Induction cetuximab, paclitaxel, and carboplatin followed by the same drug chemoradiation regimen was evaluated in 63 eligible patients with stage III/IV resectable HNSCC. Ninety percent had biopsy proven complete response. The overall and event-free 3-year survival estimates of 78% and 55% met the pre-determined end points to warrant further study of this promising treatment approach.
Keywords: cetuximab, induction chemotherapy, chemoradiation, head–neck
Abstract
Background
E2303 evaluated cetuximab, paclitaxel, and carboplatin used as induction therapy and concomitant with radiation therapy in patients with stage III/IV head and neck squamous cell carcinoma (HNSCC) determining pathologic complete response (CR), event-free survival (EFS), and toxicity.
Patients and methods
Patients with resectable stage III/IV HNSCC underwent induction therapy with planned primary site restaging biopsies (at week 8 in clinical complete responders and at week 14 if disease persisted). Chemoradiation (CRT) began week 9. If week 14 biopsy was negative, patients completed CRT (68–72 Gy); otherwise, resection was carried out. p16 protein expression status was correlated with response/survival.
Results
Seventy-four patients were enrolled; 63 were eligible. Forty-four (70%) were free of surgery to the primary site, progression, and death 1-year post-treatment. Following induction, 41 (23 CR) underwent week 8 primary site biopsy and 24 (59%) had no tumor (pathologic CR). Week 14 biopsy during chemoradiation (50 Gy) in 34 (15 previously positive biopsy; 19 no prior biopsy) was negative in 33. Thus 90% of eligible patients completed CRT. Overall survival and EFS were 78% and 55% at 3 years, respectively. Disease progression in 23 patients (37%) was local only in 10 (16%), regional in 5 (8%), local and regional in 2 (3%), and distant in 5 patients (8%). There were no treatment-related deaths. Toxicity was primarily hematologic or radiation-related. p16 AQUA score was not associated with response/survival.
Conclusions
Induction cetuximab, paclitaxel, and carboplatin followed by the same drug CRT is safe and induces high primary site response and promising survival.
Clinical trials number
introduction
Non-operative management of locally advanced squamous carcinoma of the head and neck (HNSCC) is commonly used to reduce the morbidity of primary resection. Concomitant cisplatin and radiation therapy (RT) for cancers of the larynx and oropharynx achieves excellent local control of disease. Sequential induction chemotherapy and chemoradiation (CRT) may further improve disease control and survival [1].
The Brown University Oncology Group evaluated induction paclitaxel and carboplatin followed by the same drugs concomitant with RT using primary site biopsy to evaluate response to induction [2, 3]. Promising results led the Eastern Cooperative Oncology Group (ECOG) to design a phase II trial, E2303, evaluating the combination of cetuximab, paclitaxel, and carboplatin followed by CRT with biopsy to document response. This report provides 3-year outcome data.
patients and methods
Eligible patients had biopsy confirmed, resectable stage III or IV squamous carcinoma of the oral cavity, oropharynx, larynx, or hypopharynx, measureable disease, ECOG performance status 0–1, normal values for neutrophil and platelet counts, serum creatinine, and bilirubin. All patients provided written informed consent, in accordance with institutional guidelines.
Patients with unresectable disease were ineligible. This was defined by: fixed nodal metastases or carotid encasement; tumor involvement of the root of the tongue or pharyngeal musculature; fixation to prevertebral fascia; penetration through laryngeal cartilage into strap muscles; or >1 cm tracheal invasion.
study design and treatment
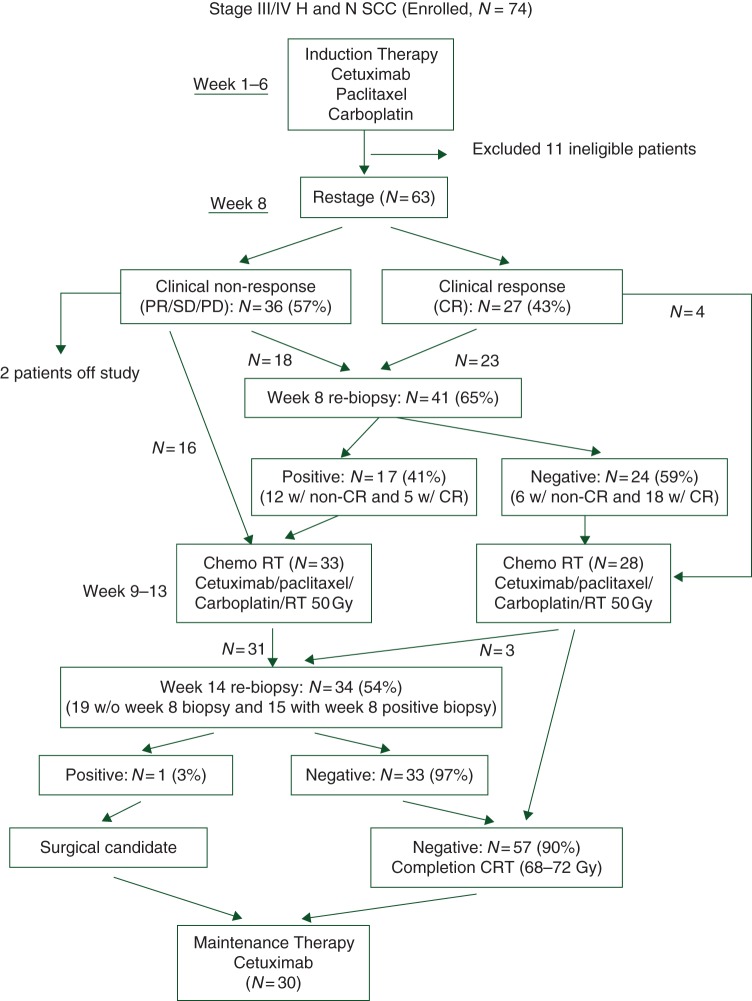
Before treatment, all patients underwent panendoscopy and percutaneous gastrostomy tube placement. All had CT or MR imaging of the head, neck, and chest (Figure 1).
Figure 1.
Treatment schema and outcome results.
induction chemotherapy
Induction chemotherapy consisted of cetuximab 400 mg/m2 IV day 1, week 1, then 250 mg/m2/week, weeks 2–6; paclitaxel 90 mg/m2/week infused over 1 h followed by carboplatin AUC = 2 (Cockcroft–Gault formula) IV weekly for 6 weeks. During weeks 7–8, patients exhibiting a clinical complete response (CR) had biopsy of the primary site.
CRT (weeks 9–13)
RT was once daily, 200 cGy/fraction, total 68–72 Gy. Two-dimensional, three-dimensional, or IMRT could be used. Chemotherapy consisted of cetuximab 250 mg/m2/week followed by paclitaxel 30 mg/m2/week infused over 1 h and carboplatin AUC 1 IV weekly throughout RT. At 50 Gy, primary site response was assessed and a biopsy carried out in those who had not yet achieved pathologic CR. Those attaining one completed CRT. If the biopsy showed cancer, resection was recommended. A planned neck dissection for patients presenting with N1–3 disease was carried out at surgeon discretion.
maintenance therapy
At the completion of CRT or following surgery, patients were to receive cetuximab 250 mg/m2/week for 6 months or until progression of disease.
study design and target accrual
The primary objective was estimation of the 1-year post-treatment event-free survival (EFS) rate. This was estimated by the proportion of patients alive and free of surgery to the primary site, or disease progression 16 months (12 months after finishing 4 months of sequential therapy) after registration [4–6]. Patients without documented events were censored at the time of the last disease evaluation.
Based on comparable studies, the null 1-year post-treatment EFS rate was estimated to be 59% [2, 3]. The treatment regimen was intended to increase the EFS rate at 1-year post-treatment from 59% to 74% (15% absolute increase). The exact one-sided binomial test suggested a minimum sample size of 65 eligible patients was required to detect the proposed difference with 10% type I error rate and 90% power. Thus, if 44 or more patients among 65 eligible were free of surgery to the primary site, disease progression, recurrence, or death at 1-year post-treatment, the null hypothesis would be rejected and the regimen considered worthy of further pursuit. Against a projected 10% ineligibility rate, a total of 72 patients were to be enrolled in order to yield 65 eligible patients.
tissue microarray construction
Paraffin-embedded specimens were collected at baseline, and in patients undergoing biopsy at weeks 8 and 14. Hematoxylin and eosin-stained slides were reviewed for the presence of squamous cell carcinoma, and marked for 0.6-mm core extraction. Cores were placed on the recipient microarray block using a Tissue Microarrayer (Beecher Instrument, Silver Spring, MD) at ECOG Pathology Center at Northwestern University. Cores from HPV18-positive HeLa and HPV16-positive Caski cell lines fixed in formalin and embedded in paraffin included in the array as controls.
fluorescent immunohistochemistry and automated quantitative protein expression analysis (AQUA)
Automated image acquisition and analysis using AQUA (HistoRx, New Haven, CT) has been described previously [7]. Monochromatic, high-resolution images were obtained of each histospot after immunofluorescent staining. Areas of tumor were distinguished from stromal elements by creating a mask from the cytokeratin signal. A tumor nuclei-specific compartment was created by using DAPI signal to identify nuclei, and the cytokeratin signal to define cytoplasm/membrane. The target signal (AQUA score) was expressed as pixel intensity divided by the target area (tumor nuclei compartment). Histospots containing <10% tumor were excluded from further analysis.
statistical analysis
In addition to EFS, other end points included progression-free survival (PFS) and overall survival (OS). PFS was defined as the time from registration to documented progression or death, or patients were censored at the time of the last disease assessment. OS was defined by the time from registration to death from any cause or censored at last contact. For the n-year event-time rate, the rate was computed exactly at n years for OS and PFS. For EFS (n years post-treatment), the rate was computed at n years plus 4 months. All survival end points were analyzed by the Kaplan–Meier method [4] and significance was tested by log-rank tests [5].
Objective response was evaluated by modified RECIST criteria with separate consideration of radiographic and clinical examination. Toxicity was assessed using the NCI Common Terminology Criteria for Adverse Events Version 3.0. Confidence limits for response rates were estimated using exact binomial confidence intervals [5]. Fisher's exact test was used to compare objective response rates between the groups. An 8-week landmark analysis [6] was carried out to compare biopsy status (yes/no) and biopsy results (positive/negative) after induction on event-time distributions to minimize lead-time bias. A 4-month landmark analysis was carried out to evaluate the effect of maintenance therapy on survival. The event time was computed forward from the landmark. Patients whose events transpired earlier were excluded from the landmark analysis. All P-values were two-sided and a level of 5% was considered statistically significant.
p16 analysis
AQUA scores for duplicate tissue cores were averaged to obtain a mean AQUA score for each tumor. For survival analysis, the mean automated AQUA scores were converted to binomial variables of high versus low expression around AQUA score 80 [8].
results
A total of 74 patients were enrolled between 15 December 2004 and 6 February 2006. Eleven were classified as ineligible due to: prior treatment (n = 2), baseline evaluations or labs not obtained within 4 weeks of registration (n = 4), metastatic aspect of staging unknown (n = 1), concurrent thyroid cancer (n = 1), unresectable (n = 2), and no baseline pathology report confirming primary disease (n = 1). A total of 63 patients were eligible and analyzable. Patient demographic and disease characteristics are shown in Table 1. Seventy-six percent had a history of tobacco use and 77% of these had a >20 pack-year smoking history. Among the 63 eligible patients, 30 (48%) received maintenance cetuximab with 25 completing the planned 6-month therapy. Reasons for discontinuing maintenance were patient refusal (n = 2), toxicity (n = 2), and disease progression (n = 1). For those not starting maintenance, the reasons were: considered as planned treatment completed (n = 11), patient refusal (n = 10), toxicity (n = 7), disease progression/relapse during treatment (n = 1), death on study (n = 1), and other (n = 3).
Table 1.
Characteristics of eligible patients (n = 63)
| n | % | |
|---|---|---|
| Age at study entry (years) | ||
| Median: 57 | ||
| Range: 31–76 | ||
| Gender | ||
| Male | 49 | 78 |
| Female | 14 | 22 |
| Race | ||
| White | 56 | 89 |
| Black | 7 | 11 |
| ECOG performance status | ||
| 0 | 39 | 62 |
| 1 | 24 | 38 |
| Smoking history | ||
| Never smoked | 14 | 24 |
| Cigarette smoker (pack years) | ||
| <20 | 10 | 18 |
| 20–40 | 17 | 29 |
| >40 | 17 | 29 |
| Unknown/other | 5 | — |
| Cancer stage | ||
| Stage III | 21 | 33 |
| Stage IV | 42 | 67 |
| Tumor stage (Clinical) | ||
| T1–2 | 22 | 35 |
| T3–4 | 41 | 65 |
| Primary site | ||
| Oral–oral cavity | 3 | 5 |
| Oropharynx, NOS | 7 | 11 |
| Tonsillar fossa, tonsil | 13 | 21 |
| Posterior pharyngeal wall | 3 | 5 |
| Base of tongue | 12 | 19 |
| Hypopharynx, NOS | 4 | 6 |
| Pyriform fossa | 3 | 5 |
| Larynx, NOS | 6 | 9 |
| Supraglottic larynx | 7 | 11 |
| Glottic larynx | 2 | 3 |
| >1 primary site | 3 | 5 |
Toxicity
The median number of weeks of treatment received for the 63 eligible patients was: induction—6 weeks (range 4–6) and CRT—7 weeks (range 0–9) (Table 2).
Table 2.
Treatment-related toxicities for treated patients (n = 70)
| Toxicity type | Grade |
|
|---|---|---|
| 3 (n) | 4 (n) | |
| Hematologic | ||
| Hemoglobin | 1 | 0 |
| Leukocytes | 22 | 6 |
| Febrile neutropenia | 2 | 0 |
| Lymphopenia | 3 | 1 |
| Neutrophils | 17 | 14 |
| Generalized | ||
| Allergic reaction | 2 | 1 |
| Fatigue | 6 | 0 |
| Weight loss | 7 | 0 |
| Burn | 0 | 1 |
| Rash/desquamation/acneiform | 11 | 0 |
| Radiation dermatitis/burn | 15 | 1 |
| Neuropathy | 1 | 0 |
| Thrombosis | 1 | 0 |
| Dehydration | 7 | 0 |
| Head and neck/GI | ||
| Anorexia | 12 | 1 |
| Diarrhea | 3 | 0 |
| Dry mouth | 4 | 0 |
| Dysphagia | 20 | 0 |
| Esophagitis | 1 | 0 |
| Muco/stomatitis (symptom) oropharynx | 34 | 2 |
| Nausea | 2 | 0 |
| Vomiting | 2 | 0 |
| Oral cavity pain | 4 | 0 |
| Throat/pharynx/larynx pain | 2 | 0 |
| Tumor pain | 1 | 0 |
| Pulmonary airway | ||
| Bronchospasm, wheezing | 0 | 1 |
| Dyspnea | 2 | 0 |
| Obstruction—airway larynx | 0 | 1 |
| Voice changes/dysarthria | 3 | 0 |
| Infection with Gr 0–2 neutrophils | ||
| Bladder | 1 | 0 |
| Colon | 2 | 0 |
| Urinary tract/catheter | 3 | 0 |
| Oropharynx mucosa | 6 | 0 |
| Foreign body/skin | 3 | 0 |
| Anal canal | 1 | 0 |
| Electrolyte—abnormalities | 0 | |
| Potassium | 3 | 0 |
| Sodium | 1 | 0 |
| Phosphorus | 3 | 0 |
| Magnesia | 2 | 1 |
| Glucose | 1 | 0 |
| Bilirubin | 1 | 1 |
| Calcium | 0 | 1 |
| Creatinine | 1 | 0 |
| Worst degree | 43 | 21 |
The protocol was originally written with an induction paclitaxel dose of 135 mg/m2/week. However, the occurrence of febrile neutropenia in the initial 40 patients necessitated a permanent dose reduction of paclitaxel to 90 mg/m2/week. Among the 70 treated patients, 21 experienced grade 4 treatment-related toxicity and 43 had grade 3 toxicity; there were no treatment-related deaths. Toxicity was primarily hematologic (leukopenia and neutropenia) and radiation-related (mucositis, dysphagia, dermatitis); 11 patients had grade 3 rash.
response
best response at last follow-up
Among the 63 eligible patients, the objective clinical response rate (54 CR, 0 PR) at the primary site was 86% [95% confidence interval (CI) 75%–93%]. The objective radiological response rate (38 CR, 6 PR) at the primary site was 70% (95% CI 57%–81%).
post-induction (week 8) and post-50 Gy CRT (week 14)
Of 60 patients undergoing biopsies at week 8 and/or week 14, only one had persistent tumor to week 14. The proportion of pathologic CRs among those biopsied was 59% (24/41) and 97% (33/34) at weeks 8 and 14, respectively. Thus, 57 of 60 (95%) biopsied patients and 90% (57/63) of all eligible patients had pathologic CR at the primary site.
treatment outcome
Among the 63 eligible patients, 20 had died and 43 were alive at the time of the analysis. The median follow-up of surviving patients was 45 months (range: 20–52). Twenty-three (37%) had documented recurrence: 10 local only, 5 nodal, 2 loco-regional, 5 distant, and 1 unknown site. Three patients underwent primary site resection, one with progression after completing 70 Gy CRT, and two after induction based upon clinical decision by the treating surgeon. Neither of the latter patients had residual tumor. Neck dissections were carried out in 30 patients with tumor found in 11.
relation of recurrence to primary site induction response
Among the 10 patients with local recurrence, 5 had a clinical CR and of these, 3 were biopsy negative at week 8. Among five patients progressing in the neck, one had a clinical CR that was biopsy negative.
survival outcome
Among the 63 eligible patients, 44 were free of surgery to the primary, progression, or death at 16 months after registration. The criterion for a minimum of 44 patients to be event-free was met and the null hypothesis was rejected. The EFS rate (Figure 2A) was estimated to be 73% (95% CI 62%–84%) and OS (Figure 2B) 97% (95% CI 92%–100%). The PFS rate was 82% (95% CI 73%–92%). OS by week 8 biopsy status (no versus yes) and by week 8 biopsy results (negative versus positive) was carried out via the landmark analysis. Patients biopsied after induction had superior OS to those not biopsied (median OS not reached versus 45.1 months, 0 = 0.04), but no difference in PFS or EFS. No difference in OS was observed between patients with negative biopsy and those with positive biopsy after induction (median OS not reached versus 47.5 months, P = 0.93).
Figure 2.
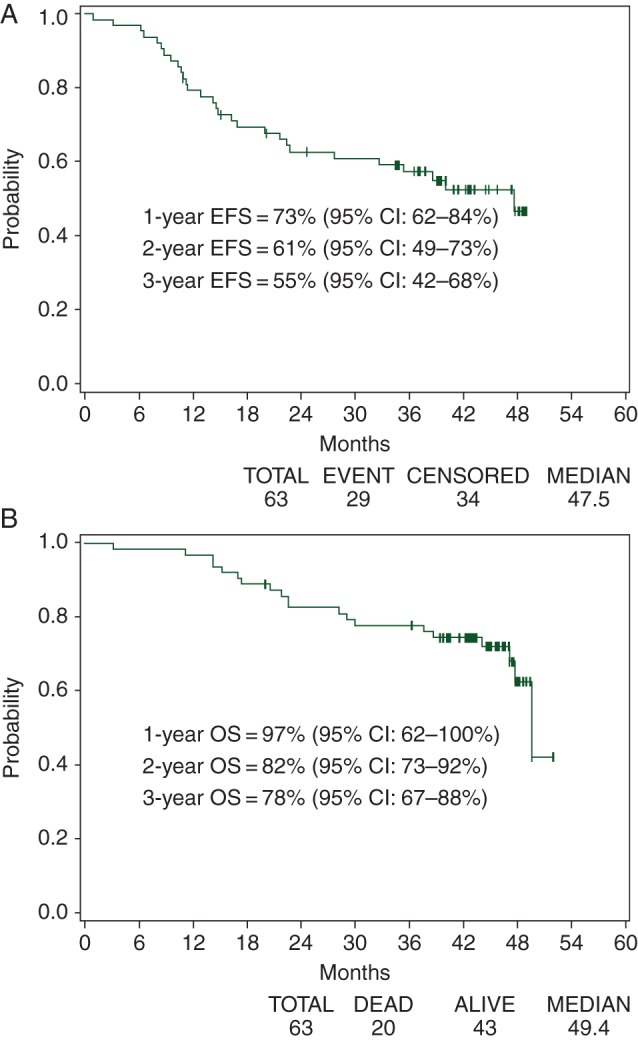
Survival outcomes: (A) event-free survival and (B) overall survival.
To evaluate the effect of maintenance cetuximab, overall survival was examined. The 4-month landmark analysis showed no difference between patients treated with maintenance and those not treated (median OS: not reached versus 45.4 months, respectively, P = 0.79), but the study was not powered to detect one.
p16 status and outcome
Thirty-four patients had sufficient tissue for analysis of p16 protein expression by AQUA. Twenty patients were classified as low expressors/negative and 14 were classified as high expressors/positive. No significant association could be demonstrated between p16 status and response or any other outcome in this small cohort (supplementary Table S1, available at Annals of Oncology online).
discussion
ECOG 2303 evaluated induction cetuximab, paclitaxel, and carboplatin followed by the same regimen concurrent with RT focusing on pathologic response, EFS, and toxicity. The 16-month EFS rate was estimated to be 73% and thus the treatment met the primary end point and is considered worthy of further study.
Others have reported their experience utilizing induction paclitaxel (135 mg/m2/week), carboplatin, and cetuximab observing a 19% clinical CR and 77% PR rate [9]. Toxicity included skin rash in nearly half and neutropenia without fever in 20%; delays in weekly treatment occurred in 60% even with allowance for use of filgrastim. Post-induction treatment included RT for T1–2 disease and either CRT or surgery for T3 cancers. The 3-year PFS and OS rates were 87% and 91%, respectively, for a recognizably more favorable population.
The docetaxel, cisplatin, 5-fluorouracil regimen (TPF) for induction is superior to cisplatin/5-fluorouracil, but a benefit from adding induction to CRT has not been demonstrated [1]. The severity of toxicity associated with TPF and the requirement for growth factor support contrasts with the tolerability of the paclitaxel, carboplatin, and cetuximab regimen tested in E2303. It has been suggested that the toxicity of TPF may be blunted by elimination of 5-fluorouracil. A trial of induction docetaxel, cisplatin, and cetuximab showed a clinical response rate of 86% after induction and 100% after sequential CRT; however, grade 3/4 neutropenia occurred in 77% and febrile neutropenia in 10% [10].
In E2303, primary site biopsy was utilized in patients exhibiting an apparent CR to induction therapy. Incomplete responders to induction therapy as confirmed by clinical and/or pathologic exam had a restaging biopsy after 50 Gy. For the total of 60 patients who had biopsy, pathologic CR was confirmed in 57 (95%), demonstrating the high level of activity of cetuximab, paclitaxel, and carboplatin in this sequential approach.
Notably, one-third of patients believed on clinical grounds to harbor tumor had no cancer on biopsies carried out after completion of induction chemotherapy; however, sampling error may account for some of these cases. Neither clinical response nor the biopsy result after induction correlated with subsequent local failure or EFS (data not shown). At a median follow-up of 45 months, approximately half of the failures (12/23) included disease recurrence at the primary (10 local, 2 locoregional). Recurrence ensued in 18 of 54 patients with a recorded clinical CR (including seven with pathologic CR after induction).
No relationship was observed between efficacy measures and p16 status. However, the study was not powered to detect such an association. Further, HPV association may interact with other risk factors such as stage and tobacco exposure [11, 12]. Our trial, in which 76% of patients were current or former smokers and which was confined to higher stage patients, may not have included an adequate proportion of true favorable risk HPV-associated oropharynx cancers for analysis.
Maintenance cetuximab was delivered in 48% of patients, and no difference in response rate and overall survival was noted. The rationale for cetuximab maintenance therapy arose from two principal considerations. The first is the relationship between EGFR signaling and poor outcome, including after radiation [13]. Residual microscopic local or distant disease might be EGFR expressing and amenable to maintenance therapy with cetuximab. The second consideration arose because of the demonstration of the role of nuclear EGFR in HNSCC. Nuclear EGFR interacts with DNA-dependent protein kinase and PCNA to foster repair of radiation-induced DNA damage, and preclinical models have demonstrated that radiation increases nuclear translocation, whereas cetuximab can decrease nuclear trafficking of EGFR [14, 15]. Thus, continuing cetuximab during the critical period of potential DNA repair might improve locoregional control. The proportion who received maintenance therapy was likely not sufficient to evaluate the worth of this intervention.
In summary, the combination of paclitaxel, carboplatin, and cetuximab sequential therapy tested in E2303 met the primary end point with an EFS rate of 73% and demonstrated a high pathologic CR rate. This regimen merits further evaluation.
funding
The work was sponsored by the Eastern Cooperative Oncology Group (Robert L. Comis, MD, Chair) and supported in part by Public Health Service Grants (CA23318, CA66636, CA21115, CA27525, CA16116) and from the National Cancer Institute, NIH, and the Department of Health and Human Services. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
disclosure
BAB was a consultant to Bristol Myers Squibb and Imclone Systems. All remaining authors have declared no conflicts of interest.
Supplementary Material
acknowledgements
The authors thank Ms Olga Grant for her assistance with the manuscript preparation.
references
- 1.Forastiere AA, Adelstein DJ, Manola J. Induction chemotherapy meta-analysis in head and neck cancer: right answer, wrong question. J Clin Oncol. 2013;31:2844–2846. doi: 10.1200/JCO.2013.50.3136. [DOI] [PubMed] [Google Scholar]
- 2.Wanebo HJ, Chougule P, Ready N, et al. Preoperative chemotherapy with paclitaxel and carboplatin and radiation achieves high rates of local regional control. Proc ASCO. 2001;20 abstr 937. [Google Scholar]
- 3.Ready N, Chougule P, Nadeen H, et al. Induction weekly paclitaxel carboplatin followed by concurrent paclitaxel, carboplatin and radiotherapy in advanced head and neck squamous cancers. Proc ASCO. 2002;21 abstr 943. [Google Scholar]
- 4.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 5.Cox DR, Snell EJ. Analysis of Binary Data. 2nd Edition. London: Chapman and Hall; 1989. [Google Scholar]
- 6.Anderson JR, Cain KC, Gelber RD, et al. Analysis and interpretation of the comparison of survival by treatment outcome variables in Cancer Clinical Trials. Cancer Treat Rep. 1985;69:1139–1144. [PubMed] [Google Scholar]
- 7.Camp RL, Chung GG, Rim DL. Automated subcellular localization and quantification of protein expression in tissue microarrays. Nat Med. 2002;8:1323–1327. doi: 10.1038/nm791. [DOI] [PubMed] [Google Scholar]
- 8.Weinberger PM, Ziwei Y, Haffty BG, et al. Molecular classification identifies a subset of human papillomavirus-associated oropharyngeal cancers with favorable prognosis. J Clin Oncol. 2006;24:736–747. doi: 10.1200/JCO.2004.00.3335. [DOI] [PubMed] [Google Scholar]
- 9.Kies MS, Holsinger FC, Lee J, et al. Induction chemotherapy and cetuximab for locally advanced squamous cell carcinoma of the head and neck: results from a Phase II prospective trial. J Clin Oncol. 2010;28:8–14. doi: 10.1200/JCO.2009.23.0425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Argiris AE, Gibson MK, Heron DE, et al. Phase II trial of neoadjuvant docetaxel (T), cisplatin (P), and cetuximab (E) followed by concurrent radiation, P and E in locally advanced head and neck cancer. J Clin Oncol. 2010;28:5294–5300. doi: 10.1200/JCO.2010.30.6423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gillison ML, Zhang Q, Jordan R, et al. Tobacco smoking and increased risk of death and progression for patients with p16-positive and p16-negative oropharyngeal cancer. J Clin Oncol. 2012;30:2102–2111. doi: 10.1200/JCO.2011.38.4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ang KK, Berkey BA, Tu X, et al. Impact of epidermal growth factor receptor expression on survival and pattern of relapse in patients with advanced head and neck carcinoma. Cancer Res. 2002;62:7350–7356. [PubMed] [Google Scholar]
- 14.Psyrri A, Yu Z, Weinberger PM, Sasaki C, et al. Quantitative determination of nuclear and cytoplasmic epidermal growth factor receptor expression in oropharyngeal squamous cell cancer by using automated quantitative analysis. Clin Cancer Res. 2005;11:5856–5862. doi: 10.1158/1078-0432.CCR-05-0420. [DOI] [PubMed] [Google Scholar]
- 15.Dittmann K, Mayer C, Fehrenbacher B, et al. Radiation-induced epidermal growth factor receptor nuclear import is linked to activation of DNA-dependent protein kinase. J Biol Chem. 2005;280:31182–31189. doi: 10.1074/jbc.M506591200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.