Abstract
Purpose.
Oxygen extraction fraction (OEF), defined by the ratio of oxygen metabolism (MO2) to delivery (DO2), determines the level of compensation of MO2 by DO2. In the current study, we tested the hypothesis that inner retinal OEF remains unchanged during light flicker under systemic normoxia and hypoxia in rats due to the matching of MO2 and DO2.
Methods.
Retinal vascular oxygen tension (PO2) measurements were obtained in 10 rats by phosphorescence lifetime imaging. Inner retinal OEF was derived from vascular PO2 based on Fick's principle. Measurements were obtained before and during light flicker under systemic normoxia and hypoxia. The effects of light flicker and systemic oxygenation on retinal vascular PO2 and OEF were determined by ANOVA.
Results.
During light flicker, retinal venous PO2 decreased (P < 0.01, N = 10), while inner retinal OEF increased (P = 0.02). Under hypoxia, retinal arterial and venous PO2 decreased (P < 0.01), while OEF increased (P < 0.01). The interaction effect was not significant on OEF (P = 0.52), indicating the responses of OEF to light flicker were similar under normoxia and hypoxia. During light flicker, OEF increased from 0.46 ± 0.13 to 0.50 ± 0.11 under normoxia, while under hypoxia, OEF increased from 0.67 ± 0.16 to 0.74 ± 0.14.
Conclusions.
Inner retinal OEF increased during light flicker, indicating the relative change in DO2 is less than that in MO2 in rats under systemic normoxia and hypoxia. Inner retinal OEF is a potentially useful parameter for assessment of the relative changes of MO2 and DO2 under physiologic and pathologic conditions.
Keywords: retina, oxygen extraction fraction, vascular oxygen tension, light flicker, hypoxia
Inner retinal oxygen extraction fraction increased during light flicker under systemic normoxia and hypoxia in rats, indicating the relative change in oxygen delivery was lower than that in oxygen metabolism.
Introduction
Stimulation of the retinal tissue with light flicker increases the inner retinal energy metabolism.1,2 This increase in energy metabolism is compensated by an augmented supply of oxygen and glucose due to increased blood flow in a process known as functional hyperemia.3,4 Impaired functional hyperemia, as demonstrated by attenuated vasodilation during light flicker, has been reported in diabetic retinopathy5–8 and glaucoma,9,10 suggesting an incomplete compensatory blood flow response to increased energy metabolism. However, since to our knowledge no clinical methods are available for measurements of inner retinal energy metabolism, it is unknown if changes in oxygen metabolism and delivery (product of blood flow and arterial oxygen content) are matched in health and disease.
The relative changes of inner retinal oxygen metabolism (MO2) and oxygen delivery (DO2) during light flicker can be quantified by measurement of inner retinal oxygen extraction fraction (OEF), which is defined by the ratio of MO2 to DO2.11 Without direct measurements of either MO2 or DO2, inner retinal OEF can be derived from measurements of arterial and venous oxygen levels based on Fick's principle.11,12 During light flicker, if OEF remains unchanged, then changes in MO2 and DO2 are matched, while an increase or decrease in OEF with light flicker would indicate either under- or overcompensation of MO2 by DO2, respectively.
In the current study, we formulated the hypothesis that inner retinal OEF remains unchanged with light flicker, under systemic normoxia and hypoxia. Under normal physiology (normoxia), the increase in MO2 with light flicker should be matched by an increase in DO2 due to vasodilation, thus resulting in no change in inner retinal OEF. Under a severe hypoxic challenge, even without light flicker, MO2 and DO2 are significantly reduced.13 With light flicker, no increase in DO2 is expected due to hypoxia-induced maximized vasodilation. Additionally, since the tissue under hypoxia is already deficient of oxygen, MO2 is unlikely to increase. Consequently, inner retinal OEF is not expected to change with light flicker under hypoxia. To test this hypothesis, we measured the response of OEF to light flicker in rats under systemic normoxia and hypoxia by vascular oxygen tension (PO2) imaging.
Methods
Animals
Ten Long Evans pigmented rats (weight 444 ± 99 g, mean ± SD; N = 10) were used in this study. Rats were cared for in compliance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Anesthesia was induced with intraperitoneal injections of ketamine (100 mg·kg−1) and xylazine (5 mg·kg−1), and maintained with supplemental doses of ketamine (20 mg·kg−1) and xylazine (1 mg·kg−1). The body temperatures of rats were maintained at 37°C using a heated animal holder. Blood pressure and heart rate were monitored continuously with a data acquisition system (Biopac Systems, Goleta, CA, USA) linked to a pressure transducer connected to a catheter placed in a femoral artery.
Rats were ventilated first with room air (21% oxygen, normoxia) and then with 10% oxygen (hypoxia) through an endotracheal tube connected to a small animal ventilator (Harvard Apparatus, Inc., South Natick, MA, USA). Mechanical ventilation prevented any anesthetic depressant effect on respiration. Blood was drawn from the femoral arterial catheter to measure systemic arterial oxygen tension (PaO2), carbon dioxide tension (PaCO2), and pH with a blood gas analyzer (Radiometer, Westlake, OH, USA) and determine hemoglobin (Hgb) concentration with the use of a hematology system (Siemens, Tarrytown, NY, USA). Rats were maintained normocapnic by adjusting the respiratory minute volume and performing blood gas analysis 5 minutes after each adjustment until PaCO2 was within the range of 35 to 45 mm Hg.
An oxygen sensitive molecular probe, Pd-porphine (Frontier Scientific, Logan, UT, USA), was dissolved (12 mg·mL−1) in bovine serum albumin solution (60 mg·mL−1) and administered (20 mg·kg−1) through the femoral arterial catheter, typically 10 minutes before imaging. The pupil was dilated with 2.5% phenylephrine and 1% tropicamide. During imaging, a glass cover slip and 1% hydroxypropyl methylcellulose were applied to the cornea to minimize the refractive power and prevent dehydration.
Retinal Vascular PO2 Imaging
Retinal vascular PO2 was measured with our previously established optical section phosphorescence lifetime imaging system.14 In brief, a laser line was projected on the retina at an angle with respect to the imaging path. This allowed depth-resolved imaging of phosphorescence emission within the major retinal arteries and veins. Phosphorescence lifetimes were measured by a frequency-domain approach as described previously.15 The lifetimes then were used to calculate the PO2 values using the Stern-Volmer relationship, defined as PO2 = (1/κQ)·(1/τ − 1/τ0), where κQ (mm Hg−1·μs−1) is the quenching constant for the triplet-state of Pd-porphine, τ (μs) is the measured phosphorescence lifetime, and τ0 (μs) is the lifetime in a zero-oxygen environment.15 A red-free retinal image was acquired for documenting the locations of PO2 measurements. Vascular PO2 images were obtained at a nasal and temporal sector relative to the optic disc. Each sector was bounded by 2 major arteries with a major vein between them. In each sector, 3 repeated images were acquired. For each image, the mean PO2 of the 2 major arteries (PO2A) and the PO2 of the vein (PO2V) between the arteries were used for calculation of inner retinal OEF. The laser power at the cornea was approximately 40 μW, which is safe for 1 hour of continuous viewing according to the American National Standard Institute for Safety Standards.16
OEF Calculation
According to our previously established method for quantifying inner retinal OEF,12 vascular PO2 measurements (PO2A and PO2V) in retinal sectors were used to calculate OEF based on the following equation,
 |
where O2A and O2V are the arterial and venous O2 contents, respectively. The O2 contents were calculated as O2max* Hgb * SO2 + k * PO2, where O2max is the maximum oxygen carrying capacity of Hgb (1.39 mLO2·g−1),17 k is the oxygen solubility in blood (0.003 mLO2·dL−1·mm Hg−1),17 and SO2 is the vascular oxygen saturation. Vascular SO2 was calculated using the rat oxygen dissociation curve: SO2 = (PO2/P50)n/[1+ (PO2/P50)n], where n is an empirical constant taken to be 2.6,18 and P50 is the PO2 when SO2 is 50% at the experimentally measured blood pH value.18,19 Because O2V cannot exceed O2A, inner retinal OEF ranges from 0 to 1, following the equation above. The repeatability of OEF measurements was assessed previously by calculating the standard deviation of repeated measurements and was on average 0.08.12
Retinal Stimulation by Light Flicker
Using our previously established protocol for stimulating the retina with light flicker,20,21 imaging was performed first under continuous light illumination (before flicker) and then under flickering light (during flicker) for each systemic oxygenation condition (normoxia and hypoxia). For light flicker stimulation, a filter with a transmission wavelength of 568 ± 5 nm was placed in front of the illumination housing of a slit-lamp biomicroscope. A shutter attached to a solenoid was placed in the light path to flicker light at a frequency of 10 Hz. The light wavelength and flickering frequency were selected based on previous studies that showed a maximal or near maximal vascular response under these conditions.20,21 The time-averaged light powers before and during flicker were matched by doubling the light intensity during flicker. Imaging was performed before and 2 minutes after the initiation of light flicker.
Statistical Analysis
Three repeated measurements of retinal oxygenation parameters (PO2A, PO2V, and OEF) were averaged for each sector. Values in nasal and temporal sectors then were averaged in each rat. A 2-way repeated measures ANOVA was used to determine the effects of light flicker (before and during) and systemic oxygenation condition (normoxia and hypoxia) on each retinal oxygenation parameter. Statistical significance was accepted at P < 0.05.
Results
Systemic Physiological Status
The systemic physiological status of rats under normoxia and hypoxia have been reported previously.12 As expected, the systemic PaO2 under hypoxia (34 ± 4 mm Hg) was significantly lower compared to that under normoxia (93 ± 8 mm Hg; P < 0.01, N = 10). Since the ventilation was controlled, systemic PaCO2 was not different under the two systemic oxygenation conditions (P = 0.76). However, under hypoxia, arterial blood pH, blood pressure, and heart rate decreased significantly (P < 0.01).
Retinal Oxygenation Parameters
Retinal oxygenation parameters before and during light flicker, and their differences (during minus before) under normoxia and hypoxia are listed in the Table. There was a significant effect of light flicker on PO2V (P < 0.01, N = 10), but not on PO2A (P = 0.71). During light flicker, PO2V decreased on average by 1.3 and 1.2 mm Hg under normoxia and hypoxia, respectively. As expected, there were significant effects of systemic oxygenation on retinal PO2A and PO2V (P < 0.01), as we reported previously.12
Table.
Retinal Oxygenation Parameters (Mean ± SD, N = 10) Before and During Light Flicker, and Their Difference (During Minus Before) Under Two Systemic Oxygenation Conditions (SOC), Normoxia (N) and Hypoxia (H)
|
SOC |
Before Flicker* |
During Flicker |
Difference |
ANOVA
P
Value |
|||
|
Flicker |
SOC |
Flicker × SOC |
|||||
| PO2A, mm Hg | N | 44 ± 4 | 44 ± 4 | 0.1 ± 1.9 | 0.71 | <0.01 | 0.92 |
| H | 19 ± 4 | 20 ± 3 | 0.1 ± 0.7 | ||||
| PO2V, mm Hg | N | 28 ± 5 | 27 ± 4 | −1.3 ± 1.0 | <0.01 | <0.01 | 0.80 |
| H | 11 ± 4 | 10 ± 5 | −1.2 ± 1.8 | ||||
| OEF | N | 0.46 ± 0.13 | 0.50 ± 0.11 | 0.05 ± 0.04 | 0.02 | <0.01 | 0.52 |
| H | 0.67 ± 0.16 | 0.74 ± 0.14 | 0.07 ± 0.11 | ||||
Retinal oxygenation parameters before light flicker have been reported previously.12
Measurements of inner retinal OEF in individual rats before and during light flicker under normoxia and hypoxia are plotted in the Figure. During light flicker, OEF increased in 8 of 10 and 9 of 10 rats under normoxia and hypoxia, respectively. There were significant main effects of light flicker and systemic oxygenation on OEF (P ≤ 0.02, N = 10). However, the interaction effect was not significant (P = 0.52), indicating similar responses of OEF to light flicker under the two systemic oxygenation conditions. Before light flicker, mean inner retinal OEF measurements were 0.46 and 0.67 under normoxia and hypoxia, respectively. During light flicker, inner retinal OEF increased to 0.50 (11%) and 0.74 (10%) under normoxia and hypoxia, respectively.
Figure.
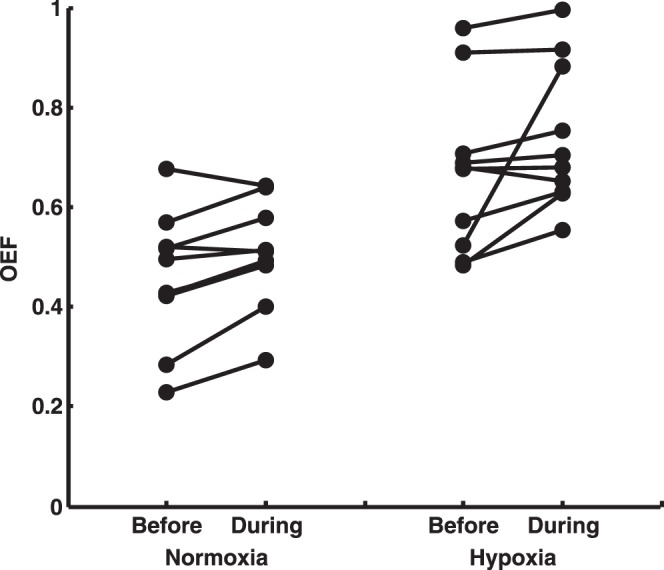
Measurements of inner retinal OEF in individual rats before and during light flicker under systemic normoxia and hypoxia. During flicker, OEF increased in 8 of 10 and 9 of 10 rats under normoxia and hypoxia, respectively. Inner retinal OEF measurements before light flicker have been reported previously.12
Discussion
Measurement of inner retinal OEF provides information about the matching of oxygen metabolism and delivery. In the current study, we demonstrated that inner retinal OEF increased during light flicker under normoxia and hypoxia. This increase in OEF was due to a smaller relative change in DO2 compared to MO2, indicating MO2 was undercompensated by DO2 during light flicker.
Under normoxia, the response of inner retinal OEF to light flicker indicated the increase in MO2 was not completely matched by an increase in DO2. In agreement with findings of the present study, undercompensation of MO2 by DO2 during light flicker has also been shown by decreases in inner retinal or optic nerve head tissue PO2 in rats and cats (1–7 mm Hg),22–25 and an increase in arteriovenous PO2 difference in rats (4 mm Hg).20 Conversely, in humans, light flicker caused an increase in retinal venous SO2 (4%), while arterial SO2 did not change,26 thereby suggesting overcompensation of MO2 by DO2. The different findings of these studies are likely attributable to variations between species and experimental protocols. Nevertheless, the complimentary findings of only slight alterations in retinal OEF and tissue PO2 with light flicker in rats suggest that DO2 almost fully compensated for MO2 and, thus, the energy-dependent neural activity in the retina was unlikely to be limited by oxygen availability. Additionally, our finding of increased OEF coupled with the known increase in blood flow (and DO2) confirms MO2 increases with light flicker.
Under hypoxia, inner retinal OEF increased with light flicker, contrary to our hypothesis that OEF would remain unchanged, which was based on the following line of reasoning. Under this severe hypoxic challenge, inner retinal MO2 was found previously to be reduced to approximately one-third of that under normoxia13; thus, the tissue was already deficient in oxygen and MO2 would not be able to increase with light flicker. Furthermore, vasodilation was likely maximized due to hypoxia; thus, precluding an increase in DO2. As a result, with no change in MO2 and DO2, OEF was expected to remain unchanged. Nonetheless, we found inner retinal OEF increased with light flicker, which is most likely attributed to an increase in MO2, since DO2 is unlikely to decrease with light flicker. The question then arises as to how oxygen extraction and MO2 could increase with light flicker, given the existing oxygen deficiency in the retina under hypoxia. We offer the following explanation for our observation. Even under systemic hypoxia, tissue near blood vessels still may be adequately supplied with oxygen and, thus, could respond to light flicker with increased MO2, whereas tissue farther away from blood vessels is unable to respond to light flicker because of hypoxia. According to Fick's laws of diffusion, with an increase in MO2, the oxygen gradient from the vessel wall into the tissue would steepen, thereby increasing the rate of oxygen loss from blood and OEF.27 Simultaneously, the lower oxygen content of blood under hypoxia coupled with the steeper oxygen gradient with light flicker will cause less tissue to be oxygenated, and, thus, exacerbate overall tissue hypoxia.
Although the responses of inner retinal OEF to light flicker were similar under normoxia and hypoxia, we speculated that the corresponding increases in MO2 may have been different. Under hypoxia, compensation by DO2 was likely lower due to maximized vasodilation; hence, a given increase in OEF would result from a smaller change in MO2. The light flicker-induced increase in MO2 can be estimated based on Fick's principle (MO2 = OEF*O2A*blood flow).11 Under normoxia, the measured increase in OEF (from 0.46 to 0.50) and unchanged O2A (unchanged PO2A), along with a previously reported increase in blood flow (from 9.9 to 13.5 μL/min),28 yielded an estimated light flicker-induced MO2 increase of 48%. This value is in agreement with an estimated 37% increase in MO2 based on PO2 and blood flow measurements at the optic nerve head in cats.24 Under hypoxia, based on the measured increase in OEF (from 0.67 to 0.74), unchanged O2A (unchanged PO2A), and a presumed negligible change in blood flow, the light flicker-induced MO2 increase was estimated to be 10%. The smaller increase in MO2 under hypoxia compared to that under normoxia indicates systemic hypoxia suppressed the ability of MO2 and, presumably, energy-dependent neural activity, to respond to light flicker. Our estimation of a reduced MO2 response to light flicker agrees with a previous report of attenuated electrical activity in the cat retina under a similar systemic hypoxic condition.29 The estimated MO2 changes due to light flicker were limited by a lack of direct blood flow measurements in the current study. Future studies are needed to establish the magnitude of MO2 increase with light flicker by simultaneous measurements of retinal blood flow and vascular oxygen contents.
In conclusion, the finding of increased inner retinal OEF substantiates that inner retinal MO2 is increased with light flicker, and that this increase may be attenuated due to limited oxygen availability under hypoxia. With impaired functional hyperemia, changes in OEF are expected to be dominated by alterations in MO2 in response to light flicker. Overall, inner retinal OEF can be used for quantitative assessment of functional hyperemia with respect to the relative changes of oxygen delivery and metabolism under physiologic and pathologic conditions.
Acknowledgments
Supported by National Eye Institute (Bethesda, MD, USA) Grants EY017918 (MS) and EY001792 (UIC), and Research to Prevent Blindness (New York, NY, USA) senior scientific investigator award (MS), and an unrestricted departmental award.
Disclosure: P.-Y. Teng, None; J. Wanek, None; N.P. Blair, None; M. Shahidi, P
References
- 1. Bill A, Sperber GO. Aspects of oxygen and glucose consumption in the retina: effects of high intraocular pressure and light. Graefes Arch Clin Exp Ophthalmol. 1990; 228: 124–127 [DOI] [PubMed] [Google Scholar]
- 2. Wang L, Bill A. Effects of constant and flickering light on retinal metabolism in rabbits. Acta Ophthalmol Scand. 1997; 75: 227–231 [DOI] [PubMed] [Google Scholar]
- 3. Newman EA. Functional hyperemia and mechanisms of neurovascular coupling in the retinal vasculature. J Cereb Blood Flow Metab. 2013; 33: 1685–1695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Riva CE, Logean E, Falsini B. Visually evoked hemodynamical response and assessment of neurovascular coupling in the optic nerve and retina. Prog Retin Eye Res. 2005; 24: 183–215 [DOI] [PubMed] [Google Scholar]
- 5. Bek T, Hajari J, Jeppesen P. Interaction between flicker-induced vasodilatation and pressure autoregulation in early retinopathy of type 2 diabetes. Graefes Arch Clin Exp Ophthalmol. 2008; 246: 763–769 [DOI] [PubMed] [Google Scholar]
- 6. Garhofer G, Zawinka C, Resch H, Kothy P, Schmetterer L, Dorner GT. Reduced response of retinal vessel diameters to flicker stimulation in patients with diabetes. Br J Ophthalmol. 2004; 88: 887–891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hammer M, Heller T, Jentsch S, et al. Retinal vessel oxygen saturation under flicker light stimulation in patients with non-proliferative diabetic retinopathy. Invest Ophthalmol Vis Sci. 2012; 26: 4063–4068 [DOI] [PubMed] [Google Scholar]
- 8. Lasta M, Pemp B, Schmidl D, et al. Neurovascular dysfunction precedes neural dysfunction in the retina of patients with type 1 diabetes. Invest Ophthalmol Vis Sci. 2013; 54: 842–847 [DOI] [PubMed] [Google Scholar]
- 9. Garhofer G, Zawinka C, Resch H, Huemer KH, Schmetterer L, Dorner GT. Response of retinal vessel diameters to flicker stimulation in patients with early open angle glaucoma. J Glaucoma. 2004; 13: 340–344 [DOI] [PubMed] [Google Scholar]
- 10. Riva CE, Salgarello T, Logean E, Colotto A, Galan EM, Falsini B. Flicker-evoked response measured at the optic disc rim is reduced in ocular hypertension and early glaucoma. Invest Ophthalmol Vis Sci. 2004; 45: 3662–3668 [DOI] [PubMed] [Google Scholar]
- 11. Pittman RN. Oxygen transport in normal and pathological situations: defects and compensations. In: Pittman RN. ed Regulation of Tissue Oxygenation. San Rafael, CA: Morgan & Claypool Life Sciences; 2011: 47–50 [PubMed] [Google Scholar]
- 12. Teng PY, Wanek J, Blair NP, Shahidi M. Inner retinal oxygen extraction fraction in rat. Invest Ophthalmol Vis Sci. 2013; 54: 647–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wanek J, Teng PY, Blair NP, Shahidi M. Inner retinal oxygen delivery and metabolism under normoxia and hypoxia in rat. Invest Ophthalmol Vis Sci. 2013; 54: 5012–5019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shahidi M, Shakoor A, Blair NP, Mori M, Shonat RD. A method for chorioretinal oxygen tension measurement. Curr Eye Res. 2006; 31: 357–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shonat RD, Kight AC. Oxygen tension imaging in the mouse retina. Ann Biomed Eng. 2003; 31: 1084–1096 [DOI] [PubMed] [Google Scholar]
- 16. ANSI. American National Standard for Safe Use of Lasers ANSI Z136.1-2007. Orlando, FL: Laser Institute of America; 2007. [Google Scholar]
- 17. Crystal GJ. Principles of cardiovascular physiology. In: Estafanous FG, Barash PG, Reves JG. eds Cardiac Anesthesia: Principles and Clinical Practice. Philadelphia, PA: Lippincott Williams & Wilkins; 2001: 37–57 [Google Scholar]
- 18. Cartheuser CF. Standard and pH-affected hemoglobin-O2 binding curves of Sprague-Dawley rats under normal and shifted P50 conditions. Comp Biochem Physiol Comp Physiol. 1993; 106: 775–782 [DOI] [PubMed] [Google Scholar]
- 19. Chen J, Edwards A, Layton AT. Effects of pH and medullary blood flow on oxygen transport and sodium reabsorption in the rat outer medulla. Am J Physiol Renal Physiol. 2010; 298: F1369–F1383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shakoor A, Blair NP, Mori M, Shahidi M. Chorioretinal vascular oxygen tension changes in response to light flicker. Invest Ophthalmol Vis Sci. 2006; 47: 4962–4965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shih YY, De la Garza BH, Muir ER, et al. Lamina-specific functional MRI of retinal and choroidal responses to visual stimuli. Invest Ophthalmol Vis Sci. 2011; 52: 5303–5310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ahmed J, Linsenmeier RA, Dunn R Jr. The oxygen distribution in the prelaminar optic nerve head of the cat. Exp Eye Res. 1994; 59: 457–465 [DOI] [PubMed] [Google Scholar]
- 23. Buerk DG, Atochin DN, Riva CE. Simultaneous tissue PO2, nitric oxide, and laser Doppler blood flow measurements during neuronal activation of optic nerve. Adv Exp Med Biol. 1998; 454: 159–164 [DOI] [PubMed] [Google Scholar]
- 24. Buerk DG, Riva CE. Adenosine enhances functional activation of blood flow in cat optic nerve head during photic stimulation independently from nitric oxide. Microvasc Res. 2002; 64: 254–264 [DOI] [PubMed] [Google Scholar]
- 25. Lau JC, Linsenmeier RA. Oxygen consumption and distribution in the Long-Evans rat retina. Exp Eye Res. 2012; 102: 50–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hammer M, Vilser W, Riemer T, et al. Retinal venous oxygen saturation increases by flicker light stimulation. Invest Ophthalmol Vis Sci. 2011; 52: 274–277 [DOI] [PubMed] [Google Scholar]
- 27. Tsai AG, Johnson PC, Intaglietta M. Oxygen gradients in the microcirculation. Physiol Rev. 2003; 83: 933–963 [DOI] [PubMed] [Google Scholar]
- 28. Shih YY, Wang L, De La Garza BH, et al. Quantitative retinal and choroidal blood flow during light, dark adaptation and flicker light stimulation in rats using fluorescent microspheres. Curr Eye Res. 2013; 38: 292–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kang Derwent J, Linsenmeier RA. Effects of hypoxemia on the a- and b-waves of the electroretinogram in the cat retina. Invest Ophthalmol Vis Sci. 2000; 41: 3634–3642 [PubMed] [Google Scholar]


