenous coagonists. Cell 150, 633–646. [DOI] [PubMed] [Google Scholar]
- Rosenberg D.; Artoul S.; Segal A. C.; Kolodney G.; Radzishevsky I.; Dikopoltsev E.; Foltyn V. N.; Inoue R.; Mori H.; Billard J. M.; Wolosker H. (2013) Neuronal d-serine and glycine release via the Asc-1 transporter regulates NMDA receptor-dependent synaptic activity. J. Neurosci. 33, 3533–3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balu D. T.; Li Y.; Puhl M. D.; Benneyworth M. A.; Basu A. C.; Takagi S.; Bolshakov V. Y.; Coyle J. T. (2013) Multiple risk pathways for schizophrenia converge in serine racemase knockout mice, a mouse model of NMDA receptor hypofunction. Proc. Natl. Acad. Sci. U.S.A. 110, E2400–E2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y.; Imai K. (1983) Liquid chromatographic determination of amino and imino acids and thiols by postcolumn derivatization with 4-fluoro-7-nitrobenzo-2,1,3-oxadiazole. Anal. Chem. 55, 1786–1791. [DOI] [PubMed] [Google Scholar]