Abstract
Classic functions of the actin cytoskeleton include control of cell size and shape and the internal organisation of cells. These functions are manifest in cellular processes of fundamental importance throughout biology such as the generation of cell polarity, cell migration, cell adhesion and cell division. However, studies in the unicellular model eukaryote Saccharomyces cerevisiae (Baker's yeast) are giving insights into other functions in which the actin cytoskeleton plays a critical role. These include endocytosis, control of protein translation and determination of protein 3-dimensional shape (especially conversion of normal cellular proteins into prions). Here we present a concise overview of these new "moonlighting" roles for the actin cytoskeleton and how some of these roles might lie at the heart of important molecular switches. This is an exciting time for researchers interested in the actin cytoskeleton. We show here how studies of actin are leading us into many new and exciting realms at the interface of genetics, biochemistry and cell biology. While many of the pioneering studies have been conducted using yeast, the conservation of the actin cytoskeleton and its component proteins throughout eukaryotes suggests that these new roles for the actin cytoskeleton may not be restricted to yeast cells but rather may reflect new roles for the actin cytoskeleton of all eukaryotes.
Conservation of the actin cytoskeleton from yeast to humans
Baker's yeast (Saccharomyces cerevisiae) was first used for genetic studies in the 1930s (1), however it was only in 1984 that S. cerevisiae was first shown to possess a cytoskeleton comprising actin and tubulin (2). It is now evident that S. cerevisiae possesses a set of cytoskeleton components that is analogous to, albeit less extensive than, that found in human cells (Table 1). Yeast cells do not physically resemble human cells which are not ellipsoid, lack a cell wall, and do not grow by budding. Nevertheless, many regulatory pathways that control the cytoskeleton have been conserved throughout the evolution of eukaryotes. There are numerous regulatory pathways that control the cytoskeleton and most key pathways are conserved between S. cerevisiae and humans: G-protein-coupled receptors, Ras GTPases, Rho GTPases, MAP kinase cascades, cyclin-dependent kinases, protein kinases A and C, phosphatidylinositol 4,5-bisphosphate (PIP2), etc (for a comprehensive database see the Saccharomyces Genome Database (SGD), www.yeastgenome.org, (3)).
Table 1.
Examples of functionally related actin cytoskeleton proteins in the yeast Saccharomyces cerevisiae and humans (assembled from the Saccharomyces Genome Database (SGD), www.yeastgenome.org, (3))
| Human protein(s) | S. cerevisiae protein(s) | Function |
|---|---|---|
| actin | Act1p (End7p) | Filament component/ polarity signalling/ endocytosis/ cytokinesis/ regulation of protein translation/ prion amyloid formation |
| Arp2, Arp3, p40, p35, p19, p18, p14 |
Arp2p, Arp3p, Arc40p, Arc35p (End9p), Arc19p, Arc18p, Arc15p |
Filament nucleation/filament binding/ polarity signalling/endocytosis |
| Type I myosin (role in filament nucleation is likely fungal-specific) |
Myo3p, Myo5p | Filament nucleation/ filament binding/motor/ polarity signalling/ endocytosis/cytokinesis |
| Type II myosin | Myo1p | Filament binding/motor/polarity signalling/ cytokinesis |
| Type V myosin | Myo2p, Myo4p | Filament binding/motor/ polarity signaling |
| Wiskott-Aldrich Syndrome Protein (WASP and N-WASP) |
Las17p (Bee1p) | Filament nucleation/binds actin monomers/ polarity signalling/ endocytosis/cytokinesis |
| WASP-Interacting Protein (WIP) (role in cytokinesis remains to be shown) |
Vrp1p (End5p) | Filament nucleation/binds actin monomers/ polarity signalling/ endocytosis/cytokinesis |
| tropomyosin | Tpm1p, Tpm2p | Filament binding/ filament stabilisation/polarity signaling |
| profilin | Pfy1p | Filament nucleation/ binds actin monomers/polarity signalling |
| capping protein (CP α/β) (role in endocytosis remains to be shown) |
Cap1p (α), Cap2p (β) | Filament binding/ filament end capping/polarity signalling/ endocytosis |
| cofilin | Cof1p | Filament binding/ filament severing/binds actin monomers/ polarity signalling/ endocytosis |
| fimbrin/plastin(role in endocytosis remains to be shown) |
Sac6p | Filament binding/ filament bundling/ polarity signalling/ endocytosis |
| twinfilin | Twf1p | Filament binding/ filament severing/ binds actin monomers |
| coronin | Crn1p | Filament nucleation/ filament binding/ filament bundling |
| formin | Bni1p, Bnr1p | Filament nucleation/filament binding/polarity signaling/ cytokinesis |
| calmodulin | Cmd1p | polarity signalling/ endocytosis |
| Cdc42 | Cdc42p, Rho5p | filament nucleation/ polarity signalling |
| RhoA | Rho1p, Rho2p, Rho3p, Rho4p |
Polarity signalling |
| Eps15 (role in filament nucleation remains to be shown) |
Pan1p | Filament nucleation/ polarity signalling/endocytosis |
| CIN85 | Sla1p | Polarity signalling/ endocytosis/ interactions with prions in yeast |
| amphiphysin/endophilin/Bin1/Bin2 | Rvs167p | Polarity signalling/ endocytosis |
| Bin3 (role in endocytosis remains to be shown) |
Rvs161p (End6p) | Polarity signalling/ endocytosis |
| mAbp1(role in filament nucleation remains to be shown) |
Abp1p | Filament binding/ filament nucleation/ polarity signalling/endocytosis |
| Hip1/Hip1R | Sla2p (End4p) | Polarity signalling/ endocytosis/ interactions with prions and polyglutamines |
| Eps15 | End3p | Polarity signalling/ endocytosis/ Interactions with prions in yeast |
| cyclase associated protein (CAP) | Srv2p (End14p) | Binds actin monomers/polarity signalling/ endocytosis |
| eEF1A | eEF1A(Tef1p/ Tef2p) | Filament binding/ filament bundling/ translation elongation factor |
| IMPACT (direct actin association remains to be shown) |
Yih1p | Regulation of the key translational regulator Gcn2p, binds actin monomers |
Advantages of yeast as an experimental tool for studying the cytoskeleton
There are six major advantages of S. cerevisiae as an experimental tool for exploring fundamental aspects of cytoskeleton function: 1) its short doubling time (90 min) (4); 2) its ability to be maintained as either a stable haploid or stable diploid (4); 3) the ability of haploids to be mated and put through genetic crosses and that one can collect and analyse all four meiotic products (4); 4) that it has only one (rarely two) member of each family of cytoskeleton component (www.yeastgenome.org); 5) gene knockout is easy and fast (3 days) (4) and a genome-wide collection of clean gene knockouts and conditional gene knockouts is widely available (e.g. (5, 6)); and 6) regulation of the cytoskeleton is not already compromised by pre-existing genetic changes prior to experimentation (which can be an issue with cancer cell lines and immortalised primary animal cell lines). In fact, yeast is being frequently used as model organism for biomedical research (e.g. (7)).
Yeast studies contribute to a better understanding of the human actin cytoskeleton
One of the many major contributions of S. cerevisiae and other yeasts (e.g. Schizosaccharomyces pombe) to the cytoskeleton field was the discovery in genetic screens of actin cytoskeleton components, most of which were subsequently found to have human homologues, e.g. Arp2p and Arp3p (8) (human Arp2 and Arp3 (8)) (Table 1). Arp2 and Arp3 are components of a conserved multisubunit complex known as Arp2/3 that initiates the assembly of branched actin filaments (8). These branched actin filaments form extensive dendritic arrays (9). Among the many other actin cytoskeleton components discovered in yeast but with mammalian homologs are: Rvs167p, Rvs161p (human amphiphysins/endophilins), Abp1p (mammalian Abp1), Sla2p (mammalian Hip1R), Sla1p (human CIN85), and Vrp1p/verprolin (human WIP) (10, 11) (Table 1).
Studies using S. cerevisiae have also assigned functions to human cytoskeleton proteins. For example, the proteins known as formins were first discovered in vertebrates as proteins with critical roles in limb development (12). However, that formins function to initiate assembly of linear (i.e. non-branched) actin filaments emerged from studies on the equivalent formins Bni1p and Bnr1p in S. cerevisiae (13) with subsequent confirmation that mammalian formins also function in initiation of linear actin filament assembly (14) . Another example is the role of the actin cytoskeleton in endocytosis, the process by which cells internalise cell surface receptors, other membrane material, and extracellular fluids and particles (Fig. 1). Studies with a variety of mammalian cells in culture gave conflicting results as to whether a functional actin cytoskeleton is essential for endocytosis (15–17). The first decisive evidence for a generalised role for the actin cytoskeleton in endocytosis came from genetic screens in yeast that identified numerous actin cytoskeleton components as proteins essential for uptake of both cell surface receptors and extracellular fluids (18, 19). It is now clear that although there are many different pathways of endocytosis in mammalian cells, most, if not all of these, are dependent on a functional actin cytoskeleton (20).
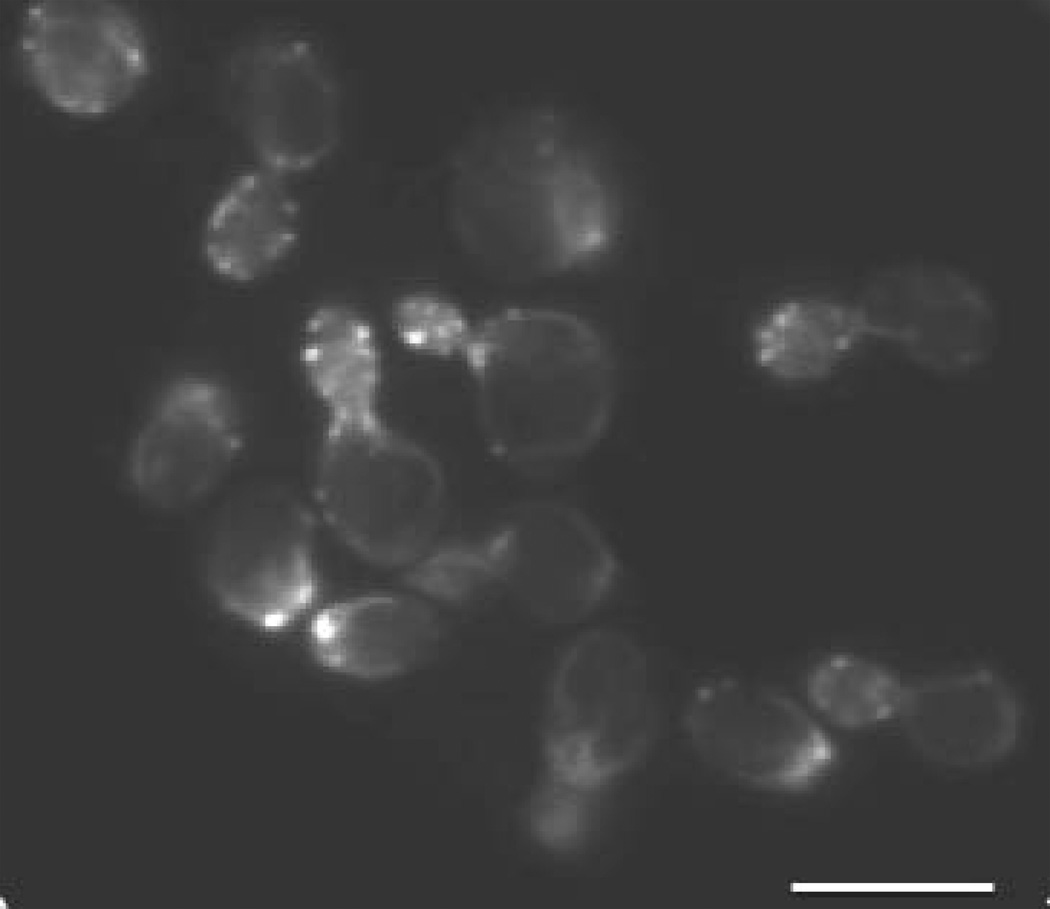
Figure 1. The yeast actin cytoskeleton and its importance for endocytosis.
A. Visualisation of actin cytoskeleton via fluorescence microscopy of S. cerevisiae cells, fixed and stained with fluorophore-conjugated phalloidin (F-actin specific reagent). Scale bar, 5 µm. B. Internalisation and vacuolar accumulation of the fluorescent endocytic dye Lucifer Yellow by endocytosis in S. cerevisiae cells. Scale bar, 5 µm.
Structures that comprise the yeast actin cytoskeleton
S. cerevisiae cells possess several recognisable types of actin filament (F-actin)-containing structures (Fig. 1). Underlying the plasma membrane are numerous small spots of F-actin. These are cortical actin patches and comprise branched actin filaments assembled by the Arp2/3 complex. The cortical actin patches have a distribution during the cell cycle that is polarised towards the site of polarised growth and have been proposed to be sites of endocytosis (2, 18, 21) (Fig. 1). Another type of F-actin-containing structure are cytoplasmic actin cables. These are fibres that extend the length of the cell and comprise thousands of linear actin filaments assembled by formins. Actin cables align along the mother cell-bud axis with their tips near the clustered cortical actin patches. Actin cables have been proposed to serve as tracks for movement of vesicles and organelles to sites of polarised growth (2, 18, 21). Finally, during mitosis a continuous ring of F-actin forms precisely at the bud neck. This ring comprises linear actin filaments assembled by formins and a conventional non-muscle myosin (myosin II). This is the contractile actomyosin ring and is equivalent to the actomyosin contractile ring found in mammalian cells. The actomyosin ring does not alter its subcellular distribution, however, it contracts to a small dot as cells divide (22).
A new role for actin in protein synthesis/translation
Spatial and temporal regulation of protein synthesis is a central theme throughout biology, e.g. for development, cellular migration and differentiation, adaptation, and long term memory formation. An intriguing idea, now supported by an increasing number of studies, is that the actin cytoskeleton is a key contributor to spatial and temporal regulation of translation. In several organisms it was found that a significant proportion of mRNAs, ribosomes, aminoacyl-tRNA synthetases, and some translation factors are anchored to the actin cytoskeleton (23), suggesting that the actin cytoskeleton acts as a scaffold for the organisation of the translation machinery components. In fact, perturbation of the actin cytoskeleton is associated with a dramatic reduction in the rate of global protein synthesis in yeast and mammalian cells (23). Direct evidence for efficient translation requiring an intact actin cytoskeleton was finally provided via genetic studies in yeast. For example, deletion of TPM1 or MDM20 leading to fewer actin cables led to a drastic reduction in global translation, and/or in the step of translation initiation (23, 24). As both actin and translation factors are conserved among eukaryotes, roles for actin in translation are also likely conserved.
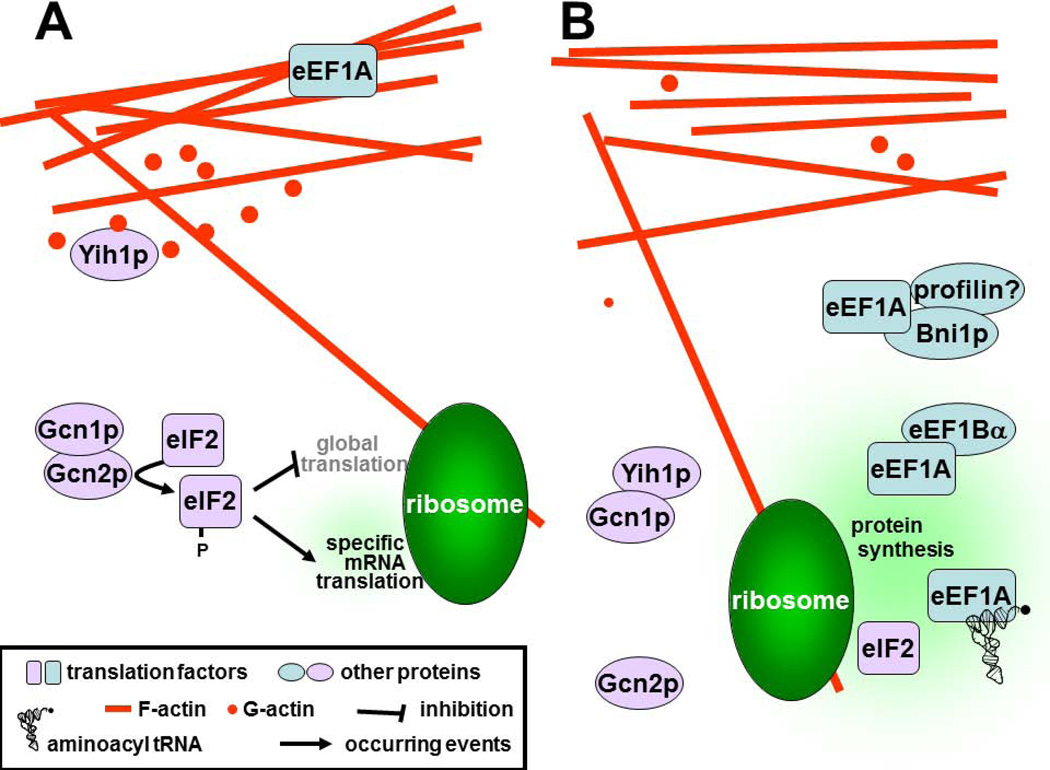
The molecular mechanisms by which actin regulates translation are, in general, still not well understood. The best studied example so far is the regulation of eEF1A, the translation factor that in its GTP-bound form delivers aminoacyl-tRNAs to the ribosome (24). Binding of filamentous (F-) actin to eEF1A promotes GTP hydrolysis and prevents eEF1A from binding GTP, suggesting that actin drives eEF1A into its translation-inactive form (21). Another example has come from recent findings suggesting that the actin monomer (G-actin) binding protein Yih1p links translational regulation with actin filament assembly (25). The general amino acid control (GAAC) regulatory network controls the response of yeast cells to amino acid starvation. Upon recognition of amino acid starvation, a protein kinase component of the GAAC network (Gcn2p) phosphorylates the alpha subunit of translation initiation factor 2 (eIF2), resulting in reduced global translation but concomitant increased specific translation of a transcriptional activator necessary to respond and adapt to starvation (26). To sense starvation, Gcn2p must directly bind to an effector protein (Gcn1p) (27). Overexpression studies suggest that Yih1p acts to bind and sequester Gcn1p (thereby diminishing Gcn1p-Gcn2p interaction and consequently Gcn2p activity). Furthermore, genetic studies suggest that Yih1p can only do this after being released from the complex with G-actin (25, 26) (Fig. 2). The finding that the GAAC response is not enhanced in yih1Δ strains suggests that Yih1p only inhibits Gcn2p under specific physiological conditions, e.g. when Gcn2p activity is deleterious to the cell. Alternatively, Yih1p may inhibit Gcn2p only at specific intracellular sites and this inhibition escapes detection when using conventional experimental procedures (that involve cell breakage and mixing of the cell content) (26). Yih1p orthologues are found in mammals (called IMPACT). When overexpressed in yeast, IMPACT inhibits Gcn2p function and co-precipitates actin. Furthermore, overexpression of IMPACT in mammalian cells impairs GCN2 function as found in yeast, suggesting that this regulatory mechanism is conserved (26). Interestingly, local regulation of GCN2 activity is very likely a contributing factor for long term memory formation in neurons, and in neuron-like N2a cells the GCN2-IMPACT module is involved in modulating neurite outgrowth (26). Recently, it was discovered that in yeast eEF1A binds and inhibits Gcn2p under nutrient-replete conditions (28). In addition, considering that actin regulates eEF1A activity (see below) in yeast and mammals, this raises the possibility that actin also utilises eEF1A to modulate Gcn2p/ GCN2 activity.
Figure 2. Actin-Translation connections found in yeasts.
A. eEF1A bound to F-actin and Yih1p bound to G-actin do not participate in translation and in controlling Gcn2p-mediated translational regulation, respectively. B. eEF1A can be released from F-actin by either Bni1p or the α subunit of the guanine nucleotide exchange factor eEF1B, and may then participate in protein synthesis. Studies support the idea that Yih1p released from G-actin sequesters Gcn1p thereby inhibiting Gcn2p function. For more detailed explanation please see text.
Conversely, translation factors are required for proper actin cytoskeleton function. For example, eEF1A binds and bundles actin filaments in all eukaryotes (23, 24). Taking into account that eEF1A is the second most abundant cellular protein after actin, and according to work in the slime mold Dictyostelium, eEF1A has high affinity for actin and more than 70% of eEF1A is actin bound (29), one may suggest that eEF1A is a major contributor in regulating the actin cytoskeleton. Supporting this idea, specific eEF1A mutations, or eEF1A overexpression, disrupt the actin cytoskeleton in S. cerevisiae (24). Furthermore, eEF1A overexpression leads to synthetic growth defects when combined with mutations in actin. In mammals, eEF1A has been implicated in tumour metastasis and it has been shown that eEF1A from metastatic cells has reduced F-actin affinity (30).
The relationship between translation factors and actin is dynamic and regulated. A good example is the interaction between eEF1A and actin. Studies have shown that eEF1A binds exclusively to either F-actin or aminoacyl tRNA, and to either F-actin or its activation factor eEF1Bα (23, 24). While bound to F-actin, eEF1A does not participate in translation, possibly due to its inability to bind aminoacyl-tRNAs. In vitro and genetic studies in yeast suggest that the alpha (catalytic) subunit of the guanine nucleotide exchange factor complex (eEF1B) promotes dissociation of eEF1A from F-actin, thereby switching eEF1A function from F-actin bundling to translation (23, 24). Together, this suggests that the sub-compartmental stoichiometric balance between aminoacyl-tRNA, eEF1A, eEF1Bα and F-actin is crucial for determining the rate of translation elongation as well as actin bundling, illustrating a complex relationship between the eEF1A function in translation and actin organisation (Fig. 2).
Adding to the complexity, several proteins have been found to modulate eEF1A-actin interaction directly or indirectly. For example, the F-actin binding and bundling activity of eEF1A is inhibited by association with the formin Bni1p (21). Bni1p regulates the actin cytoskeleton through its ability to nucleate the assembly of linear actin filaments, interacts with the actin-monomer-binding protein profilin, and is also a downstream target of Rho1p, a protein belonging to the Rho family of small GTPase proteins involved in important signalling processes such as regulation of the actin cytoskeleton. In Dictyostelium, it was found that the eEF1A-F-actin interaction is pH-dependent. Chemoattractants such as cAMP lead to an increased intracellular pH thereby dissociating eEF1A from actin, while pH changes do not affect eEF1A-aminoacyl-tRNA interaction (31, 32), suggesting that pH alterations can stimulate protein synthesis. Interestingly, in mammals intracellular alkalinisation has been associated with the growth and metastasis of tumor cells (33). It was found that intracellular alkalinisation correlated with increased dissociation of eEF1A from actin, and that siRNA-mediated knockdown of eEF1A reverted the effects of alkalinisation-induced cell growth (34). Furthermore, eEF1A is subject to regulation by key signalling molecules or enzymes. For example, the actin bundling activity of Tetrahymena eEF1A is inhibited by the Ca2+/calmodulin complex (35).
Actin-mediated mRNA transport to specific intracellular sites is another powerful and fast mechanism to spatially and temporally control translation of specific proteins. Interestingly, eEF1A is involved in anchoring beta-actin mRNA to F-actin in the protrusions of motile cells (36), thereby allowing local synthesis and efficient polymerisation of actin at the site of demand. Studies suggest that actin-dependent mRNA transport and its local translation are also important for yeast pseudohyphal growth and the filamentation and virulence of Candida albicans (37).
Another way to regulate protein synthesis is to translationally silence mRNAs in RNA granules. RNA granules are macromolecular aggregates found in all eukaryotes that in addition to mRNA may contain components of the translation machinery. RNA granules may require microfilaments for their dynamics (38). Of the RNA granules one type referred to as processing bodies exist constitutively and take up or release mRNAs in response to particular stimuli. Processing bodies are also involved in RNA degradation. In contrast, the RNA granules known as stress granules occur only under certain stress conditions.
Actin-mediated spatial organisation of translation machinery components would increase the local concentration of these components and thus enhance the efficiency of protein synthesis. The actin-translation linkage may also allow the cytoskeleton to convey internal/external cues (e.g. environmental stress) to the translation machinery for optimal and quick adaptation. Conversely, translation factors, or their regulators, may convey information to and thereby regulate the actin cytoskeleton. Taken together, it becomes increasingly evident that the actin cytoskeleton and protein synthesis machinery reciprocally regulate and require each other, which in turn allows an optimal cellular adaptation to any given condition. More work is necessary to gain a full understanding of this complex interdependency and reciprocal regulation.
A new role for actin in protein folding
There is emerging evidence that the actin cytoskeleton also plays a critical role in the folding of certain proteins, in particular proteins that can form cross-beta fibrous aggregates known as amyloids. Self-perpetuating amyloids termed prions transmit heritable traits in yeast (39) and neurodegenerative diseases in mammals (including humans), e.g. Creutzfeldt-Jakob Disease (CJD), scrapie, and bovine spongiform encephalopathy (BSE) (40). Many other amyloid diseases, including Alzheimer’s, Parkinson’s and Huntington diseases, possess at least some prion properties. Similar to mammalian prions, yeast prions propagate by immobilizing and converting other polypeptides of the same amino acid sequence into an amyloid form and generate aberrant protein aggregates. However, mammalian prions are transmitted via extracellular infection, whereas yeast prions are inherited via the cytoplasm in cell divisions, mating or cytoplasm exchange (cytoduction) (39, 41). Some yeast prions are pathogenic to the yeast host (reviewed in (39)). However the recent discovery of traits controlled in a prion-like fashion in at least about 1/3 of the natural and industrial isolates of Saccharomyces cerevisiae confirm that prions are widespread and suggest that some of them are adaptive (42). Traits controlled by prions are usually associated with the decrease or loss of protein function, although gain of function has also been reported. Prion formation by regulatory proteins may result in altering a range of cellular processes, including metabolism and gene expression pathways (39). Yeast prions provide a robust, but dynamic system for epigenetic regulation of phenotype controlled by the cellular and environmental factors (41).
Yeast heritable element [PSI+] is a prion isoform of the yeast translation termination factor (eRF3/Sup35p) (39). Conformational change of soluble Sup35p into the amyloid-like aggregated form leads to reduced efficiency in termination of the polypeptide synthesis at stop codons that may potentially result in production of elongated polypeptide chains (39). Several lines of evidence link the actin cytoskeleton to the yeast prion [PSI+]. First, the prion-forming domain of Sup35p physically interacts with a number of cortical actin patch proteins (43, 44). Furthermore, during the initial transition from normal protein to [PSI+] prion (induced by Sup35p over-expression) Sup35p forms filamentous aggregates that exhibit subcellular colocalization with several cortical actin patch components (43). These aggregates appear to be an intermediate found only in cells converting from [psi−] to [PSI+] and colocalization with cortical actin patch components appears to specifically occur in this conversion phase. Mutations that affect the actin cytoskeleton reduce the ability of Sup35p to form visible intracellular aggregates required for conversion to the [PSI+] prion and increase the toxicity of Sup35p when over-expressed in cells that harbour [PSI+], suggesting that the assembly of protein polymers into the large visible aggregates may counteract cytotoxicity (43). Interestingly, actin assembly proteins also interact with the polyglutamines, expressed in yeast in the yeast model of Huntington’s disease. These interactions might be mediated by prion form of another yeast protein, Rnq1, that promotes polyglutamine aggregation in yeast, and result in the inhibition of endocytosis (apparently due to sequestration of the actin assembly proteins by polyglutamines) and respectively, in cytotoxicity (reviewed in (39)).
The assembly of amyloid fibres in some ways resembles the assembly of actin filaments in that both require a rate-limiting nucleation event followed by elongation and final breaking up of existing polymers in order to nucleate new polymers (43). This, together with the finding that the conversion of Sup35p to [PSI+] appears to occur at sites that also contain cortical actin patch components and is perturbed by mutation of cortical actin patch components, has led to the proposal that amyloid fibre assembly may be regulated by the same machinery that functions in actin filament assembly and actin-dependent endocytosis (43). Indeed, depletion of actin associated Las17p (WASP) binding protein Lsb2 results in the destabilization of [PSI+] prion aggregates during and after thermal stress (45). At the same time, high levels of Lsb2p trigger conversion of Sup35p into the prion form (45). Levels of Lsb2p protein are dramatically increased by heat shock, while its prion inducing ability is strictly dependent on its association with cortical actin patches (45). These findings directly implicate actin cytoskeleton in regulating prions during the environmental changes. Interactions of actin cytoskeleton with the prion aggregates somewhat parallel the proposed role for the actin cytoskeleton in asymmetric segregation of aggregated oxidatively damaged protein, resulting in generation of the aggregate-free daughter cells (buds) in the cell divisions following stress (46).
The actin cytoskeleton itself may contain amyloidogenic proteins. Lsb2p as well as some other actin assembly proteins interacting with Sup35p, e. g. Sla2p (homolog of human HIP1R), possess QN-rich domains similar to prion domains of yeast prion proteins (reviewed in (39)). In addition, many actin cytoskeleton components in yeast (including Lsb2p) and mammals possess Src Homology 3 (SH3) domains - a type of small (~60 amino acids long) protein-protein interaction module first found in the non-receptor tyrosine kinase Src (47). Evidence suggests that SH3 domains can be converted to fibrous amyloids in specific conditions in vitro (48). The amyloid form of SH3 domains is highly cytotoxic when taken up by cultured mammalian cells (49), however it is not known whether SH3 domains naturally switch to amyloid in vivo. Some specific yeast SH3 domains become highly cytotoxic in vivo following either mutation or loss of their binding site in a partner protein (a cytotoxicity that is effectively cured by deletion of the offending SH3 domain) (50–52). Although the molecular basis for this SH3-domain-dependent cytotoxicity is not understood, one possibility is that association with a binding partner prevents the SH3 domain from undergoing conversion to an amyloid. An ability to form amyloid may reflect some as yet over-looked physiological signalling function of SH3 domains. Much more work is needed to test these hypotheses. The ability of the actin cytoskeleton to affect stable and heritable changes in protein conformation and activity may play important roles in long-term adaptation of cells to changes in the environment with similar effects to (but not necessarily with the permanency of) mutation of DNA.
Taken together, strong evidence is accumulating to support the possibility that the cellular roles of actin go far beyond its well-studied skeletal functions and may range from control of vital cellular processes like protein translation to promotion of disease development and in particular those diseases attributable to protein aggregation. More studies are necessary to fully understand the crosstalk between the actin cytoskeleton and specific cellular processes, which simultaneously will provide insight into how certain diseases may develop and how they may be treated or prevented in the future. Clearly, several studies have demonstrated that the amenable yeast eukaryotic model is a prime tool for contributing to the further decipherment of the many new roles actin plays in the cell.
ACKNOWLEDGEMENTS
This work was supported in parts by the grant DP110100389 from the Australian Research Council (A.L.M. and Y.O.C.), Griffith University Short Term Visiting Fellowship (Y.O.C.), NIH grant R01GM093294 (Y.O.C. and T.A.C.), the St. Petersburg State University research project 1.50.2218.2013 (Y.O.C.), and the Health Research Council (HRC) of New Zealand Emerging Researcher First Grant #06/410 and Massey University Research Fund (E.S.).
Footnotes
The authors have no conflict of interest to declare.
REFERENCES
We apologize to those whose work was not cited or discussed because of space limitations.
- 1.Hall M, Linder P, editors. The early days of yeast genetics. Cold Spring Harbor, NY, USA: Cold Spring Harbor Laboratory Press; 1993. [Google Scholar]
- 2.Adams AEM, Pringle JR. Relationship of actin and tubulin distribution to bud growth in wild-type and morphogenetic-mutant Saccharomyces cerevisiae. Journal of Cell Biology. 1984;98:934–945. doi: 10.1083/jcb.98.3.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cherry JM, Hong EL, Amundsen C, Balakrishnan R, Binkley G, Chan ET, Christie KR, Costanzo MC, Dwight SS, Engel SR, Fisk DG, Hirschman JE, Hitz BC, Karra K, Krieger CJ, Miyasato SR, Nash RS, Park J, Skrzypek MS, Simison M, Weng S, Wong ED. Saccharomyces Genome Database: the genomics resource of budding yeast. Nucleic Acids Research. 2012;40:D700–D705. doi: 10.1093/nar/gkr1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adams A, Gottschling D, Kaiser C, Stearns T, editors. Methods in yeast genetics: a laboratory course manual. Cold Spring Harbor, NY, USA: Cold Spring Harbor Laboratory Press; 1997. [Google Scholar]
- 5.Giaever G, Chu A, Ni L, Connelly C, Riles L, Veronneau S, Dow S, Lucau-Danila A, Anderson K, Andre B. Functional profiling of the Saccharomyces cerevisiae genome. Nature. 2002;418:387–391. doi: 10.1038/nature00935. [DOI] [PubMed] [Google Scholar]
- 6.Mnaimneh S, Davierwala AP, Haynes J, Moffat J, Peng WT, Zhang W, Yang X, Pootoolal J, Chua G, Lopez A, Trochesset M, Morse D, Krogan NJ, Hiley SL, Li Z, Morris Q, Grigull J, Mitsakakis N, Roberts CJ, Greenblatt JF, Boone C, Kaiser CA, Andrews BJ, Hughes TR. Exploration of essential gene functions via titratable promoter alleles. Cell. 2004;118:31–44. doi: 10.1016/j.cell.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 7.Mager WH, Winderickx J. Yeast as a model for medical and medicinal research. Trends in Pharmacological Sciences. 2005;26:265–273. doi: 10.1016/j.tips.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 8.Higgs HN, Pollard TD. Regulation of actin filament network formation through Arp2/3 complex: Activation by a diverse array of proteins. Annual Review of Biochemistry. 2001;70:649–676. doi: 10.1146/annurev.biochem.70.1.649. [DOI] [PubMed] [Google Scholar]
- 9.Blanchoin L, Amann KJ, Higgs HN, Marchand J-B, Kaiser DA, Pollard TD. Direct observation of dendritic actin filament networks nucleated by Arp2/3 complex and WASP/Scar proteins. Nature. 2000;404:1007–1011. doi: 10.1038/35010008. [DOI] [PubMed] [Google Scholar]
- 10.Munn AL. Molecular requirements for the internalisation step of endocytosis: insights from yeast. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease. 2001;1535:236–257. doi: 10.1016/s0925-4439(01)00028-x. [DOI] [PubMed] [Google Scholar]
- 11.Robertson AS, Smythe E, Ayscough KR. Functions of actin in endocytosis. Cellular and Molecular Life Sciences. 2009;66:2049–2065. doi: 10.1007/s00018-009-0001-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maas RL, Zeller R, Woychik RP, Vogt TF, Leder P. Disruption of formin-encoding transcripts in two mutant limb deformity alleles. Nature. 1990;346:853–855. doi: 10.1038/346853a0. [DOI] [PubMed] [Google Scholar]
- 13.Evangelista M, Pruyne D, Amberg DC, Boone C, Bretscher A. Formins direct Arp2/3-independent actin filament assembly to polarize cell growth in yeast. Nature cell biology. 2002;4:32–41. doi: 10.1038/ncb718. [DOI] [PubMed] [Google Scholar]
- 14.Kovar DR, Harris ES, Mahaffy R, Higgs HN, Pollard TD. Control of the assembly of ATP- and ADP-actin by formins and profilin. Cell. 2006;124:423–435. doi: 10.1016/j.cell.2005.11.038. [DOI] [PubMed] [Google Scholar]
- 15.Gottlieb TA, Ivanov IE, Adesnik M, Sabatini DD. Actin microfilaments play a critical role in endocytosis at the apical but not the basolateral surface of polarized epithelial cells. Journal of Cell Biology. 1993;120:695–710. doi: 10.1083/jcb.120.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lamaze C, Fujimoto LM, Yin HL, Schmid SL. The actin cytoskeleton is required for receptor-mediated endocytosis in mammalian cells. Journal of Biological Chemistry. 1997;272:20332–20335. doi: 10.1074/jbc.272.33.20332. [DOI] [PubMed] [Google Scholar]
- 17.Fujimoto LM, Roth R, Heuser JE, Schmid SL. Actin assembly plays a variable, but not obligatory role in receptor-mediated endocytosis in mammalian cells. Traffic. 2000;1:161–171. doi: 10.1034/j.1600-0854.2000.010208.x. [DOI] [PubMed] [Google Scholar]
- 18.Engqvist-Goldstein ÃEY, Drubin DG. Actin Assembly and Endocytosis: From Yeast to Mammals. Annual Review of Cell and Developmental Biology. 2003;19:287–332. doi: 10.1146/annurev.cellbio.19.111401.093127. [DOI] [PubMed] [Google Scholar]
- 19.Munn AL, Stevenson BJ, Geli MI, Riezman H. end5, end6, and end7: Mutations that cause actin delocalization and block the internalization step of endocytosis in Saccharomyces cerevisiae. Molecular Biology of the Cell. 1995;6:1721–1742. doi: 10.1091/mbc.6.12.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kirkham M, Parton RG. Clathrin-independent endocytosis: new insights into caveolae and non-caveolar lipid raft carriers. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research. 2005;1746:349–363. doi: 10.1016/j.bbamcr.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 21.Moseley JB, Goode BL. The yeast actin cytoskeleton: From cellular function to biochemical mechanism. Microbiology and Molecular Biology Reviews. 2006;70:605–645. doi: 10.1128/MMBR.00013-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bi E, Maddox P, Lew DJ, Salmon ED, McMillan JN, Yeh E, Pringle JR. Involvement of an actomyosin contractile ring in Saccharomyces cerevisiae cytokinesis. Journal of Cell Biology. 1998;142:1301–1312. doi: 10.1083/jcb.142.5.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim S, Coulombe PA. Emerging role for the cytoskeleton as an organizer and regulator of translation. Nature Reviews Molecular Cell Biology. 2010;11:75–81. doi: 10.1038/nrm2818. [DOI] [PubMed] [Google Scholar]
- 24.Mateyak MK, Kinzy TG. eEF1A: Thinking outside the ribosome. Journal of Biological Chemistry. 2010;285:21209–21213. doi: 10.1074/jbc.R110.113795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sattlegger E, Swanson MJ, Ashcraft EA, Jennings JL, Fekete RA, Link AJ, Hinnebusch AG. YIH1 is an actin-binding protein that inhibits protein kinase GCN2 and impairs general amino acid control when overexpressed. Journal of Biological Chemistry. 2004;279:29952–29962. doi: 10.1074/jbc.M404009200. [DOI] [PubMed] [Google Scholar]
- 26.Castilho B, Shanmugam R, Cardoso R, Himme B, Ramesh R, Sattlegger E. Keeping the eIF2 alpha kinase Gcn2 in check. Biochimica et Biophysica Acta - Molecular Cell Research. 2014;1843:1948–1968. doi: 10.1016/j.bbamcr.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 27.Sattlegger E, Hinnebusch AG. Separate domains in GCN1 for binding protein kinase GCN2 and ribosomes are required for GCN2 activation in amino acid-starved cells. EMBO J. 2000;19:6622–6633. doi: 10.1093/emboj/19.23.6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Visweswaraiah J, Lageix S, Castilho BA, Izotova L, Kinzy TG, Hinnebusch AG, Sattlegger E. Evidence that eukaryotic translation elongation factor 1A (eEF1A) binds the Gcn2 protein C terminus and inhibits Gcn2 activity. Journal of Biological Chemistry. 2011;286:36568–36579. doi: 10.1074/jbc.M111.248898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Condeelis J. Elongation factor 1alpha, translation and the cytoskeleton. Trends in Biochemical Sciences. 1995;20:169–170. doi: 10.1016/s0968-0004(00)88998-7. [DOI] [PubMed] [Google Scholar]
- 30.Lamberti A, Caraglia M, Longo O, Marra M, Abbruzzese A, Arcari P. The translation elongation factor 1A in tumorigenesis, signal transduction and apoptosis: Review article. Amino Acids. 2004;26:443–448. doi: 10.1007/s00726-004-0088-2. [DOI] [PubMed] [Google Scholar]
- 31.Liu G, Edmonds BT, Condeelis J. pH, EF-1alpha and the cytoskeleton. Trends in Cell Biology. 1996;6:168–171. doi: 10.1016/0962-8924(96)20013-3. [DOI] [PubMed] [Google Scholar]
- 32.Liu G, Tang J, Edmonds BT, Murray J, Levin S, Condeelis J. F-actin sequesters elongation factor 1alpha from interaction with aminoacyl-tRNA in a pH-dependent reaction. Journal of Cell Biology. 1996;135:953–963. doi: 10.1083/jcb.135.4.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cardone RA, Casavola V, Reshkin SJ. The role of disturbed pH dynamics and the Na+/H+ exchanger in metastasis. Nature Reviews Cancer. 2005;5:786–795. doi: 10.1038/nrc1713. [DOI] [PubMed] [Google Scholar]
- 34.Kim J, Namkung W, Yoon JS, Jo MJ, Lee SH, Kim KH, Kim JY, Lee MG. The role of translation elongation factor eEF1A in intracellular alkalinization-induced tumor cell growth. Laboratory Investigation. 2009;89:867–874. doi: 10.1038/labinvest.2009.53. [DOI] [PubMed] [Google Scholar]
- 35.Kurasawa Y, Hanyu K, Watanabe Y, Numata O. F-actin bundling activity of Tetrahymena elongation factor 1alpha is regulated by Ca2+/calmodulin. Journal of Biochemistry. 1996;119:791–798. doi: 10.1093/oxfordjournals.jbchem.a021309. [DOI] [PubMed] [Google Scholar]
- 36.Meignin C, Davis I. Transmitting the message: intracellular mRNA localization. Current Opinion in Cell Biology. 2010;22:112–119. doi: 10.1016/j.ceb.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 37.Zarnack K, Feldbrugge M. mRNA trafficking in fungi. Molecular Genetics and Genomics. 2007;278:347–359. doi: 10.1007/s00438-007-0271-8. [DOI] [PubMed] [Google Scholar]
- 38.Thomas MG, Loschi M, Desbats MA, Boccaccio GL. RNA granules: The good, the bad and the ugly. Cellular Signalling. 2011;23:324–334. doi: 10.1016/j.cellsig.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liebman SW, Chernoff YO. Prions in yeast. Genetics. 2012;191:1041–1072. doi: 10.1534/genetics.111.137760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Watts JC, Balachandran A, Westaway D. The expanding universe of prion diseases. PLoS pathogens. 2006;2:e26. doi: 10.1371/journal.ppat.0020026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chernova TA, Wilkinson KD, Chernoff YO. Physiological and environmental control of yeast prions. FEMS Microbiology Reviews. 2014;38:326–344. doi: 10.1111/1574-6976.12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Halfmann R, Jarosz DF, Jones SK, Chang A, Lancaster AK, Lindquist S. Prions are a common mechanism for phenotypic inheritance in wild yeasts. Nature. 2012;482:363–368. doi: 10.1038/nature10875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ganusova EE, Ozolins LN, Bhagat S, Newnam GP, Wegrzyn RD, Sherman MY, Chernoff YO. Modulation of prion formation, aggregation, and toxicity by the actin cytoskeleton in yeast. Molecular and Cellular Biology. 2006;26:617–629. doi: 10.1128/MCB.26.2.617-629.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bailleul PA, Newnam GP, Steenbergen JN, Chernoff YO. Genetic Study of Interactions Between the Cytoskeletal Assembly Protein Sla1 and Prion-Forming Domain of the Release Factor Sup35 (eRF3) in Saccharomyces cerevisiae. Genetics. 1999;153:81–94. doi: 10.1093/genetics/153.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chernova Tatiana A, Romanyuk Andrey V, Karpova Tatiana S, Shanks John R, Ali M, Moffatt N, Howie Rebecca L, O'Dell A, McNally James G, Liebman Susan W, Chernoff Yury O, Wilkinson Keith D. Prion Induction by the Short-Lived, Stress-Induced Protein Lsb2 Is Regulated by Ubiquitination and Association with the Actin Cytoskeleton. Molecular Cell. 2011;43:242–252. doi: 10.1016/j.molcel.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu B, Larsson L, Caballero A, Hao X, Oling D, Grantham J, Nystrom T. The Polarisome Is Required for Segregation and Retrograde Transport of Protein Aggregates. Cell. 2010;140:257–267. doi: 10.1016/j.cell.2009.12.031. [DOI] [PubMed] [Google Scholar]
- 47.Ren R, Mayer BJ, Cicchetti P, Baltimore D. Identification of a ten-amino acid proline-rich SH3 binding site. Science. 1993;259:1157–1161. doi: 10.1126/science.8438166. [DOI] [PubMed] [Google Scholar]
- 48.Guijarro JI, Sunde M, Jones JA, Campbell ID, Dobson CM. Amyloid fibril formation by an SH3 domain. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:4224–4228. doi: 10.1073/pnas.95.8.4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bucciantini M, Giannoni E, Chiti F, Baroni F, Formigli L, Zurdo J, Taddei N, Ramponi G, Dobson CM, Stefani M. Inherent toxicity of aggregates implies a common mechanism for protein misfolding diseases. Nature. 2002;416:507–511. doi: 10.1038/416507a. [DOI] [PubMed] [Google Scholar]
- 50.Naqvi SN, Feng Q, Boulton VJ, Zahn R, Munn AL. Vrp1p functions in both actomyosin ring-dependent and Hof1p-dependent pathways of cytokinesis. Traffic. 2001;2:189–201. doi: 10.1034/j.1600-0854.2001.020305.x. [DOI] [PubMed] [Google Scholar]
- 51.Ren G, Wang J, Brinkworth R, Winsor B, Kobe B, Munn AL. Verprolin cytokinesis function mediated by the Hof one trap domain. Traffic. 2005;6:575–593. doi: 10.1111/j.1600-0854.2005.00300.x. [DOI] [PubMed] [Google Scholar]
- 52.Thanabalu T, Munn AL. Functions of Vrp1p in cytokinesis and actin patches are distinct and neither requires a WH2/V domain. EMBO Journal. 2001;20:6979–6989. doi: 10.1093/emboj/20.24.6979. [DOI] [PMC free article] [PubMed] [Google Scholar]