Abstract
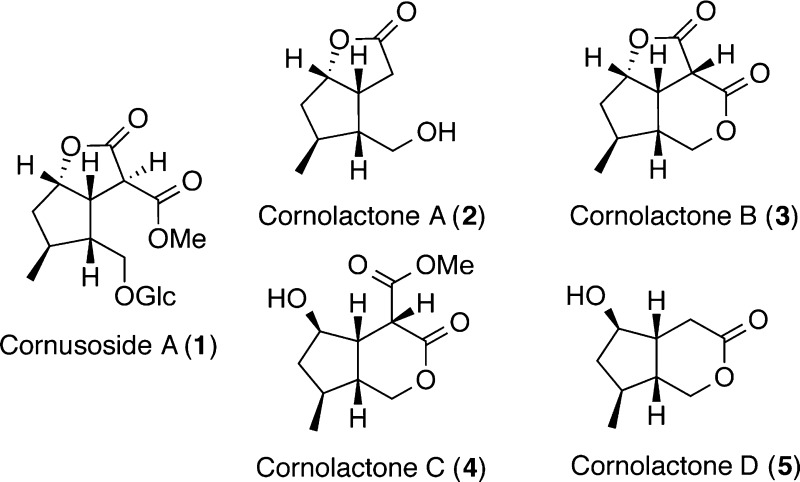
A new iridoid glucoside, cornusoside A (1), and four new natural product iridoid aglycones, cornolactones A–D (2–5), together with 10 known compounds were isolated from the leaves of Cornus florida. The structures of compounds 1–5 were established by interpretation of their spectroscopic data. Cornolactone B (3) is the first natural cis-fused tricyclic dilactone iridoid containing both a five- and a six-membered lactone ring. A biosynthesis pathway is proposed for cornolactones C (4) and D (5), the C-6 epimers of compounds 1–3.
The plant genus Cornus (dogwood) belongs to the family Cornaceae and consists of approximately 55 species distributed mainly in the northern hemisphere, eastern Asia, and eastern and northern parts of the United States.1 This genus is a rich source of diverse iridoid glucosides, which have raised interest because of their wide range of promising bioactivities. These include antidiabetic,2,3 antioxidant,4 anti-inflammatory,5 antiamnesic,6 and immunesuppressive effects.7
Cornus florida L., commonly known as flowering dogwood, is a tree native to eastern North America that has been traditionally used for the treatment of malaria.8,9 Previous chemical investigations of C. florida have resulted in the isolation of a number of compounds including anthocyanins and other flavonoids, triterpenoids, and sterols.9,10 The anthocyanins impart bright colors to several fruits and vegetables and possess anti-inflammatory,10−12 antioxidative,11,12 antineoplastic,10,13 and antidiabetic activities.14
In the present study the chemical constituents of C. florida collected from Oxford, Mississippi, were investigated. A large-scale extraction of the leaves of C. florida yielded a new iridoid glucoside, cornusoside A (1), and four new natural product iridoid aglycones, cornolactones A–D (2–5). Cornolactone A (2) was previously reported as a synthetic intermediate in the enantioselective synthesis of semperoside A;15 however, this is the first report of this compound from a natural source. In addition, 10 known compounds were also isolated, which included five iridoids, two megastigmane compounds, and two ellagic acid derivatives, together with a flavonoid. The structures of the new compounds were assigned by detailed spectroscopic analysis and those of the known compounds by comparison with literature data. Cornusoside A (1) is one of a small number of C10 iridoid glucosides with a ring-opening between C-1 and O-2 and a γ-lactone linkage between C-6 and C-11. Cornolactone B (3) is the first natural cis-fused tricyclic dilactone iridoid containing both a five- and a six-membered lactone ring. Herein are reported the isolation and structure elucidation of 1–5 and a possible biosynthetic pathway to cornolactones C (4) and D (5) as C-6 epimers of compounds 1–3.
A 90% aqueous ethanol extract of the dried leaves of C. florida (15 kg) was fractionated initially on silica gel (step gradient elution of hexane to EtOAc to MeOH). The 20% MeOH in EtOAc was then subjected to column chromatography on polymeric HP-20 (step gradient elution 10% Me2CO in H2O to 100% Me2CO). The 20% Me2CO fraction was then subjected to repeated fractionation on either polymeric HP-20ss, reversed-phase C18, normal-phase silica gel, or molecular exclusion Sephadex LH-20 column chromatography followed by a series of HPLC separations on either a PRP-1 column or a C8 or C18 column to yield cornusoside A (1), cornolactones A–D (2–5), and the known compounds alternosides A,16 hastatoside,17 cornin,17 dihydrocornin,18 cornalternoside,16 lauroside A,19 (5S*,6R*)-9-hydroxymegastigm-7-en-3-one,20 3,3′-dimethyl-4-O-β-d-glucopyranosylellagic acid,21 3,4,3′-trimethyl-4′-O-β-d-glucopyranosylellagic acid,22 and isoquercitrin.23
Cornusoside A (1) was isolated as a colorless gum. Its molecular formula, C17H26O10, determined from the HRESIMS of the [M + Na]+ at m/z 413.1417, required five degrees of unsaturation. A preliminary analysis of the 1H and 13C NMR data (Table 1) revealed two ester carbonyl groups (δC 172.8 and 169.0; IR 1730 cm–1), an oxygenated methine [δH 5.03 (1H, t, J = 6.8 Hz, H-6); δC 83.6 (C-6)], an oxymethylene [δH 3.98 (1H, dd, J = 10.4, 3.6 Hz, H-1a), δH 3.38 (1H, m, H-1b); δC 66.7 (C-1)], a methoxy [δH 3.68 (3H, s); δC 52.7], and signals that could be attributed to a glucopyranosyl group. The coupling constant of the anomeric proton (δH 4.05, 1H, d, J = 7.8 Hz) suggested a β-configuration of the glucose. The presence of the β-d-glucopyranosyl group was confirmed by acid hydrolysis. These data led to the preliminary conclusion that 1 is an iridoid glucoside and accounted for three of the five double-bond equivalents, indicating the compound to be bicyclic.
Table 1. NMR Spectroscopic Data for Cornusoside A (1) and Cornolactone A (2)a.
|
1b |
2c |
|||
|---|---|---|---|---|
| position | δC, mult. | δH (J in Hz) | δC, mult. | δH (J in Hz) |
| 1a | 66.7, CH2 | 3.98, dd (10.4, 3.6) | 61.4, CH2 | 3.87, dd (11.0, 4.0) |
| 1b | 3.38, m | 3.58, dd (11.0, 9.0) | ||
| 3 | 169.0, qC | |||
| 4α | 47.8, CH | 3.94, d (5.6) | 29.7, CH2 | 2.66, dd (18.8, 4.7) |
| 4β | 2.59, dd (18.8, 9.8) | |||
| 5 | 45.5, CH | 3.37, q (7.2) | 40.2, CH | 3.14, m |
| 6 | 83.6, CH | 5.03, t (6.8) | 84.9, CH | 5.00, t (6.0) |
| 7α | 40.6, CH2 | 1.99, dd (13.6, 6.0) | 41.9, CH2 | 2.20, dd (14.0, 5.0) |
| 7β | 1.47, ddd (13.6, 11.6, 6.0) | 1.44, ddd (14.0, 11.8, 5.5) | ||
| 8 | 32.0, CH | 1.90, m | 32.8, CH | 1.82, m |
| 9 | 48.0, CH | 1.83, m | 50.6, CH | 1.79, m |
| 10 | 17.3, CH3 | 0.95, d (5.6) | 17.6, CH3 | 1.02, d (5.6) |
| 11 | 172.8, qC | 178.3, qC | ||
| OMe | 52.7, CH3 | 3.68, s | ||
| 1′ | 103.0, CH | 4.05, d (7.8) | ||
| 2′ | 73.3, CH | 2.92, td (8.0, 4.4) | ||
| 3′ | 76.7, CH | 3.10, m | ||
| 4′ | 70.1, CH | 3.03, m | ||
| 5′ | 76.9, CH | 3.07, d (3.9) | ||
| 6a′ | 61.1, CH | 3.64, m | ||
| 6b′ | 3.43, m | |||
| OH-2′ | 4.72, d (4.3) | |||
| OH-3′ | 4.94, d (5.0) | |||
| OH-4′ | 4.93, d (5.5) | |||
| OH-6′ | 4.45, t (5.9) | |||
1H NMR measured at 400 MHz, 13C NMR measured at 100 MHz.
Measured in DMSO-d6.
Measured in CDCl3.
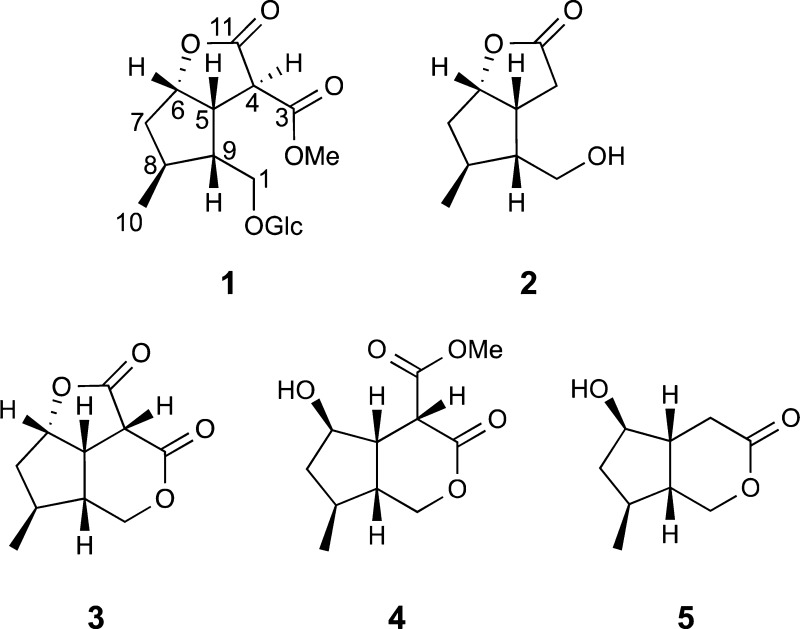
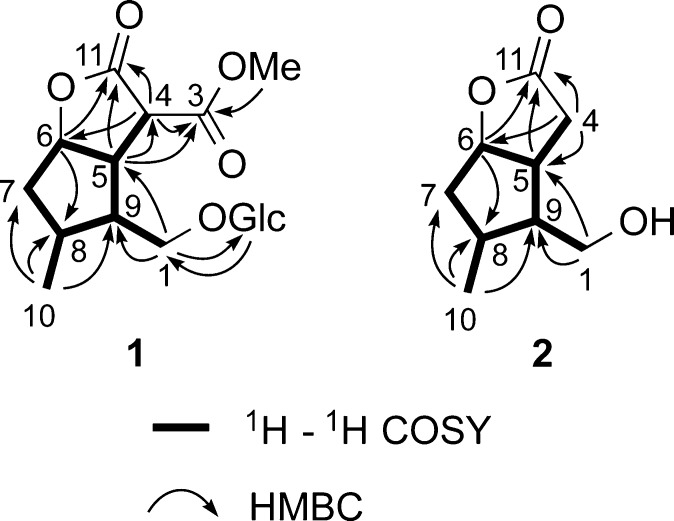
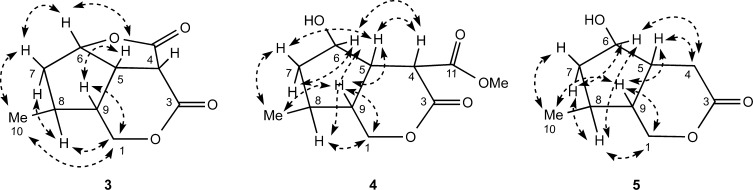
The terpenoid portion of 1 was determined by detailed analysis of its NMR data (Figure 1). The observation of 1H–1H COSY correlations from H-6 to H-5 and H-7, H-7 to H-8, H-8 to H-9 and the methyl doublet H3-10, and H-9 to H-5 established the presence of a cyclopentane ring with methyl substitution at C-8. An additional COSY correlation observed between H-9 and H-1a and H-1b of the oxygenated methylene group at C-1 together with an HMBC correlation from H-1′ to C-1 and from H-1 to C-1′ confirmed the connection of the β-d-glucopyranosyl group to C-1 and the connection of C-9 to C-1. Remaining to be assigned were two ester carbonyl carbons, C-3 (δC 169.0) and C-11 (δC 172.8), a methine, H-4 (δH 3.94; δC 47.8), and a methoxy group (δH 3.68; δC 52.7). HMBC correlations observed between H-6 (δH 5.03) and the ester carbonyl carbon at δC 172.8 (C-11) and from H-5 (δH 3.37) to both C-4 and C-11 established the presence of a γ-lactone ring. A COSY correlation observed between H-5 and H-4 and an HMBC correlation from H-4 to C-11 further supported this assignment. Finally, HMBC correlations observed from H-4, H-5, and the methoxy signal at δH 3.68 (OMe-3) to the remaining ester carbonyl carbon at δC 169.0 (C-3) established the connection of C-4 to C-3 and the presence of a methyl ester at C-3.
Figure 1.
Selected 2D NMR correlations for cornusoside A (1), and cornolactone A (2).
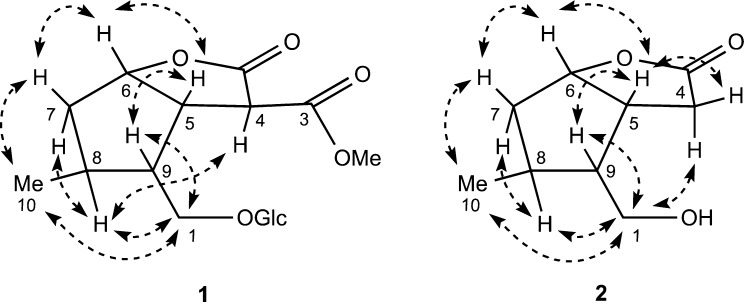
The relative configuration of 1 was determined by NOE correlations observed in a NOESY experiment and by scalar coupling (Figure 2). NOE correlations from H-5 to H-6 and H-9 together with correlations from H-7β to H-6 and H3-10 indicated that H-5, H-6, H-9, and Me-10 are on the same side of the cyclopentane ring in a β-orientation. NOE correlations from H-8 to H-7α and H-1 confirmed the α-orientation of H-8 and the glucosylated side chain. A long-range W-coupling in the COSY spectrum between Me-10 and H-7α was consistent with the 1,2-diaxial arrangement of these two groups. Finally, the α-orientation of H-4 was indicated by a NOE correlation observed between H-4 and H-8. This was substantiated by a small coupling (5.6 Hz) observed between H-4 and H-5 that was similar to the coupling constants (ca. 5.0 Hz in both cases) of the previously reported C-1 to O-2 ring-opened iridoids gelsemiol24 and borreriagenin.25 The absolute configuration of 1 was determined based on biogenetic grounds in that nearly all iridoids found in Nature have a configuration of 5S and 9R and by analogy to the known co-isolated compounds that were found to have closely comparable NMR data and similar optical rotation values. Thus, the configuration of cornusoside A (1) was defined as 4S,5S,6S,8S,9R. Additional support for the absolute stereochemistry came from the isolation of cornolactone A (2), previously reported as a synthetic intermediate in the total synthesis of the iridoid semperoside A.15 Since this is the first report of 2 from a natural source, the isolation, structure elucidation, and full spectroscopic data are reported.
Figure 2.
Selected NOE correlations observed for cornusoside A (1) and cornolactone A (2).
Cornolactone A (2), isolated as a colorless gum, showed a [M + H]+ ion at m/z 171.1010 in the HRESIMS corresponding to the molecular formula C9H14O3, requiring four degrees of unsaturation. An initial inspection of the NMR data revealed 2 to be similar to 1, except for the absence of the signals for the methine at C-4, the methyl ester at C-3, and the glucopyranosyl group and the appearance of signals for a diastereotopic methylene at δH 2.66 (1H, dd, J = 18.8, 4.7 Hz, H-4α) and δH 2.59 (1H, dd, J = 18.8, 9.8 Hz, H-4β). This suggested that 2 is an iridoid aglycone missing a C-3 methyl ester moiety. The observation of 1H–1H COSY correlations from both H2-4 to H-5 and HMBC correlations from H2-4 to C-5, C-6, and C-11 and from H-6 to C-11 further supported this assignment (Figure 1). The similarity of proton–proton coupling constants and 1H and 13C NMR chemical shifts together with the NOESY spectrum of 2 showed the same relative configuration as 1 at the four chiral centers (Figure 2). Additional NOE correlations from H-4α to H-1b of the oxygenated methylene and from H-4β to H-5 confirmed this assignment. Compound 2 was found to have near-identical NMR data and a comparable optical rotation value ([α]18D +3.2) (lit. [α]D +14.7) to that of the reported synthetic compound.15
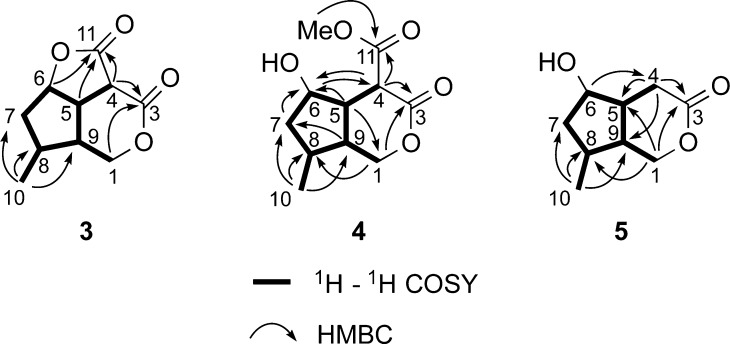
Cornolactone B (3) was isolated as a colorless gum. The molecular formula of cornolactone B (3), C10H12O4, as determined from the HRESIMS of the [M + H]+ ion at m/z 197.0811, required five degrees of unsaturation. Analysis of the NMR data (Figure 3) revealed 3 to be similar to 2 except for the absence of the signals for the methylene group at C-4 and the addition of signals for an ester carbonyl carbon at δC 164.7 (C-3) and a methine δH 3.78 (1H, d, J = 9.6 Hz, H-4). Having accounted for all protons, carbons, and oxygens in the molecule and four of the five double-bond equivalents, as required by the molecular formula, this indicated that cornolactone B (3) is tricyclic. The only possible connection was between the C-1 oxygen and the ester carbonyl carbon C-3 to form a δ-lactone unit. The connection was confirmed with HMBC correlations observed between both H2-1 (δH 4.47, 4.05) and H-4 (δH 3.80) to the ester carbonyl carbon at δC 170.2 (C-3). The relative configuration of 3 at C-5, C-6, C-8, and C-9 was determined to be identical to that of 1 and 2 by NOE correlations observed in a NOESY experiment and scalar coupling (Figure 4). The absence of any NOE correlations observed to or from H-4 made it difficult to assign the configuration at C-4. However, the presence of a large 1H NMR coupling constant (J = 9.6 Hz) between H-4 and H-5 suggested the cis-relationship of these two protons and the β-orientation of H-4. This coupling constant is consistent with that observed for the cis-fused tricyclic iridoids semperoside (J = 10.5 Hz), 9-hydroxysemperoside (J = 11.4 Hz), and dihydrobrasoside (J = 10.5 Hz),24 together with the dilactone compounds, asperuloside tetraacetate lactone (J = 9.8 Hz) and dihydroasperuloside tetraacetate lactone (J = 10.0 Hz), produced from the oxidation of the iridoid glucoside asperuloside.26 Thus, the configuration of cornolactone B (3) was defined as 4S,5S,6S,8S,9R.
Figure 3.
Selected 2D NMR correlations for cornolactones B–D (3–5).
Figure 4.
Selected NOE correlations observed for cornolactones B–D (3–5).
Cornolactone C (4) was isolated as a colorless gum. The molecular formula of this compound, C11H16O5, as determined from the HRESIMS of the [M + Na]+ ion at m/z 251.0884, required four degrees of unsaturation. The NMR data of 4 were similar to those of cornolactone B (3), except that the addition of a methoxy group (δH 3.80; δC 53.4) was shown and H-6 [δH 5.04, t (5.5)] was shifted upfield by 1.22 ppm (Table 2) as compared to that of 3. This suggested 4 is the product from methanolysis of the γ-lactone ring in 3. An HMBC correlation from the methoxy signal at δH 3.80 (OMe-11) to δC 169.2 (C-11) confirmed the presence of a methyl ester at C-11. Additional 1H–1H COSY and HMBC correlations (Figure 3) further supported this assignment. The relative configuration of 4 was determined by NOE correlations observed in a NOESY experiment and scalar coupling (Figure 4). NOE correlations from H-5 to H-4, H-7β, and H-9, together with correlations from H3-10 to H-7β and H-9, established the cis-fusion of the cyclopenta[c]pyran skeleton with H-4, H-5, H-7β, and H-9 in the β-orientation. Finally, the α-orientation of H-6 was indicated by an NOE correlation observed from H-6 to H-7α and H-8 on the underside of the cyclopentane ring. Thus, the configuration of cornolactone C (4) was defined as 4R,5S,6R,8S,9R.
Table 2. NMR Spectroscopic Data for Cornolactones B–D (3–5) in CDCl3a.
|
3 |
4 |
5 |
||||
|---|---|---|---|---|---|---|
| position | δC, mult. | δH (J in Hz) | δC, mult. | δH (J in Hz) | δC, mult. | δH (J in Hz) |
| 1a | 68.8, CH2 | 4.47, dd (12.2, 4.8) | 69.3, CH2 | 4.27, dd (12.0, 5.6) | 68.6, CH2 | 4.18, dd (11.6, 4.0) |
| 1b | 4.05, dd (12.2, 8.0) | 4.00, dd (11.6, 6.0) | 4.08, dd (12.0, 3.2) | |||
| 3 | 164.7, qC | 169.0, qC | 174.0, qC | |||
| 4 | 45.7, CH | 3.80, d (9.6) | 50.4, CH | 3.58, d (6.6) | 32.4, CH2 | 2.57, d (5.2) |
| 5 | 42.3, CH | 3.35, dt (9.5, 6.4) | 46.1, CH | 2.76, dt (11.6, 7.2) | 42.6, CH | 2.39, m |
| 6 | 85.1, CH | 5.04, t (5.5) | 77.4, CH | 3.82, ddd (10.2, 7.4, 6.0) | 77.8, CH | 3.71, ddd (10.2, 7.8, 5.5) |
| 7α | 41.2, CH2 | 2.36, dd (14.8, 6.8) | 43.2, CH2 | 2.12, td (11.7, 6.0) | 42.6, CH2 | 2.01, m |
| 7β | 1.68, ddd (14.8, 9.4, 5.6) | 1.38, dt (12.1, 10.2) | 1.27, dt (12.1, 11.2) | |||
| 8 | 34.2, CH | 2.04, m | 33.9, CH | 1.78, m | 33.1, CH | 1.79, m |
| 9 | 43.1, CH | 2.10, m | 42.8, CH | 2.20, ddd (11.7, 9.4, 6.3) | 43.2, CH | 1.97, m |
| 10 | 19.4, CH3 | 1.12, d (6.7) | 18.9, CH3 | 1.07, d (6.3) | 18.7, CH3 | 1.03, d (6.4) |
| 11 | 170.2, qC | 169.2, qC | ||||
| OMe | 53.4, CH3 | 3.80, s | ||||
1H NMR measured at 400 MHz, 13C NMR measured at 100 MHz.
Cornolactone D (5) was isolated as a colorless gum. The molecular formula, C9H14O3, was determined from the HRESIMS of the [M + H]+ ion at m/z 193.0834 (calcd 193.0835) and required three degrees of unsaturation. An initial inspection of the NMR data revealed that 5 is similar to 4, except for the absence of the signals for the methine at C-4 and the methyl ester at C-3 and the appearance of a methylene group at δH 2.57 (2H, d, J = 5.2 Hz, H-4). This suggested that 5 did not have a C-11 methyl ester unit. The observation of 1H–1H COSY correlations from H2-4 to H-5 and HMBC correlations from H2-4 to C-3, C-5, and C-9, together with a correlation from H-6 to C-4, confirmed this assignment (Figure 3). The similarity of proton–proton coupling constants and 1H and 13C NMR chemical shifts together with a NOESY spectrum of 5 showed the same relative configuration as 4 at the four chiral centers (Figure 4). Thus, the configuration of cornolactone D (5) was defined as 5R,6R,8S,9R.
Compounds 1–5 and cornin were evaluated for cell growth inhibitory activities against human embryonic stem cells (BG02) and human breast cancer cell lines (MCF-7 and MDA-MB-231). No cytotoxicity was observed for any of the compounds at 100 μM except for slight cytotoxicty being observed for compound 5 against the MDA-MB-231 cell line. The compounds were also examined for agonistic activity against peroxisome proliferator-activated receptor γ (PPARγ), but no activity was observed.
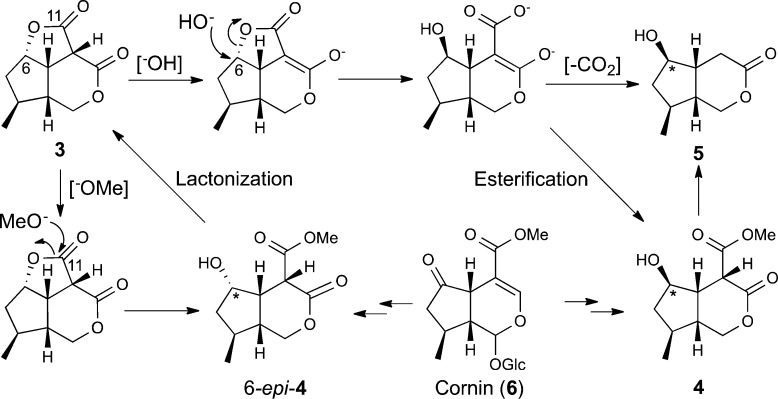
Cornusoside A (1) is one of a small number of C10 iridoid glucosides where the δ-lactone is ring-opened between the C-1 and O-2 positions and contains a γ-lactone linkage between C-6 and C-11. Other examples reported include gelsemiol 3-O-β-d-glucoside,24 gelsemiol 6′-trans-caffeoyl-1-glucoside,27 and verbenabrasides A and B.28 Cornolactone B (3) is the first natural cis-fused tricyclic dilactone iridoid containing both a five- and a six-membered lactone ring. Interestingly, cornolactones C (4) and D (5) have an opposite configuration at C-6 compared to that of compounds 1–3. This suggests that rather than cleavage of the γ-lactone in 3 by methanolysis at C-11, to give the C-6 epimer of 4 (6-epi-4) with retention of configuration, an alternative biosynthetic pathway is necessary (Figure 5). A plausible pathway leading to inversion of configuration at C-6 could occur through the SN2 hydrolysis of 3 at C-6, followed by esterification to give cornolactone C (4) or decarboxylation to give cornolactone D (5). Alternatively, cornolactones C (4) and D (5) could also have been formed from the reduction followed by oxidation of the co-isolated iridoid cornin (6). Previously it has been shown that reduction of 6 with NaBH4 gives approximately a 1:1 mixture of both epimers at C-6.18 It is also conceivable that 6-epi-4 could undergo a lactonization reaction to give cornolactone B (3) and could provide an explanation of why no 6-epi-4 was isolated.
Figure 5.
Plausible biosynthetic route to 3 and potential pathways to inversion of configuration at C-6 in 4 and 5.
Experimental Section
General Experimental Procedures
Optical rotations were measured on a JASCO P-2000 polarimeter (c g/100 mL) equipped with a halogen lamp (589 nm) and a 1 mL microcell. IR spectra were recorded on a Thermo Electronic Corporation Nicolet IR-100 spectrophotometer. All NMR spectra were acquired with a Varian MercuryPlus 400 spectrometer using solvent signals (DMSO-d6: 1H, δH 2.50; 13C, δC 39.52; CDCl3: 1H, δH 7.24 ppm; 13C, δC 77.23 ppm) as references. Short- and long-range 1H–13C correlations were determined with gradient-enhanced inverse-detected HSQC and HMBC experiments, respectively. NOE correlations were detected with NOESY experiments with a 0.5 s mixing time. The HRESIMS were obtained using an Agilent 6220 series TOF mass spectrometer. HPLC was performed on a Shimadzu LC-20AT instrument with a Shimadzu SPD-M20A UV/vis photodiode detector and a Shimadzu ELSD-LTII detector.
Plant Material
The leaves of Cornus florida L. were collected from a one-mile radius around Timber Lake, Oxford, Mississippi, during the spring and summer of 2011 by M.T.H. Voucher specimens are kept in the Hamann Laboratory at the University of Mississippi, School of Pharmacy, Oxford, MS (Cf2011).
Extraction and Purification Procedures
The leaves of C. florida (15.0 kg, dry weight) were extracted with 90% aqueous ethanol and dried under reduced pressure to give a crude extract (900 g). A portion of this crude extract (400 g) was separated on a silica gel column (20 × 70 cm) using a stepwise gradient of hexanes/EtOAc (100:0, 80:20, 50:50, and 0:100, v/v, each 3 L) and EtOAc/MeOH mixtures (80:20, 60:40, 50:50, and 0:100, v/v, each 3 L) to afford eight fractions. Fraction E (220 g) was then chromatographed on HP-20 (8 × 50 cm) using a stepwise gradient of acetone/water (10:90, 20:80, 50:50, 60:40, 80:20, and 100:0, v/v, each 2 L) to give six subfractions (Fr. E1–E6). Fraction E2 (40 g) was chromatographed on a preparative C18 reversed-phase MPLC column (20 × 250 mm; 20–35% CH3OH/H2O over 40 min, 35–65% CH3OH/H2O over 20 min; flow rate: 12 mL/min) to afford eight subfractions (Fr. E2a–E2h). Fraction E2d (4.0 g) was chromatographed on a preparative C18 reversed-phase HPLC column (Shim-pak RP-C18 column; 5 μm; 20 × 250 mm; 8–45% CH3CN/H2O over 120 min, 7 mL/min) to yield eight subfractions (Fr. E2d-1–E2d-8). Fraction E2d-4 (50 mg) was purified by C8 reversed-phase HPLC (Polar-C8; 5 μm; 10 × 250 mm; 15–45% CH3OH/H2O over 90 min, 5 mL/min) to yield cornusoside A (1, 5.0 mg, tR 76.2 min). Fraction E2d-6 (832 mg) was subjected to silica gel column chromatography using CH2Cl2/MeOH (90:10 to 0:100, v/v) to afford nine fractions (Fr. E2d-6-a–Fr. E2d-h). Fraction E2d-6-a (320 mg) was further purified by C8 reversed-phase HPLC (Polar-C8; 5 μm; 10 × 250 mm; 5–25% CH3CN/H2O over 70 min, 5 mL/min) to yield two fractions. The first fraction (60 mg) was purified on a polymeric HPLC column (Hamilton PRP-1; 5 μm; 20 × 250 mm; 10–40% CH3CN/H2O over 50 min, 7 mL/min) to yield cornolactone C (4, 8.0 mg, tR 41.5 min). The second fraction (40 mg) was chromatographed on a polymeric HPLC column (Hamilton PRP-1; 5 μm; 20 × 250 mm; 15–45% CH3CN/H2O over 70 min, 7 mL/min) to yield cornolactone B (3, 4.0 mg, tR 37.9 min). Cornolactones A (2, 15.0 mg) and D (5, 12 mg) were purified from the remaining crude extract (500 g) using a similar procedure (see Supporting Information).
Cornusoside A (1):
colorless gum; [α]18D −13.8 (c 0.01, MeOH); UV (MeOH) λmax (log ε) 230 (3.4) nm; IR (KBr) νmax 3355, 2922, 1730, 1073, 1020 cm–1; 1H and 13C NMR (400 MHz, d6-DMSO), see Table 1; HRESIMS m/z 413.1417 [M + Na]+ (calcd for C17H26O10Na, 413.1418).
Cornolactone A (2):
colorless gum; [α]18D +3.3 (c 0.2, MeOH); UV (MeOH) λmax (log ε) 237 (3.2) nm; IR (KBr) νmax 3355, 2922, 1730, 1073, 1020 cm–1; 1H and 13C NMR (400 MHz, CDCl3), see Table 1; HRESIMS m/z 171.1010 [M + H]+ (calcd for C9H15O3, 171.1016).
Cornolactone B (3):
colorless gum; [α]18D −7.5 (c 0.02, MeOH); UV (MeOH) λmax nm 243 (3.1) nm; IR (KBr) νmax 2944, 1658, 1023 cm–1; 1H and 13C NMR (400 MHz, CDCl3), see Table 2; HRESIMS m/z 197.0811 [M + H]+ (calcd for C10H13O4, 197.0808).
Cornolactone C (4):
colorless gum; [α]18D +13.3 (c 0.03, MeOH); UV (MeOH) λmax (log ε) 241 (3.1) nm; IR (KBr) νmax 3337, 2952, 2937, 1733, 1650, 1017 cm–1; 1H and 13C NMR (400 MHz, CDCl3), see Table 2; HRESIMS m/z 215.0884 [M + Na]+ (calcd for C11H16O5Na, 215.0890).
Cornolactone D (5):
colorless gum; [α]18D +18.5 (c 0.02, MeOH); UV (MeOH) λmax (log ε) 244 (2.8) nm; IR (KBr) νmax 3401, 2952, 1733, 1089, 1068 cm–1; 1H and 13C NMR (400 MHz, CDCl3), see Table 2; HRESIMS m/z 193.0834 [M + Na]+ (calcd for C9H14O3Na, 193.0835).
Acid Hydrolysis and Sugar Analysis
Cornusoside A (1) (2 mg) was hydrolyzed by using 1 M HCl (0.4 mL) at 100 °C for 2 h under argon and neutralized with Amberlite IR400. After drying under reduced pressure, the residue was dissolved in pyridine (0.4 mL) containing l-cysteine ethyl ester hydrochloride (2 mg) and heated at 60 °C for 1 h. A 0.4 mL solution of 3,5-dichlorophenyl isothiocyanate (2 mg) in pyridine was added to the mixture, which was heated at 60 °C for 1 h. The reaction mixture was directly analyzed by analytical HPLC on a Shim-pak RP-C18 column, 5 μm, 4.6 × 250 mm, by eluting with a gradient of 30–80% CH3CN in H2O with 0.02% HCOOH for 40 min and subsequent washing of the column with 100% CH3CN at a flow rate 0.8 mL/min. In the acid hydrolysate of 1, d-glucose was confirmed by comparison of the retention times of their derivatives with those of l-glucose and d-glucose derivatives prepared in the same way, which showed retention times of 34.8 and 34.0 min, respectively.
Acknowledgments
The authors thank Mr. S. Abbas (Ole Miss) for his assistance with plant collections, together with Kraft Foods and the National Institutes of Health (1R01AT007318) for some financial support. We also thank Dr. P. Griffin at the Scripps Research Institute Florida for performing the PPARγ agonistic activity evaluation, and Drs. A. Robbins and T. Schulz at Viacyte Inc. for cytotoxicity testing. This research was supported by National Institutes of Health grants (P41GM079597 and P01GM085354) and by the Scientific Research Program Funded by Shaanxi Provincial Education Department in China (2010JK749). This research was also supported in part by Xi'an University of Technology Starting grant (108-211409), awarded to Dr. Y. He, and the Youth Scientists Innovation Team Program.
Supporting Information Available
Full isolation procedures and 1D and 2D NMR spectroscopic data of 1–5 are available including 1H, 13C, COSY, HSQC, HMBC, and NOESY. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Fan C.; Xiong Q. Y. Am. J. Bot. 2001, 88, 1131–1138. [PubMed] [Google Scholar]
- Yamabe N.; Kang K. S.; Matsuo Y.; Tanaka T.; Yokozawa T. Biol. Pharm. Bull. 2007, 30, 1289–1296. [DOI] [PubMed] [Google Scholar]
- Yokozawa T.; Yamabe N.; Kim H. Y.; Kang K. S.; Hur J. M.; Park C. H.; Tanaka T. Biol. Pharm. Bull. 2008, 31, 1422–1428. [DOI] [PubMed] [Google Scholar]
- Wang W.; Sun F. L.; An Y.; Ai H. X.; Zhang L.; Huang W. T.; Li L. Eur. J. Pharmacol. 2009, 613, 19–23. [DOI] [PubMed] [Google Scholar]
- Diaz A. M.; Abad M. J.; Fernandez L.; Recuero C.; Villaescusa L.; Silvan A. M.; Bermejo P. Biol. Pharm. Bull. 2000, 23, 1307–1313. [DOI] [PubMed] [Google Scholar]
- Lee K. Y.; Sung S. H.; Kim S. H.; Jang Y. P.; Oh T. W.; Kim Y. C. Arch. Pharm. Res. 2009, 32, 677–683. [DOI] [PubMed] [Google Scholar]
- Meng J.; Chen L.; Tang S. B.; Chen J. S.; Lin S. F.; Zhao S. B. Chin. J. Pathophysiol. 2005, 21, 927–930. [Google Scholar]
- Hasegawa G. R. Mil. Medicine 2007, 172, 650–655. [DOI] [PubMed] [Google Scholar]
- Graziose R.; Rojas-Silva P.; Rathinasabapathy T.; Dekock C.; Grace M. H.; Poulev A.; Lila M. A.; Smith P.; Raskin I. J. Ethnopharmocol 2012, 142, 456–461. [DOI] [PubMed] [Google Scholar]
- Vareed S. K.; Reddy M. K.; Schutzki R. E.; Nair M. G. Life Sci. 2006, 78, 777–784. [DOI] [PubMed] [Google Scholar]
- Seeram N. P.; Schutzki R.; Chandra A.; Nair M. G. J. Agric. Food Chem. 2002, 50, 2519–2523. [DOI] [PubMed] [Google Scholar]
- Wang H.; Nair M. G.; Strasburg B. M.; Chang Y. C.; Booren A. M. J. Nat. Prod. 1999, 62, 294–296. [DOI] [PubMed] [Google Scholar]
- Kamei H.; Kojima T.; Hasegama M.; Koide T.; Umeda T.; Yukawa T.; Terabe K. Cancer Invest. 1995, 13, 590–594. [DOI] [PubMed] [Google Scholar]
- Jayaprakasam B.; Vareed S. K.; Olson L. K.; Nair M. G. J. Agric. Food Chem. 2005, 53, 28–31. [DOI] [PubMed] [Google Scholar]
- Piccinini P.; Vidari G.; Zononi G. J. Am. Chem. Soc. 2004, 126, 5088–5089. [DOI] [PubMed] [Google Scholar]
- He Y. Q.; Ma G. Y.; Peng J. N.; Ma Z. Y.; Hamann M. T. Biochem. Biophys. Acta 2012, 1820, 1021–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzyk H.; Jensen R. S.; Stermitz F. R. Phytochemistry 1998, 49, 2025–2030. [DOI] [PubMed] [Google Scholar]
- Jensen S. R.; Kjær A.; Nielsen B. J. Acta Chem. Scand. 1973, 27, 2581–2585. [DOI] [PubMed] [Google Scholar]
- Marino S. D.; Borbone N.; Zollo F.; Ianaro A.; Meglio P. D.; Iorizzi M. J. Agric. Food Chem. 2004, 52, 7525–7531. [DOI] [PubMed] [Google Scholar]
- Sefton M. A.; Francis L.; Williams P. J. J. Agric. Food Chem. 1990, 38, 2045–2049. [Google Scholar]
- Nawwar M. A.; Buddrus J.; Bauer H. Phytochemistry 1982, 21, 1755–1758. [Google Scholar]
- Hillis W. E.; Yazaki Y. Phytochemistry 1973, 12, 2969–2977. [Google Scholar]
- Xie X. Y.; Wang R.; Shi Y. P. Biochem. Syst. Ecol. 2012, 45, 120–123. [Google Scholar]
- Jensen S. S.; Kirk O.; Nielsen B. J.; Norrestam R. Phytochemistry 1987, 26, 1725–1731. [Google Scholar]
- Vieira I. J.; Mathias L.; Braz-Filho R.; Schripsema J. Org. Lett. 1999, 1, 1169–1171. [Google Scholar]
- Berkowitz W. F.; Choudhry S. C.; Hrabie J. A. J. Org. Chem. 1982, 47, 824–829. [Google Scholar]
- Li Y.; Ishibashi M.; Satake M.; Oshima Y.; Ohizumi Y. Chem. Pharm. Bull. 2003, 51, 1103–1105. [DOI] [PubMed] [Google Scholar]
- Ono M.; Oishi K.; Abe H.; Masuoka C.; Okawa M.; Ikeda T.; Nohara T. Chem. Pharm. Bull. 2006, 54, 1421–1424. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.