Abstract
Objective
Intestinal failure is a rare, devastating condition associated with significant morbidity and mortality. We sought to determine if ethnic and racial differences were associated with patient survival and likelihood of receiving an intestinal transplant in a contemporary cohort of children with intestinal failure.
Methods
This was an analysis of a multicenter cohort study with data collected from chart review conducted by the Pediatric Intestinal Consortium (PIFCon). Entry criteria included infants < 12 mo receiving parenteral nutrition (PN) for > 60 continuous days and followed for at least 2 years. Outcomes included death and intestinal transplant (ITx). Race and ethnicity were recorded as they were in the medical record. For purposes of statistical comparisons and regression modeling, categories of race were consolidated into “white” and “non-white” children.
Results
Of 272 subjects enrolled, 204 white and 46 non-white children were available for analysis. The 48 month cumulative incidence probability (CIP) of death without ITx was 0.40 for non-white and 0.16 for white children (p<0.001); the CIP of ITx was 0.07 for non-white vs 0.31 for white children (p=0.003). The associations between race and outcomes remained after accounting for low-birth weight, diagnosis, and being seen at a transplant center.
Conclusion
Race is associated with death and receiving an ITx in a large cohort of children with intestinal failure. This study highlights the need to investigate reasons for this apparent racial disparity in outcome among children with intestinal failure.
Keywords: race, intestinal failure, outcome, intestinal transplant
Introduction
Intestinal failure (IF), broadly defined, is the inability of the native intestine to sustain adequate weight gain and growth in the absence of parenteral nutrition (PN).1, 2 Causes of intestinal failure in infants include short bowel syndrome, congenital enterocyte disorders, and intestinal dysmotility.3, 4 Management is largely supportive and has improved with establishment of multidisciplinary teams focused on strategies to minimize complications and enhance intestinal adaptation.5-9 Mortality associated with IF may be improving over time. In a single-site retrospective chart review of 171 children with IF, Hess, et. al. compared children with onset of IF between 1990-1994 with those whose onset was between 2005-2009 and reported that mortality fell during those time intervals from 29.4% to 6.7%, respectively.10 Months and often years of PN are necessary before enteral autonomy (EA) is established.4 For children unable to achieve EA or who develop life-threatening complications associated with IF and its management, intestinal transplantation (ITx) can be life-saving.11, 12
Children with IF suffer significant morbidity and mortality.4 Factors reported to be associated with morbidity and mortality include prematurity, absence of the ileocecal valve, age-adjusted small bowel length, PN associated liver disease, catheter-related blood stream infections, presence of a motility disorder, lack of enteral feedings, not receiving breast milk, and receiving more than 3.5 grams/kg/day of soybean-based fat emulsion.13-18 Race has not been previously assessed for its association with outcome in children with IF.
Racial disparities in prenatal care utilization, premature birth, and infant mortality are well documented.19-22 Hispanic and Black non-Hispanic women are less likely to access or receive adequate prenatal care and are more likely to deliver a preterm infant than are white women.22 MacDorman reported infants born to non-Hispanic black women in 2006 were 2.4 times more likely to die than infants born to non-Hispanic white women.21 Similarly, racial and ethnic disparities in access to and undergoing, liver transplantation have been reported.23-25 Methodologies to identify factors that appear to disadvantage racial and ethnic minorities from undergoing a liver transplantation typically do not include important factors that include biological differences, access to health insurance, referral bias, patient knowledge, and access to quality medical care.24
As the relationship of ethnicity and race with outcomes of infants with IF has not been previously explored, we sought to determine if ethnic and racial differences were associated with patient survival or likelihood of receiving an ITx in a large contemporary cohort of children with IF.
Materials and Methods
The Pediatric Intestinal Failure Consortium (PIFCon) consists of 14 sites with established multi-disciplinary pediatric intestinal rehabilitation programs comprised of medical, surgical, nutritional, nursing and other specialists. Of the 14 clinical sites, 9 were established ITx centers. Study methods have been previously described.4
This was a multicenter cohort study with data collected by chart review. Inclusion criteria included infants with IF, no more than 12 months of age and prolonged PN as a consequence of IF. Prolonged PN was defined as receiving an intravenous solution containing protein, carbohydrate, electrolytes, vitamins, and trace elements on 60 out of 74 consecutive days at any time in the first year of life. The range was established to allow for brief interruptions in PN for loss of intravenous access or surgery. Participants at 13 of the 14 sites met age and PN criteria between January 1, 2000 and December 31, 2004. At one site, children who met criteria until December 31, 2005 were included. Data were collected through December 31, 2006 for 13 sites and December 31, 2007 at the latter site to allow at least a two year follow-up for all children.
Following approval by the Institutional Review Board at each site, data of patients with IF who were followed by a site investigator were collected retrospectively by chart review. Data were entered into de-identified case report forms and electronically transmitted to a central database at the University of Pittsburgh Graduate School of Public Health. Time intervals for collecting data after entry criteria were met were 1, 3, 6, 9, and 12 months and annually thereafter. Windows to record data were contiguous to ensure maximum data collection. If data elements were available on more than one occasion within a time interval, those closest to the designated month were selected.
Data elements included baseline demographics, diagnosis leading to IF, residual small bowel length as determined by operative reports, enteral and parenteral feeding regimens, and clinical laboratory tests. Diagnosis was determined by the attending physician at each site. If more than one diagnosis contributed to IF, patients were categorized with multiple diagnoses. Data at follow-up included clinical laboratory tests, sentinel events, and whether an outcome occurred during the interval of observation. Sentinel events included bacteremia, cholestasis (defined as serum total bilirubin > 5 mg/dl or conjugated or direct bilirubin > 2 mg/dl), intestinal bleeding, admission to hospital, medical or surgical interventions, and referral for ITx evaluation. Outcome variables included death, ITx, and EA defined as the discontinuation of PN for more than 3 consecutive months.
Race and ethnicity were recorded as they were in the medical record at the time entry criteria were met. For purposes of analysis, race was consolidated into “white” and “non-white”.
Statistical Analysis
Baseline characteristics were summarized using frequencies and proportions for categorical variables and medians and quartiles for continuous variables. Pearson’s chi-square test or its exact version was used to assess statistical significance of categorical characteristics between white and non-white children, and Wilcoxon rank-sum test was used for continuous characteristics. Methods for competing risks26 were used to estimate cumulative probabilities from study enrollment to ITx or death without ITx . The cumulative incidence probabilities (CIP) between subgroups (e.g., white children vs. non-white children) were tested for statistical significance using Gray’s K-sample test27. Fine and Gray’s proportional hazards regression model28 was used to evaluate the relationship with race after adjusting for other potentially confounding baseline characteristics birth weight birth weight (< 1.5 kg vs. otherwise), diagnosis of NEC, whether the site is a transplant center. The descriptive analyses were performed in SAS (version 9.2, SAS Institute), and the competing risks analyses were performed in R (version 3.0.1) using “cmprsk”.
Results
Among the 272 subjects enrolled in the PIFCon cohort 22 subjects did not have their race recorded. Baseline characteristics of the 204 white and 46 non-white children are shown in Table 1. Compared to white children, non-white children were more likely to die without ITx (p<0.001) and less likely to receive an ITx (p=0.003) (Figure 1). Specifically, at 48 months after entry criteria were met, the CIP of death without ITx was 0.40 for non-white children compared to 0.16 for white children and the CIP of ITx was 0.07 for non-white children and 0.31 for white children.
Table 1.
Comparison of Patients’ Characteristics between White and Non-White Children
| All N=272 |
White N=204 |
Non-White N=46 |
p-value | |
|---|---|---|---|---|
| Age at study entry (day) ** | N=272 63(61,74) |
N=204 63(61,72) |
N=46 65(61,88) |
0.07 |
|
| ||||
| Gender * | N=272 | N=204 | N=46 | 0.8 |
| Male | 156(57.4) | 116(56.9) | 25(54.3) | |
|
| ||||
| Gestation <37 wk (premature) * | N=264 202(76.5) |
N=199 151(75.9) |
N=45 37(82.2) |
0.4 |
|
| ||||
| Birth weight<= 1.5 kg * | N=221 66(29.9) |
N=169 42(24.9) |
N=37 22(59.5) |
<.001 |
|
| ||||
| Hispanic * | N=272 | N=204 | N=46 | 0.004 |
| Yes | 42(15.4) | 40(19.6) | 1(2.2) | |
|
| ||||
| Small Bowel Length at study entry ** | N=143 43(25,70) |
N=112 41(25,69) |
N=24 45(34,70.5) |
0.4 |
|
| ||||
| ALT>=80 IU/mL * | N=163 77(47.2) |
N=126 61(48.4) |
N=26 12(46.2) |
0.8 |
|
| ||||
| AST>=80 IU/mL * | N=138 92(66.7) |
N=110 72(65.5) |
N=21 15(71.4) |
0.6 |
|
| ||||
| Albumin<2.8g/dL * | N=167 82(49.1) |
N=127 64(50.4) |
N=29 14(48.3) |
0.8 |
|
| ||||
| INR>1.5 * | N=65 6(9.2) |
N=51 5(9.8) |
N=11 1(9.1) |
1 |
|
| ||||
| Total bilirubin >=5 mg/dl * | N=131 68(51.9) |
N=103 55(53.4) |
N=17 10(58.8) |
0.7 |
|
| ||||
| Adjusted Weight for age z-score<-2*# | N=174 37(21.3) |
N=133 27(20.3) |
N=32 9(28.1) |
0.3 |
|
| ||||
| Adjusted Height for age z-score<-2*# | N=111 38(34.2) |
N=83 28(33.7) |
N=23 10(43.5) |
0.4 |
|
| ||||
| Weight for height z-score<-2 * | N=89 10(11.2) |
N=71 10(14.1) |
N=13 0(0.0) |
0.3 |
|
| ||||
| Diagnosed with NEC * | N=272 | N=204 | N=46 | 0.005 |
| Yes | 97(35.7) | 66(32.4) | 25(54.3) | |
|
| ||||
| Ever at an ITx Site * | N=272 | N=204 | N=46 | 0.7 |
| Yes | 186(68.4) | 135(66.2) | 32(69.6) | |
summarized by freq(%)
summarized by median (q1-q3).
FIGURE 1.
The CIP of death without transplant and transplant, separately for white and non-white children are presented. The x-axis represents time (months) since the enrollment criteria were met, and the y-axis represents the estimated cumulative incidence probabilities.
To determine if race was independently associated with these outcomes, we examined other baseline characteristics. Children were more likely to die without ITx if they had a birth weight < 1.5 kg (p <0.001), a baseline aspartate aminotransferase (AST) level > 80 (p=0.05), a diagnosis of NEC (p=0.05), a small bowel length ≤43cm (p=0.03), or a baseline International Normalization Ratio (INR) > 1.5 (p=0.02). Subjects were more likely to undergo ITx if they met entry criteria < 63 days of age (p=0.001), had a total bilirubin > 5mg/dl (p=0.02), or went to a PIFcon ITx center (p<.001). Subjects were less likely to undergo ITx if they had a birth weight < 1.5 kg (p=0.002), a small bowel length > 43cm (p=<0.001), or a diagnosis of NEC (p<.001). Characteristics that were not significantly associated with either ITx or death without ITx included gender, Hispanic ethnicity, gestational age <37 weeks, baseline adjusted weight and height for age z-score < -2, baseline alanine aminotransferase (ALT) level >80 and baseline albumin <2.8 gm/dL. Because low birth weight and NEC were more common in non-white than in white children, we performed analyses within strata defined by presence or absence of NEC diagnosis and birth weight. To examine whether receiving medical care at an ITx center was related to incidence of ITx, we also examined the relationship of race with outcome in strata defined by ITx center.
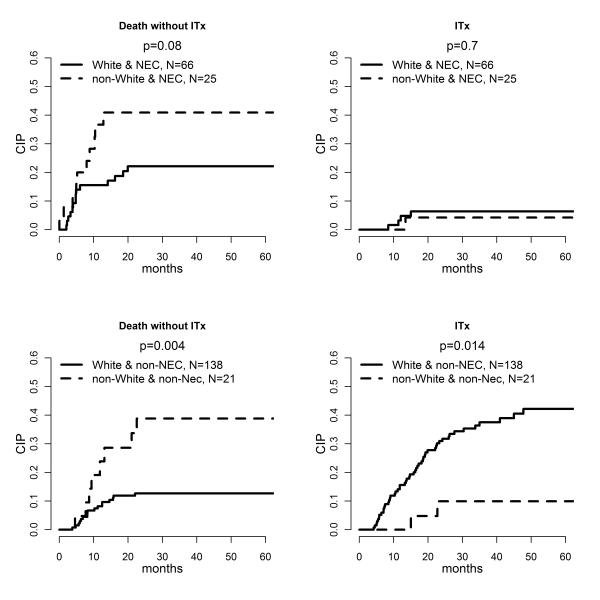
Among 91 participants diagnosed with NEC with known race, there were 66 white and 25 non-white children. The associations of race with both death and ITx were not statistically significant, but the numbers were small and the point estimates for non-white children were higher than for white children (Figure 2). For the 159 children with a diagnosis other than NEC, non-white children (n=21) were more likely to die without ITx (p=0.004) and less likely to receive an ITx (p=0.014) than were white children (n = 138) (Figure 2). The CIP of death without ITx 48 months after enrollment criteria were met was 0.13 for white children without NEC and 0.39 for non-white children without NEC. Furthermore, the CIP of ITx at 48 months was 0.42 for white vs. 0.10 for non-white children without NEC.
FIGURE 2.
The plots on the first row correspond to CIP among children diagnosed with NEC, and the two plots on the second row correspond to CIP among children with a diagnosis other than NEC. The x-axis represents time (months) since the enrollment criteria were met, and the y-axis represents the estimated cumulative incidence probabilities.
Among the 64 participants with a birth weight < 1.5 kg and known race, there were 42 white and 22 non-white infants. There were no significant racial differences in the CIP for death or for ITx in those subjects < 1.5 kg (Figure 3). However, among 127 white and 15 non-white infants > 1.5 kg, non-white children were significantly more likely to die without ITx (p=0.003) (Figure 3).
FIGURE 3.
The plots on the first row correspond to CIP among children with birth weight ≤ 1.5 kg, and the two plots on the second row correspond to CIP among children with birth weight over 1.5 kg. The x-axis represents time (months) since the enrollment criteria were met, and the y-axis represents the estimated cumulative incidence probabilities.
Finally, we evaluated the association of race with outcome within the strata defined by whether or not a participant received medical care at a transplantation center participating in PIFCon. Among 83 known-race children who did not receive medical care at a transplant center (69 white and 14 non-white) non-white children were more likely to die (p=0.036) (Figure 4). For 167 participants who received care at a PIFCon ITx center, there were 135 white and 32 non-white children and similarly, non-white children were more likely to die (p=0.003) and less likely to undergo ITx (p=0.005) (Figure 4).
FIGURE 4.
The plots on the first row correspond to CIP among children not evaluated at a PIFCon ITx center, and the plots on the second row correspond to CIP among children seen at a PIFCon ITx center. The x-axis represents time (months) since the enrollment criteria were met, and the y-axis represents the estimated cumulative incidence probabilities.
Race and birth weight are associated with the CIP of death without ITx so, recognizing the limited power we had to detect a significant interaction, we examined the interaction between race and birth weight due to its clinically meaningful effect, and to identify a large effect if it existed. The interaction was not statistically significant (p=0.10) which needs to be interpreted in the light of low statistical power to detect meaningful differences in the relationship between birth weight and outcome by race. Using the estimates from the model with interaction, however, the estimated hazard for death without ITx among non-white infants with birth weights greater than 1.5kg is 3.9 (95% CI 1.6 – 9.5, p=0.002) times that among white infants with birth weights > 1.5kg.
With respect to ITx, the association of race is statistically significant after adjusting for NEC and whether the patient was ever seen at a PIFCon ITx center. The hazard for undergoing ITx for non-white children is 0.3 (95% CI 0.1—0.8, p=0.02) times that for whites children, suggesting that non-white children were less likely to receive an ITx after adjusting for NEC diagnosis and whether the patient was seen at a transplant center.
Discussion
In this retrospective analysis of 272 infants with IF, we found that non-white children were significantly more likely to die and less likely to receive an ITx than white children, even when the data were stratified by birth weight, diagnosis and site of care. This report is the first to suggest that, for infants with IF, race may be a risk factor for survival and for the likelihood of receiving an ITx. It is important to note that race encompasses social, economic, and cultural issues, among others, and its association with outcome is an important finding for further investigating what it is about race that is responsible for the association.
We sought to determine if factors other than race might account for these differences in outcome. Among the many factors examined, we found birth weight, evidence of liver disease (e.g., elevated total bilirubin, AST and INR), length of remaining small bowel, diagnosis, and whether the child was ever cared for at an ITx center to be associated with death and/or receiving an ITx. When we examined these baseline characteristics among white and non-white infants, birth weight and diagnosis were significantly different between the two groups and we queried whether the effect of race could be explained by these two factors. However, the racial effect persisted for those with a birth weight over 1.5 kg and those with a diagnosis other than NEC.
Overall infant mortality is higher among non-Hispanic black, American Indian/Alaskan, and Puerto Rican infants than non-Hispanic white infants.20 Many factors lead to these discrepancies, but highest among them are increased rates of prematurity and congenital malformations; although gastrointestinal malformations, such as gastroschisis or rotational abnormalities appear not to be a major cause of infant mortality.29 Gastroschisis was the most common diagnosis in the non-NEC category among PIFCon participants and could disadvantage non-white infants if it were to occur more commonly among minorities. However, among 72 infants with gastroschisis identified at one site, only 3% were black.30
Factors that influence the likelihood of EA or are associated with death or eligibility for ITx may have influenced our findings. Breast feeding is thought to be advantageous to infants with short bowel syndrome.18 The Center for Disease Control reported 50.1% of black children were ever breast fed compared to 71.5% of white children.31 It is unlikely that exposure to human milk sufficiently advantaged white infants to account for their improved survival as we found the likelihood of receiving breast milk was similar among white and non-white infants. Blood stream infections contribute to morbidity and mortality. Being African American was a risk factor for developing a blood stream infection in a cohort of cancer patients.32 In our study, the frequency of sepsis episodes per person month was similar between white and non-white children. Venous thrombosis and loss of venous access is an indication for ITx. However, the incidence of venous thrombosis appears to be similar in African-American and white pediatric patients.33
Barriers to solid organ transplantation in adult patients include race, older age, insurance status and income.34 Racial barriers also extend to children seeking access to renal transplantation.35 To our knowledge, racial disparities have not been reported in ITx for adults or children. Myriad factors account for racial barriers in adult liver transplantation include prevalence and severity of the liver disease, access to primary care, health insurance, social support, socioeconomic status, spiritual beliefs, and geographic differences.24 Likewise, our study identifying race as being associated with death and transplantation does not imply a causal relationship, but may simply reflect complex relationships between patient characteristics and outcomes.
The study has several important limitations. It utilized data collected through chart review, so is limited to information available for clinical care. While variations in practice were present between sites, we found no difference in the likelihood of death among non-white children seen at an ITx center versus those who were not. Race could have been miscoded. It is possible that eligible patients were not identified due to incomplete or un-retrievable paper records. While ours is the largest single cohort of infants with IF, numbers remain too small to assess potential confounding factors other than race that may have influenced outcomes. This study was not able to address the many disease specific, environmental, socioeconomic, cultural, geographic, medical, and behavioral factors that might have also influenced our findings.
Conclusions
Race is associated with death and ITx in a large cohort of children with IF. We believe this study highlights the need for a prospective, geographically distributed, multi-center registry to investigate reasons for this apparent racial disparity in outcome among children with IF.
Acknowledgement
Funding for the project is from the National Institutes of Health (NIHNIDDK R21DK081059). CD was supported in part by NICHD K24 HD058795. Key individuals who actively participated in the establishment of the Pediatric Intestinal Failure Consortium as well as portions of this study include (by site): Children’s Hospital of Pittsburgh: Robert H. Squires MD, Cartland Burns, MD, George Mazariegos,, MD, Anita Nucci, PhD, RD, Jane Anne Yaworski, RN, Danielle Sebbens, DNP, CRNP, Rhonda Cunningham. Boston Children’s Hospital: Christopher Duggan, MD, Daniel Kamin, MD, Tom Jaksic, MD, PhD, Hueng Bae Kim, MD, Sharon Collier, RD, LDN, Melanie Connolly, RD. University of Michigan: Daniel H. Teitelbaum, MD, Pamela Brown, M.D., Michele Johnson, LD, Robert Drongowski, MA, Research Coordinator. Nationwide Children’s Hospital: Jane Balint, MD, Christina. Valentine, MD, Steven Teich, MD, Beth Skaggs, Clinical Research Coordinator. UCLA: Robert Venick, MD, Martin G. Martin, MD, M.P.P., Patty Beckwith, RD, CDE, James Dunn, MD, Ph.D, Douglas G. Farmer, MD, F.A.C.S.. Laurie Reyen, RN, MN. UCSF: Susan Rhee, MD, Diana Farmer, MD, Sang-Mo Kang, MD, Lane Bower, Dietician. University of Nebraska: Debra Sudan, MD, David Mercer, MD, Dean L. Antonson, MD, Steve C. Raynor, MD, Brandy Sunderman, RD, Kris Seipel, Clinical Studies Program Coordinator. Vanderbilt: J. Andres Martinez, MD, Brent Polk, MD, Martha Ballew, MEd, RD. Texas Children’s Hospital: Beth A. Carter, MD, Mary Brandt, MD, Saul Karpen, MD, PhD, Sara Philips, RD, Kristin Brown, RD, Alejandro De La Torre. Children’s Hospital of Colorado, Denver: Jason Soden, MD, Sara Fidanza, NP, Kristin Brown, RD. Seattle Children’s Hospital: Simon Horslen, MD, Frances Malone, ARNP, Ph.D, Patrick Healey, MD, Jorge Reyes, MD, Cheryl Davis, RD. Cincinnati Children’s Hospital: Jeffrey A. Rudolph, MD, Samuel Kocoshis, MD, Greg Tiao, MD, Jacqueline Wessel, Nurse Coordinator. Children’s Memorial Hospital: Riccardo Superina, MD, Valeria Cohran, MD, Kimberley Kazmerski, RD, Lisa Keys, BSN, RN, CCTN, Margaret “Peggy” Richard, RN. University of Calgary: David Sigalet, MD, PhD. Emory University: Conrad Cole, MD University of Pittsburgh, School of Public Health: Steven H. Belle, PhD, MScHyg, Ruosha Li, PhD, Sharon Lawlor, MBA, Tamara Haller, BS, Marcia Kurs-Lasky, MS,
Funding: All phases of this study were supported by the NIH/NIDDK: 1 R21 DK081059-01, C.D. received funding from NICHD (K24 HD058795).
Abbreviations
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- CIP
cumulative incidence probability
- EA
enteral autonomy
- INR
International Normalized Ratio
- IF
intestinal failure
- ITx
intestinal transplant
- NEC
necrotizing enterocolitis
- PN
parenteral nutrition
- PIFCon
Pediatric Intestinal Failure Consortium
Footnotes
Financial Disclosure: All authors have no financial relationships relevant to this article to disclose.
Conflict of Interest Statement: All authors have no conflicts of interest relevant to this article.
Contributors Statement Page Robert H Squires: Dr. Squires helped conceptualize the study, aided in the study design, interpretation of the date, drafting the initial and subsequent drafts of the manuscript, and approved the final manuscript
Jane Balint, Simon Horslen, Paul W. Wales, Jason Soden, Christopher Duggan: Drs. Balint, Horslen, Wales, Soden and Duggan aided in the study design, interpretation of the data, drafting the initial and subsequent drafts of the manuscript, and approved the final manuscript
Ruosha Li: Carried out the initial analysis, aided in drafting the initial and subsequent drafts of the manuscript, and approved the final manuscript
Steven H Belle: Dr. Belle helped conceptualize the study, aided in the study design, interpretation of the date, drafting the initial and subsequent drafts of the manuscript, and approved the final manuscript
Members of the Pediatric Intestinal Failure Consortium who contributed to design of the data collection forms, identified eligible patients, and completed data collection forms at each of the sites listed in the Acknowledgement section.
References
- 1.D’Antiga L, Goulet O. Intestinal failure in children: the European view. J Pediatr Gastroenterol Nutr. 2013;56:118–26. doi: 10.1097/MPG.0b013e318268a9e3. [DOI] [PubMed] [Google Scholar]
- 2.Kocoshis SA, Beath SV, Booth IW, et al. Intestinal failure and small bowel transplantation, including clinical nutrition: Working Group report of the second World Congress of Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. 2004;39(Suppl 2):S655–61. doi: 10.1097/00005176-200406002-00012. [DOI] [PubMed] [Google Scholar]
- 3.Salvia G, Guarino A, Terrin G, et al. Neonatal onset intestinal failure: an Italian Multicenter Study. J Pediatr. 2008;153:674–6. 676, e1–2. doi: 10.1016/j.jpeds.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 4.Squires RH, Duggan C, Teitelbaum DH, et al. Natural history of pediatric intestinal failure: initial report from the Pediatric Intestinal Failure Consortium. J Pediatr. 2012;161:723–8. e2. doi: 10.1016/j.jpeds.2012.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goulet O, Ruemmele F. Causes and management of intestinal failure in children. Gastroenterology. 2006;130:S16–28. doi: 10.1053/j.gastro.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 6.Rudolph JA, Squires R. Current concepts in the medical management of pediatric intestinal failure. Curr Opin Organ Transplant. 2010;15:324–9. doi: 10.1097/MOT.0b013e32833948be. [DOI] [PubMed] [Google Scholar]
- 7.De Marco G, Barabino A, Gambarara M, et al. Network approach to the child with primary intestinal failure. J Pediatr Gastroenterol Nutr. 2006;43(Suppl 1):S61–7. doi: 10.1097/01.mpg.0000226392.09978.6d. [DOI] [PubMed] [Google Scholar]
- 8.Diamond IR, de Silva N, Pencharz PB, et al. Neonatal short bowel syndrome outcomes after the establishment of the first Canadian multidisciplinary intestinal rehabilitation program: preliminary experience. J Pediatr Surg. 2007;42:806–11. doi: 10.1016/j.jpedsurg.2006.12.033. [DOI] [PubMed] [Google Scholar]
- 9.Stanger JD, Oliveira C, Blackmore C, et al. The impact of multi-disciplinary intestinal rehabilitation programs on the outcome of pediatric patients with intestinal failure: a systematic review and meta-analysis. J Pediatr Surg. 2013;48:983–92. doi: 10.1016/j.jpedsurg.2013.02.070. [DOI] [PubMed] [Google Scholar]
- 10.Hess RA, Welch KB, Brown PI, et al. Survival outcomes of pediatric intestinal failure patients: analysis of factors contributing to improved survival over the past two decades. J Surg Res. 2011;170:27–31. doi: 10.1016/j.jss.2011.03.037. [DOI] [PubMed] [Google Scholar]
- 11.Mazariegos GV, Squires RH, Sindhi RK. Current perspectives on pediatric intestinal transplantation. Curr Gastroenterol Rep. 2009;11:226–33. doi: 10.1007/s11894-009-0035-1. [DOI] [PubMed] [Google Scholar]
- 12.Wada M, Kato T, Hayashi Y, et al. Intestinal transplantation for short bowel syndrome secondary to gastroschisis. J Pediatr Surg. 2006;41:1841–5. doi: 10.1016/j.jpedsurg.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 13.Cole CR, Hansen NI, Higgins RD, et al. Very low birth weight preterm infants with surgical short bowel syndrome: incidence, morbidity and mortality, and growth outcomes at 18 to 22 months. Pediatrics. 2008;122:e573–82. doi: 10.1542/peds.2007-3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pakarinen MP, Koivusalo AI, Rintala RJ. Outcomes of intestinal failure--a comparison between children with short bowel and dysmotile intestine. J Pediatr Surg. 2009;44:2139–44. doi: 10.1016/j.jpedsurg.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 15.Spencer AU, Neaga A, West B, et al. Pediatric short bowel syndrome: redefining predictors of success. Ann Surg. 2005;242:403–9. doi: 10.1097/01.sla.0000179647.24046.03. discussion 409-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wales PW, de Silva N, Kim JH, et al. Neonatal short bowel syndrome: a cohort study. J Pediatr Surg. 2005;40:755–62. doi: 10.1016/j.jpedsurg.2005.01.037. [DOI] [PubMed] [Google Scholar]
- 17.Beath S, Pironi L, Gabe S, et al. Collaborative strategies to reduce mortality and morbidity in patients with chronic intestinal failure including those who are referred for small bowel transplantation. Transplantation. 2008;85:1378–84. doi: 10.1097/TP.0b013e31816dd513. [DOI] [PubMed] [Google Scholar]
- 18.Andorsky DJ, Lund DP, Lillehei CW, et al. Nutritional and other postoperative management of neonates with short bowel syndrome correlates with clinical outcomes. J Pediatr. 2001;139:27–19. doi: 10.1067/mpd.2001.114481. [DOI] [PubMed] [Google Scholar]
- 33.Cullen MR, Cummins C, Fuchs VR. Geographic and racial variation in premature mortality in the U.S.: analyzing the disparities. PLoS One. 2012;7:e32930. doi: 10.1371/journal.pone.0032930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hauck FR, Tanabe KO, Moon RY. Racial and ethnic disparities in infant mortality. Semin Perinatol. 2011;35:209–20. doi: 10.1053/j.semperi.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 21.MacDorman MF. Race and ethnic disparities in fetal mortality, preterm birth, and infant mortality in the United States: an overview. Semin Perinatol. 2011;35:200–8. doi: 10.1053/j.semperi.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 22.Partridge S, Balayla J, Holcroft CA, et al. Inadequate prenatal care utilization and risks of infant mortality and poor birth outcome: a retrospective analysis of 28,729,765 U.S. deliveries over 8 years. Am J Perinatol. 2012;29:787–93. doi: 10.1055/s-0032-1316439. [DOI] [PubMed] [Google Scholar]
- 23.Mathur AK, Schaubel DE, Gong Q, et al. Racial and ethnic disparities in access to liver transplantation. Liver Transpl. 2010;16:1033–40. doi: 10.1002/lt.22108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mathur AK, Sonnenday CJ, Merion RM. Race and ethnicity in access to and outcomes of liver transplantation: a critical literature review. Am J Transplant. 2009;9:2662–8. doi: 10.1111/j.1600-6143.2009.02857.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moylan CA, Brady CW, Johnson JL, et al. Disparities in liver transplantation before and after introduction of the MELD score. JAMA. 2008;300:2371–8. doi: 10.1001/jama.2008.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Putter H, Fiocco M, Geskus RB. Tutorial in biostatistics: Competing risks and multi-state models. Statistics in Medicine. 2007;26:2389–2430. doi: 10.1002/sim.2712. [DOI] [PubMed] [Google Scholar]
- 27.Gray RJ. A Class of K-Sample Tests for Comparing the Cumulative Incidence of a Competing Risk. Annals of Statistics. 1988;16:1141–1154. [Google Scholar]
- 28.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. Journal of the American Statistical Association. 1999;94:496–509. [Google Scholar]
- 29.Lee K, Khoshnood B, Chen L, et al. Infant mortality from congenital malformations in the United States, 1970-1997. Obstet Gynecol. 2001;98:620–7. doi: 10.1016/s0029-7844(01)01507-1. [DOI] [PubMed] [Google Scholar]
- 30.Eggink BH, Richardson CJ, Malloy MH, et al. Outcome of gastroschisis: a 20-year case review of infants with gastroschisis born in Galveston, Texas. J Pediatr Surg. 2006;41:1103–8. doi: 10.1016/j.jpedsurg.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 31.Racial and Socioeconomic Disparities in Breastfeeding--United States, 2004. MMWR. 2006 Mar 31;55:335–339. [PubMed] [Google Scholar]
- 32.Doganis D, Asmar B, Yankelevich M, et al. Predictive factors for blood stream infections in children with cancer. Pediatr Hematol Oncol. 2013;30:403–15. doi: 10.3109/08880018.2013.778379. [DOI] [PubMed] [Google Scholar]
- 33.Wright JM, Watts RG. Venous thromboembolism in pediatric patients: epidemiologic data from a pediatric tertiary care center in Alabama. J Pediatr Hematol Oncol. 2011;33:261–4. doi: 10.1097/MPH.0b013e3182134111. [DOI] [PubMed] [Google Scholar]
- 34.Schold JD, Gregg JA, Harman JS, et al. Barriers to evaluation and wait listing for kidney transplantation. Clin J Am Soc Nephrol. 2011;6:1760–7. doi: 10.2215/CJN.08620910. [DOI] [PubMed] [Google Scholar]
- 35.Patzer RE, Amaral S, Klein M, et al. Racial disparities in pediatric access to kidney transplantation: does socioeconomic status play a role? Am J Transplant. 2012;12:369–78. doi: 10.1111/j.1600-6143.2011.03888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]