Analysis of tumor samples from the ZODIAC study showed that EGFR gene mutation or EGFR gene amplification predicted greater benefits from vandetanib plus docetaxel treatment compared with docetaxel treatment alone. In contrast, KRAS mutations were associated with poor outcomes to docetaxel therapy, and addition of vandetanib did not appear to confer any additional benefit over docetaxel alone.
Keywords: vandetanib, docetaxel, EGFR, KRAS, lung cancer
Abstract
Background
ZODIAC was a randomized phase III study of second-line treatment in patients with advanced non-small cell lung cancer (NSCLC) that evaluated the addition of vandetanib to docetaxel. The study showed a statistically significant improvement in progression-free survival and objective response rate, but not in overall survival for unselected patients. This study evaluated epidermal growth factor receptor (EGFR) gene mutation, copy number gain, and protein expression, and KRAS gene mutation, in pretreatment tumor samples as potential biomarkers predicting benefit from vandetanib as second-line treatment of NSCLC.
Patients and methods
After progression following first-line chemotherapy, 1391 patients with locally advanced or metastatic (stage IIIB/IV) NSCLC were randomized 1 : 1 to receive vandetanib (100 mg/day) plus docetaxel (75 mg/m2 every 21 days) or placebo plus docetaxel in the ZODIAC study. Archival tumor samples (n = 570) were collected from consenting patients (n = 958) for predefined, prospective biomarker analyses.
Results
Of evaluable samples, 14% were EGFR mutation positive, 35% were EGFR FISH positive, 88% were EGFR protein expression positive, and 13% were KRAS mutation positive. Compared with the overall study population, in which progression-free survival (PFS) [hazard ratio (HR) = 0.79] but not OS (HR = 0.91) were significantly improved with vandetanib, there was greater relative clinical benefit for patients with EGFR mutation-positive tumors [PFS HR 0.51, confidence interval (CI) 0.25–1.06 and OS HR 0.46, CI 0.14–1.57] and EGFR FISH-positive tumors (PFS HR 0.61, CI 0.39–0.94 and OS HR 0.48, CI 0.28–0.84). Similarly, patients with EGFR mutation or FISH-positive tumor samples who received vandetanib had an increased chance of objective tumor response (odds ratios 3.34, CI 0.8–13.89, and 3.90, CI 1.02–14.82, respectively). There did not appear to be benefit for vandetanib in patients with KRAS mutation-positive tumors.
Conclusions
High EGFR gene copy number or activating EGFR mutations may identify patient subgroups who receive increased clinical benefit from vandetanib in combination with docetaxel in second-line NSCLC.
ClinicalTrials.gov
introduction
Vandetanib is a once-daily, oral anticancer agent that selectively targets the vascular endothelial growth factor receptor (VEGFR), epidermal growth factor receptor (EGFR) and REarranged during Transfection (RET) tyrosine kinases [1, 2], and is currently approved for the treatment of symptomatic or progressive medullary thyroid cancer [3, 4].
ZODIAC (NCT00312377), a randomized, double-blind, multicenter, placebo-controlled phase III study, investigated the efficacy of vandetanib (100 mg daily) plus docetaxel versus docetaxel alone in patients with previously treated advanced non-small cell lung cancer (NSCLC). The addition of vandetanib to docetaxel showed a statistically significant improvement in the primary end point of progression-free survival (PFS) and in objective response rate (ORR), but not in overall survival (OS) [5]. The focus of the prospective biomarker analyses in the ZODIAC study was on as potential markers of differential benefit from EGFR tyrosine kinase inhibitors (TKIs), including EGFR protein expression, EGFR gene amplification, EGFR gene mutation [6, 7], and KRAS gene mutation [8], since, at the study outset, no tumor biomarkers predicting sensitivity to VEGFR2 inhibitors in NSCLC had been identified. The results of these analyses are reported here.
patients and methods
study design
Full details of the ZODIAC study have been published [5]. The primary objective of the trial was to determine whether vandetanib added to docetaxel prolonged PFS compared with placebo plus docetaxel. Secondary efficacy end points included OS and ORR. Patients were given the option of providing an additional informed consent to allow archival tumor samples to be used for the purposes of biomarker research. All biomarker analyses were completed before the study was unblinded.
sample analysis
Biomarker analyses methods and the demographics and baseline characteristics for patients with evaluable tumor samples are described in supplementary Methods (available at Annals of Oncology online).
statistical analysis
The ZODIAC study had two co-primary analysis populations [5] and, therefore, the conventional two-sided 5% significance level was adjusted to 2.5% for all subgroup analyses, and further adjusted to 2.42% for PFS and 2.48% for OS due to a single interim analysis. All P values are two-sided. The subgroup analyses presented here were prospective and due to their exploratory nature, no adjustment for multiple testing was used. PFS and OS were analyzed using the log-rank test (unadjusted model with treatment factor only) and ORR was analyzed using logistical regression. Due to the number of patients and number of variables, multivariate analyses were not carried out. All statistical analyses were done using SAS version 9.1.
results
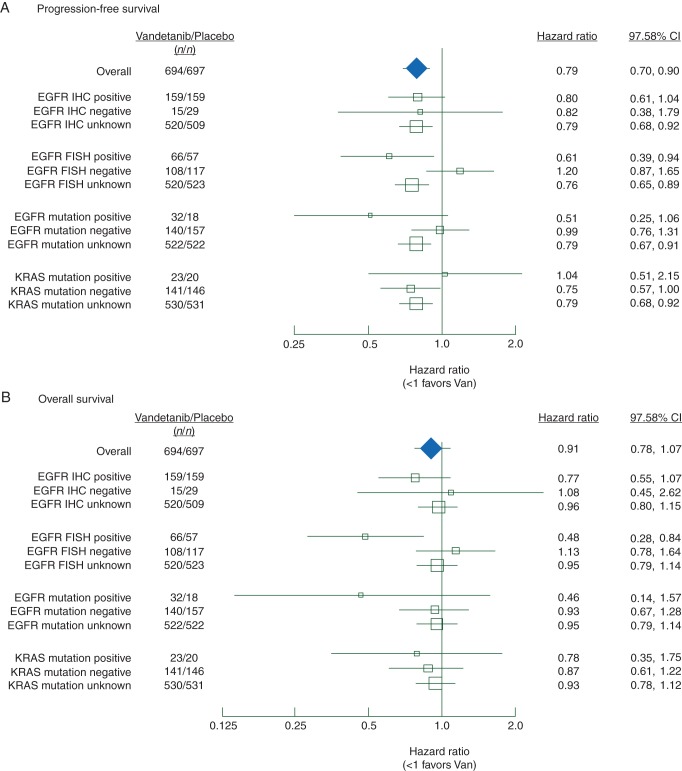
The benefits of vandetanib treatment did not appear to be different in patients with EGFR protein expression positive (IHC+) or negative (IHC−) tumor samples compared with unselected patients in terms of the primary end point (PFS), or secondary efficacy end points (OS, ORR) (Figure 1, supplementary Tables S1–S3, available at Annals of Oncology online).
Figure 1.
Forest plots of (A) progression-free survival, and (B) overall survival by biomarker subgroups. Patients who did not consent to biomarker analyses, or where there was no suitable tumor sample available, or where the sample was of insufficient quality, or where a sample was unevaluable were categorized as biomarker status unknown.
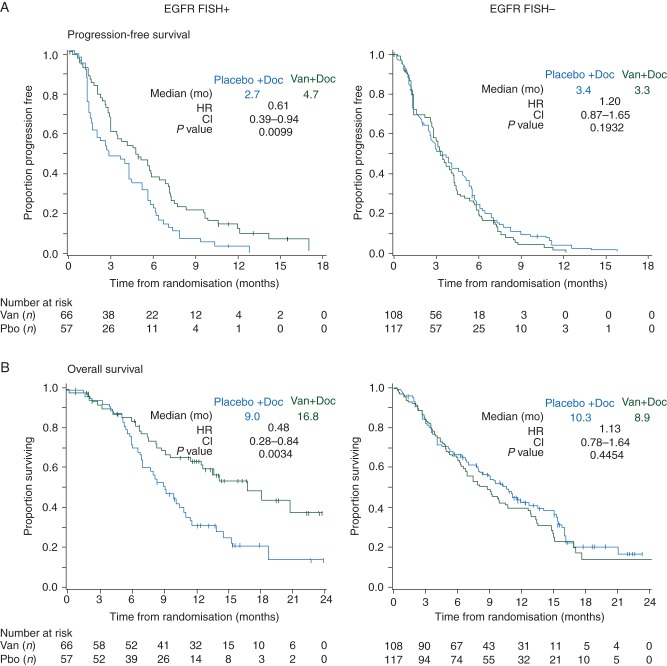
There were increased proportions of Asians (46% versus 24%), nonsmokers (21% versus 11%) and adenocarcinoma histology (67% versus 52%) among patients with EGFR gene amplification-positive (FISH+) tumor samples compared with patients with EGFR gene amplification-negative (FISH−) tumor samples. In the EGFR FISH+ patient subgroup, the observed outcomes suggest benefit for vandetanib over placebo in terms of PFS [hazard ratio (HR) 0.61; 97.58% confidence interval (CI) 0.39–0.94; P = 0.010; median 4.7 versus 2.7 months], OS (HR 0.48; 97.52% CI 0.28–0.84; P = 0.003; median 16.8 versus 9.0 months), and ORR (22.7 versus 7.0%; odds ratio 3.90; 97.5% CI 1.02–14.82; P = 0.023). In contrast, for the EGFR FISH− patient subgroup, the data suggest a lack of benefit of vandetanib treatment compared with placebo (Figures 1 and 2, supplementary Tables S1–S3, available at Annals of Oncology online).
Figure 2.
Kaplan–Meier curves for (A) progression-free survival, and (B) overall survival by EGFR gene amplification (EGFR FISH) status. Van (vandetanib), Doc (docetaxel), Pbo (placebo), mo (months), HR (hazard ratio), CI (confidence interval). HR <1.0 favors vandetanib + docetaxel versus placebo + docetaxel.
EGFR mutation status
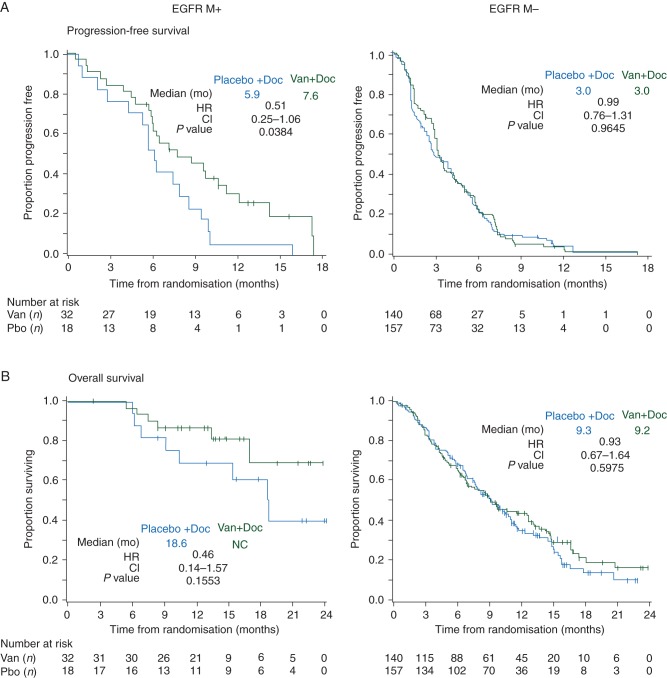
A greater proportion of patients with EGFR mutation-positive (MT+) tumor samples were female (42% versus 25%), East Asian (74% versus 29%), nonsmokers (42% versus 12%), stage IV (96% versus 84%), or had adenocarcinoma histology (92% versus 50%) compared with patients with EGFR mutation negative (MT−) tumors. Patients with EGFR MT+ tumor samples had outcomes suggesting improved PFS in the vandetanib arm compared with the placebo arm (HR 0.51; 97.58% CI 0.25–1.06; P = 0.038; median 7.6 versus 5.9 months), OS (HR 0.46; 97.52% CI 0.14–1.57; P = 0.155; median not reached versus 18.6 months) and ORR (56.3 versus 27.8%; odds ratio 3.34; 97.5% CI 0.80–13.89; P = 0.058). There did not appear to be a benefit of vandetanib compared with placebo in patients with EGFR MT− tumor samples (Figures 1 and 3, supplementary Tables S1–S3, available at Annals of Oncology online).
Figure 3.
Kaplan–Meier curves for (A) progression-free survival, and (B) overall survival by EGFR gene mutation (EGFR MT) status. Van (vandetanib), Doc (docetaxel), Pbo (placebo), mo (months), HR (hazard ratio), CI (confidence interval). HR <1.0 favors vandetanib + docetaxel versus placebo + docetaxel.
Although the majority of EGFR M+ tumor samples were also EGFR FISH+, post hoc analyses suggested that patients with EGFR M− tumors benefited from vandetanib treatment if the tumors were EGFR FISH+ (HR 0.78; 97.58% CI 0.46–1.33) but not if they were EGFR FISH− (HR 1.17; 97.58% CI 0.82–1.66) indicating that the benefits of vandetanib in EGFR FISH+ tumors are not restricted to patients with concurrent EGFR mutations (supplementary Tables S4, available at Annals of Oncology online).
KRAS mutation status
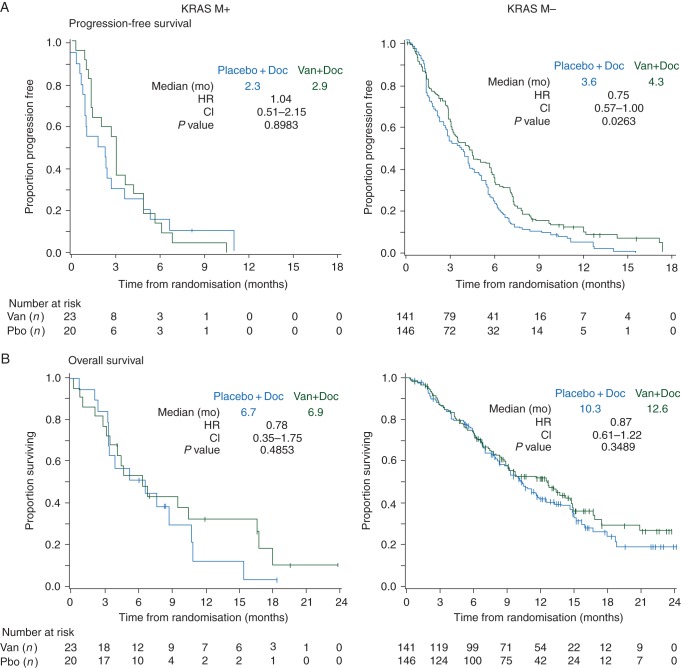
A smaller proportion of patients with KRAS MT+ tumors were Asian (12% versus 38%), nonsmokers (5% versus 19%) or had squamous histology (9% versus 33%) compared with patients with KRAS MT− tumors. In patients with KRAS MT+ tumors, the data do not suggest a clear benefit for the vandetanib arm compared with placebo in terms of PFS (HR 1.04; 97.58% CI 0.51–2.15; P = 0.898; median 2.9 versus 2.3 months), OS (HR 0.78; 97.52% CI 0.35–1.75; P = 0.485; median 6.9 versus 6.7 months), or ORR (8.7% versus 5.0%; odds ratio 1.81; 97.5% CI 0.11–30.83; P = 0.639). There was benefit of vandetanib in KRAS MT− tumors but this was similar to that seen in unselected patients (Figures 1 and 4, supplementary Tables S1–S3, available at Annals of Oncology online).
Figure 4.
Kaplan–Meier curves for (A) progression-free survival, and (B) overall survival by KRAS gene mutation (KRAS MT) status. Van (vandetanib), Doc (docetaxel), Pbo (placebo), mo (months), HR (hazard ratio), CI (confidence interval). HR <1.0 favors vandetanib + docetaxel versus placebo + docetaxel.
The focus of the planned analyses was to evaluate potential predictive markers for vandetanib plus docetaxel, but it was also of interest to consider how the biomarker subgroups were associated with docetaxel response. For patients in the docetaxel alone arm, median PFS (5.9 months) and OS (18.6 months), and ORR (28%) were higher in the EGFR MT+ subgroup than in EGFR MT− (3.0 and 9.3 months, and 10%, respectively), In contrast, docetaxel treatment alone was associated with lower median PFS and OS, and ORR values in KRAS MT+ patients (2.3and 6.7 months, and 5%, respectively) than in KRAS MT− patients (3.6 and 10.3 months, and 12%, respectively). The EGFR FISH+ and FISH− subgroups appeared to have similar clinical efficacy outcomes when treated with docetaxel alone (Figures 2–4, supplementary Tables S3, available at Annals of Oncology online).
discussion
Vandetanib was the first shown to have additional efficacy when added to docetaxel chemotherapy in patients with previously treated NSCLC, in a randomized phase II study [9]. Nonetheless, in the subsequent phase III trial in a biomarker unselected population, the therapeutic impact of adding vandetanib to docetaxel yielded modest advantages in efficacy for PFS and ORR, but not OS [5, 10]. A recent meta-analysis of six combination studies with chemotherapy in advanced NSCLC concluded that although vandetanib has clinical activity, identification of predictive biomarkers is required to select subsets of patients with who may benefit from vandetanib [11]. This prospective, exploratory study was undertaken to evaluate which prespecified tumor biomarkers might identify subgroups of patients who received greatest relative benefit from vandetanib treatment in combination with docetaxel in the ZODIAC trial.
In the EGFR FISH+ patient subgroup, PFS, OS, and ORR had better observed outcomes in the vandetanib arm compared with placebo. The effect size (based on HR values) appeared large, with P values <0.025, despite the small subgroup size (n = 123) compared with the full study population (n = 1391), although no adjustment was made for multiplicity. Of note, the data suggest a benefit for vandetanib in patients with EGFR FISH+ tumors even when the tumor was EGFR M−, but no benefit for vandetanib treatment in patients with EGFR FISH− tumors. Other studies have suggested tumor EGFR FISH status as a potential predictive biomarker for EGFR tyrosine kinases in NSCLC, when used as monotherapy [12, 13] or in combination with chemotherapy [14]. In other studies, however, this association was not observed [15] or only occurred in EGFR FISH+ patients who also harbored a concurrent EGFR mutation [16].
We also saw that patients with EGFR MT+ tumors had trends favoring vandetanib in terms of ORR and PFS. The small number of EGFR MT+ patients in this study is likely to have reduced our ability to detect a statistically significant effect of vandetanib even though the effect size appeared large. Nonetheless, our data do suggest that activating EGFR mutations may be a marker for vandetanib benefit when used in combination with chemotherapy. Consistent with earlier studies, our data also suggest that EGFR MT+ tumor status is a marker of good prognosis for advanced NSCLC patients [17, 18]. The current data provided no evidence for any differential benefit of vandetanib treatment based on KRAS gene mutation status, although the small number of patients with KRAS MT+ samples means that only very marked differences would have been detectable. Patients with KRAS MT+ tumors appeared to do poorly irrespective of treatment arm supporting previous suggestions that KRAS MT+ tumor status may be a marker of poor prognosis for advanced NSCLC patients [19, 20].
Since vandetanib simultaneously inhibits both EGFR and VEGFR2 signaling [21], it is not possible in the present study to determine whether the biomarkers are of benefit from pharmacological inhibition of one or both of these targets, although the association between benefit from vandetanib and EGFR biomarkers suggests that the activity of the drug is mediated, at least in part, through inhibition of EGFR. However, in the randomized phase III BETA study comparing the combination of erlotinib and bevacizumab with erlotinib alone, the EGFR mutant subgroup appeared to derive greater benefit from the addition of bevacizumab suggesting that dual blockade of the VEGF and EGFR pathways may be particularly active in EGFR MT+ tumors [22]. Nonetheless, the present study raises the possibility that the combination of docetaxel chemotherapy and an EGFR TKI in patients with EGFR FISH+ and/or EGFR MT+ tumors may be worthy of further investigation since previous studies that suggested no benefit of EGFR TKIs in combination with chemotherapy were in unselected patients. [23–26].
In the docetaxel arm alone, it is noteworthy that the EGFR MT+ subgroup had a higher ORR compared with the EGFR MT− subgroup (28% versus 10%). This is consistent with earlier studies, such as the first-line IPASS study in which ORRs of 47.3% and 23.5% were observed in the EGFR MT+ and MT− subgroups, respectively, following first-line doublet chemotherapy [27]. Interestingly, we did not see the same trend in patients with EGFR FISH+ tumors, where ORR rates for docetaxel alone were not appreciably different from EGFR FISH− tumors (7% versus 10%). This suggests that while there is some overlap in the EGFR MUT+ and FISH+ tumors, the two groups differ in their responsiveness to chemotherapy. In the current study, patients with KRAS MT+ tumors treated with docetaxel alone had a response rate of 5% (1/20), a PFS of 2.3 months, and an OS of 6.7 months; consistent with a previous study [28]. Taken together, these two studies suggest that NSCLC patients with KRAS mutations treated with docetaxel alone may have particularly poor outcomes.
The current study focused on certain prespecified biomarkers but additional tumor biomarkers associated with the other molecular targets of vandetanib might further refine the identification of patients who get the greatest benefit from vandetanib in NSCLC. For example tumor cell expression of VEGFR2 has been demonstrated in lung cancer [29, 30], and although its biological role is not yet known, it may impact response to vandetanib [31] and other VEGFR signaling inhibitors. In addition, somatic gene fusions leading to RET kinase activation have been reported recently in NSCLC patients [32–34], and these may also represent an additional patient subgroup with the potential to gain differential benefit from RET inhibitors, including vandetanib [35] although the low frequency of RET mutations among NSCLC patients makes it unlikely that this would have significantly influenced the present study.
In conclusion, these biomarker analyses suggest that EGFR gene copy number (EGFR FISH+) and possibly EGFR mutation status (EGFR MT+) may be useful in identifying those patients who receive the most clinical benefit from vandetanib plus docetaxel as a second-line treatment of advanced NSCLC. Additional, prospective studies in biomarker-selected patients will be required to confirm these observations.
funding
The study was funded by AstraZeneca.
disclosure
Consultant or advisory role for AstraZeneca (JVH, BEJ, AJR). Employee or former employee of AstraZeneca (SJL, AJR).
Supplementary Material
acknowledgements
The authors acknowledge AstraZeneca, the Medical Research Council, and Cancer Research UK for their support.
references
- 1.Wedge SR, Ogilvie DJ, Dukes M, et al. ZD6474 inhibits vascular endothelial growth factor signaling, angiogenesis, and tumor growth following oral administration. Cancer Res. 2002;62:4645–4655. [PubMed] [Google Scholar]
- 2.Ryan AJ, Wedge SR. ZD6474—a novel inhibitor of VEGFR and EGFR tyrosine kinase activity. Br J Cancer. 2005;92(Suppl 1):S6–13. doi: 10.1038/sj.bjc.6602603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thornton K, Kim G, Maher VE, et al. Vandetanib for the treatment of symptomatic or progressive medullary thyroid cancer in patients with unresectable locally advanced or metastatic disease: U.S. Food and Drug Administration drug approval summary. Clin Cancer Res. 2012;18:3722–3730. doi: 10.1158/1078-0432.CCR-12-0411. [DOI] [PubMed] [Google Scholar]
- 4.Langmuir PB, Yver A. Vandetanib for the treatment of thyroid cancer. Clin Pharmacol Ther. 2012;91:71–80. doi: 10.1038/clpt.2011.272. [DOI] [PubMed] [Google Scholar]
- 5.Herbst RS, Sun Y, Eberhardt WE, et al. Vandetanib plus docetaxel versus docetaxel as second-line treatment for patients with advanced non-small-cell lung cancer (ZODIAC): a double-blind, randomised, phase 3 trial. Lancet Oncol. 2010;11:619–626. doi: 10.1016/S1470-2045(10)70132-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cappuzzo F, Hirsch FR, Rossi E, et al. Epidermal growth factor receptor gene and protein and gefitinib sensitivity in non-small-cell lung cancer. J Natl Cancer Inst. 2005;97:643–655. doi: 10.1093/jnci/dji112. [DOI] [PubMed] [Google Scholar]
- 7.Cappuzzo F. Predictive factors for response and for resistance to tyrosine kinase inhibitor therapy in lung cancer. J Thorac Oncol. 2007;2:S12–S14. doi: 10.1097/01.JTO.0000268634.17956.5e. [DOI] [PubMed] [Google Scholar]
- 8.Pao W, Wang TY, Riely GJ, et al. KRAS mutations and primary resistance of lung adenocarcinomas to gefitinib or erlotinib. PLoS Med. 2005;2:e17. doi: 10.1371/journal.pmed.0020017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heymach JV, Paz-Ares L, De Braud F, et al. Randomized phase II study of vandetanib alone or with paclitaxel and carboplatin as first-line treatment for advanced non-small-cell lung cancer. J Clin Oncol. 2008;26:5407–5415. doi: 10.1200/JCO.2008.17.3138. [DOI] [PubMed] [Google Scholar]
- 10.Stinchcombe TE, Govindan R. One more fallen star—ZODIAC and its implications. Lancet Oncol. 2010;11:604–605. doi: 10.1016/S1470-2045(10)70149-2. [DOI] [PubMed] [Google Scholar]
- 11.Tian W, Ding W, Kim S, et al. Efficacy and safety profile of combining vandetanib with chemotherapy in patients with advanced non-small cell lung cancer: a meta-analysis. PLoS One. 2013;8:e67929. doi: 10.1371/journal.pone.0067929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu CQ, da Cunha Santos G, Ding K, et al. Role of KRAS and EGFR as biomarkers of response to erlotinib in National Cancer Institute of Canada Clinical Trials Group Study BR.21. J Clin Oncol. 2008;26:4268–4275. doi: 10.1200/JCO.2007.14.8924. [DOI] [PubMed] [Google Scholar]
- 13.Hirsch FR, Varella-Garcia M, Bunn PA, Jr, et al. Molecular predictors of outcome with gefitinib in a phase III placebo-controlled study in advanced non-small-cell lung cancer. J Clin Oncol. 2006;24:5034–5042. doi: 10.1200/JCO.2006.06.3958. [DOI] [PubMed] [Google Scholar]
- 14.Hirsch FR, Varella-Garcia M, Dziadziuszko R, et al. Fluorescence in situ hybridization subgroup analysis of TRIBUTE, a phase III trial of erlotinib plus carboplatin and paclitaxel in non-small cell lung cancer. Clin Cancer Res. 2008;14:6317–6323. doi: 10.1158/1078-0432.CCR-08-0539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crino L, Cappuzzo F, Zatloukal P, et al. Gefitinib versus vinorelbine in chemotherapy-naive elderly patients with advanced non-small-cell lung cancer (INVITE): a randomized, phase II study. J Clin Oncol. 2008;26:4253–4260. doi: 10.1200/JCO.2007.15.0672. [DOI] [PubMed] [Google Scholar]
- 16.Fukuoka M, Wu YL, Thongprasert S, et al. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS) J Clin Oncol. 2011;29:2866–2874. doi: 10.1200/JCO.2010.33.4235. [DOI] [PubMed] [Google Scholar]
- 17.Riely GJ, Politi KA, Miller VA, Pao W. Update on epidermal growth factor receptor mutations in non-small cell lung cancer. Clin Cancer Res. 2006;12:7232–7241. doi: 10.1158/1078-0432.CCR-06-0658. [DOI] [PubMed] [Google Scholar]
- 18.da Cunha Santos G, Shepherd FA, Tsao MS. EGFR mutations and lung cancer. Annu Rev Pathol. 2011;6:49–69. doi: 10.1146/annurev-pathol-011110-130206. [DOI] [PubMed] [Google Scholar]
- 19.Eberhard DA, Johnson BE, Amler LC, et al. Mutations in the epidermal growth factor receptor and in KRAS are predictive and prognostic indicators in patients with non-small-cell lung cancer treated with chemotherapy alone and in combination with erlotinib. J Clin Oncol. 2005;23:5900–5909. doi: 10.1200/JCO.2005.02.857. [DOI] [PubMed] [Google Scholar]
- 20.Douillard JY, Shepherd FA, Hirsh V, et al. Molecular predictors of outcome with gefitinib and docetaxel in previously treated non-small-cell lung cancer: data from the randomized phase III INTEREST trial. J Clin Oncol. 2010;28:744–752. doi: 10.1200/JCO.2009.24.3030. [DOI] [PubMed] [Google Scholar]
- 21.Brave SR, Odedra R, James NH, et al. Vandetanib inhibits both VEGFR-2 and EGFR signalling at clinically relevant drug levels in preclinical models of human cancer. Int J Oncol. 2011;39:271–278. doi: 10.3892/ijo.2011.1022. [DOI] [PubMed] [Google Scholar]
- 22.Herbst RS, Ansari R, Bustin F, et al. Efficacy of bevacizumab plus erlotinib versus erlotinib alone in advanced non-small-cell lung cancer after failure of standard first-line chemotherapy (BeTa): a double-blind, placebo-controlled, phase 3 trial. Lancet. 2011;377:1846–1854. doi: 10.1016/S0140-6736(11)60545-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herbst RS, Prager D, Hermann R, et al. TRIBUTE: a phase III trial of erlotinib hydrochloride (OSI-774) combined with carboplatin and paclitaxel chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol. 2005;23:5892–5899. doi: 10.1200/JCO.2005.02.840. [DOI] [PubMed] [Google Scholar]
- 24.Gatzemeier U, Pluzanska A, Szczesna A, et al. Phase III study of erlotinib in combination with cisplatin and gemcitabine in advanced non-small-cell lung cancer: the Tarceva Lung Cancer Investigation Trial. J Clin Oncol. 2007;25:1545–1552. doi: 10.1200/JCO.2005.05.1474. [DOI] [PubMed] [Google Scholar]
- 25.Herbst RS, Giaccone G, Schiller JH, et al. Gefitinib in combination with paclitaxel and carboplatin in advanced non-small-cell lung cancer: a phase III trial—INTACT 2. J Clin Oncol. 2004;22:785–794. doi: 10.1200/JCO.2004.07.215. [DOI] [PubMed] [Google Scholar]
- 26.Giaccone G, Herbst RS, Manegold C, et al. Gefitinib in combination with gemcitabine and cisplatin in advanced non-small-cell lung cancer: a phase III trial—INTACT 1. J Clin Oncol. 2004;22:777–784. doi: 10.1200/JCO.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 27.Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 28.Janne PA, Shaw AT, Pereira JR, et al. Selumetinib plus docetaxel for KRAS-mutant advanced non-small-cell lung cancer: a randomised, multicentre, placebo-controlled, phase 2 study. Lancet Oncol. 2013;14:38–47. doi: 10.1016/S1470-2045(12)70489-8. [DOI] [PubMed] [Google Scholar]
- 29.Koo PJ, Morgensztern D, Boyer JL, Herbst RS. Targeting vascular endothelial growth factor in patients with squamous cell lung cancer. J Clin Oncol. 2012;30:1137–1139. doi: 10.1200/JCO.2011.40.4053. [DOI] [PubMed] [Google Scholar]
- 30.Pajares MJ, Agorreta J, Larrayoz M, et al. Expression of tumor-derived vascular endothelial growth factor and its receptors is associated with outcome in early squamous cell carcinoma of the lung. J Clin Oncol. 2012;30:1129–1136. doi: 10.1200/JCO.2011.37.4231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim ES, Herbst RS, Wistuba II, et al. The BATTLE trial: personalizing therapy for lung cancer. Cancer Discov. 2011;1:44–53. doi: 10.1158/2159-8274.CD-10-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lipson D, Capelletti M, Yelensky R, et al. Identification of new ALK and RET gene fusions from colorectal and lung cancer biopsies. Nat Med. 2012;18:382–384. doi: 10.1038/nm.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ju YS, Lee WC, Shin JY, et al. A transforming KIF5B and RET gene fusion in lung adenocarcinoma revealed from whole-genome and transcriptome sequencing. Genome Res. 2012;22:436–445. doi: 10.1101/gr.133645.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takeuchi K, Soda M, Togashi Y, et al. RET, ROS1 and ALK fusions in lung cancer. Nat Med. 2012;18:378–381. doi: 10.1038/nm.2658. [DOI] [PubMed] [Google Scholar]
- 35.Gautschi O, Zander T, Keller FA, et al. A patient with lung adenocarcinoma and RET fusion treated with vandetanib. J Thorac Oncol. 2013;8:e43–e44. doi: 10.1097/JTO.0b013e31828a4d07. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.