Abstract
Certain nematodes are common soilborne organisms found in turfgrass in the United States that cause significant economic damage to golf course turf. One of the most prevalent plant-parasitic nematodes infesting turfgrass are root-knot nematodes (Meloidogyne spp.). Chemical treatment options for root-knot nematodes in turfgrass are limited, and there is a need for new nematicidal active ingredients to address this problem. In this study, we evaluated the use of silver nanoparticles (AgNP) as a potential nematicide in laboratory and field experiments. AgNP was synthesized by a redox reaction of silver nitrate with sodium borohydride using 0.2% starch as a stabilizer. When J2 of M. incognita were exposed to AgNP in water at 30 to 150 μg/ml, >99% nematodes became inactive in 6 hr. When turfgrass and soil composite samples infested with M. graminis were treated with 150 μg/ml AgNP, J2 were reduced in the soil samples by 92% and 82% after 4- and 2-d exposures, respectively, in the treated compared to the nontreated soil samples. Field trials evaluating AgNP were conducted on a bermudagrass (Cynodon dactylon × C. transvaalensis) putting green infested with M. graminis. Biweekly application of 90.4 mg/m2 of AgNP improved turfgrass quality in one year and reduced gall formation in the roots in two years without phytotoxicity. The AgNP application did not significantly reduce the number of M. graminis J2 in plots during the growing season. The laboratory assays attested to the nematicidal effect of AgNP, and the field evaluation demonstrated its benefits for mitigating damage caused by root-knot nematode in bermudagrass.
Keywords: bermudagrass, management, Meloidogyne, nematicide, root-knot nematode, silver nanoparticle, turfgrass
Nematodes are common soilborne organisms found in turfgrass in the United States. Plant-parasitic nematodes are known to cause significant damage to turfgrass (Crow, 2007). Subtropical turfgrasses, particularly ultradwarf bermudagrass (Cynodon dactylon × C. transvaalensis) used in golf courses, where the turfgrass is intensively managed and grown in sand-based soil, are most vulnerable to nematode damage. Turfgrass damage caused by nematodes can be economically important to golf courses because the damage can cause loss of aesthetic quality and playability on fairways and putting greens where most play activities occur (Crow, 2005).
The current major issue concerning nematode damage to turfgrass is the lack of effective chemical treatment methods for established golf course turfgrass in the United States. The most widely used turfgrass nematicide, Nemacur (fenamiphos, Bayer Environmental Science, Research Triangle Park, NC), was cancelled in 2008 because of environmental concerns. Since then, effective alternatives have been limited. Biological control agents have disadvantages of possessing a narrow range of treatment effects and a lack of reliability under varying environmental conditions. For example, a Pasteuria sp. that is a bacterial parasite of sting nematodes (Belonolaimus longicaudatus) (Luc et al., 2010) was commercially developed as biological nematicide. However, the commercial formulation of Pasteuria sp. was ineffective for sting nematode management on golf course turf (Crow et al., 2011) and does not work for the other species of plant-parasitic nematodes such as root-knot nematodes (Meloidogyne spp.).
There are few synthetic chemicals, other than botanical derivatives, that are labeled for nematicides on turfgrass in the U.S. market. 1,3-Dicholoropropene (Curfew Soil Fumigant, Dow AgroSciences, Indianapolis, IN) was documented to be effective in controlling nematodes in turfgrass (Crow et al., 2003; Starr et al., 2007). It is a soil fumigant injected into the soil under established turf in a liquid form which converts to a gas to kill nematodes. Because of its unconventional method of application and high risk to chemical applicators and handlers, only certified applicators administer the product.
The lack of options for managing nematodes poses a serious problem in turfgrass management. It is of the utmost importance that new nematicidal active ingredients be sought to combat this challenge to the turfgrass industry. Silver nanoparticles (AgNP) are a prominent example of nano-sized materials being applied as a means of controlling human pathogenic microbes. As active ingredients, AgNP possesses bactericidal and fungicidal effects and has been frequently used in the field of medicine (Wright et al., 1999; Yin et al., 1999; Furno et al., 2004; Kim et al., 2007). AgNP has also shown evidence of being a potentially effective nematicide (Roh et al., 2009), and its toxicity is associated with induction of oxidative stress in the cells of targeted nematodes (Lim et al., 2012).
The goal of this study was to investigate the potential of AgNP as a nematicide for turfgrass. In this study, AgNP was evaluated against root-knot nematodes (Meloidogyne spp.) via laboratory assays and field trials.
Materials and Methods
Silver nanoparticle preparation:
AgNP used in this study was chemically produced (Fan et al., 2009). For synthesizing 1.015 L of AgNP, 15 ml of 0.1 M AgNO3 were added to 970 ml of 0.2% starch solution, and then 30 ml of 0.1 M NaBH4 was added into the reaction solution. After stirring for 30 min, 150 μg/ml of dark brown AgNP solution resulted.
Direct exposure of M. incognita to silver nanoparticles in water:
J2 of M. incognita isolated from tomato plants (Solanum lycopersicum) were used for this direct exposure assay to AgNP, because of their availability and the difficulty in collecting J2 of root-knot nematodes from turfgrass. Approximately 20 nematodes were added to 10 ml of solutions containing 0, 1.5, 3, 15, 30, or 150 μg/ml of AgNP, with three replications of each solution. They were arranged in a completely randomized design and incubated at room temperature (∼25°C). Nematode health or activity was measured with an inverted compound microscope (Leeds Instruments, Inc., Minneapolis, MN) at 1, 3, or 6 hr after the AgNP treatments to determine the effective dose required for affecting nematodes. Healthy nematodes were defined as those that were curled whereas unhealthy nematodes were defined as those that appeared stiff- or straight-bodied.
After 6 hr of the AgNP treatment, the nematodes were filtered with a sieve (pore size = 25 μm; Newark Wire Cloth Co., Clifton, NJ) and resuspended in tap water. Healthy nematodes were counted after 1-hr incubation at room temperature to determine if AgNP killed or intermittently inactivated the movement of nematodes.
Soil treatment with silver nanoparticle:
Composites of turfgrass and soil were collected from a bermudagrass cv. TifEagle putting green in the Texas A&M University Turfgrass Center, which possessed a high infestation of M. graminis, identified by their mitochondrial DNA sequences (McClure et al., 2012). The water saturation level of the 100-cm3 soil sample was predetermined to be 25 ml. The total soil was homogenized, divided into 100 cm3, placed into plastic containers, and then saturated with 25-ml AgNP solution at 0, 1.5, 3, 15, 30, or 150 μg/ml. The samples were arranged in a completely randomized design with four replicates (soil samples) and incubated at room temperature for 1, 2, 4, or 8 d. After the designated exposure time, nematodes were extracted from the samples using Baermann tray system. After 48-hr submergence in water, the samples were then poured into a sieve (pore size = 25 μm). Filtered J2 root-knot nematodes were counted using an inverted compound microscope.
Field evaluation:
Field trials with AgNP were carried out on a bermudagrass cv. TifEagle putting green at the Texas A&M University Turfgrass Center that was maintained by conventional management practices except forgoing pesticide use in 2012 and 2013. Plots were established on one putting green where different locations were used in 2012 and 2013. Plots (1.66 m2) were arranged as a completely randomized block design with four replicates. Two treatments tested in the field trial were 90.4 mg/m2 of AgNP [0.5 liter of 150 μg/ml AgNP per plot mixed with a surfactant (0.1% v/v), Duplex, Precision Laboratories, Waukegan, IL] applied biweekly; and 78.5 kg/ha of Nortica 5 WP (Bacillus firmus, a biological nematicide labeled for turfgrass, Bayer Environmental Science, Research Triangle Park, NC) applied monthly. Nontreated control plots were included. Treatments were applied at 275.79 kPa using a carbon dioxide boom sprayer equipped with two TeeJet 8002 nozzles (Springfield, IL). The spray solution was delivered at 81.5 ml/m2. Immediately after application, 6 to 12 mm of water was applied to all treated plots until the soil was saturated for delivery of active ingredients into the soil. The treatments were initiated in April and continued until November each year.
Composite samples of turfgrass roots and soil were collected monthly from each plot with a soil probe (2.5 cm in diam. and 6-cm deep) for examining nematode populations. Root-knot nematode J2 were isolated from 100 cm3 of the soil samples with the aforementioned Baermann tray method. Turf quality was visually measured monthly at a 1 to 9 scale with a rating of 6 = acceptable and 9 = best possible.
In addition, turfgrass roots were separated from the soil samples, washed with tap water, and examined for presence of galls (symptoms of root-knot nematodes) with a dissecting microscope. Five 3.5-cm root segments were randomly selected from each sample representative of a plot, and the number of root galls per sample was recorded.
Statistical analyses:
Both types of laboratory experiments were conducted twice. Homogeneity of variance between two datasets from experimental replicates was tested with Bartlett's test (Snedecor and Cochran, 1989). Upon no significant difference between two experiments (P > 0.05), the two datasets were combined for data presentation and further analysis.
In the direct exposure assay and the soil treatment with AgNP, its concentration and exposure time were the predetermined source of two-way analysis of variance (ANOVA) for the activity or mortality of J2. The analysis was carried out using the GLM procedure with SAS 9.1 statistical software (SAS Institute, Cary, NC). In the field trial, the number of J2, turf quality, and the number of root galls collected from the plots were subjected to ANOVA to determine any treatment effects during the growing season. When an ANOVA indicated a significant treatment effect, pair-wise comparisons between treatment means were conducted using Fisher’s least significant difference (LSD) test at P ≤ 0.05.
Results
Direct exposure of M. incognita to silver nanoparticles in water:
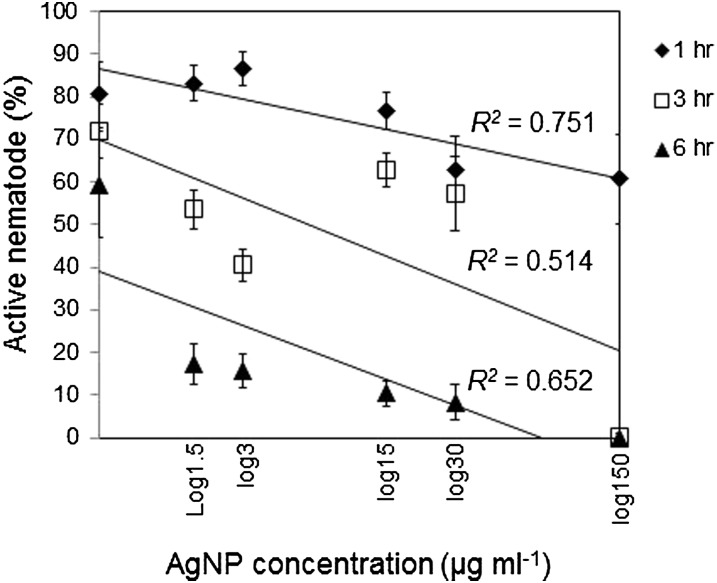
AgNP effectively and consistently decreased the activity of M. incognita in the repeated experiments (Bartlett’s test for homogeneity, P = 0.931). As exposure time and concentration of AgNP increased, the percentage of healthy J2 decreased (Fig. 1; ANOVA: source = exposure time, P ≤ 0.0001; source = AgNP concentration, P ≤ 0.0001). An exposure time of longer than 1 hr to AgNP was needed to inactivate more than 50% of nematodes. All nematodes treated with 150 μg/ml AgNP were unhealthy after 3 hr. After 6-hr exposure to AgNP at 30 or 150 μg/ml, > 99% nematodes were unhealthy. At the 6-hr exposure time, 15 or 30 μg/ml AgNP inactivated more than 50% nematodes, but neither concentration affected nematode activity at 3-hr exposure. After 1 hr of resuspension in water following a 6-hr AgNP treatment, the proportion of healthy and unhealthy nematodes was similar to that before resuspension for all AgNP concentrations (P > 0.05).
Fig. 1.
Effects of concentration and time of exposure to silver nanoparticles (AgNP) on activity of Meloidogyne incognita J2.
Soil treatment with silver nanoparticles:
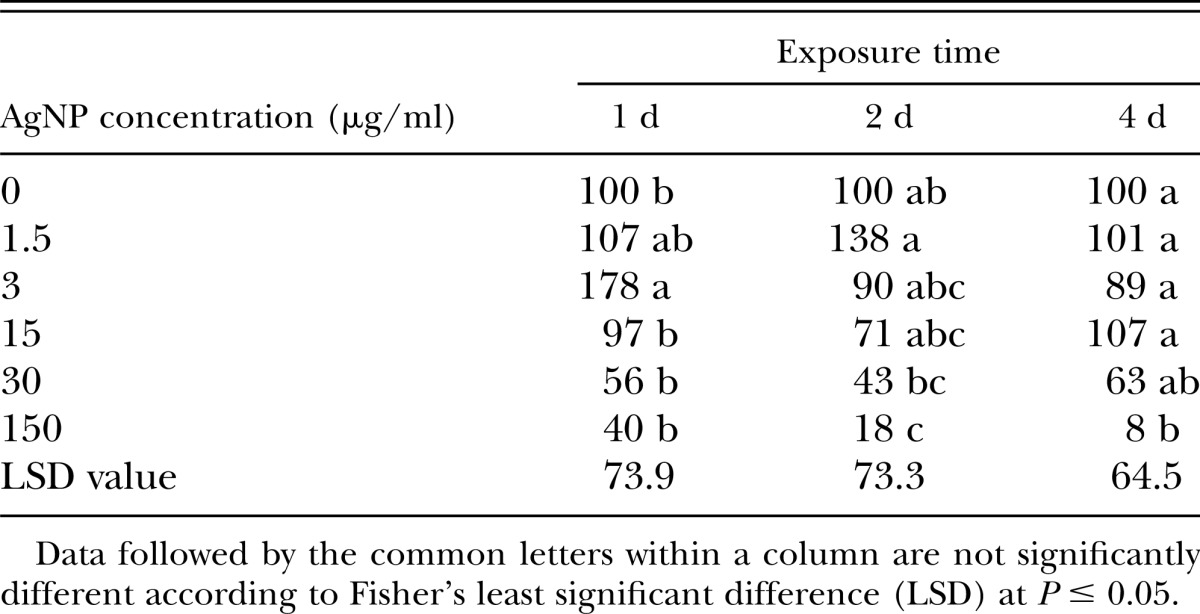
Numbers of M. graminis J2 extracted from soil samples were reduced when the soil was treated with AgNP (ANOVA: source = AgNP concentration, P = 0.0005) in repeated laboratory experiments (Bartlett’s test for homogeneity, P = 0.955). Exposure time between 1 and 4 hr did not affect the efficacy of AgNP (ANOVA: source = experiment, P > 0.05). There was an interaction between exposure time and AgNP concentration (P ≤ 0.0001). AgNP reduced the number of J2 recovered after 2- or 4-d exposure at 150 μg/ml compared to the nontreated control (Table 1). Lower concentrations (30, 15, 3, or 1.5 μg/ml) of AgNP did not reduce the number of J2 extracted from the soil when exposed up to 4 d (Table 1).
Table 1.
Relative number (%) of Meloidogyne incognita second-stage juveniles isolated from soil samples after the treatment with silver nanoparticle (AgNP) compared to the nontreated control.
Field evaluation:
AgNP reduced gall formation caused by M. graminis in turfgrass roots, compared to the nontreated or Nortica-treated plots in the later season, August and October in 2012 and December in 2013 (Table 2). However, the treatment of AgNP or Nortica resulted in no significant change in the number of M. graminis J2 (P = 0.10 in 2012 and 0.17 in 2013) (Table 3). The treatment significantly improved the turf quality in 2012 (P = 0.005) but not in 2013 (P = 0.68) (Table 3).
Table 2.
Effects of silver nanoparticles and a commercial preparation of Bacillus firmus (Nortica) on the number of galls in a bermudagrass putting green.
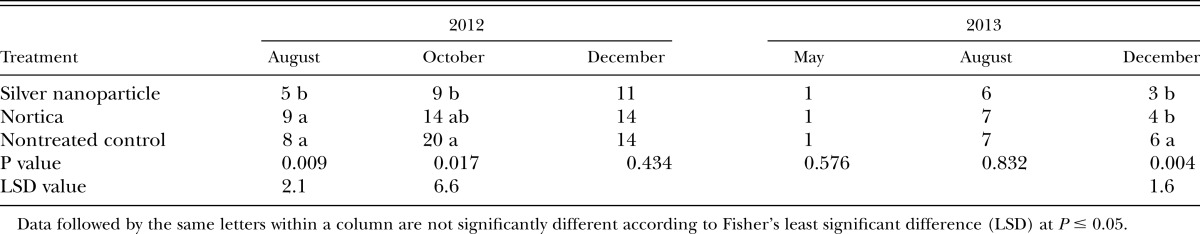
Table 3.
Analysis of variance (ANOVA) of the effect of treatment with silver nanoparticles or a commercial preparation of Bacillus firmus (Nortica) and the month effect on the turf quality and the number of Meloidogyne graminis J2 in a bermudagrass putting green.
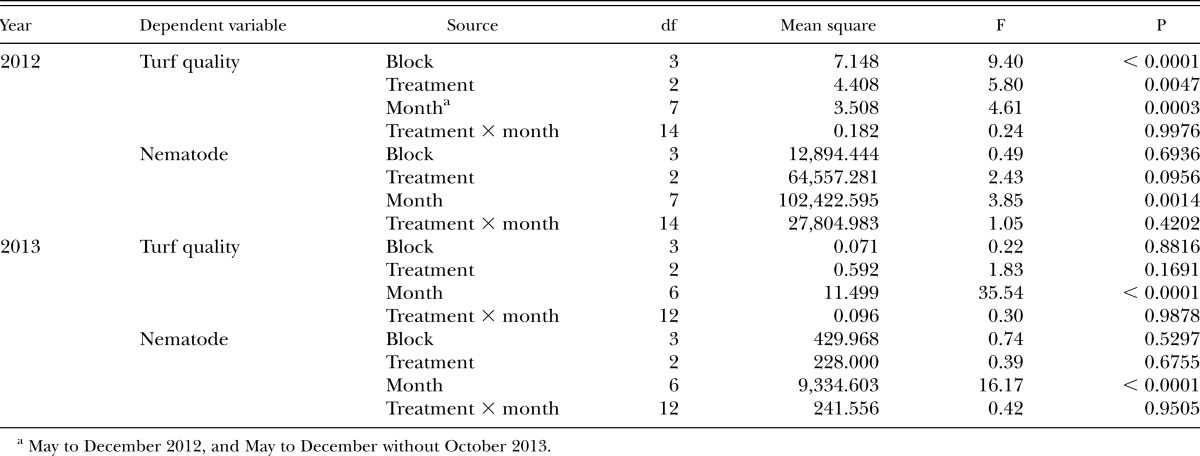
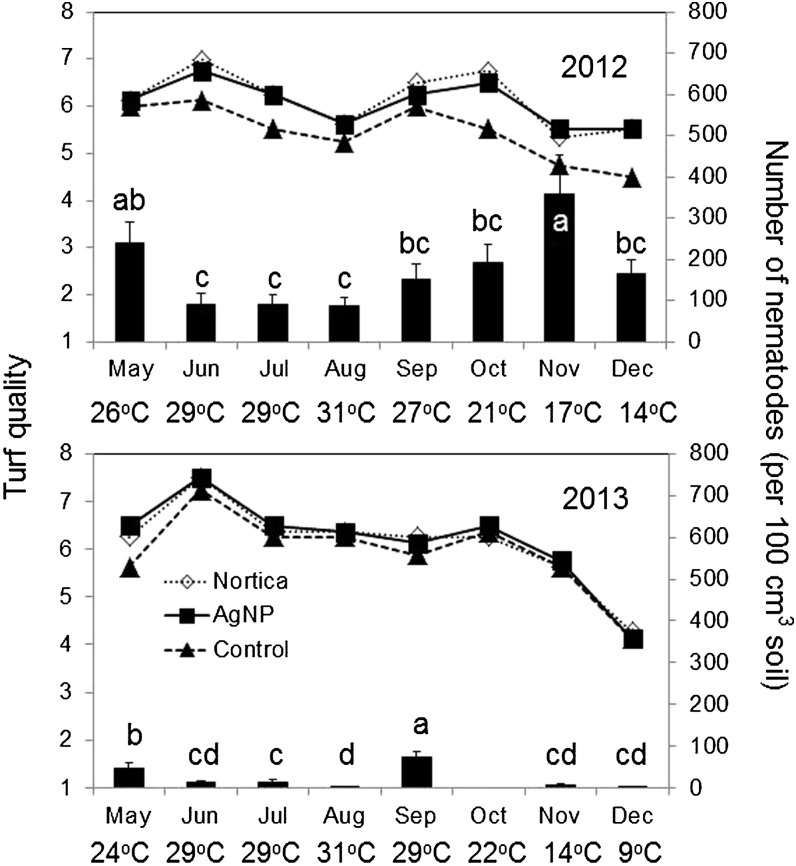
The number of J2 and the turf quality varied depending on the month in both 2012 and 2013 (Table 3). There was no interaction between treatment and month on turf quality or number of J2 (Table 3). More J2 were detected in 2012 than 2013. The highest numbers of J2 were detected in May and November, and J2 numbers decreased in the summer between June and August in 2012. In 2013, higher numbers were detected in May and September than in the other months (Fig. 2). Turf quality was the best in June and declined in the middle of summer (August) and the winter (November and December) consistently in both years (Fig. 2). There was no correlation observed between turf quality and number of J2 (r2 = 0.026 in 2012 and 0.008 in 2013) or between turf quality and number of galls (r2 = 0.014 in 2012 and 0.289 in 2013).
Fig. 2.
Effects of silver nanoparticles (AgNP) and a commercial preparation of Bacillus firmus (Nortica) on turf quality (line graph), and seasonal fluctuation of Meloidogyne graminis J2 (bar graph) in a bermudagrass putting green. Turf quality was rated on a scale of 1 to 9 with 6 = acceptable and 9 = the best. In the bar graph, J2 numbers are monthly averages per plot collected from all treatments and nontreated control, and the same letters indicates that the average nematode numbers are not different according to Fisher’s least significant difference at P ≤ 0.05. Monthly averages of air temperature are presented. No data of nematode counts in October 2013.
Discussion
This study provided evidence that AgNP may have utility for management of root-knot nematodes in bermudagrass. Inactivation of M. incognita J2 via direct exposure, and reduction of M. graminis J2 by soil treatment, demonstrated strong nematicidal effects of AgNP. Subsequently, the field trial demonstrated the beneficial effects of AgNP for intensively managed bermudagrass by improving turfgrass quality and decreasing root galling. AgNP was also determined to be safe for bermudagrass, causing no detectible phytotoxicity even with frequent (biweekly) applications during the growing season.
The nematicidal effect of AgNP was first tested against plant-parasitic nematodes in this study, compared with previous toxicological studies based on a nonparasitic nematode, Caenorhabditis elegans (Meyer et al., 2010; Lim et al., 2012). The effect of AgNP on J2 root-knot nematodes was remarkable under the direct exposure in water. Given the observation that the percentage of unhealthy nematodes after 6 hr of direct exposure remained unchanged after their filtration and resuspension in water, the phenotype appears to be an accurate measure of nematode mortality in this study. The nematicidal effect was further confirmed by the soil treatment with AgNP (150 μg/ml for 2 to 4 d) that effectively reduced J2 that could be extracted from the soil.
However, reduction of M. graminis J2 as a result of the AgNP treatment was not observed in the field trial, even though turf quality was improved by the AgNP treatment during one growing season in 2012, and root gall formation was reduced in the later season in both 2012 and 2013. The number of M. graminis J2 detected in the field plots was highly variable, making it difficult to detect any possible effects of treatments on nematode population densities. The J2 numbers in the research field greatly fluctuated with the changing environmental conditions depending on month. Particularly, temperature was considered as an important contributing factor. During the hottest summer months between June and August when average ambient air temperature in College Station, TX, was 30 ± 6°C, there was a notable decrease in the number of J2 in the field (Fig. 2). In addition, the site used in 2012 was more severely infested by root-knot nematodes than the one used in 2013.
Another observation related to the difficulty in the AgNP nematicide field evaluation was that there was no direct correlation of turfgrass quality with J2 counts or gall formation. High numbers of J2 or galls did not always result in noticeably low quality of turfgrass, as long as the turfgrass was well maintained with conventional management practices. For this same reason, significant turf quality improvement or reduction of gall formation by AgNP was not translated into a decrease of J2 in a single growing season. A long-term monitoring may be required before a change in the J2 population density becomes apparent under field conditions.
Another reason for the failure of AgNP to immediately reduce J2 in the field plots might arise from a dilution effect of AgNP. The AgNP soil treatment in the lab assay indicated 2- to 4-d exposure with 150 μg/ml AgNP was required to cause the reduction of J2 counts. There was a difficulty of complete saturation with 150 μg/ml AgNP in the soil profile in the field, and substantially lower doses of AgNP were applied in comparison to those used in the laboratory assay. Without sufficient concentration levels of AgNP being applied in the field, nematodes might remain alive without imminent mortality or required longer exposure time to be affected. According to previous reports, toxicity of sublethal doses of AgNP to nematodes can result in reproduction inhibition [with 0.05 to 0.5 μg/ml of AgNP for 72 hr (Roh et al., 2009; Lim et al., 2012)] or growth inhibition [with 5 to 50 μg/ml of AgNP for 1 to 3 d (Meyer et al., 2010)]. This suggests the AgNP effect may be subtle and chronic at low concentrations applied in the field.
Nematicidal effect of AgNP against root-knot nematodes likely applies to other genera of plant-parasitic nematodes and also to plant-pathogenic fungi, because its mode of action is not specific but associated with disrupting multiple cellular mechanisms including membrane permeability, ATP synthesis, and response to oxidative stress in both eukaryotic (Roh et al., 2009; Ahamed et al., 2010; Lim et al., 2012) and prokaryotic cells (Sondi and Salopek-Sondi, 2004; Morones et al., 2005; Lok et al., 2006; Choi and Hu, 2008). For this reason, AgNP is a broad-spectrum antimicrobial agent capable of affecting plant-pathogenic bacteria and fungi (Park et al., 2006; Jo et al., 2009). As such, it is possible that AgNP displays an antifungal effect on common root-associated fungal pathogens (for example, Gaeumannomyces graminis and Rhizoctonia solani) in warm-season turfgrass. Turfgrass treated with AgNP can become more tolerant to root-knot nematode damage because of its protection from additional weakness and stress brought on by these other pathogens. Therefore, AgNP may provide an additional benefit of managing multiple turfgrass pathogens and contribute to turf quality improvement.
AgNP possess nematicidal activity that may provide an alternative to high-risk synthetic nematicides or inconsistent biological control agents. To our knowledge, this is the first study to show positive efficacy of AgNP against root-knot nematodes in turfgrass without phytotoxicity. High frequency (biweekly) and high application doses (≥ 90.4 mg/m2) of AgNP may be required to achieve effective field efficacy for root-knot nematode problems in turfgrass. Combining AgNP with an irrigation system such as fertigation or tank-mixture with compatible chemicals that supplement the AgNP nematicidal effect may increase applicability of AgNP. Further understanding of the mechanism in the nematicidal action of AgNP also warrants improvement of AgNP efficacy.
Literature Cited
- Ahamed M, Posgai R, Gorey TJ, Nielsen M, Hussain SM, Rowe JJ. Silver nanoparticles induced heat shock protein 70, oxidative stress and apoptosis in Drosophila melanogaster. Toxicology and Applied Pharmacology. 2010;242:263–269. doi: 10.1016/j.taap.2009.10.016. [DOI] [PubMed] [Google Scholar]
- Choi O, Hu Z. Size dependent and reactive oxygen species related nanosilver toxicity to nitrifying bacteria. Environmental Science and Technology. 2008;42:4583–4588. doi: 10.1021/es703238h. [DOI] [PubMed] [Google Scholar]
- Crow WT. Plant-parasitic nematodes on golf course turf. Outlooks on Pest Management. 2005;16:10–15. [Google Scholar]
- Crow WT. 2007. Understanding and managing parasitic nematodes on turfgrasses. Pp. 351–374 in M. Pessarakli, ed. Handbook of turfgrass management & physiology. Boca Raton, FL: CRC Press.
- Crow WT, Giblin-Davis RM, Lickfeldt DW. Slit injection of 1,3-dichloropropene for management of Belonolaimus longicaudatus on established bermudagrass. Journal of Nematology. 2003;35:302–305. [PMC free article] [PubMed] [Google Scholar]
- Crow WT, Luc JE, Giblin-Davis RM. Evaluation of EconemTM, a formulated Pasteuria sp. bionematicide, for management of Belonolaimus longicaudatus on golf course turf. Journal of Nematology. 2011;43:101–109. [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Liu Z, Wang L, Zhan J. Synthesis of starch-stabilized Ag nanoparticles and Hg2+ recognition in aqueous media. Nanoscale Research Letters. 2009;4:1230–1235. doi: 10.1007/s11671-009-9387-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furno F, Morley KS, Wong B, Sharp BL, Arnold PL, Howdle SM, Bayston R, Brown PD, Winship PD, Reid HJ. Silver nanoparticles and polymeric medical devices: A new approach to prevention of infection? Journal of Antimicrobial Chemotherapy. 2004;54:1019–1024. doi: 10.1093/jac/dkh478. [DOI] [PubMed] [Google Scholar]
- Jo Y-K, Kim BH, Jung G. Antifungal activity of silver ions and nanoparticles on phytopathogenic fungi. Plant Disease. 2009;93:1037–1043. doi: 10.1094/PDIS-93-10-1037. [DOI] [PubMed] [Google Scholar]
- Kim JS, Kuk E, Yu KN, Kim J-H, Park SJ, Lee HJ, Kim SH, Park YK, Park YH, Hwang C-Y, Kim Y-K, Lee Y-S, Jeong DH, Cho M-H. Antimicrobial effects of silver nanoparticles. Nanomedicine: Nanotechnology, Biology, and Medicine. 2007;3:95–101. doi: 10.1016/j.nano.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Lim D, Roh J-Y, Eom H-J, Hyun JW, Choi J. Oxidative stress-related PMK-1 P38 MAPK activation as a mechanism for toxicity of silver nanoparticles to reproduction in the nematode Caenorhabditis elegans. Environmental Toxicology and Chemistry. 2012;31:585–592. doi: 10.1002/etc.1706. [DOI] [PubMed] [Google Scholar]
- Lok CN, Ho CM, Chen R, He QY, Yu WY, Sun H, Tam PK, Chiu JF, Che CM. Proteomic analysis of the mode of antibacterial action of silver nanoparticles. Journal of Proteome Research. 2006;5:916–924. doi: 10.1021/pr0504079. [DOI] [PubMed] [Google Scholar]
- Luc JE, Pang W, Crow WT, Giblin-Davis RM. Effects of formulation and host density on the ability of in vitro-produced Pasteuria endospores to control its host Belonolaimus longicaudatus. Journal of Nematology. 2010;42:87–90. [PMC free article] [PubMed] [Google Scholar]
- McClure MA, Nischwitz C, Skantar AM, Schmitt ME, Subbotin SA. Root-knot nematodes in golf course greens of the western United States. Plant Disease. 2012;96:635–647. doi: 10.1094/PDIS-09-11-0808. [DOI] [PubMed] [Google Scholar]
- Meyer JN, Lord CA, Yang XY, Turner EA, Badireddy AR, Marinakos SM, Chilkoti A, Wiesner MR, Auffan M. Intracellular uptake and associated toxicity of silver nanoparticles in Caenorhabditis elegans. Aquatic Toxicology. 2010;100:140–150. doi: 10.1016/j.aquatox.2010.07.016. [DOI] [PubMed] [Google Scholar]
- Morones JR, Elechiguerra JL, Camacho A, Holt K, Kouri JB, Ramirez JT, Yacaman MJ. The bactericidal effect of silver nanoparticles. Nanotechnology. 2005;16:2346–2353. doi: 10.1088/0957-4484/16/10/059. [DOI] [PubMed] [Google Scholar]
- Park H-J, Kim SH, Kim HJ, Choi S-H. A new composition of nanosized silica-silver for control of various plant diseases. Plant Pathology Journal. 2006;22:295–302. [Google Scholar]
- Roh J-Y, Sim SJ, Yi J, Park K, Chung KH, Ryu D-Y, Choi J. Ecotoxicity of silver nanoparticles on the soil nematode Caenorhabditis elegans using functional ecotoxicogenomics. Environmental Science and Technology. 2009;43:3933–3940. doi: 10.1021/es803477u. [DOI] [PubMed] [Google Scholar]
- Snedecor GW, Cochran WG. 8th ed. Iowa State University Press; 1989. Statistical methods. [Google Scholar]
- Sondi I, Salopek-Sondi B. Silver nanoparticles as antimicrobial agent: A case study on E. coli as a model for Gram-negative bacteria. Journal of Colloid Interface Science. 2004;275:177–182. doi: 10.1016/j.jcis.2004.02.012. [DOI] [PubMed] [Google Scholar]
- Starr JL, Ong KL, Huddleston M, Handoo ZA. Control of Meloidogyne marylandi on bermudagrass. Nematropica. 2007;37:43–49. [Google Scholar]
- Wright JB, Lam K, Hansen D, Burrell RE. Efficacy of topical silver against fungal burn wound pathogens. American Journal of Infection Control. 1999;27:344–350. doi: 10.1016/s0196-6553(99)70055-6. [DOI] [PubMed] [Google Scholar]
- Yin HQ, Langford R, Burrell RE. Comparative evaluation of the antimicrobial activity of ACTICOAT antimicrobial barrier dressing. Journal of Burn Care & Rehabilitation. 1999;20:195–200. doi: 10.1097/00004630-199905000-00006. [DOI] [PubMed] [Google Scholar]