Abstract
OBJECTIVE
Lactation is associated with reduction in maternal metabolic disease and hypertension later in life; however, findings in humans may be confounded by socioeconomic factors. We sought to determine the independent contribution of lactation on cardiovascular parameters and adiposity in a murine model.
STUDY DESIGN
Following delivery, CD-1 female mice were randomly divided into two groups: lactated (L, nursed pups for 3 weeks, n=10), and nonlactated (NL, pups were removed after birth, n=12). Blood pressure (BP) was assessed prepregnancy and at 1 and 2 months postpartum. Visceral (VAT) and subcutaneous adipose tissue (SAT) determined by computed tomography, and left ventricular ejection fraction (EF), cardiac output (CO) and the E/A ratio determined by micro-ultrasound were evaluated at 1 and 2 months postpartum. Results were analyzed using Student’s t-test (significance: P<0.05).
RESULTS
We observed a significantly different maternal BP at 2 months postpartum with relatively greater BP in NL (systolic BP: NL 122.2±7.2 vs L 96.8±9.8 mmHg; P=0.04; diastolic BP NL 87.0±6.8 vs L 65.9±6.2 mmHg; P=0.04). VAT was significantly increased in NL mice at 1 (22.0±4.1 vs 10.7±1.8 %, P=0.04) and 2 months postpartum (22.9±3.5 vs 11.2±2.2 %, P=0.02), while SAT did not differ between the groups. At 2 months postpartum, EF (51.8±1.5 vs 60.5±3.8%; P=0.04), CO (14.2±1.0 vs 18.0±1.3ml/min; P=0.02) and MV E/A (1.38±0.06 vs 1.82±0.13; P=0.04) were significantly lower in NL mice than L mice.
CONCLUSION
Our data provide evidence that interruption of lactation adversely affects postpartum maternal cardiovascular function and adiposity.
Keywords: adipose, blood pressure, CD-1 mouse, lactation, maternal health
1. INTRODUCTION
The benefits of breastfeeding for the health of the child are well described, including a reduction in infectious disease, childhood obesity, and may improve neurocognitive function.1–3 Animal models and intensive studies of human milk components have elucidated many of the mechanisms through which breast milk affects infectious morbidity risk; however, the mechanisms underlying associations between lactation and maternal health remain to be determined. Multiple observational studies have linked lactation with reduced maternal metabolic disease in later life. Longer duration of lactation is associated with reduced risk of type 2 diabetes,4–6 hypertension,4,7,8 breast cancer,9 hyperlipidemia,4,10 myocardial infarction,4,11 cardiovascular disease,4 and metabolic syndrome.12,13
Yet these observational studies have several confounding factors. Mothers that breastfeed differ from mothers who formula feed: they are wealthier, better educated, less likely to smoke, and more likely to engage in other beneficial health behaviors.14–15 In addition, obesity is associated with decreased breastfeeding initiation and duration, and women who are prone to develop metabolic syndrome may have difficulty with lactogenesis.16,17 Breastfeeding may therefore be a marker for maternal metabolic health, rather than a mechanism conferring reduced risk of disease. Thus, residual or unmeasured confounding factors, rather than breastfeeding itself, may explain observed associations between breastfeeding and maternal health outcomes. Currently, no data exist from randomized clinical trials in US populations to support or refute a causal association between breastfeeding and cardiovascular disease or adiposity in mothers.
Animal models are essential to quantify effects of lactation on later maternal health because lactation duration is assigned by experimental design and is therefore not confounded by other maternal behaviors. The very few studies in rodents have demonstrated that pregnancy without subsequent lactation results in an increase in fat content, lipoprotein lipase activity, larger adipocytes, altered glucose levels, and insulin resistance.18–20
Our objective was to determine the independent associations of lactation with subsequent blood pressure, cardiac function and adiposity in an in vivo mouse model.. We hypothesized that interruption of lactation would lead to elevated blood pressure, increase in visceral adipose tissue, and diminished cardiac function.
2. MATERIALS AND METHODS
2.1 Animals
The Institutional Animal Care and Use Committee (IACUC) at the University of Texas Medical Branch at Galveston approved the study protocol. Three to four week old CD-1 mice were obtained from Charles Rivers Laboratories (Wilmington, MA). The animals were housed in a temperature and humidity controlled facility with automatically controlled 12:12-hour light and dark cycles. Mice were allowed to consume regular chow and drinking solution ad libitum. Certified personnel and veterinary staff provided regular maintenance and animal care according to IACUC guidelines. The animals were sacrificed by carbon dioxide inhalation according to the IUCUC and American Veterinary Medical Association guidelines.
CD-1 mice have an average lifespan of 2 years. They are ready for breeding at about 5–8 weeks of age. Estrus occurs every 4 to 5 days. Pregnancy lasts about 20–21 days. Mice deliver somewhere between 10 to 16 fetuses. Females could be bred right after delivery or 1 week after weaning pups. Pups are typically weaned by 21 days of age.
2.2 Breeding
Male CD1 mice were placed with individual 6 week old females overnight for breeding. Pregnant female CD1 mice were randomized to lactated (L group, n=10) or nonlactated (NL group, n=12) groups. Litter mate status at the time of randomization of animals to NL versus L was unknown. Numbers of animals were chosen based on our previous studies.21 In NL group, pups were removed after delivery and the pups in the L group weaned at 21 day of age.
2.3 Maternal data
Since this was an exploratory study, maternal parameters were obtained before pregnancy and in immediate postpartum period – 1 and 2 months post delivery. Because of constricted time frame for obtaining maternal parameters and to avoid stress due to multiple manipulations, the estrus cycle during measurements was not assessed. Maternal weight was recorded at baseline (before pregnancy), 1- and 2-months postpartum. Tail vein blood (100 μL) was collected from all dams after overnight fasting. Blood was centrifuged for 20 minutes at 3000 rpm and serum was collected and stored at −80°C until the time of testing. Glucose was measured with an OneTouch Ultra glucometer (LifeScan Inc, Milipitas, CA) after overnight fasting. Fasting serum LDL, HDL, and triglycerides were determined using a quantitative colorimetric assay (BioAssay Systems, Hayward, CA) according to the manufacturer’s instructions and were interpreted by an automated spectrophotometer (Fusion 5.0; Ortho Clinical Diagnostics, Rochester, NY). All samples were run in duplicate.
2.4 Blood Pressure
Blood pressure was determined in vivo using CODA non-invasive system (Kent Scientific, Torrington, CT) before pregnancy and 1 and 2 months postpartum. In vivo volume-pressure recording (VPR) via tail following previously validated methods22 obtains measurement of systolic and diastolic blood pressure with ability to detect changes in tail blood volume in up to eight mice simultaneously.23 To obtain these measures, mice were first acclimated to restraints followed by 20 cycles of blood pressure measurement. Systolic blood pressure (SBP) was determined from the point in which tail blood volume increases. Diastolic blood pressure (DBP) was indicated by the occlusion cuff pressure at which the blood flow into the tail equilibrates. CODA has been validated with telemetry with 99% correlation.24
2.5 Cardiac Function
Echocardiography using Vevo 770 high-resolution system (VisualSonics, Toronto, Ontario, Canada) with 30 MHz transducers was performed at 1 and 2 months postpartum. Animals were anesthetized using 1–2% isoflurane in oxygen delivered by face mask. Abdominal hair was removed with chemical hair removal lotion prior to ultrasound. Animals were placed on a warmed platform for maintaining optimal physiological conditions integrated with electrocardiogram monitoring, heart rate, core temperature, and respiration. M-mode, B-mode, and pulsed Doppler were used with a 30-micrometer resolution and an ability to capture 240 frames per second.
Systolic function was evaluated by left ventricular ejection fraction obtained at the short axis cardiac view using M-mode by obtaining the end diastolic diameter (EDD) and end systolic diameter (ESD).25–27 Ejection fraction was equal to (EDD3 − ESD3)/EDD3. Diastolic function was assessed with mitral valve E/A ratio from the 4-chamber cardiac view where E represents peak velocity and A - the atrial kick during diastole. In early stage diastolic dysfunction, the E peak velocity decreases due to left ventricular stiffness, which results in a reduced E/A ratio.
Cardiac output was determined by measuring the left ventricular outflow diameter from the parasternal long axis view to get the cross-sectional area. Then, the blood flow per unit time through the aorta was used to determine the velocity time integral. The product of these and heart rate resulted in cardiac output (CO=CSA x VTI x HR). Total peripheral resistance (TPR) was calculated by mean arterial pressure divided by cardiac output.
2.6 Visceral and Subcutaneous Adiposity
In-vivo computed tomography with Inveon (Siemens Preclinical Solutions, Knoxville, TN) was used at 1 and 2 months postpartum to assess maternal visceral adipose tissue (VAT) and subcutaneous adipose tissue (SAT).28–31 Mice were anesthetized with ketamine (Ketalar, Parke-Davis, Morris Plains, NJ) and xylazine (Gemini, Rugby, Rockville Center, NY) intraperitoneally. Imaging parameters were: voltage 70 kV, current 500 μA, resolution 0.107 mm, exposure time 1000 ms, 520 steps, and 360 degrees of rotation. Scan time was approximately 15 minutes per mouse. Mice were given supplemental oxygen via nose cone during scanning. The transverse views on micro-CT images (1 per animal) at the level of the 5th lumbar vertebra were selected for VAT analysis, and images at the level of the 6th were used for SAT analysis.30–31 The total body area in cross-section and areas of VAT and SAT were measured using Inveon Research Workplace software. The % VAT and SAT was calculated from the total body area imaged in cross-section.
2.7 Statistical Analysis
For statistical analysis Student t or Mann-Whitney U tests were performed as appropriate using GraphPad Prism version 6.0c for Mac OSX (GraphPad Software, San Diego, CA, www.graphpad.com). Since this was an exploratory study, comparisons were only performed between the lactated and nonlacted groups at either two (1 and 2 months pospartum) or three (prepgregnancy, 1, and 2, months postpartum) time points. Due to technical problems, such as failed blood pressure, CT or US recordings and death of animals during longitudinal study, numbers of mice in final analyses varied between 5 and 12. A probability value (P-value) of less than or equal to 0.05 was considered statistically significant.
3. RESULTS
3.1 Weights
There was no difference in prepregnancy weight between L and NL mice (Table 1, P=0.39). At 1 month postpartum, NL mice weighed significantly less (Table 1, P=0.004). By 2 months postpartum, this difference in weight resolved (Table 1, P=0.2).
Table 1.
Characteristics of dams
| Characteristic | Lactated (n=10) | Nonlactated (n=12) | P value |
|---|---|---|---|
| Maternal weight, g | |||
| Baseline | 18.9 ± 1.3 | 17.6 ± 0.7 | 0.39 |
| 1 month postpartum | 37.8 ± 1.3 | 31.8 ± 1.3 | 0.004 |
| 2 months postpartum | 31.5 ± 1.3 | 29.4 ± 0.9 | 0.20 |
| Glucose, mg/dL | |||
| 1 month postpartum | 104.5 ± 3.8 | 97.1 ± 3.8 | 0.19 |
| 2 months postpartum | 103.5 ± 2.6 | 99.3 ± 3.3 | 0.34 |
| HDL, mmol/L | |||
| 1 month postpartum | 123.1 ± 8.8 | 111.9 ± 9.5 | 0.40 |
| 2 months postpartum | 127.5 ± 11.4 | 110.6 ± 6.4 | 0.20 |
| LDL, mmol/L | |||
| 1 month postpartum | 33.9 ± 2.8 | 30.6 ± 1.9 | 0.35 |
| 2 months postpartum | 21.5 ± 2.0 | 22.9 ± 2.0 | 0.64 |
| Triglycerides, mmol/L | |||
| 1 month postpartum | 1.12 ± 0.15 | 1.09 ± 0.12 | 0.86 |
| 2 months postpartum | 1.13 ± 0.16 | 0.97 ± 0.1 | 0.41 |
Glucose and lipids are fasting serum values. Data expressed as mean ± standard deviation.
3. 2 Blood Analytes
There was no difference in glucose, HDL, LDL, or triglycerides at 1 or 2 months postpartum (Table 1).
3.3 Blood Pressure
There was no difference in SBP or DBP at baseline between the two groups (Figure 1A and B, P=0.65 and P=0.39, respectively). At 1 month postpartum, a trend was seen for higher SBP and DBP in NL group (Figure 1,A and B, P=0.53 and P=0.51, respectively). At 2 months postpartum, the SBP and DBP became significantly different with a relatively greater BP in NL mice (Figure 1, A and B, P=0.04 for both).
Figure 1. Blood Pressure.
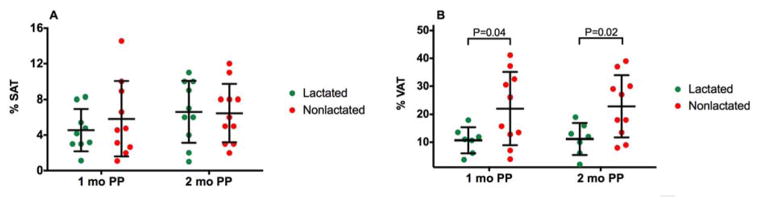
Systolic blood pressure (A, SBP), diastolic blood pressure (B, DBP) and total peripheral resistance (C, TPR) in dams that lactated and did not lactate. Data are expressed as dot plot with a line at the mean and whiskers showing standard deviation.
PP, postpartum
3.4 Cardiac Function
Cardiac output, while the same at 1 month, was also lower among the nonlactated group at 2 months postpartum (Figure 4, P=0.27 and P=0.02, respectively). There was no statistically significant difference in heart rate between lactated and nonlactated mice (370±12 vs 348±14 bpm, P=0.29). Total peripheral resistance, while the same at 1 month postpartum, was different and relatively higher in the nonlactated mice at 2 months postpartum (Figure 1C, P=0.95 and P=0.05, respectively).
Systolic function, as evaluated with ejection fraction, was similar between groups at 1 month postpartum, but, while it remained the same in the lactated group, it was lower in the nonlactated group at 2 months postpartum (Figure 2B, P=0.85 and P=0.04, respectively). Mitral valve E/A ratio, similarly, was no different at 1 month postpartum and significantly lower at 2 months postpartum in the nonlactated group, indicative of diastolic dysfunction (Figure 2C, P=0.48 and P=0.04, respectively).
Figure 2. Ejection Fraction.
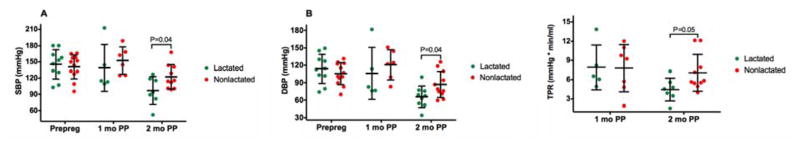
Cardiac output (A), feft ventricular ejection fraction (B) and left ventricular E/A ratio at mitral valve (C) of dams that lactated and did not lactate. Data are expressed as dot plot with a line at the mean and whiskers showing standard deviation. PP, postpartum
3.5 Visceral and Subcutaneous Adiposity
Subcutaneous adipose tissue determined via micro-CT was not different at 1 or 2 months postpartum (Figure 3A, P=0.44 and P=0.92 respectively) between the groups. However, the percent of visceral adipose tissue was significantly higher at 1 and 2 months postpartum in the nonlactated group (Figure 3B, P=0.04 and P=0.02, respectively).
Figure 3. Subcutaneous and Visceral Adipose Tissue.
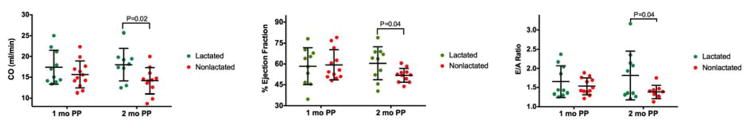
A. % subcutaneous adipose tissue and B, % visceral adipose tissue of dams at 1 and 2 months postpartum that lactated and did not lactate. Data are expressed as dot plot with a line at the mean and whiskers showing standard deviation. PP, postpartum; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue
4. COMMENT
In an animal model, we found that interruption of lactation adversely affected maternal cardiovascular function and increased visceral adipose tissue in the immediate postpartum period. In this study we demonstrate that mice that did not lactate had relatively greater systolic and diastolic blood pressure and concomitant total peripheral resistance at two months postpartum when compared to lactated animals. Cardiac function was preserved after pregnancy and lactation while a diminished systolic and diastolic function was noted in mice that did not lactate. The increase in mean arterial pressure and reduction in cardiac output are both indicative of higher total peripheral resistance in the mice that did not lactate. Moreover, a relatively greater accumulation of visceral adipose tissue was found in postpartum nonlactated mice. Thus, our data suggest a direct harmful effect of interrupting lactation on immediate postpartum maternal cardiovascular function and adiposity.
Several limitations of our study need to be noted. First, this is an animal model involving lactating dams with a multiple pups rather than a single infant as in humans. The greater metabolic load in this animal model likely intensifies differences between lactated and nonlactated animals compared to humans. Secondly, our model used complete separation from pups in the nonlactated condition, and thus did not evaluate the effect of separation from the effects of suckling on maternal physiology. Studies among breastfeeding women have shown that lactation immediately before a stressor presentation reduces HPA axis activation more than holding an infant without breastfeeding, suggesting that the act of lactation impacts stress reactivity independent of physical contact with the infant.32 Thus, differences seen between two animal groups in our model could be confounded by the stress of separation in maternal physiology. Thirdly, the follow up only during immediate postpartum period in our study limits extrapolation of results to long-term maternal effect.. Fourth, although several parameters were similar at 1 month postpartum, relatively greater or lesser values in the non lactated group were noted only at 2 months postpartum. We propose that differences become evident due to delayed effect of lactation on cardiovascular function and adiposity. Further investigations are needed, to address limitations of the current study.
It had been proposed that lactation plays a central role in mobilizing accumulated fat stores and “resetting” maternal metabolism.33–35 The “reset hypothesis” links lactation with a protective effect on maternal health.33 During pregnancy, dramatic changes occur in a woman’s metabolism as she accommodates the demands of “metabolizing for two.”36 These well-described increases in visceral fat, insulin production, insulin resistance, and circulating lipid levels both support the developing fetus and allow accumulation of energy stores for lactation.37–38 However, if no lactation occurs, maternal metabolism does not return to prepregnancy state and results in a negative impact on the mother’s health. Our study is a first step towards providing evidence for the “reset hypothesis”. Further studies are needed to support or refute this theory.
Although there is an initial increase in weight in the mice that lactated at 1 month postpartum, we did not find difference in weight at 2 months postpartum. This was likely an initial effect of lactation as it had only been 1 week since the animals had weaned. The association between lactation and postpartum weight loss varies among human studies.1,39–44 After birth, lactation utilizes the fat stores and maternal metabolism returns to baseline. However, if lactation does not occur, the metabolic changes of pregnancy persist leading to cardiometabolic disease later in life. Visceral adiposity was decreased in the lactated group both at 1 and 2 months postpartum. Thus, the increased visceral adipose tissue among nonlactated animals in our model due to lactation may increase risk for metabolic disease later in life.33 Visceral adipose tissue has been demonstrated to correlate strongly with metabolic syndrome.45–47 In the absence of the caloric demands of breastfeeding, our results suggest that visceral adipose tissue accumulated during pregnancy persists, with adverse affects on long-term maternal health.37, 48, 49
Although we found no difference in HDL at 2 months postpartum, there appears to be a trend with higher HDL among the group that lactated. Pregnancy is a state of relative hyperlipidemia, and cholesterol and triglyceride levels fall postpartum. Compared with their nonlactating peers, breastfeeding women have lower insulin, glucose, and triglyceride along with higher HDL levels.11, 50–54 In a small human study, breastfeeding was associated with favorable changes in HDL that persisted after weaning.11
The echocardiographic changes seen in our model would unlikely lead to overt disease; however, these changes could contribute to future cardiovascular disease. Mothers who do not breastfeed have been shown to have an increase in subclinical heart disease compared with those who lactated, including an increase in aortic calcification and coronary artery calcification.55
The results of this study suggest an effect of lactation on immediate postpartum maternal cardiac function and adiposity. Further study is required to demonstrate a long-term effect of lactation on maternal health. Mechanisms involved in this protective effect could lead to novel preventive strategies to decrease cardiovascular disease in women. In addition, enabling more women to achieve their breastfeeding goals may improve health across two generations56.
Acknowledgments
FINANCIAL SUPPORT:
Dr. Egle Bytautiene, is supported by a research career development award (K12HD052023: Building Interdisciplinary Research Careers in Women’s Health Program-BIRCWH) from the National Institute of Allergy and Infectious Diseases (NIAID), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), and the Office of the Director (OD), National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIAID, NICHD, OD, or the National Institutes of Health.
This study was conducted with the support of the Institute for Translational Sciences at the University of Texas Medical Branch, supported in part by a Clinical and Translational Science Award (UL1RR029876) from the National Center for Research Resources, National Institutes of Health
We would like to express gratitude to Phyllis Gamble and Esther Tamayo for their assistance with breeding, blood pressure assessment, weight, and blood draws. We thank Talar Kechichian, BS, MS for her expertise and assistance with HDL/LDL and triglycerides and Jingna Wei, MD, for her help with computed tomography.
We would also like to recognize Maged Costantine, MD for his help with the development of the manuscript and Massoud Motamedi, PhD for the unlimited access to the Bioengineering Center Facilities and equipment. All acknowledged are University of Texas Medical Branch employees at Galveston, Texas. Funding was received from the National Institute of Health NIH UL1RR029876 AND K12HD052023 (BIRCWH) grants.
Footnotes
DISCLOSURE: The authors report no conflict of interest.
DISCLAIMER: The views expressed in this presentation are those of the author and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, or the United States Government.
PREVIOUS PRESENTATION: Presented at the 34th annual meeting of the Society for Maternal-Fetal Medicine, New Orleans, LA, Feb. 3–8, 2014.
CONTACT INFORMATION: reprints will not be available
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ip S, Chung M, Raman G, et al. Breastfeeding and maternal and infant health outcomes in developed countries. Evid Rep Technol Assess. 2007 Apr;(153):1–186. [PMC free article] [PubMed] [Google Scholar]
- 2.Horwood LJ, Darlow BA, Mogridge N. Breast milk feeding and cognitive ability at 7–8 years. Arch Dis Child Fetal Neonatal Ed. 2001 Jan;84(1):F23–7. doi: 10.1136/fn.84.1.F23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Von Kries R, Koetzko B, Sauerwald T, et al. Breast feeding and obesity: cross sectional study. BMJ. 1999 Jul 17;319(7203):147–50. doi: 10.1136/bmj.319.7203.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwarz EB, Ray RM, Stuebe AM, et al. Duration of lactation and risk factors for maternal cardiovascular disease. Obstet Gynecol. 2009;113(5):974–982. doi: 10.1097/01.AOG.0000346884.67796.ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stuebe AM, Rich-Edwards JW, Willett WC, Manson JE, Michels KB. Duration of lactation and incidence of type 2 diabetes. JAMA. 2005;294(20):2601–2610. doi: 10.1001/jama.294.20.2601. [DOI] [PubMed] [Google Scholar]
- 6.Villegas R, Gao YT, Yang G, et al. Duration of breast-feeding and the incidence of type 2 diabetes mellitus in the Shanghai Women’s Health Study. Diabetologia. 2008;51(2):258–266. doi: 10.1007/s00125-007-0885-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee SY, Kim MT, Jee SH, Yang HP. Does long-term lactation protect premenopausal women against hypertension risk? A Korean women’s cohort study. Prev Med. 2005;41(2):433–438. doi: 10.1016/j.ypmed.2004.11.025. [DOI] [PubMed] [Google Scholar]
- 8.Lupton SJ, Chiu CL, Lujic S, Hennessy A, Lind JM. Association between parity and breastfeeding with maternal high blood pressure. Am J Obstet Gynecol. 2013 Jun;208(6):454.e1–7. doi: 10.1016/j.ajog.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 9.Newcomb PA, Egan KM, Titus-Ernstoff L, et al. Lactation in relation to postmenopausal breast cancer. Am J Epid. 1999 Jul 15;150(2):174–82. doi: 10.1093/oxfordjournals.aje.a009977. [DOI] [PubMed] [Google Scholar]
- 10.Gunderson EP, Lewis CE, Wei GS, Whitmer RA, Quesenberry CP, Sidney S. Lactation and changes in maternal metabolic risk factors. Obstet Gynecol. 2007;109(3):729–738. doi: 10.1097/01.AOG.0000252831.06695.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stuebe AM, Michels KB, Willett WC, Manson JE, Rexrode K, Rich-Edwards JW. Duration of lactation and incidence of myocardial infarction in middle to late adulthood. Am J Obstet Gynecol. 2009;200(2):138. doi: 10.1016/j.ajog.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gunderson EP, Jacobs DR, Chiang V, et al. Duration of Lactation and Incidence of the Metabolic Syndrome in Women of Reproductive Age According to Gestational Diabetes Mellitus Status: A 20-Year Prospective Study in CARDIA (Coronary Artery Risk Development in Young Adults) Diabetes. 2010;59(2):495–504. doi: 10.2337/db09-1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ram KT, Bobby P, Hailpern SM, et al. Duration of lactation is associated with lower prevalence of the metabolic syndrome in midlife-SWAN, the study of women’s health across the nation. Am J Obstet Gynecol. 2008;198(3):268. doi: 10.1016/j.ajog.2007.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beck LF, Morrow B, Lipscomb LE, et al. Prevalence of selected maternal behaviors and experiences, Pregnancy Risk Assessment Monitoring System (PRAMS), 1999. MMWR Surveillance summaries. 2002;51(2):1–27. [PubMed] [Google Scholar]
- 15.Pesa JA, Shelton MM. Health-Enhancing Behaviors Correlated with Breastfeeding Among a National Sample of Mothers. Public Health Nursing. 1999;16(2):120–124. doi: 10.1046/j.1525-1446.1999.00120.x. [DOI] [PubMed] [Google Scholar]
- 16.Rasmussen KM, Kjolhede CL. Prepregnant overweight and obesity diminish the prolactin response to suckling in the first week postpartum. Pediatrics. 2004;113(5):e465–471. doi: 10.1542/peds.113.5.e465. [DOI] [PubMed] [Google Scholar]
- 17.Baker JL, Michaelsen KF, Sorensen TI, Rasmussen KM. High prepregnant body mass index is associated with early termination of full and any breastfeeding in Danish women. Am J Clin Nutr. 2007;86(2):404–411. doi: 10.1093/ajcn/86.2.404. [DOI] [PubMed] [Google Scholar]
- 18.Steingrimsdottir L, Brasel JA, Greenwood MRC. Diet, pregnancy, and lactation: Effects on adipose tissue, lipoprotein lipase, and fat cell size. Metabolism. 1980;29(9):837–841. doi: 10.1016/0026-0495(80)90122-5. [DOI] [PubMed] [Google Scholar]
- 19.Moore BJ, Brasel JA. One Cycle of Reproduction Consisting of Pregnancy, Lactation or No Lactation, and Recovery: Effects on Carcass Composition in Ad Libitum-Fed and Food-Restricted Rats. The Journal of Nutrition. 1984;114(9):1548–1559. doi: 10.1093/jn/114.9.1548. [DOI] [PubMed] [Google Scholar]
- 20.Jones RG, Ilic V, Williamson DH. Physiological significance of altered insulin metabolism in the conscious rat during lactation. Biochem J. 1984;220(2):455–460. doi: 10.1042/bj2200455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bytautiene E, Lu F, Tamayo EH, Hankins GD, Long M, Kublickiene K, Saade GR. Long-term maternal cardiovascular function in a mouse model of sFlt-1-induced preeclampsia. Am J Physiol Heart Circ Physiol. 2010 Jan;298(1):H189–93. doi: 10.1152/ajpheart.00792.2009. [DOI] [PubMed] [Google Scholar]
- 22.Daugherty A, Rateri D, Hong L, Balakrishnan A. Measuring Blood Pressure in Mice using Volume Pressure Recording, a Tail-cuff Method. J Vis Exp. 2009;(27):e1291. doi: 10.3791/1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feng M, Dipetrillo K. Non-invasive blood pressure measurement in mice. 573. 2009. pp. 45–55. [DOI] [PubMed] [Google Scholar]
- 24.Feng M, Whitesall S, Zhang Y, Beibel M, D’Alecy L, DiPetrillo K. Validation of volume-pressure recording tail-cuff blood pressure measurements. Am J Hypertens. 2008 Dec;21(12):1288–91. doi: 10.1038/ajh.2008.301. [DOI] [PubMed] [Google Scholar]
- 25.Collins KA, Korcarz CE, Lang RM. Use of echocardiography for the phenotypic assessment of genetically altered mice. Physiol Genomics. 2003;13:227–239. doi: 10.1152/physiolgenomics.00005.2003. [DOI] [PubMed] [Google Scholar]
- 26.Vinhas M, Araújo AC, Ribeiro S, Rosário LB, Belo JA. Transthoracic echocardiography reference values in juvenile and adult 129/Sv mice. Cardiovascular Ultrasound. 2013;11:12. doi: 10.1186/1476-7120-11-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stypmann J, Engelen MA, Troatz C, Rothenburger M, Eckardt L, Tiemann K. Echocardiographic assessment of global left ventricular function in mice. Laboratory Animals. 2009;43:127–137. doi: 10.1258/la.2007.06001e. [DOI] [PubMed] [Google Scholar]
- 28.Hildebrandt AL, Kelly-Sullivan DM, Black SC. Validation of a high-resolution X-ray computed tomography system to measure murine adipose tissue depot mass in situ and longitudinally. J Pharmacol Toxicol Methods. 2002;47(2):99–106. doi: 10.1016/s1056-8719(02)00208-3. [DOI] [PubMed] [Google Scholar]
- 29.Luu YK, Lublinsky S, Ozcivici E, et al. In vivo quantification of subcutaneous and visceral adiposity by micro-computed tomography in a small animal model. Medical Engineering & Physics. 2009;31(1):34–41. doi: 10.1016/j.medengphy.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lublinsky S, Luu Y, Rubin C, Judex S. Automated separation of visceral and subcutaneous adiposity in in vivo microcomputed tomographies of mice. J Digit Imaging. 2009;22(3):222–231. doi: 10.1007/s10278-008-9152-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Judex S, Luu YK, Ozcivici E, Adler B, Lublinsky S, Rubin CT. Quantification of adiposity in small rodents using micro-CT. Methods. 2010;50(1):14–19. doi: 10.1016/j.ymeth.2009.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heinrichs M, Meinlschmidt G, Neumann I, Wagner S, Kirschbaum C, Ehlert U, Hellhammer DH. Effects of Suckling on Hypothalamic-Pituitary-Adrenal Axis Responses to Psychosocial Stress in Postpartum Lactating Women. J Clin Endocrinol Metab. 2001;86(10):4798–4804. doi: 10.1210/jcem.86.10.7919. [DOI] [PubMed] [Google Scholar]
- 33.Stuebe AM, Rich-Edwards JW. The reset hypothesis: lactation and maternal metabolism. Am J Perinatol. 2009;26(1):81–88. doi: 10.1055/s-0028-1103034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Butte NF, Garza C, Stuff JE, Smith EO, Nichols BL. Effect of maternal diet and body composition on lactational performance. Am J Clin Nutr. 1984;39(2):296–306. doi: 10.1093/ajcn/39.2.296. [DOI] [PubMed] [Google Scholar]
- 35.Butte NF, Wong WW, Hopkinson JM. Energy Requirements of Lactating Women Derived from Doubly Labeled Water and Milk Energy Output. The Journal of Nutrition. 2001;131(1):53–58. doi: 10.1093/jn/131.1.53. [DOI] [PubMed] [Google Scholar]
- 36.Ellison PT. On Fertile Ground. Cambridge: Harvard University Press; 2001. [Google Scholar]
- 37.Einstein FH, Fishman S, Muzumdar RH, Yang XM, Atzmon G, Barzilai N. Accretion of visceral fat and hepatic insulin resistance in pregnant rats. American Journal of Physiology - Endocrinology And Metabolism. 2008;294(2):E451–E455. doi: 10.1152/ajpendo.00570.2007. [DOI] [PubMed] [Google Scholar]
- 38.Dall SR, Boyd IL. Evolution of mammals: lactation helps mothers to cope with unreliable food supplies. Proceedings of the Royal Society of London Series B: Biological Sciences. 2004;271(1552):2049–2057. doi: 10.1098/rspb.2004.2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baker JL, Gamborg M, Heitmann BL, Lissner L, Sørensen TI, Rasmussen KM. Breastfeeding reduces postpartum weight retention. Am J Clin Nutr. 2008;88(6):1543–1551. doi: 10.3945/ajcn.2008.26379. [DOI] [PubMed] [Google Scholar]
- 40.Brewer MM, Bates MR, Vannoy LP. Postpartum changes in maternal weight and body fat depots in lactating vs nonlactating women. Am J Clin Nutr. 1989;49(2):259–265. doi: 10.1093/ajcn/49.2.259. [DOI] [PubMed] [Google Scholar]
- 41.Dewey KG, Heinig MJ, Nommsen LA. Maternal weight-loss patterns during prolonged lactation. Am J Clin Nutr. 1993;58(2):162–166. doi: 10.1093/ajcn/58.2.162. [DOI] [PubMed] [Google Scholar]
- 42.Dugdale AE, Eaton-evans J. The effect of lactation and other factors on post-partum changes in body-weight and triceps skinfold thickness. British Journal of Nutrition. 1989;61(02):149–153. doi: 10.1079/bjn19890105. [DOI] [PubMed] [Google Scholar]
- 43.Gigante DP, Victora CG, Barros FC. Breast-Feeding Has a Limited Long-Term Effect on Anthropometry and Body Composition of Brazilian Mothers. The Journal of Nutrition. 2001;131(1):78–84. doi: 10.1093/jn/131.1.78. [DOI] [PubMed] [Google Scholar]
- 44.Sichieri R, Field AE, Rich-Edwards J, Willett WC. Prospective assessment of exclusive breastfeeding in relation to weight change in women. International Journal of Obesity & Related Metabolic Disorders. 2003;27(7):815. doi: 10.1038/sj.ijo.0802285. [DOI] [PubMed] [Google Scholar]
- 45.Wajchenberg BL, Giannella-Neto D, da Silva ME, Santos RF. Depot-specific hormonal characteristics of subcutaneous and visceral adipose tissue and their relation to the metabolic syndrome. Horm Metab Res. 2002 Nov-Dec;34(11–12):616–21. doi: 10.1055/s-2002-38256. [DOI] [PubMed] [Google Scholar]
- 46.Neeland IJ, Ayers CR, Rohatgi AK, et al. Associations of visceral and abdominal subcutaneous adipose tissue with markers of cardiac and metabolic risk in obese adults. Obesity. 2013 Sep;21(9):E439–47. doi: 10.1002/oby.20135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Akagiri S, Naito Y, Ichikawa H, et al. A mouse model of metabolic syndrome; increase in visceral adipose tissue precedes the development of fatty liver and insulin resistance in high-fat diet-fed male KK/Ta mice. J Clin Biochem Nutr. 2008;42(2):150–157. doi: 10.3164/jcbn.2008022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McClure C, Catov J, Ness R, Schwarz E. Maternal Visceral Adiposity by Consistency of Lactation. Matern Child Health J. 2012;16(2):316–321. doi: 10.1007/s10995-011-0758-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McClure CK, Schwarz EB, Conroy MB, Tepper PG, Janssen I, Sutton-Tyrrell KC. Breastfeeding and Subsequent Maternal Visceral Adiposity. Obesity Res. 2011;19(11):2205–2213. doi: 10.1038/oby.2011.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Butte NF, Hopkinson JM, Mehta N, Moon JK, Smith EO. Adjustments in energy expenditure and substrate utilization during late pregnancy and lactation. Am J Clin Nutr. 1999;69(2):299–307. doi: 10.1093/ajcn/69.2.299. [DOI] [PubMed] [Google Scholar]
- 51.Kjos SL, Henry O, Lee R, Buchanan TA, Mishell DRJ. The effect of lactation on glucose and lipid metabolism in women with recent gestational diabetes. Obstet Gynecol. 1993;82(3):451–455. [PubMed] [Google Scholar]
- 52.Darmady JM, Postle AD. Lipid metabolism in pregnancy. BJOG. 1982;89(3):211–215. doi: 10.1111/j.1471-0528.1982.tb03616.x. [DOI] [PubMed] [Google Scholar]
- 53.Erkkola R, Viikari J, Irjala K, Solakivi-Jaakkola T. One-year follow-up of lipoprotein metabolism after pregnancy. Bio Res Pregnancy Perinatol. 1986;7(2):47–51. [PubMed] [Google Scholar]
- 54.Knopp RH, Walden CE, Wahl PW, et al. Effect of Postpartum Lactation on Lipoprotein Lipids and Apoproteins. Journal of Clinical Endocrinology & Metabolism. 1985;60(3):542–547. doi: 10.1210/jcem-60-3-542. [DOI] [PubMed] [Google Scholar]
- 55.Schwarz EB, McClure CK, Tepper PG, et al. Lactation and maternal measures of subclinical cardiovascular disease. Obstet Gynecol. 2010 Jan;115(1):41–8. doi: 10.1097/AOG.0b013e3181c5512a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stuebe AM. Enabling Women to Achieve Their Breastfeeding Goals. Obstet Gynecol. 2014 Mar;123(3):643–52. doi: 10.1097/AOG.0000000000000142. [DOI] [PubMed] [Google Scholar]


