This study confirms a twofold excess risk of pancreatic cancer in diabetics and shows that a 30% excess risk persists more than two decades after diabetes diagnosis, supporting a causal role of diabetes in pancreatic cancer. Oral antidiabetics may decrease pancreatic cancer risk, whereas insulin showed an inconsistent time-related association.
Keywords: oral antidiabetics, case–control study, diabetes, insulin, pancreatic cancer, pooled analysis
Abstract
Background
Type 2 diabetes mellitus has been associated with an excess risk of pancreatic cancer, but the magnitude of the risk and the time–risk relationship are unclear, and there is limited information on the role of antidiabetic medications.
Patients and methods
We analyzed individual-level data from 15 case–control studies within the Pancreatic Cancer Case-Control Consortium, including 8305 cases and 13 987 controls. Pooled odds ratios (ORs) were estimated from multiple logistic regression models, adjusted for relevant covariates.
Results
Overall, 1155 (15%) cases and 1087 (8%) controls reported a diagnosis of diabetes 2 or more years before cancer diagnosis (or interview, for controls), corresponding to an OR of 1.90 (95% confidence interval, CI, 1.72–2.09). Consistent risk estimates were observed across strata of selected covariates, including body mass index and tobacco smoking. Pancreatic cancer risk decreased with duration of diabetes, but a significant excess risk was still evident 20 or more years after diabetes diagnosis (OR 1.30, 95% CI 1.03–1.63). Among diabetics, long duration of oral antidiabetic use was associated with a decreased pancreatic cancer risk (OR 0.31, 95% CI 0.14–0.69, for ≥15 years). Conversely, insulin use was associated with a pancreatic cancer risk in the short term (OR 5.60, 95% CI 3.75–8.35, for <5 years), but not for longer duration of use (OR 0.95, 95% CI 0.53–1.70, for ≥15 years).
Conclusion
This study provides the most definitive quantification to date of an excess risk of pancreatic cancer among diabetics. It also shows that a 30% excess risk persists for more than two decades after diabetes diagnosis, thus supporting a causal role of diabetes in pancreatic cancer. Oral antidiabetics may decrease the risk of pancreatic cancer, whereas insulin showed an inconsistent duration–risk relationship.
introduction
Pancreatic cancer is the fifth most frequent cause of cancer death in high-income countries [1] and is one of the most aggressive neoplasms with a 5-year relative survival rate of <5% [2]. Besides tobacco smoking, other recognized risk factors are overweight/obesity, high alcohol consumption, history of pancreatitis and diabetes, family history of pancreatic cancer, and possibly selected dietary factors [3].
Type 2 diabetes mellitus has been associated with an excess risk of pancreatic cancer in a number of epidemiological studies [4–9]. The excess risk has been shown to be greater for a recent diagnosis of diabetes and to decline with increasing time since diagnosis, thus supporting the hypothesis that diabetes may be—at least in part—a consequence or an early manifestation of the disease. However, some increased risks for pancreatic cancer have been reported among individuals who had diabetes for 10 or more years [5, 7, 10, 11], indicating that diabetes may also play a causal role in pancreatic carcinogenesis.
Diabetes medications may also influence the risk of pancreatic cancer, although epidemiologic data are scanty. In particular, it has been reported that insulin might further increase pancreatic cancer risk, metformin might reduce it [6, 12], while sulfonylureas [12] or thiazolidinediones [13] have no consistent role on pancreatic cancer.
The availability of a large dataset within the Pancreatic Cancer Case-Control Consortium (PanC4; http://www.panc4.org) gave a unique opportunity to further investigate the association of diabetes and its medications with the risk of pancreatic cancer.
methods
The present pooled analysis included 8305 cases of adenocarcinoma of the exocrine pancreas and 13 987 controls from 15 case–control studies within the PanC4 consortium, providing information on history of diabetes and medications for diabetes [14–28]. The main characteristics of the studies are described in supplementary Table S1, available at Annals of Oncology online. In most studies, cases and controls were interviewed or surveyed in-person, with the exception of 537 cases (6.5%) and 332 of controls (2.4%).
The original datasets were restructured either by the study investigators or by our central coordinators using a uniform format for data harmonization. From each study, we collected individual data on sociodemographic factors, study characteristics, anthropometric measures, tobacco smoking, alcohol consumption, and history of pancreatitis. History of diabetes was based on self-reported information from questionnaire and included age at first diagnosis. Some of the studies provided additional information on diabetes medications (see supplementary Text S1, available at Annals of Oncology online for details).
statistical analysis
To estimate the association between diabetes, antidiabetics and pancreatic cancer, we conducted an aggregate analysis pooling individual-level data from all the PanC4 studies into a single dataset, and we computed pooled odds ratios (ORs) and the corresponding 95% confidence intervals (CIs), using unconditional logistic regression models [29]. These included terms for study, center (for multicenter studies), age, sex, education, race/ethnicity, body mass index (BMI), tobacco, alcohol consumption, and history of pancreatitis. Dummy variables were used in all analyses for variables with missing data. Study-specific and pooled ORs and corresponding 95% CIs were also plotted for visual comparison. We investigated whether the effect of history of diabetes was homogeneous in strata of selected covariates by conducting stratified analyses. We also carried out sensitivity analyses excluding proxy-respondents and participants with a history of pancreatitis.
results
Cases and controls had similar distributions by sex and alcohol consumption; cases were somewhat older than controls, had a higher level of education, were more frequently obese, white and ever smokers, and reported a history of pancreatitis more frequently (supplementary Table S2, available at Annals of Oncology online).
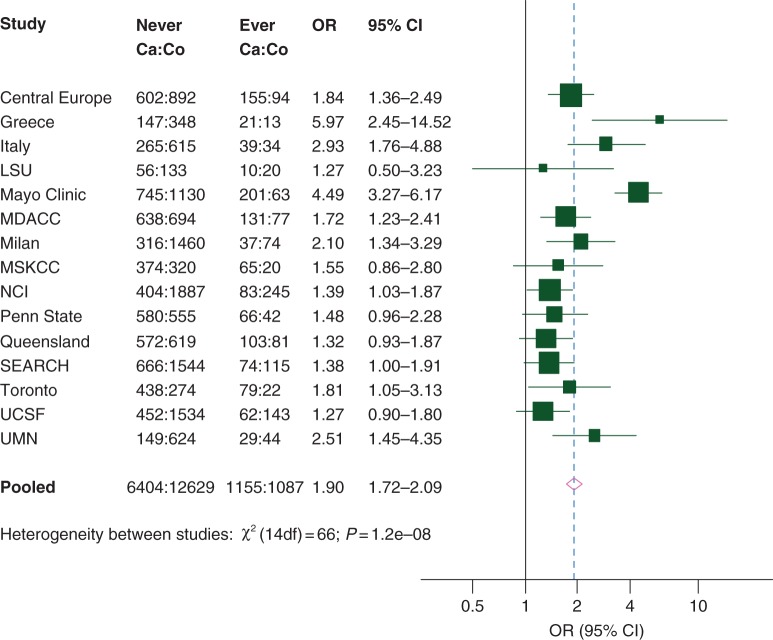
Overall, 1767 (21%) cases and 1284 (9%) controls reported a history of diabetes, corresponding to an OR of 2.39 (95% CI 2.19–2.61); 1155 (15%) cases and 1087 (8%) controls had a history of diabetes 2 or more years before cancer diagnosis (or interview), with a corresponding OR of 1.90 (95% CI 1.72–2.09, Table 1). Significant heterogeneity between study results was observed, the ORs varying between 1.3 and 6.0 (Figure 1). Pancreatic cancer risk decreased with a duration of diabetes, but a 54% excess risk was still evident 15 to <20 years since diabetes diagnosis and a 30% excess risk 20 or more years since diabetes diagnosis (Table 1). The OR was 1.21 (95% CI 0.91–1.61) for 20 to <30 years since diabetes diagnosis and 1.48 (95% CI 1.01–2.16) for ≥30 years since diabetes diagnosis (data not shown). The OR for diabetes 2 or more years before cancer diagnosis/interview was 1.46 for age at diagnosis <30 years and 1.91 for age at diagnosis ≥30 years.
Table 1.
Distribution of 8305 pancreatic cancer cases and 13 987 controls, and corresponding odds ratios (ORs) and 95% confidence intervals (CIs), by history of diabetes. Data from Pancreatic Cancer Case-Control Consortium (PanC4)
| Cases |
Controls |
ORa (95% CI) | |||
|---|---|---|---|---|---|
| N | % | N | % | ||
| History of diabetes | |||||
| No | 6404 | 77.1 | 12 629 | 90.3 | 1b |
| Yes | 1767 | 21.3 | 1284 | 9.2 | 2.39 (2.19–2.61) |
| Missing | 134 | 1.6 | 74 | 0.5 | |
| Duration of diabetes (years) | |||||
| <1 | 307 | 3.7 | 48 | 0.3 | 10.32 (7.48–14.23) |
| 1 to <2 | 218 | 2.6 | 95 | 0.7 | 3.68 (2.84–4.77) |
| 2 to <5 | 383 | 4.6 | 236 | 1.7 | 2.92 (2.44–3.50) |
| 5 to <10 | 304 | 3.7 | 290 | 2.1 | 1.84 (1.54–2.20) |
| 10 to <15 | 206 | 2.5 | 211 | 1.5 | 1.69 (1.36–2.09) |
| 15 to <20 | 111 | 1.3 | 134 | 1.0 | 1.54 (1.17–2.03) |
| ≥20 | 151 | 1.8 | 216 | 1.5 | 1.30 (1.03–1.63) |
| Missing | 221 | 2.7 | 128 | 0.9 | |
| P-value for trendc | <0.0001 | ||||
| History of diabetesd | |||||
| No | 6404 | 82.3 | 12 629 | 91.2 | 1b |
| Yes | 1155 | 14.9 | 1087 | 7.9 | 1.90 (1.72–2.09) |
| Missing | 221 | 2.8 | 128 | 0.9 | |
| Age at diabetes diagnosisd | |||||
| <30 years | 28 | 0.4 | 38 | 0.3 | 1.46 (0.85–2.48) |
| ≥30 years | 1127 | 14.9 | 1049 | 7.6 | 1.91 (1.73–2.12) |
| Missing | 221 | 2.8 | 128 | 0.9 | |
aORs were computed from logistic regression models adjusted for study, center (for multicenter studies), age, sex, race/ethnicity, education, body mass index, tobacco smoking, alcohol drinking, and history of pancreatitis.
bReference category.
cTests for linear trend of the OR were based on ordinal coding of the categories and the corresponding χ2 statistic.
dExcluding patients with a diagnosis of diabetes within 2 years before cancer diagnosis (or interview for controls).
Figure 1.
Study-specific and pooled odd ratiosa (ORs) for pancreatic cancer by history of diabetes 2 or more years before cancer diagnosis (or interview for controls) when compared with no history. Data from Pancreatic Cancer Case-Control Consortium (PanC4). 95% CI, 95% confidence interval; LSU, Louisiana State University; MDACC, MD Anderson Cancer Center; MSKCC, Memorial Sloan Kettering Cancer Center; NCI, National Cancer Institute; SEARCH, Surveillance of Environmental Aspects Related to Cancer in Humans; UCSF, University of California, San Francisco; UMN, University of Minnesota. aStudy-specific ORs were computed from logistic regression models adjusted for center (for multicenter studies), age, sex, race/ethnicity, education, body mass index, tobacco smoking, alcohol drinking (except for the MSKCC study), and history of pancreatitis (except for the Italy, Mayo Clinic, and Penn State studies). Pooled ORs were further adjusted for study. Homogeneity of ORs between studies was based on likelihood ratio tests and the resulting χ2 statistic.
Risk estimates for a diagnosis of diabetes 2 or more years before cancer diagnosis/interview were consistent across strata of sex, age, race/ethnicity, education, BMI, tobacco smoking, and study areas, whereas they were slightly higher in no/low drinkers (OR 2.09) than in moderate (OR 1.79) and high (OR 1.57) drinkers, and in hospital-based (OR 2.55) than in population-based (OR 1.50) studies (Table 2). For diabetes diagnoses <2 years before cancer diagnosis/interview, risk estimates were consistent across the strata of most covariates considered, but they were significantly higher in men (OR 7.14) than in women (OR 4.86), in those with higher (OR 9.05) than lower (OR 4.53) level of education, and in hospital-based (OR 7.98) than in population-based (OR 4.52) studies. The ORs for diabetes 2 or more years before cancer diagnosis/interview were 1.91 (95% CI 1.73–2.12) when we excluded proxy-respondents and 1.64 (95% CI 1.46–1.84) when we excluded participants with a history of pancreatitis (data not shown).
Table 2.
Odds ratios (ORs) and 95% confidence intervals (CIs) for pancreatic cancer by history of diabetes in strata of selected covariates. Data from Pancreatic Cancer Case-Control Consortium (PanC4)
| History of diabetes |
|||||
|---|---|---|---|---|---|
| No | Diabetes within 2 years before cancer diagnosis or interviewa |
Diabetes 2 years or more before cancer diagnosis or interviewa |
|||
| Cases:controls | Cases:controls | ORb (95% CI) | Cases:controls | ORb (95% CI) | |
| Sex | |||||
| Men | 3553:7223 | 315:73 | 7.14 (5.40–9.43) | 721:669 | 1.90 (1.67–2.16) |
| Women | 2851:5406 | 210:70 | 4.86 (3.63–6.51) | 434:418 | 1.87 (1.60–2.19) |
| P-value for heterogeneityc | 0.04 | 0.87 | |||
| Age (years) | |||||
| <65 | 3337:6932 | 261:71 | 5.95 (4.47–7.92) | 480:426 | 2.10 (1.79–2.45) |
| ≥65 | 3067:5697 | 264:72 | 5.91 (4.46–7.83) | 675:661 | 1.82 (1.60–2.07) |
| P-value for heterogeneityc | 0.79 | 0.22 | |||
| Race/ethnicity | |||||
| Non–Hispanic White | 5759:10 942 | 468:123 | 6.21 (5.02–7.69) | 979:835 | 1.97 (1.76–2.19) |
| Other | 496:1331 | 49:18 | 3.12 (1.65–5.92) | 148:222 | 1.48 (1.11–1.98) |
| P-value for heterogeneityc | 0.20 | 0.17 | |||
| Education | |||||
| High school graduate or less | 3509:6935 | 280:101 | 4.53 (3.54–5.80) | 682:680 | 1.87 (1.65–2.13) |
| College graduate or more | 2846:5640 | 239:42 | 9.05 (6.37–12.9) | 465:400 | 1.92 (1.64–2.26) |
| P-value for heterogeneityc | 0.001 | 0.40 | |||
| Body mass index (kg/m2)d | |||||
| <25 | 2720:5715 | 133:36 | 6.03 (4.08–8.90) | 262:274 | 1.90 (1.56–2.30) |
| ≥25 | 3410:6131 | 358:98 | 5.45 (4.28–6.95) | 847:752 | 1.84 (1.63–2.07) |
| P-value for heterogeneityc | 0.56 | 0.71 | |||
| Tobacco smoking | |||||
| Never smoker | 2362:5381 | 199:51 | 7.15 (5.15–9.93) | 404:424 | 1.81 (1.54–2.13) |
| Current smoker | 1630:2536 | 123:33 | 4.87 (3.17–7.48) | 242:149 | 2.31 (1.80–2.95) |
| Ex-smoker | 2184:4278 | 190:57 | 5.64 (4.09–7.79) | 465:462 | 1.84 (1.58–2.15) |
| P-value for heterogeneityc | 0.10 | 0.43 | |||
| Alcohol drinking (drinks/day)e | |||||
| 0 to <1 | 3568:7044 | 305:83 | 6.48 (4.98–8.43) | 743:646 | 2.09 (1.84–2.38) |
| 1 to <4 | 1508:3524 | 97:38 | 4.87 (3.20–7.41) | 188:255 | 1.79 (1.43–2.23) |
| ≥4 | 752:1486 | 42:13 | 5.04 (2.52–10.1) | 110:133 | 1.57 (1.16–2.14) |
| P-value for heterogeneityc | 0.26 | 0.06 | |||
| Study area | |||||
| North America | 3836:7151 | 331:76 | 6.11 (4.65–8.03) | 726:676 | 1.95 (1.71–2.23) |
| Other areas | 2568:5478 | 194:67 | 5.75 (4.27–7.75) | 429:411 | 1.83 (1.56–2.14) |
| P-value for heterogeneityc | 0.52 | 0.20 | |||
| Source of controls | |||||
| Hospital | 3065:5122 | 306:51 | 7.98 (5.85–10.89) | 560:323 | 2.55 (2.18–3.00) |
| Population | 3339:7507 | 219:92 | 4.52 (3.45–5.92) | 595:764 | 1.50 (1.31–1.71) |
| P-value for heterogeneityc | 0.015 | <0.001 | |||
aInterview for controls.
bPooled ORs were computed from logistic regression models adjusted for study, center (for multicenter studies), age, sex, race/ethnicity, education, body mass index, tobacco smoking, alcohol drinking, and history of pancreatitis. Reference category: no history of diabetes.
cHomogeneity of ORs between strata of the covariates was based on likelihood ratio tests and the resulting χ2 statistics.
dInformation not available in the University of Minnesota study.
eInformation not available in the Memorial Sloan Kettering Cancer Center study.
Diabetics using oral antidiabetics had a nonsignificant reduced risk of pancreatic cancer when compared with those not using them (OR 0.92, Table 3). The OR decreased with an increasing duration of oral antidiabetic use and was 0.31 for ≥15 years of use. The risk was significantly higher in diabetics who used insulin (OR 2.66) when compared with those not using insulin; it was higher for a shorter insulin use (OR 5.60, for <5 years of use) and declined with an increasing duration of use (OR 0.95, for ≥15 years of use). Risk estimates did not meaningfully change when we mutually adjusted the use of oral antidiabetics and insulin, nor when we adjusted oral antidiabetic and insulin use for the duration of diabetes.
Table 3.
Distribution of pancreatic cancer cases and controls, and corresponding odds ratios (ORs) and 95% confidence intervals (CIs), by the use of antidiabetic medications among diabetics with a diagnosis of diabetes 2 or more years before cancer diagnosis (or interview for controls). Data from Pancreatic Cancer Case-Control Consortium (PanC4)
| Cases |
Controls |
ORa (95% CI) | |||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Use of oral antidiabeticsb | |||||
| No | 184 | 23.9 | 185 | 24.4 | 1c |
| Yes | 496 | 64.3 | 543 | 71.7 | 0.92 (0.69–1.23) |
| Missing | 91 | 11.8 | 29 | 3.8 | |
| Duration of oral antidiabetic use (years)d | |||||
| No use | 61 | 17.5 | 42 | 13.4 | 1c |
| <5 | 93 | 26.7 | 94 | 29.9 | 0.79 (0.44–1.41) |
| 5 to <10 | 53 | 15.2 | 74 | 23.6 | 0.56 (0.30–1.05) |
| 10 to <15 | 33 | 9.5 | 40 | 12.7 | 0.57 (0.27–1.18) |
| ≥15 | 21 | 6.0 | 35 | 11.2 | 0.31 (0.14–0.69) |
| Missing | 87 | 25.0 | 29 | 9.2 | |
| P-value for trende | 0.002 | ||||
| Use of insulinf | |||||
| No | 407 | 50.9 | 547 | 68.3 | 1c |
| Yes | 360 | 45.0 | 237 | 29.6 | 2.66 (2.07–3.43) |
| Missing | 33 | 4.1 | 17 | 2.1 | |
| Duration of insulin use (years)g | |||||
| No use | 245 | 48.5 | 456 | 67.7 | 1c |
| <5 | 134 | 26.5 | 58 | 8.6 | 5.60 (3.75–8.35) |
| 5 to <10 | 37 | 7.3 | 43 | 6.4 | 1.87 (1.08–3.23) |
| 10 to <15 | 24 | 4.8 | 41 | 6.1 | 1.66 (0.90–3.06) |
| ≥15 | 24 | 4.8 | 56 | 8.3 | 0.95 (0.53–1.70) |
| Missing | 41 | 8.1 | 20 | 3.0 | |
| P-value for trend | 0.065 | ||||
aPooled ORs were computed from logistic regression models adjusted for study, study center (for multicenter studies), age, sex, race/ethnicity, education, body mass index, tobacco smoking, alcohol drinking, and history of pancreatitis.
bInformation available in the Central Europe, Mayo Clinic, MD Anderson Cancer Center (MDACC), Memorial Sloan Kettering Cancer Center (MSKCC), National Cancer Institute (NCI), Surveillance of Environmental Aspects Related to Cancer (SEARCH), and University of California, San Francisco (UCSF) studies.
cReference category.
dInformation available in the Central Europe, MDACC, and UCSF studies.
eTests for linear trend of the ORs were based on ordinal coding of the categories and the corresponding χ2 statistic.
fInformation available in the Central Europe, Mayo Clinic, MDACC, MSKCC, NCI, SEARCH, UCSF, and University of Minnesota studies.
gInformation available in the Central Europe, MDACC, NCI, SEARCH, and UCSF studies.
discussion
This collaborative analysis—including data on more than 8000 pancreatic cancer cases—confirms that diabetics have an almost twofold increased risk of developing pancreatic cancer and provides more definitive and precise estimates than previously available [5, 7, 9].
About 30% of diabetics in the present study had a diagnosis of diabetes close to the time of their pancreatic cancer diagnosis and the excess risk in those individuals was particularly high [5, 7]. Although the risk of pancreatic cancer tended to decline with an increasing duration of diabetes, we found that a significant 50% excess risk was still evident at 15–20 years, and a 30% excess risk for 20 or more years following diabetes diagnosis. Similarly, three meta-analyses [5, 7, 9] showed a 30%–50% increased risk of pancreatic cancer among patients who had diabetes for 10 or more years, but they were not able to estimate a pancreatic cancer risk for longer duration of diabetes.
All studies provided risk estimates above unity, but significant heterogeneity between them was observed. In particular, higher excess risk was observed in the Greece, Italy, Mayo Clinic, and University of Minnesota studies, that had a low prevalence of diabetes among controls.
The mechanisms underlying the relationship between diabetes and pancreatic cancer are complex [8, 30]. The excess pancreatic cancer risk for a recent diagnosis of diabetes is compatible with the hypothesis that diabetes is, at least in part, an early manifestation or a consequence of pre-clinical pancreatic cancer [31–33]. Pancreatic cancer has been shown to cause diabetes, the so-called Type 3c diabetes, by inducing beta-cell dysfunction and peripheral insulin resistance, suppressing insulin secretion, and impairing proinsulin conversion [34–36]. Diabetes-induced by pancreatic cancer is usually resolved after pancreatic tumor resection [32, 37]. Early symptoms of pancreatic cancer may also favor the diagnosis of diabetes (or vice versa), thus partly explaining the excess risk in patients with a recent diagnosis of diabetes. To reduce the possibility of reverse causation or diagnostic bias, we have focused our analyses on diabetes diagnosed 2 or more years before cancer diagnosis.
The increased pancreatic cancer risk observed 5, 10, and up to 20 or more years since diagnosis of diabetes cannot be due to reverse causation or diagnostic bias and supports causal role of diabetes on pancreatic cancer. Diabetes is associated with hyperglycemia, insulin resistance, compensatory hyperinsulinemia, and up-regulated levels of insulin-like growth factors (IGFs) [8, 38]. Peripheral insulin resistance 5 or 10 years before pancreatic cancer diagnosis has been related to an increased pancreatic cancer risk [39]. High insulin concentrations may directly be involved in the etiology of pancreatic cancer, as insulin is a growth promoter and mitogen in pancreatic cell lines [40, 41]. Moreover, IGF-1 and its receptors can decrease apoptosis and increase proliferation, invasion, and angiogenesis [42], and possibly increase pancreatic cancer risk [43]. The association between diabetes and pancreatic cancer might also be mediated by obesity—one of the major determinants of Type 2 diabetes mellitus and a known risk factor for pancreatic cancer [44]. However, the consistent risks observed across strata of BMI indicate that obesity is unlikely explain the observed association.
We found that pancreatic cancer risk was increased, although weakly and not significantly, among diabetics who were diagnosed with diabetes before 30 years of age. This provides support for a possible role of Type 1 diabetes in pancreatic carcinogenesis [45]. However, the number of early-onset diabetics is too limited to draw any definitive conclusion.
We observed a nonsignificant reduced risk of pancreatic cancer in diabetics using oral antidiabetics, the risk decreasing with longer duration of use. Conversely, the risk was elevated among insulin users and particularly in those with a shorter duration of use. Although the duration of antidiabetics use is likely to mirror the time between diabetes diagnosis and cancer diagnosis/interview, the addition of a term for the duration of diabetes in the models did not meaningfully affect our risk estimates. A few previous studies—most of them included in our pooled-analysis—reported a lower pancreatic cancer risk in diabetics using oral antidiabetics than in those using insulin [10, 12, 16, 18, 21, 28, 46]. Metformin, in particular, has been associated with a reduced risk of (pancreatic) cancer [12], the favorable effect being mediated by both its ability to lower glycemic and insulin levels and to its tumor growth inhibitory activity [12, 47]. However, the lower severity of diabetes and variable baseline characteristics of patients using oral antidiabetic drugs may have influenced this association. Similarly, although insulin may directly increase pancreatic cancer risk [41], the excess risk for shorter-term insulin use in the absence of a risk–duration relationship indicates that such excess risk in insulin users is likely explained by reverse causation. Insulin, in fact, tends to be prescribed in patients with more advanced diseases and its short-term excess risk may be due to a progressive worsening of diabetes after pancreatic cancer [48].
This PanC4 collaborative study has a number of strengths. These include the uniquely large dataset, the detailed information from most studies on diabetes and on antidiabetic medications, and the ability to account uniformly and carefully for study design variables and major recognized confounding factors. However, information on alcohol drinking and history of pancreatitis was not available in a few studies and this may have contributed to the heterogeneity between studies. Moreover, the large available dataset allowed us to show that risk estimates were consistent across strata of various covariates, including tobacco smoking and BMI. Among the limitations of our study is that history of diabetes was self-reported. However, diabetes is a serious chronic condition and it has been reliably recalled in other epidemiological studies [49, 50]. Furthermore, it is possible that self-reported information on medical conditions is reported more accurately in cases than in population-controls, but not necessarily in hospital-controls [49].
Furthermore, our risk estimates are consistent with those provided by cohort studies [4, 5, 7, 9], arguing against a major role of recall or a selection bias. Accuracy of medical information may also be different between in-person and proxy-respondents, although proxy-respondents were a small minority of the total dataset and restriction of our analyses to in-person respondents yielded similar results. We could not distinguish between Type 1 and Type 2 diabetes, but 97% of our diabetic patients were diagnosed after 30 years of age and were thus likely to be affected by Type 2 diabetes. Finally, medication history was also self-reported, and, in almost all studies, we had no information of the specific types of oral antidiabetic used.
funding
This work was conducted with the contribution of the Italian Association for Cancer Research (AIRC, project N. 13203, PI CB) and within the COST Action (BM1214) EU-Pancreas. The Central Europe study was supported by the Grant Agency of Ministry of Health of the Czech Republic (IGA MZ ČR 8090-3 and 9422-3). The center of Brno, Czech Republic—within the Central Europe study—was supported by MH CZ—DRO (MMCI, 00209805) and RECAMO CZ.1.05/2.1.00/03.0101. The Italy and Milan studies were supported by the Italian Association for Cancer Research. The Louisiana State University study was supported by the Louisiana Board of Regents Millennium Trust Health Excellence Fund (Project 5: HEF 2000-05, Genetics Studies in the Acadian Population). The Mayo Clinic Biospecimen Resource for Pancreas Research is partially supported by The National Institutes of Health (NIH) US (P50 CA102701). The MD Anderson Cancer Center study was supported by the NIH Research Project Grant Program (RO1 CA98380). The Pancreatic Cancer Family Registry at the Memorial Sloan Kettering Cancer Center (MSKCC) was supported by the Prevention, Control, and Population Research Goldstein Award; the Society of MSKCC; and the Geoffrey Beene Cancer Research Fund. The NCI study was supported by the Intramural Research Program of the National Institute of Health, National Cancer Institute, Division of Cancer Epidemiology and Genetics (contract numbers: N01-CP-51090, N01-CP-51089, N01-CP-51092, N01-CP-05225, N01-CP-31022, and N01-CP-05227). The Penn State study was supported by the National Institutes of Health (NIH) US (P01CA068384). The Queensland Pancreatic Cancer study was funded by the National Health and Medical Research Council (Australia) (grant 442302). The Montreal investigation in the Surveillance of Environmental Aspects Related to Cancer in Humans study was supported by the Cancer Research Society, the Toronto contribution was supported by the National Cancer Institute of Canada, and the Netherlands contribution was supported by the Dutch Ministry of Public Health, Welfare and Sports (formerly Welfare, Health and Culture). The Ontario Pancreas Cancer Study was supported by grants from the National Institutes of Health (R01 CA97075, as part of the PACGENE consortium and U01-CA74783), the Lustgarten Foundation for Pancreatic Cancer Research, and the Ontario Cancer Research Network. The University of California, San Francisco (UCSF) study work was supported in part by National Cancer Institute grants (CA59706, CA108370, CA109767, CA89726, and CA098889), and by the Rombauer Pancreatic Cancer Research Fund. Cancer incidence data collection in the UCSF study was supported by the California Department of Public Health, the National Cancer Institute's Surveillance, Epidemiology and End Results Program (contract N01-PC-35136) awarded to the Northern California Cancer Center. EJD was supported by the Red Temática de Investigación Cooperativa en Cáncer (RTICC; Rd06/0020/0091 and Rd12/0036/0018) and by the Generalitat de Catalunya. REN was funded by a National Health and Medical Research Council (Australia) Senior Research Fellowship.
disclosure
The authors have declared no conflicts of interest.
Supplementary Material
acknowledgements
The authors thank Mrs Ivana Garimoldi for editorial assistance.
references
- 1.Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Klint A, Engholm G, Storm HH, et al. Trends in survival of patients diagnosed with cancer of the digestive organs in the Nordic countries 1964–2003 followed up to the end of 2006. Acta Oncol. 2010;49:578–607. doi: 10.3109/02841861003739330. [DOI] [PubMed] [Google Scholar]
- 3.Maisonneuve P, Lowenfels AB. Epidemiology of pancreatic cancer: an update. Dig Dis. 2010;28:645–656. doi: 10.1159/000320068. [DOI] [PubMed] [Google Scholar]
- 4.Everhart J, Wright D. Diabetes mellitus as a risk factor for pancreatic cancer. A meta-analysis. JAMA. 1995;273:1605–1609. [PubMed] [Google Scholar]
- 5.Huxley R, Ansary-Moghaddam A, Berrington de Gonzalez A, et al. Type-II diabetes and pancreatic cancer: a meta-analysis of 36 studies. Br J Cancer. 2005;92:2076–2083. doi: 10.1038/sj.bjc.6602619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giovannucci E, Harlan DM, Archer MC, et al. Diabetes and cancer: a consensus report. Diabetes Care. 2010;33:1674–1685. doi: 10.2337/dc10-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ben Q, Xu M, Ning X, et al. Diabetes mellitus and risk of pancreatic cancer: a meta-analysis of cohort studies. Eur J Cancer. 2011;47:1928–1937. doi: 10.1016/j.ejca.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 8.Li D. Diabetes and pancreatic cancer. Mol Carcinog. 2012;51:64–74. doi: 10.1002/mc.20771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Batabyal P, Vander Hoorn S, Christophi C, Nikfarjam M. Association of diabetes mellitus and pancreatic adenocarcinoma: a meta-analysis of 88 studies. Ann Surg Oncol. 2014;21:2453–2462. doi: 10.1245/s10434-014-3625-6. [DOI] [PubMed] [Google Scholar]
- 10.Li D, Tang H, Hassan MM, et al. Diabetes and risk of pancreatic cancer: a pooled analysis of three large case-control studies. Cancer Causes Control. 2011;22:189–197. doi: 10.1007/s10552-010-9686-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elena JW, Steplowski E, Yu K, et al. Diabetes and risk of pancreatic cancer: a pooled analysis from the pancreatic cancer cohort consortium. Cancer Causes Control. 2013;24:13–25. doi: 10.1007/s10552-012-0078-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soranna D, Scotti L, Zambon A, et al. Cancer risk associated with use of metformin and sulfonylurea in type 2 diabetes: a meta-analysis. Oncologist. 2012;17:813–822. doi: 10.1634/theoncologist.2011-0462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bosetti C, Rosato V, Buniato D, et al. Cancer risk for patients using thiazolidinediones for type 2 diabetes: a meta-analysis. Oncologist. 2013;18:148–156. doi: 10.1634/theoncologist.2012-0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luckett BG, Su LJ, Rood JC, Fontham ET. Cadmium exposure and pancreatic cancer in south Louisiana. J Environ Public Health. 2012;2012:180186. doi: 10.1155/2012/180186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McWilliams RR, Bamlet WR, de Andrade M, et al. Nucleotide excision repair pathway polymorphisms and pancreatic cancer risk: evidence for role of MMS19L. Cancer Epidemiol Biomarkers Prev. 2009;18:1295–1302. doi: 10.1158/1055-9965.EPI-08-1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hassan MM, Bondy ML, Wolff RA, et al. Risk factors for pancreatic cancer: case-control study. Am J Gastroenterol. 2007;102:2696–2707. doi: 10.1111/j.1572-0241.2007.01510.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olson SH, Orlow I, Simon J, et al. Allergies, variants in IL-4 and IL-4R alpha genes, and risk of pancreatic cancer. Cancer Detect Prev. 2007;31:345–351. doi: 10.1016/j.cdp.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 18.Silverman DT, Schiffman M, Everhart J, et al. Diabetes mellitus, other medical conditions and familial history of cancer as risk factors for pancreatic cancer. Br J Cancer. 1999;80:1830–1837. doi: 10.1038/sj.bjc.6690607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muscat JE, Stellman SD, Hoffmann D, Wynder EL. Smoking and pancreatic cancer in men and women. Cancer Epidemiol Biomarkers Prev. 1997;6:15–19. [PubMed] [Google Scholar]
- 20.Anderson LN, Cotterchio M, Gallinger S. Lifestyle, dietary, and medical history factors associated with pancreatic cancer risk in Ontario, Canada. Cancer Causes Control. 2009;20:825–834. doi: 10.1007/s10552-009-9303-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang F, Gupta S, Holly EA. Diabetes mellitus and pancreatic cancer in a population-based case-control study in the San Francisco Bay Area, California. Cancer Epidemiol Biomarkers Prev. 2006;15:1458–1463. doi: 10.1158/1055-9965.EPI-06-0188. [DOI] [PubMed] [Google Scholar]
- 22.Henry SA, Prizment AE, Anderson KE. Duration of diabetes and pancreatic cancer in a case-control study in the Midwest and the Iowa Women's Health Study (IWHS) cohort. JOP. 2013;14:243–249. doi: 10.6092/1590-8577/1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Urayama KY, Holcatova I, Janout V, et al. Body mass index and body size in early adulthood and risk of pancreatic cancer in a central European multicenter case-control study. Int J Cancer. 2011;129:2875–2884. doi: 10.1002/ijc.25959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kalapothaki V, Tzonou A, Hsieh CC, et al. Tobacco, ethanol, coffee, pancreatitis, diabetes mellitus, and cholelithiasis as risk factors for pancreatic carcinoma. Cancer Causes Control. 1993;4:375–382. doi: 10.1007/BF00051341. [DOI] [PubMed] [Google Scholar]
- 25.Bosetti C, Rosato V, Polesel J, et al. Diabetes mellitus and cancer risk in a network of case-control studies. Nutr Cancer. 2012;64:643–651. doi: 10.1080/01635581.2012.676141. [DOI] [PubMed] [Google Scholar]
- 26.La Vecchia C, Negri E, Franceschi S, et al. A case-control study of diabetes mellitus and cancer risk. Br J Cancer. 1994;70:950–953. doi: 10.1038/bjc.1994.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tran B, Whiteman DC, Webb PM, et al. Association between ultraviolet radiation, skin sun sensitivity and risk of pancreatic cancer. Cancer Epidemiol. 2013;37:886–892. doi: 10.1016/j.canep.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 28.Maisonneuve P, Lowenfels AB, Bueno-de-Mesquita HB, et al. Past medical history and pancreatic cancer risk: results from a multicenter case-control study. Ann Epidemiol. 2010;20:92–98. doi: 10.1016/j.annepidem.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 29.Breslow NE, Day NE. Lyon, France: IARC; 1980. Statistical methods in cancer research, volume I: the analysis of case-control studies. IARC Sci Publ No. 32. [PubMed] [Google Scholar]
- 30.Sah RP, Nagpal SJ, Mukhopadhyay D, Chari ST. New insights into pancreatic cancer-induced paraneoplastic diabetes. Nat Rev Gastroenterol Hepatol. 2013;10:423–433. doi: 10.1038/nrgastro.2013.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Permert J, Ihse I, Jorfeldt L, et al. Pancreatic cancer is associated with impaired glucose metabolism. Eur J Surg. 1993;159:101–107. [PubMed] [Google Scholar]
- 32.Chari ST, Leibson CL, Rabe KG, et al. Pancreatic cancer-associated diabetes mellitus: prevalence and temporal association with diagnosis of cancer. Gastroenterology. 2008;134:95–101. doi: 10.1053/j.gastro.2007.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pannala R, Leirness JB, Bamlet WR, et al. Prevalence and clinical profile of pancreatic cancer-associated diabetes mellitus. Gastroenterology. 2008;134:981–987. doi: 10.1053/j.gastro.2008.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Permert J, Adrian TE, Jacobsson P, et al. Is profound peripheral insulin resistance in patients with pancreatic cancer caused by a tumor-associated factor? Am J Surg. 1993;165:61–66. doi: 10.1016/s0002-9610(05)80405-2. discussion 66–67. [DOI] [PubMed] [Google Scholar]
- 35.Cersosimo E, Pisters PW, Pesola G, et al. Insulin secretion and action in patients with pancreatic cancer. Cancer. 1991;67:486–493. doi: 10.1002/1097-0142(19910115)67:2<486::aid-cncr2820670228>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 36.Nakamori S, Ishikawa O, Ohigashi H, et al. Increased blood proinsulin and decreased C-peptide levels in patients with pancreatic cancer. Hepatogastroenterology. 1999;46:16–24. [PubMed] [Google Scholar]
- 37.Permert J, Ihse I, Jorfeldt L, et al. Improved glucose metabolism after subtotal pancreatectomy for pancreatic cancer. Br J Surg. 1993;80:1047–1050. doi: 10.1002/bjs.1800800841. [DOI] [PubMed] [Google Scholar]
- 38.Giovannucci E, Michaud D. The role of obesity and related metabolic disturbances in cancers of the colon, prostate, and pancreas. Gastroenterology. 2007;132:2208–2225. doi: 10.1053/j.gastro.2007.03.050. [DOI] [PubMed] [Google Scholar]
- 39.Wolpin BM, Bao Y, Qian ZR, et al. Hyperglycemia, insulin resistance, impaired pancreatic beta-cell function, and risk of pancreatic cancer. J Natl Cancer Inst. 2013;105:1027–1035. doi: 10.1093/jnci/djt123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fisher WE, Boros LG, Schirmer WJ. Insulin promotes pancreatic cancer: evidence for endocrine influence on exocrine pancreatic tumors. J Surg Res. 1996;63:310–313. doi: 10.1006/jsre.1996.0266. [DOI] [PubMed] [Google Scholar]
- 41.Ding XZ, Fehsenfeld DM, Murphy LO, et al. Physiological concentrations of insulin augment pancreatic cancer cell proliferation and glucose utilization by activating MAP kinase, PI3 kinase and enhancing GLUT-1 expression. Pancreas. 2000;21:310–320. doi: 10.1097/00006676-200010000-00014. [DOI] [PubMed] [Google Scholar]
- 42.Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer. 2008;8:915–928. doi: 10.1038/nrc2536. [DOI] [PubMed] [Google Scholar]
- 43.Rohrmann S, Giovannucci E, Smit E, Platz EA. Association of IGF-1 and IGFBP-3 with lower urinary tract symptoms in the third national health and nutrition examination survey. Prostate. 2007;67:1693–1698. doi: 10.1002/pros.20659. [DOI] [PubMed] [Google Scholar]
- 44.Renehan AG, Tyson M, Egger M, et al. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 45.Stevens RJ, Roddam AW, Beral V. Pancreatic cancer in type 1 and young-onset diabetes: systematic review and meta-analysis. Br J Cancer. 2007;96:507–509. doi: 10.1038/sj.bjc.6603571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bonelli L, Aste H, Bovo P, et al. Exocrine pancreatic cancer, cigarette smoking, and diabetes mellitus: a case-control study in northern Italy. Pancreas. 2003;27:143–149. doi: 10.1097/00006676-200308000-00007. [DOI] [PubMed] [Google Scholar]
- 47.Dowling RJ, Goodwin PJ, Stambolic V. Understanding the benefit of metformin use in cancer treatment. BMC Med. 2011;9:33. doi: 10.1186/1741-7015-9-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pannala R, Leibson CL, Rabe KG, et al. Temporal association of changes in fasting blood glucose and body mass index with diagnosis of pancreatic cancer. Am J Gastroenterol. 2009;104:2318–2325. doi: 10.1038/ajg.2009.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bosetti C, Tavani A, Negri E, et al. Reliability of data on medical conditions, menstrual and reproductive history provided by hospital controls. J Clin Epidemiol. 2001;54:902–906. doi: 10.1016/s0895-4356(01)00362-6. [DOI] [PubMed] [Google Scholar]
- 50.Cavanaugh KL, Merkin SS, Plantinga LC, et al. Accuracy of patients’ reports of comorbid disease and their association with mortality in ESRD. Am J Kidney Dis. 2008;52:118–127. doi: 10.1053/j.ajkd.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.