Abstract
In a repeated greenhouse experiment, organic soil amendments were screened for effects on population density of soybean cyst nematode (SCN), Heterodera glycines, and soybean growth. Ten amendments at various rates were tested: fresh plant material of field pennycress, marigold, spring camelina, and Cuphea; condensed distiller’s solubles (CDS), ash of combusted CDS, ash of combusted turkey manure (TMA), marigold powder, canola meal, and pennycress seed powder. Soybeans were grown for 70 d in field soil with amendments and SCN eggs incorporated at planting. At 40 d after planting (DAP), many amendments reduced SCN egg population density, but some also reduced plant height. Cuphea plant at application rate of 2.9% (amendment:soil, w:w, same below), marigold plant at 2.9%, pennycress seed powder at 0.5%, canola meal at 1%, and CDS at 4.3% were effective against SCN with population reductions of 35.2%, 46.6%, 46.7%, 73.2%, and 73.3% compared with control, respectively. For Experiment 1 at 70 DAP, canola meal at 1% and pennycress seed powder at 0.5% reduced SCN population density 70% and 54%, respectively. CDS at 4.3%, ash of CDS at 0.2%, and TMA at 1% increased dry plant mass whereas CDS at 4.3% and pennycress seed powder at 0.1% reduced plant height. For Experiment 2 at 70 DAP, amendments did not affect SCN population nor plant growth. In summary, some amendments were effective for SCN management, but phytoxicity was a concern.
Keywords: Calendula, Camelina sativa, canola meal, condensed distiller’s solubles, Cuphea, field pennycress, Glycine max, green manure, Heterodera glycines, management, marigold, organic fertilizer, organic soil amendment, soybean, soybean cyst nematode, spring camelina, Thlaspi arvense, turkey manure ash
Soybean cyst nematode (SCN), Heterodera glycines, is the major yield-limiting pathogen of soybean (Glycine max) in the United States, causing an estimated 3 billion kilograms of soybean yield loss in 2009, representing one-quarter of the total yield loss to disease (Koenning and Wrather, 2010). Current management strategies in the midwestern United States rely on SCN-resistant cultivars and crop rotation. While resistant soybean cultivars are effective (Chen et al., 2001a; Chen, 2007), sources of resistance are limited (Kim et al., 2011), and SCN populations are adapting and diversifying to overcome these sources of resistance (Zheng et al., 2006; Niblack et al., 2008). Annual rotation of SCN-susceptible soybean with corn, the primary crop in the Midwest, does not adequately manage SCN on its own (Chen et al., 2001b; Porter et al., 2001) and longer corn sequences reduce corn yield (Crookston et al., 1991; Porter et al., 1997). Additionally, environmental concerns and increased regulation have reduced availability of chemical nematicides (Noling and Becker, 1994; Rich et al., 2004), while cost and inconsistent efficacy generally excludes use of the remaining nematicides for SCN management in the Midwest (De Bruin and Pedersen, 2008; Rotundo et al., 2010). So, alternative SCN management strategies with limited negative environmental impact are needed to reduce reliance on current management strategies.
One alternative strategy is applying organic soil amendments that suppress nematode populations. Some organic soil amendments are known to be nematicidal, with constituent compounds directly impacting nematodes (Walker, 1996; Insunza et al., 2001; Riga et al., 2005; Szakiel et al., 2008; Warnke et al., 2008; Doligalska et al., 2011). Amendments may also act as green manures that suppress nematode population growth by altering the microbial community (Matthiessen and Kirkegaard, 2006; Oka, 2010; Moore, 2011). Organic soil amendments in the form of incorporated rotation or cover crops (Ploeg, 1999; Reynolds et al., 2000; Ploeg, 2002; Perez et al., 2003; Warnke et al., 2008; Owen et al., 2010; Villate et al., 2012), incorporated plant residue from outside sources (Wang et al., 2001), and processed plant products (Mazzola et al., 2009; Zasada et al., 2009) have demonstrated value for managing various plant-parasitic nematodes. Despite this, few organic soil amendments have been tested on SCN.
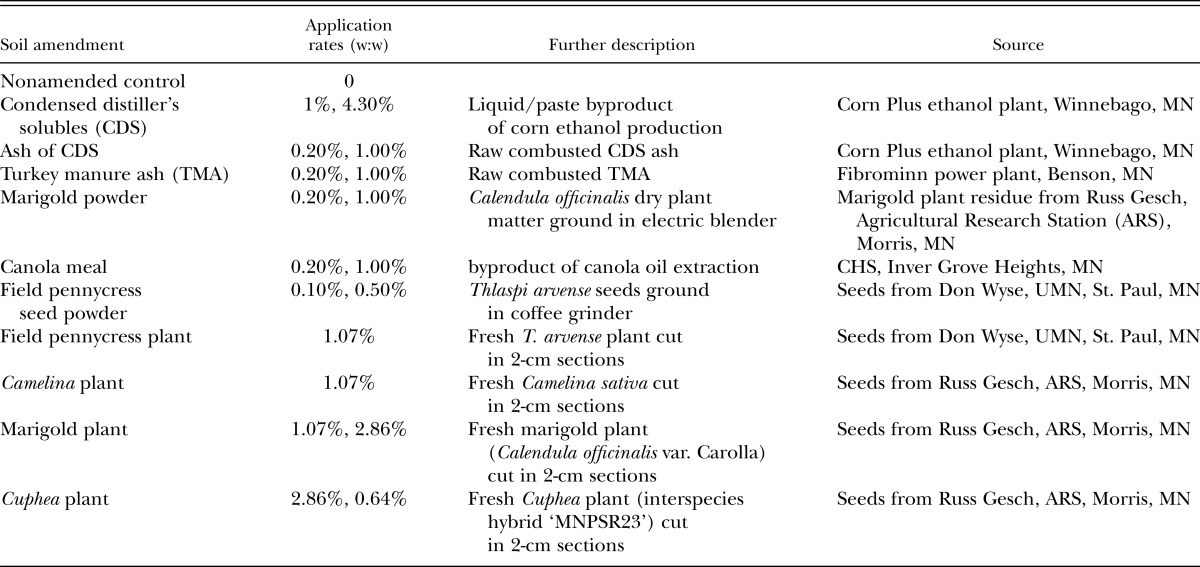
In addition to efficacy at managing plant-parasitic nematode populations, viable organic soil amendments must be readily available and their application economically feasible. Amendments used in this study (Table 1) are currently produced or are being developed for production in the Midwest and fall into three categories: (i) byproducts of corn ethanol (CDS), or electricity production (TMA); (ii) fresh plant material of alternative crops (marigold [Calendula officinalis], field pennycress [Thlaspi arvense], spring camelina [Camelina sativa], and Cuphea [interspecies hybrid ‘MNPSR23’, cigar plant]); or (iii) processed plant products (field pennycress seed powder, canola [Brassica napus] meal, and marigold plant powder).
Table 1.
Organic amendments tested in greenhouse experiments.
Many of these products can detrimentally affect nematodes, fungal pathogens, insects, or weeds. As a soil amendment, CDS has reduced fungal pathogen populations and corresponding root diseases in potato, radish, and cucumber due in part to toxicity of various organic acids (Abbasi et al., 2007, 2009). Various C. officinalis plant extracts were nematicidal or nematostatic in vitro to Xiphinema americanum sensu lato (Insunza et al., 2001), and Heligmosomoides species, an animal-parasitic nematode (Szakiel et al., 2008; Doligalska et al., 2011). Additionally, soil amendment with C. officinalis flowers effectively reduced Meloidogyne artiellia population density in growth chamber assays (Perez et al., 2003).
Plant species in the Brassicaceae family, including spring camelina, field pennycress, and canola, contain glucosinolates that are converted to isothiocyanates in the soil (Morra and Kirkegaard, 2002), which can act as biofumigants against plants, microbes, and nematodes depending on application rate (Bending and Lincoln, 1999; Morra and Kirkegaard, 2002; Balesh et al., 2005; Matthiessen and Kirkegaard, 2006; Vaughn et al., 2006; Gimsing and Kirkegaard, 2009; Oka, 2010; Hu et al., 2011). Three specific glucosinates have been identified in Camelina (Berhow et al., 2013), and camelina seed meal reduced Phymatotrichopsis omnivora (causal agent of cotton root rot) growth in field trials (Hu et al., 2011). However, crop rotation with spring camelina in greenhouse studies did not affect Xiphinema index (Villate et al., 2012) or SCN populations (Warnke et al., 2006). Similarly, pennycress seed meal has exhibited phytotoxic activity against plant seedlings in vitro (Vaughn et al., 2005, 2006) although neither pennycress plant material nor seed meal has been reported to be tested against nematodes.
Canola has been tested as a rotation crop for management of SCN in greenhouse and in vitro assays (Warnke et al., 2006, 2008). In one greenhouse assay simulating crop rotation, incorporation of canola into soil reduced SCN population density compared with incorporation of other crop residues (Warnke et al., 2008), but this effect was not consistent as it did not reduce SCN population in another greenhouse assay (Warnke et al., 2006). Similarly, canola plant residue extract was nematicidal to SCN J2 in vitro, while fresh canola plant extract had no effect (Warnke et al., 2008). Canola meal as a soil amendment has suppressed Meloidogyne arenaria on tomato (Solanum lycopersicum) in the greenhouse (Walker, 1996), Pratylenchus penetrans on apple (Malus domestica) rootstock in the greenhouse (Mazzola et al., 2009), and P. penetrans as well as Meloidogyne incognita in soil laboratory tests (Zasada et al., 2009).
The objectives of this study were to: (i) screen various organic soil amendments for utility in SCN population management, (ii) assess the impact of those amendments on soybean growth, and (iii) determine relationship between SCN population density and soybean growth in this study to elucidate potential mechanisms of amendment utility in a greenhouse setting.
Materials and Methods
Organic amendments:
Ten organic soil amendments at various application rates for a total of nineteen treatments (Table 1) were screened for efficacy in managing SCN under greenhouse conditions. Treatments included higher application rates to facilitate detection of amendment effects while also testing lower application rates more realistic for field application. Source material for fresh plant amendments were from plants grown in greenhouses in St. Paul, MN. Mature plants were harvested and all aboveground plant parts except seeds were cut into 2-cm sections and incorporated into soil within one day of harvest.
Experimental setup:
For each treatment except CDS treatments, the appropriate soil amendment in appropriate quantity (Table 1) was added to 1.4 kg of a 3:1 (soil:sand by weight) mixture of untreated SCN-free Webster clay loam field soil and sterile sand. The mixture was inoculated with SCN eggs at a rate of 2,000 eggs/100 cm3 soil, and mixed thoroughly in 7-liter plastic bags. This mixture was placed in 16-cm-diam. pots and planted with six seeds of SCN-susceptible ‘Sturdy’ soybean that were thinned to three plants per pot following germination. Because CDS is a semi-liquid paste, mixing CDS with soil in bags was not practical, so CDS was drench-applied at the soil surface after planting. Pots were arranged on greenhouse benches in a randomized complete block design with four replicates.
Data collection:
At 40 DAP, corresponding to about one SCN reproductive cycle, plant heights were recorded, and a soil sample consisting of seven 1-cm-diam. soil cores taken to a depth of 15 cm and totaling around 100-g soil were collected from each pot. Cysts were extracted from the entire soil sample by hand decanting and then sucrose centrifugation, crushed using a mechanical crusher (Faghihi and Ferris, 2000), and SCN population density (eggs/100 cm3 soil) was determined for each pot. At 70 DAP, corresponding to about two SCN reproductive cycles, plant heights were recorded, and soybeans were cut at the soil line. Aboveground soybean plant dry mass was measured after drying for 48 hr at 90°C. Soil was removed from pots and mixed thoroughly by hand. Soybean roots were rubbed by hand to dislodge cysts into the soil. Cysts were extracted from a 100-cm3 subsample of soil and crushed to determine SCN egg population density. The experiment was conducted twice, with Experiment 1 planted on 22 April 2011, and Experiment 2 planted on 11 January 2012. During Experiment 1, soil temperature was measured from 13 May to the end of the experiment. During this time, mean soil temperature was 27.7°C with standard deviation 3.34°C and range 22.3°C to 43.7°C. Soil temperature during Experiment 2 averaged 25.9°C with standard deviation 2.27°C and range 19.5 to 32°C.
Statistical analysis:
At 40 DAP, there were no significant interactions between experiment and treatment effects for SCN population or mean plant height (P > 0.1), therefore data from the two experiments were combined. At 70 DAP, there was significant interaction between experiment and treatment effect for SCN population (P ≤ 0.01), so data were analyzed separately by experiment.
SCN egg population density at 40 and 70 DAP; mean plant height at 40 and 70 DAP; and soybean shoot mass at 70 DAP were each analyzed as dependent variables using analysis of variance (ANOVA). ANOVA models were checked for homogeneity of variance using Levene’s test and for normality of residuals graphically (Levene, 1960; Cook and Weisburg, 1999). When necessary, dependent variables were transformed to meet these assumptions. Mean plant height at 40 DAP for combined experiments was transformed by loge x before analysis. For Experiment 1 at 70 DAP, no variables were transformed. For Experiment 2 at 70 DAP, plant height was transformed by x2 before analysis.
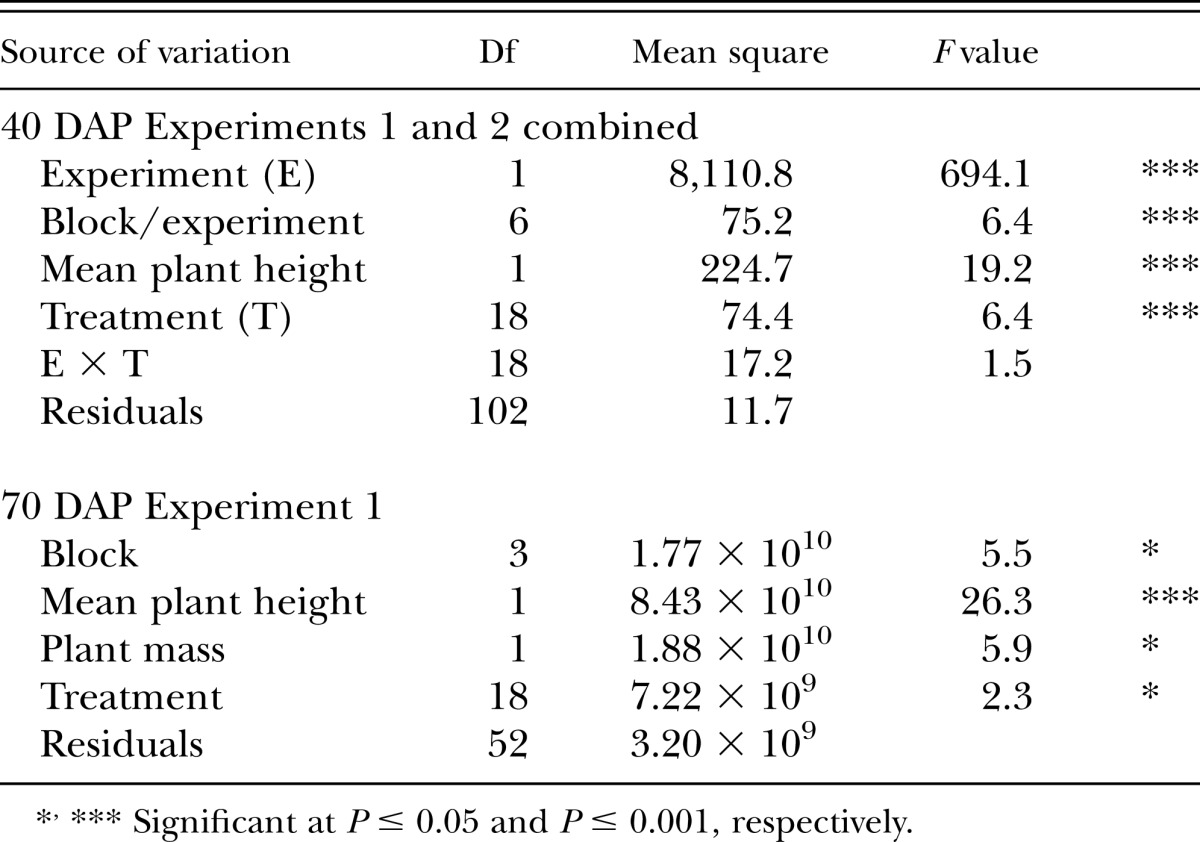
Because of substantial treatment effects on plant height and shoot dry mass, analysis of covariance (ANCOVA) was conducted to correct for plant growth effects on SCN population. For 40 DAP Experiments 1 and 2 combined data, ANCOVA was conducted with SCN egg population density as dependent variable, plant height at 40 DAP as covariate, and soil amendment treatment as treatment factor (Table 2). A similar ANCOVA model was constructed for 70 DAP Experiment 1 data except with plant height and plant mass at 70 DAP as covariates in a single model (Table 2). ANOVA models are not displayed for these ANCOVA models. For 70 DAP Experiment 2 data, ANCOVA was not conducted because treatments did not affect plant growth in previously described ANOVA (P > 0.05). SCN egg population at 40 DAP for combined experiments was transformed by x1/3 before analysis, but SCN egg density at 70 DAP was not transformed for either experiment. Treatment means compared to non-amended control were separated using Fischer’s LSD at P ≤ 0.05.
Table 2.
Analysis of covariance (ANCOVA) on soybean cyst nematode egg population density with plant height and mass as covariate preceding soil amendment treatment.
Linear regression analysis was performed to further determine the relationship between SCN egg population and plant growth in these experiments, to help determine the relationship between amendment effects on plant growth and amendment efficacy against SCN. At 40 DAP, linear regression of SCN egg population on Experiment and plant height was conducted for the two experiments combined. For 70 DAP Experiment 1 data, linear regression of SCN egg population on plant height as well as linear regression of SCN egg population density on plant mass were conducted. Linear regression of 70 DAP Experiment 2 data was not conducted because amendments did not affect plant growth. Linear regression models were checked for homogeneity of variance (using Levene’s test) and normality of residuals (graphically) and transformed when necessary. SCN egg population density at 40 DAP of the two experiments combined was transformed by x1/3 before linear regression analysis, while SCN egg population density at 70 DAP for Experiment 1 was transformed by x1/2 before analysis. Data was analyzed using R 2.15 software (The R Foundation for Statistical Computing, Vienna, Austria).
Results
Effects of soil amendments on SCN:
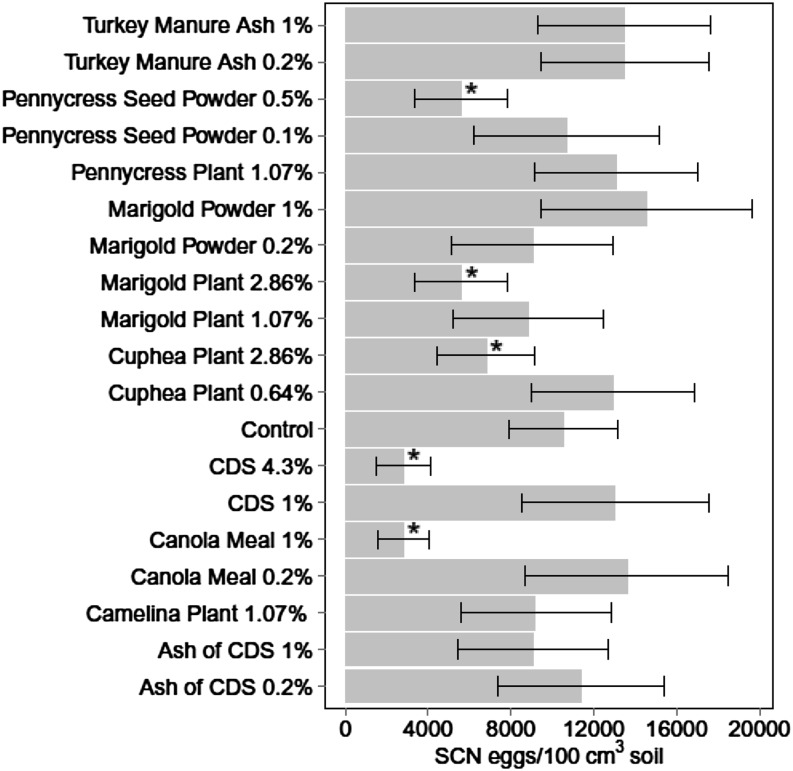
For the two experiments combined, soil amendment treatment affected SCN population density at 40 DAP based on ANCOVA (P ≤ 0.001, Table 2). Cuphea plant materials at an application rate of 2.9% (amendment:soil, w:w, same below), marigold plant materials at 2.9%, pennycress seed powder at 0.5%, canola meal at 1%, and CDS at 4.3% were effective, reducing SCN population density 35.2%, 46.6%, 46.7%, 73.2%, and 73.3% compared with control, respectively (Fig. 1).
Fig. 1.
Soybean cyst nematode (SCN) egg population densities at 40 d after planting in soil treated with various organic amendments (Experiments 1 and 2 combined). Values are means of eight replicates and bars represent standard error. CDS = condensed distiller’s soluble. *Indicates soil amendment treatment is significantly different from control (LSD, P ≤ 0.05).
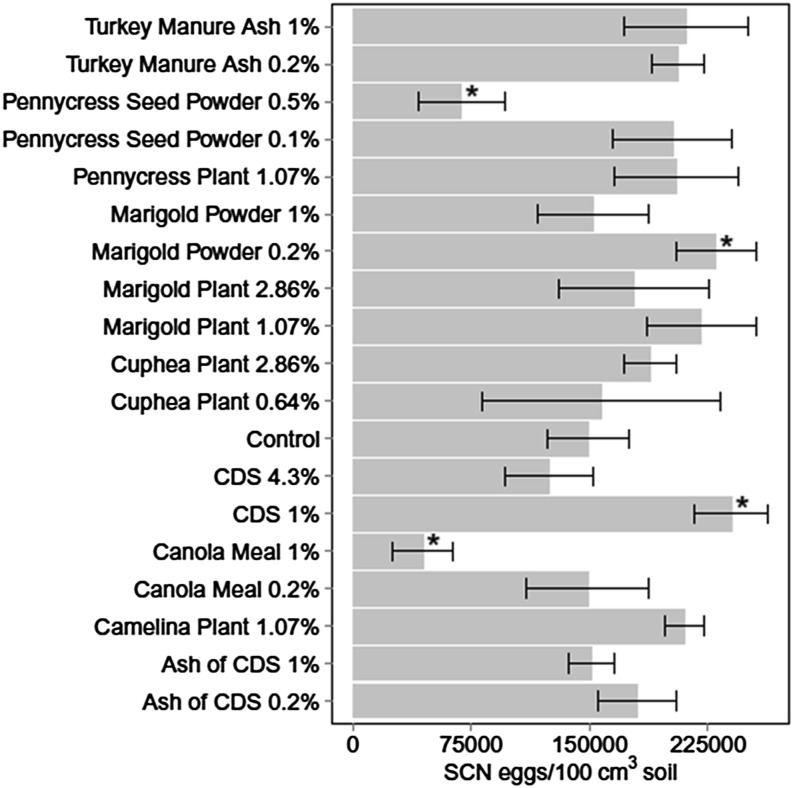
In Experiment 1, soil amendment treatment affected SCN egg population density at 70 DAP based on ANCOVA (P ≤ 0.05, Table 2). Canola meal at 1% and pennycress seed powder at 0.5% significantly reduced SCN egg population density (70% and 54%, respectively) compared with control (Fig. 2). In contrast, CDS at 1% and marigold powder at 0.2% increased egg population 61% and 54% respectively compared with control. In Experiment 2, there was no difference in SCN egg population density among treatments at 70 DAP based on ANOVA (P > 0.10). The slightly better amendment efficacy at 70 DAP in Experiment 1 than in Experiment 2 may be attributable to lower SCN population—possibly caused by higher temperature—in Experiment 1 than in Experiment 2. SCN egg population density at 70 DAP was high at 172,000 and 165,000 eggs/100 cm3 soil for Experiments 1 and 2, respectively.
Fig. 2.
Soybean cyst nematode (SCN) egg population density 70 d after planting in soil treated with various organic amendments (Experiment 1). Values are means of four replicates and bars represent standard error. CDS = condensed distiller’s soluble. *Indicates soil amendment treatment is significantly different from control (LSD, P ≤ 0.05).
Effects of soil amendments on plant growth:
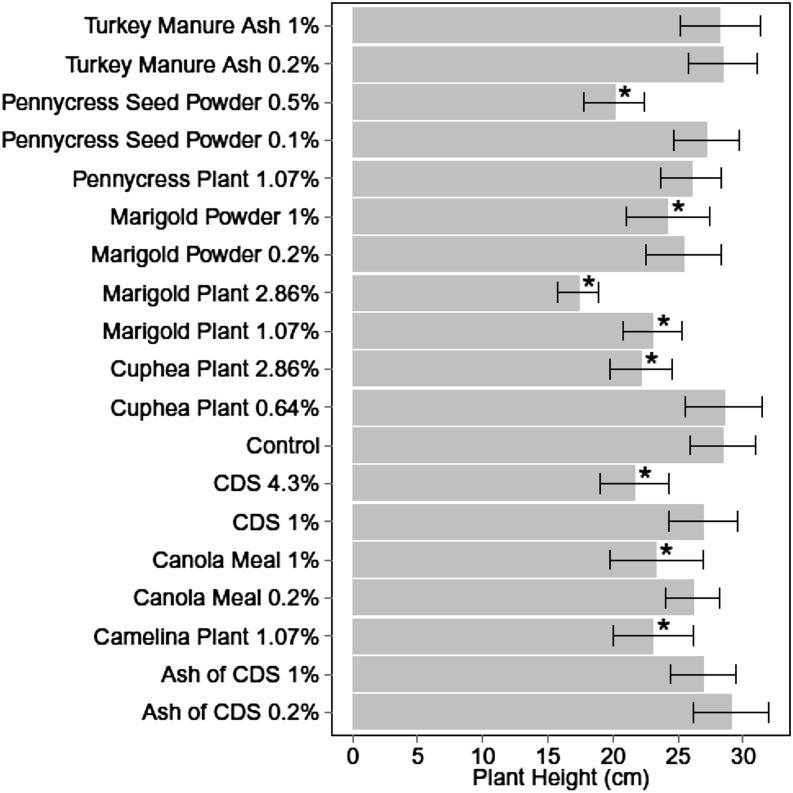
Soil amendment treatment also affected plant height at 40 DAP for combined experiments (P ≤ 0.001) resulting in similar or reduced plant height compared with control. Cuphea plant at 2.9%, CDS at 4.3%, pennycress seed powder at 0.5%, and marigold plant at 2.9% had strongest impact reducing plant height 22%, 24%, 29%, and 39% compared with control, respectively (Fig. 3).
Fig. 3.
Plant height at 40 d after planting in soil treated with various organic amendments (Experiments 1 and 2 combined). Values are means of eight replicates and bars represent standard error. CDS = condensed distiller’s soluble. *Indicates soil amendment treatment is significantly different from control (LSD, P ≤ 0.05).
Plant height at 70 DAP Experiment 1 was also affected by soil amendment treatment (P ≤ 0.05). Only pennycress seed powder at 1% and CDS at 4.3% resulted in significant differences (P ≤ 0.05) from control with 20% and 22% reductions, respectively (data not shown). In addition, soybean aboveground dry mass at 70 DAP was affected by soil amendment treatment (P ≤ 0.001). CDS at 4.3%, ash of CDS at 0.2%, and ash of turkey manure at 1% rates resulted in 42%, 34%, and 28% increases in plant mass compared with control (data not shown). At 70 DAP Experiment 2, no difference in plant mass (P > 0.10) or plant height (P > 0.05) among treatments was observed.
Relationship between plant growth and SCN:
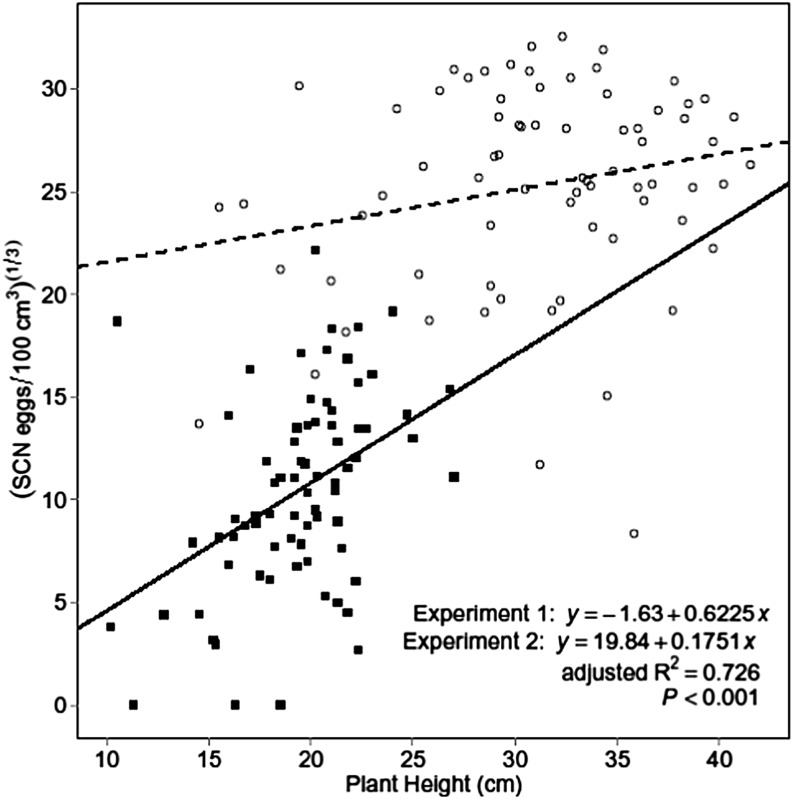
A linear model of SCN egg population based on mean plant height (both at 40 DAP), experiment (a factor with value of 0 for Experiment 1 and value of 1 for Experiment 2), and plant height by experiment interaction was fit resulting in a linear model with separate intercepts and slopes for the two experiments (Fig. 4). The model was significant (P ≤ 0.001) with the following equation:
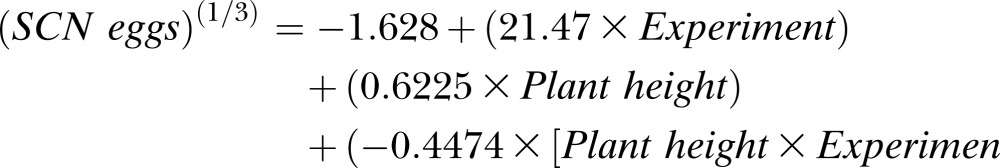 |
which, for Experiment 1, is equivalent to the following equation:
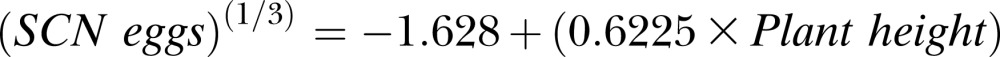 |
or for Experiment 2, to the following equation:
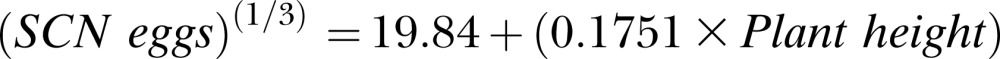 |
Fig. 4.
Linear regression of soybean cyst nematode (SCN) egg population density on mean plant height (both at 40 d after planting) with separate intercepts for each experiment. Solid and dashed lines are equations for Experiments 1 and 2, respectively. Solid square and hollow circle points represent pots from Experiments 1 and 2, respectively.
The coefficients for experiment (P ≤ 0.001), plant height (P ≤ 0.001), and experiment by plant height interaction (P ≤ 0.05) were also significant. The positive coefficient for experiment reflects the higher average SCN population density in Experiment 2 (17,923 eggs/100 cm3 soil) than Experiment 1 (1,842 eggs/100 cm3 soil) at 40 DAP, which may have been influenced by the higher temperature for Experiment 1. Additionally, the positive coefficients for plant height demonstrate a positive linear relationship between SCN population density and mean plant height at 40 DAP. Adjusted R2 was 0.73, showing experiment and mean plant height accounted for 73% of variation in SCN egg population density.
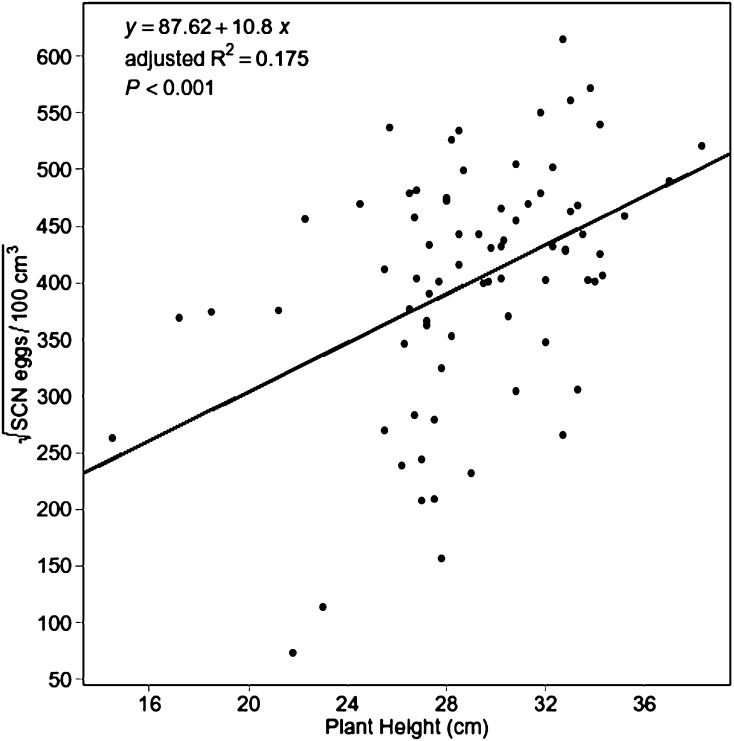
At 70 DAP Experiment 1, the linear regression of SCN population density on mean plant height produced a significant model (P ≤ 0.001) with the following equation:
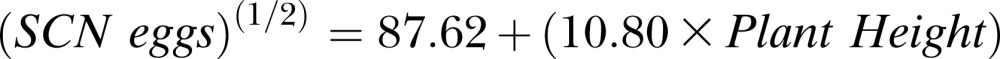 |
The positive slope coefficient for plant height was also significant (P ≤ 0.001), indicating a positive relationship between plant height and SCN population density (Fig. 5). However, the adjusted R2 was 0.175, suggesting the model accounted for only 17.5% of variation in SCN egg population.
Fig. 5.
Linear regression of soybean cyst nematode (SCN) egg population density on mean plant height (both at 70 d after planting—Experiment 1).
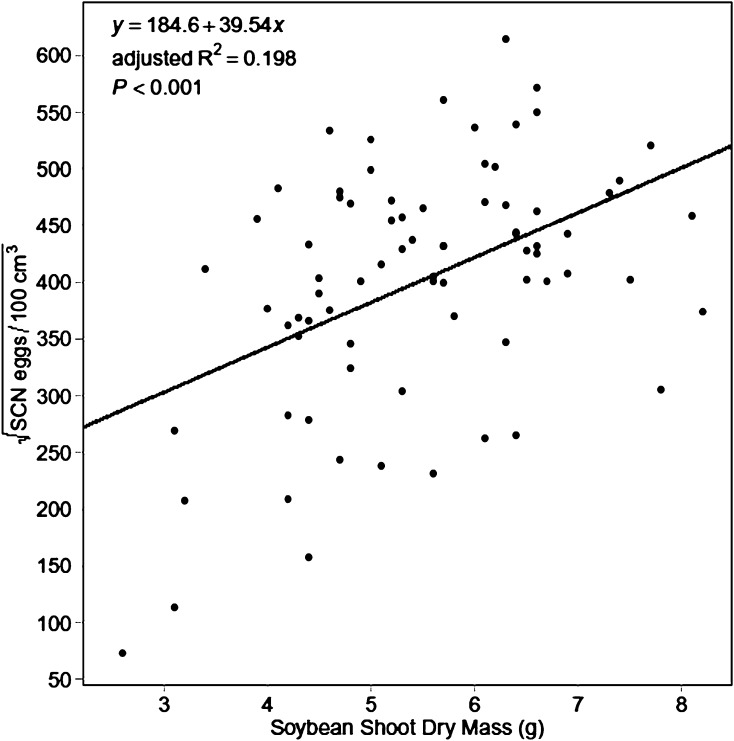
The regression of SCN population density on plant mass at 70 DAP Experiment 1 also produced a significant (P ≤ 0.001) model as follows:
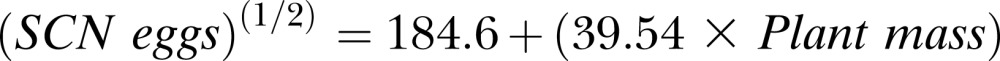 |
The positive slope coefficient for plant mass was also significant (P ≤ 0.001), indicating a positive relationship between plant mass and SCN egg population density (Fig. 6). However, adjusted R2 was 0.198, meaning the model accounted for only 19.8% of variation in SCN population density.
Fig. 6.
Linear regression of soybean cyst nematode (SCN) egg population density on plant mass (both at 70 d after planting—Experiment 1).
Discussion
Organic soil amendments—including pennycress seed powder, marigold plant material, Cuphea plant material, condensed distiller’s soluble, and canola meal—effectively reduced SCN population density in this study. However, except for canola meal and pennycress seed powder in Experiment 1, amendment efficacy against SCN dissipated by 70 DAP or approximately two generations. This suggests effective amendments caused an acute SCN population density reduction event, possibly because of nematoxic compounds, but SCN population density rebounded after two generations. Short-term amendment efficacy suggests nematicidal compounds in amendments were not persistent long term in the soil (Oka, 2010). In this greenhouse study where soybean root density was high and environmental conditions were managed, SCN egg density was very high by 70 DAP averaging 168,000 eggs/100 cm3 soil. Field conditions—including temperature extremes, water stress, lower root density—rarely support such high SCN population densities (Chen et al., 2001a; Porter et al., 2001; Chen and Liu, 2007; De Bruin and Pedersen, 2008; Rotundo et al., 2010), so amendments may suppress SCN for longer duration at lower SCN population densities that would be present in the field. Despite only short-term SCN suppression in this study, application of soil amendments may still aid in limiting soybean damage from SCN by reducing early SCN infection.
Some amendments tested in this study, or similar products, are known to be nematicidal including marigold (Insunza et al., 2001; Szakiel et al., 2008; Doligalska et al., 2011), and canola meal (Walker, 1996). This suggests SCN reduction by marigold and canola meal in this study was due partly to nematicidal activity. Other amendments may also have nematicidal activity, particularly those known to be toxic to other organisms such as CDS (Abbasi et al., 2007, 2009), and field pennycress seed meal (Vaughn et al., 2005, 2006).
Beside nematicidal activity, other mechanisms for SCN population reduction by soil amendments are possible. Mechanisms of action may also vary by amendment, and multiple mechanisms may exist for a single amendment. In particular, the observed plant growth reduction under some soil amendment treatments may have contributed to SCN population density reduction as this would decrease food resources for SCN as suggested elsewhere (Riggs et al., 2000). Linear models showed a positive relationship between SCN population density and plant growth meaning SCN population density was decreased when plant growth was decreased. This raises the possibility that reduced plant growth suppressed SCN population, but does not prove causation as the linear models show correlation only and represent trends across treatments which influenced both plant growth and SCN population.
Soil amendments that decreased plant growth—including pennycress seed powder, marigold powder, marigold plant, Cuphea plant, spring camelina plant, condensed distiller’s solubles, and canola meal—may contain chemical compounds that are phytotoxic as well as nematotoxic. No obvious phytotoxic effects such as scorching or stunting were observed in this study. However, some of the amendments in this study or related products are known to be phytotoxic at certain rates including pennycress seed meal at rates as low as 0.1% (w/w) (Vaughn et al., 2005, 2006) because of isothiocyanates (Vaughn et al., 2005), canola meal at 3% (w/w) on tomato (Walker, 1996), and corn gluten meal (similar to CDS) on perennial ryegrass (Lolium perenne) roots in vitro because of dipeptide compounds (Liu and Christians, 1994). Impact of the observed plant height and mass reductions on final yield was not determined, so the agronomic importance of this potential phytotoxicity is uncertain.
One option for avoiding phytotoxic effects is adjusting amendment rate such that application suppresses SCN population without adversely affecting plant growth. More research would be needed to determine if such an optimum application rate exists. A second option is delaying planting after amendment application so amendment concentration in soil is not phytotoxic by planting but nematode suppression still occurs. Reduction in instances of negative plant growth effects at 70 DAP suggest any amendment phytotoxicity dissipates over time. In another study, delaying planting 20 d after canola meal application successfully eliminated phytotoxic effects on tef (Eragrostis tef) (Balesh et al., 2005).
While plant growth reduction by soil amendments could have contributed to SCN population reduction in this study, evidence from ANCOVA and linear regression models suggest it was not the only cause. SCN population reductions were disproportionately large compared with plant growth reductions. Additionally, the significant soil amendment treatment effects even after accounting for plant growth in ANCOVA models and the moderate variation that plant growth accounted for in linear models support this point. This suggests that effective soil amendments reduced SCN population densities directly using nematotoxic compounds and indirectly by decreasing soybean growth, possibly because of phytotoxic compounds. However, the contribution of each mechanism for each amendment is impossible to determine precisely from this study. Other recognized mechanisms of nematode population reduction, such as stimulating microbial antagonists of nematodes or inducing host plant resistance (Oka, 2010), could have also contributed to SCN population reduction in this study.
Although most amendments reduced plant growth, some amendments showed promise as soybean fertilizers such as TMA, CDS ash, and, under some conditions, CDS. Other amendments may be useful fertilizers in certain doses or when strategies to mitigate potential phytotoxic effects are employed such as delaying planting after amendment application (Balesh et al., 2005). Additionally, increase in plant growth may explain increase in SCN population at 70 DAP Experiment 1 with CDS 1% amendment.
In summary, some of the soil amendments screened—pennycress seed powder, marigold plant material, Cuphea plant material, condensed distiller’s soluble, and canola meal—showed potential as nematode management agents although phytoxicity and duration of nematode suppression are concerns. This highlights the importance of monitoring for phytoxicity of nematode-management products, and statistical techniques for initial separation of nematotoxic and phytotoxic effects are demonstrated in this paper. Additionally, some amendments show potential as soybean fertilizers, particularly condensed distiller’s solubles, TMA, and ash of condensed distiller’s solubles. Further research is needed to determine mechanisms of SCN population reduction for specific amendments with particular emphasis on demonstrating nematotoxicity or phytotoxicity directly and identifying causal compounds. Research should also focus on application timing and rate to maximize nematode population reduction while minimizing phytotoxic effects. Eventually, the effectiveness of the amendments should be tested under field conditions.
Literature Cited
- Abbasi PA, Conn KL, Lazarovits G. Managing soilborne diseases of vegetable crops with a pre-plant soil or substrate amendment of a corn distillation product. Biocontrol Science and Technology. 2007;17:331–344. [Google Scholar]
- Abbasi PA, Lazarovits G, Weselowski B, Lalin I. Organic acids in condensed distiller's solubles: Toxicity to soilborne plant pathogens and role in disease suppression. Canadian Journal of Plant Pathology-Revue Canadienne De Phytopathologie. 2009;31:88–95. [Google Scholar]
- Balesh T, Zapata F, Aune J. Evaluation of mustard meal as organic fertiliser on tef (Eragrostis tef (Zucc) Trotter) under field and greenhouse conditions. Nutrient Cycling in Agroecosystems. 2005;73:49–57. [Google Scholar]
- Bending G, Lincoln S. Characterisation of volatile sulphur-containing compounds produced during decomposition of Brassica juncea tissues in soil. Soil Biology & Biochemistry. 1999;31:695–703. [Google Scholar]
- Berhow MA, Polat U, Glinski JA, Glensk M, Vaughn SF, Isbell T, Ayala-Diaz I, Marek L, Gardner C. Optimized analysis and quantification of glucosinolates from Camelina sativa seeds by reverse-phase liquid chromatography. Industrial Crops and Products. 2013;43:119–125. [Google Scholar]
- Chen S. Tillage and crop sequence effects on Heterodera glycines and soybean yields. Agronomy Journal. 2007;99:797–807. [Google Scholar]
- Chen S, Liu S. Effects of tillage and crop sequence on parasitism of Heterodera glycines juveniles by Hirsutella spp. and on juvenile population density. Nematropica. 2007;37:93–106. [Google Scholar]
- Chen S, Porter P, Orf J, Reese C, Stienstra W, Young N, Walgenbach D, Schaus P, Arlt T, Breitenbach F. Soybean cyst nematode population development and associated soybean yields of resistant and susceptible cultivars in Minnesota. Plant Disease. 2001a;85:760–766. doi: 10.1094/PDIS.2001.85.7.760. [DOI] [PubMed] [Google Scholar]
- Chen S, Porter P, Reese C, Stienstra W. Crop sequence effects on soybean cyst nematode and soybean and corn yields. Crop Science. 2001b;41:1843–1849. [Google Scholar]
- Cook RD, Weisburg S. 1999. Response transformations. Pp. 316–333 in Applied regression including computing and graphics. New York City, NY: Wiley-Interscience. [Google Scholar]
- Crookston R, Kurle J, Copeland P, Ford J, Lueschen W. Rotational cropping sequence affects yield of corn and soybean. Agronomy Journal. 1991;83:108–113. [Google Scholar]
- De Bruin JL, Pedersen P. Soybean cultivar and planting date response to soil fumigation. Agronomy Journal. 2008;100:965–970. [Google Scholar]
- Doligalska M, Jozwicka K, Kiersnowska M, Mroczek A, Paczkowski C, Janiszowska W. Triterpenoid saponins affect the function of P-glycoprotein and reduce the survival of the free-living stages of Heligmosomoides bakeri. Veterinary Parasitology. 2011;179:144–151. doi: 10.1016/j.vetpar.2011.01.053. [DOI] [PubMed] [Google Scholar]
- Faghihi J, Ferris JM. An efficient new device to release eggs from Heterodera glycines. Journal of Nematology. 2000;32:411–413. [PMC free article] [PubMed] [Google Scholar]
- Gimsing AL, Kirkegaard JA. Glucosinolates and biofumigation: Fate of glucosinolates and their hydrolysis products in soil. Phytochemistry Reviews. 2009;8:299–310. [Google Scholar]
- Hu P, Wang AS, Engledow AS, Hollister EB, Rothlisberger KL, Matocha JE, Zuberer DA, Provin TL, Hons FM, Gentry TJ. Inhibition of the germination and growth of Phymatotrichopsis omnivora (cotton root rot) by oilseed meals and isothiocyanates. Applied Soil Ecology. 2011;49:68–75. [Google Scholar]
- Insunza V, Aballay E, Macaya J. In vitro nematicidal activity of aqueous plant extracts on Chilean populations of Xiphinema americanum sensu lato. Nematropica. 2001;31:47–54. [Google Scholar]
- Kim M, Hyten DL, Niblack TL, Diers BW. Stacking resistance alleles from wild and domestic soybean sources improves soybean cyst nematode resistance. Crop Science. 2011;51:934–943. [Google Scholar]
- Koenning SR, Wrather JA. Suppression of soybean yield potential in the continental United States by plant diseases from 2006 to 2009. Plant Health Progress doi:10.1094/PHP-2010-1122-01-RS. 2010 [Google Scholar]
- Levene H. 1960. Robust tests for equality of variances. Pp. 278–292 in I. Olkin, ed. Contributions to probability and statistics. Palo Alto, CA: Stanford University Press. [Google Scholar]
- Liu DLY, Christians NE. Isolation and identification of root inhibiting compounds from corn gluten hydrolysate. Journal of Plant Growth Regulation. 1994;13:227–230. [Google Scholar]
- Matthiessen JN, Kirkegaard JA. Biofumigation and enhanced biodegradation: Opportunity and challenge in soilborne pest and disease management. Critical Reviews in Plant Sciences. 2006;25:235–265. [Google Scholar]
- Mazzola M, Brown J, Zhao X, Izzo AD, Fazio G. Interaction of brassicaceous seed meal and apple rootstock on recovery of Pythium spp. and Pratylenchus penetrans from roots grown in replant soils. Plant Disease. 2009;93:51–57. doi: 10.1094/PDIS-93-1-0051. [DOI] [PubMed] [Google Scholar]
- Moore A. 2011. Fertilizer potential of biofuel byproducts. Pp. 437–450 in M. A. S. Bernardes, ed. Biofuel production—recent developments and prospects. Rijeka, Croatia: InTech. [Google Scholar]
- Morra M, Kirkegaard J. Isothiocyanate release from soil-incorporated brassica tissues. Soil Biology & Biochemistry. 2002;34:1683–1690. [Google Scholar]
- Niblack TL, Colgrove AL, Colgrove K, Bond JP. Shift in virulence of soybean cyst nematode is associated with use of resistance from PI 88788. Plant Health Progress doi/10.1094/PHP-2008-0118-01-RS. 2008 [Google Scholar]
- Noling JW, Becker JO. The challenge of research and extension to define and implement alternatives to methyl bromide. Journal of Nematology. 1994;26:573–586. [PMC free article] [PubMed] [Google Scholar]
- Oka Y. Mechanisms of nematode suppression by organic soil amendments—a review. Applied Soil Ecology. 2010;44:101–115. [Google Scholar]
- Owen KJ, Clewett TG, Thompson JP. Pre-cropping with canola decreased Pratylenchus thornei populations, arbuscular mycorrhizal fungi, and yield of wheat. Crop & Pasture Science. 2010;61:399–410. [Google Scholar]
- Perez MP, Navas-Cortes JA, Pascual-Villalobos MJ, Castillo P. Nematicidal activity of essential oils and organic amendments from Asteraceae against root-knot nematodes. Plant Pathology. 2003;52:395–401. [Google Scholar]
- Ploeg AT. Greenhouse studies on the effect of marigolds (Tagetes spp.) on four Meloidogyne species. Journal of Nematology. 1999;31:62–69. [PMC free article] [PubMed] [Google Scholar]
- Ploeg AT. Effects of selected marigold varieties on root-knot nematodes and tomato and melon yields. Plant Disease. 2002;86:505–508. doi: 10.1094/PDIS.2002.86.5.505. [DOI] [PubMed] [Google Scholar]
- Porter P, Chen S, Reese C, Klossner L. Population response of soybean cyst nematode to long term corn-soybean cropping sequences in Minnesota. Agronomy Journal. 2001;93:619–626. [Google Scholar]
- Porter P, Lauer J, Lueschen W, Ford J, Hoverstad T, Oplinger E, Crookston R. Environment affects the corn and soybean rotation effect. Agronomy Journal. 1997;89:442–448. [Google Scholar]
- Reynolds L, Potter J, Ball-Coelho B. Crop rotation with Tagetes sp. is an alternative to chemical fumigation for control of root-lesion nematodes. Agronomy Journal. 2000;92:957–966. [Google Scholar]
- Rich JR, Dunn R, Noling J. 2004. Nematicides: Past and present uses. Pp. 1041–1082 in Z. X. Chen, S. Y. Chen, and D. W. Dickson, eds. Nematology: Advances and perspectives, vol. 2. Nematode management and utilization. Oxfordshire, UK: CABI Publishing.
- Riga E, Hooper C, Potter J. In vitro effect of marigold seed exudates on plant parasitic nematodes. Phytoprotection. 2005;86:31–35. [Google Scholar]
- Riggs R, Wrather J, Mauromoustakos A, Rakes L. Planting date and soybean cultivar maturity group affect population dynamics of Heterodera glycines, and all affect yield of soybean. Journal of Nematology. 2000;32:334–342. [PMC free article] [PubMed] [Google Scholar]
- Rotundo JL, Tylka GL, Pedersen P. Source of resistance affect soybean yield, yield components, and biomass accumulation in Heterodera glycines–infested fields. Crop Science. 2010;50:2565–2574. [Google Scholar]
- Szakiel A, Ruszkowski D, Grudniak A, Kurek A, Wolska KI, Doligalska M, Janiszowska W. Antibacterial and antiparasitic activity of oleanolic acid and its glycosides isolated from marigold (Calendula officinalis) Planta Medica. 2008;74:1709–1715. doi: 10.1055/s-0028-1088315. [DOI] [PubMed] [Google Scholar]
- Vaughn S, Isbell T, Weisleder D, Berhow M. Biofumigant compounds released by field pennycress (Thlaspi arvense) seedmeal. Journal of Chemical Ecology. 2005;31:167–177. doi: 10.1007/s10886-005-0982-4. [DOI] [PubMed] [Google Scholar]
- Vaughn SF, Palmquist DE, Duval SM, Berhow MA. Herbicidal activity of glucosinolate-containing seedmeals. Weed Science. 2006;54:743–748. [Google Scholar]
- Villate L, Morin E, Demangeat G, Van Helden M, Esmenjaud D. Control of Xiphinema index populations by fallow plants under greenhouse and field conditions. Phytopathology. 2012;102:627–634. doi: 10.1094/PHYTO-01-12-0007. [DOI] [PubMed] [Google Scholar]
- Walker J. Crambe and rapeseed meal as soil amendments: Nematicidal potential and phytotoxic effects. Crop Protection. 1996;15:433–437. [Google Scholar]
- Wang KH, Sipes BS, Schmitt DP. Suppression of Rotylenchulus reniformis by Crotalaria juncea, Brassica napus, and Tagetes erecta. Nematropica. 2001;31:235–249. [Google Scholar]
- Warnke SA, Chen SY, Wyse DL, Johnson GA, Porter PM. Effect of rotation crops on Heterodera glycines population density in a greenhouse screening study. Journal of Nematology. 2006;38:391–398. [PMC free article] [PubMed] [Google Scholar]
- Warnke SA, Chen S, Wyse DL, Johnson GA, Porter PM. Effect of rotation crops on hatch, viability and development of Heterodera glycines. Nematology. 2008;10:869–882. [Google Scholar]
- Zasada IA, Meyer SLF, Morra MJ. Brassicaceous seed meals as soil amendments to suppress the plant-parasitic nematodes Pratylenchus penetrans and Meloidogyne incognita. Journal of Nematology. 2009;41:221–227. [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Li Y, Chen S. Characterization of the virulence phenotypes of Heterodera glycines in Minnesota. Journal of Nematology. 2006;38:383–390. [PMC free article] [PubMed] [Google Scholar]