Abstract
Incentive contrast effects include changes in behavioral responses after a reward upshift (positive contrast) or downshift (negative contrast). Proposed influences on these behavioral changes are emotional state reactions after experiencing or anticipating a change in reward outcome. Rat ultrasonic vocalizations have been shown to be indicators of emotional state during behavior and anticipatory periods. The objective of the present study was to monitor rodent ultrasounds during incentive contrast using a classical runway procedure called instrumental successive negative contrast. The procedure is one that has been used often to examine incentive relativity because of its reliability in measuring negative contrast effects. Rats were trained to run in the alleyway to receive a high (12 pellets) or low magnitude (1 pellet) outcome. The high magnitude was then shifted to the low and running speeds in the alleyway for the reward and USV emission were compared. Replicating previous work, a negative contrast effect was observed with postshift running speeds significantly slower in the shifted group compared to the unshifted group. USVs did not follow the same pattern with an apparent lack of significant differences between the groups following the reward downshift. We also tested another group of animals using a visual predictive cue in the same runway test. When visual cues predicted high or low magnitude outcome, no incentive contrast was found for the running speeds following an outcome downshift, but a weak contrast effect was observed for the USV emission. These results demonstrate a separation between USVs and behavioral indicators of incentive contrast suggesting that concomitant shifts in negative affect may not be necessary for anticipatory relative reward processes.
Keywords: communication, emotion, expectancy, incentive contrast, motivation
Introduction
In general, motivation and emotion work together as ‘emotionally-motivational states’ (Jürgens, 1977) by integrating internal states and external events to produce goal-directed action (Trowill et al., 1969; Panksepp, 1998). These interactive states has been examined in a wide array of incentive contrast paradigms. These utilize systematic alterations of reward value to examine the impact of different forms of relative reward comparisons on behavior (Reynolds, 1961; Flaherty, 1996). Emotional-motivational states have been monitored in animal models by investigating vocalizations in diverse species (Jürgens, 1979; Sales and Pye, 1974; Panksepp, 1998). Affective state influences on behavior in rodent models have been examined by recording and measuring ultrasonic vocalizations (USVs) (Amsel et al., 1977; Panksepp and Burgdorf, 2000; Brudzynski, 2001; Brudzynski et a., 2002).
One behavioral paradigm used to examine iSNC has been a runway procedure (Gonzalez et al., 1962). The animal is trained to run down the alley in order to obtain a reward outcome, typically a set of food pellets. Early work on reward processing and behavior used a similar methodology to examine relative reward effects and motivation. Runways have been used as a traditional means to examine basic factors that influence motivation such as levels of food deprivation (Kintsch, 1962), incentive amount (Di Lollo, 1962) and goal box experience (Rosen and Freedman, 1971). Crespi (1942) emphasized the value of the runway as a powerful procedure because of the ability to measure time in running speed as the main indication of contrast. Other dependent measures such as error-difference were found to not be as sensitive and thought to be unable to track contrast effects on performance after extensive training. Two other characteristics of the runway were noted as important and they included the simplicity of the training regimen as well as the ease with which the behavior could be observed and tracked.
The behavioral paradigm of iSNC is reliable in producing behavioral instrumental contrast. Interestingly, the changes in instrumental behavior have been shown to be dissociated from consummatory measures of contrast such as licking rate to sucrose solutions (Flaherty and Caprio, 1976). This is interesting in that the approach behavior (i.e., running speed) is thought to be more intimately linked to emotional state influences when guiding flexible behavior (Panksepp, 1998). Emotions have been proposed as a potential influence on contrast effects since early work in the area. Crespi (1942) actually used the emotion-laden term depression to describe the decrease in running speed after outcome downshift. A process involving negative affect has been proposed to be a part of incentive contrast as animals would be suppressing behavior potentially because of the activation of a relative negative emotional state (Flaherty, 1991; Scull et al., 1970; Daly, 1967). Relative hedonic value of sucrose solutions can influence anticipatory contrast (Flaherty et al, 1994) supporting a role for value hierarchy involved in outcome comparisons. Consummatory and instrumental behavior can be triggered more intensely or maintained over time because of a link to emotional state shifts (Williams, 1991); however, direct relationships between preferences and contrast are sometimes lacking (Williams, 1992). Monitoring emotional output in the rodent model can be difficult especially during active instrumental responses. Recent research on rodent motivation has included potential measures of affect by incorporating ultrasonic vocalizations (USVs) as a dependent variable (Burgdorf et al., 2013). Monitoring USVs has proven to be highly useful in revealing new relationships and understanding affective styles of the animal subjects (Panksepp, 2005; Harmon et al., 2008).
50 kHz USVs have been found to be linked to positive affect and 22 kHz calls to negative affect (Sales and Pye, 1974; Portfors, 2007; Brudzynski, 2001). The higher frequency calls are seen in conjunction with positive rewards such as social interactions like play and somatosensory handplay (e.g., tickling see Burgdorf et al., 2000; Panksepp and Burgdorf, 2000). Studies on sensorimotor gating have used USV detection to decipher concurrent emotional states that could be regulating startle and anxiety while gating sensory input (Webber et al., 2013; Tunstall et al., 2009). Other work has used USVs for a phenotype to select animals that emit high or low levels of USVs (Harmon et al., 2008; Burgdorf et al., 2013; Burgdorf et al., 2009). This work has illustrated a potential underlying genetic component involved in USV emission and the shared traits that are related to emotional behavior (Webber et al., 2012).
One reason the incentive contrast and USVs could be related is that they can depend upon anticipatory states. 50 kHz USVs have been shown to be linked to expectations for reward outcomes. Animals gradually increase 50 kHz call emission prior to electrical stimulation of the brain (Burgdorf et al., 2000) and reception of a drug reinforcer (Knutsen et al, 1999; Ahrens et al., 2013). These are powerful outcomes when delivered in a predictable manner produce anticipatory states that regulate motivation. A second part of this study modified the original paradigm to enhance anticipatory states prior to reward acquisition. We implemented visual cues for each trial that reliably signaled the reward outcome magnitude. We enhanced the involvement of anticipation by using distinct visual cues to signal different reward magnitude outcomes. Previous work has already found that cues or predictive stimuli can have significant effects on contingency and incentive contrast (Williams, 1988, 1991). One influence is that they can increase the breath of outcomes which can be used to obtain contrast effects (e.g., both food pellets and sucrose solution see Flaherty et al., 1973). The situation with cues could increase emissions of USVs by increasing predictability thereby reducing overall risk (Burgdorf et al., 2008). Since reward outcome value has been hypothesized to interact with predictability, we predicted that USVs and contrast effects could be more highly related to one another when certainty of outcome was enhanced using discriminative cues. Using both paradigms (uncued and cued versions) enables a more complete analysis for how anticipatory states might incorporate emotional information prior to reward outcome acquisition and during incentive contrast. The aim of the present work is to examine how these emotional-motivational states could impact reward contrast effects by measuring rat running speeds and USVs during instrumental successive negative contrast (iSNC). The results of this work could be very important in highlighting new, possible ways to study complex motivational-emotional state functions in animal models such as disappointment or frustration and translating this work to human emotional impairment during reward processing proposed to play a role in different mental illnesses (Gold et al., 2008).
Methods
Subjects
For this study, adult, male Sprague-Dawley rats (Rattus norvegicus; n= 80; 8-12 months old) were used. These animals were kept on a 12:12 light: dark cycle. Lights turned on at 8AM. Animals were individually housed in polycarbonate shoebox style cages (65cm × 24cm × 15cm) and given ad libitum water and food upon arrival. Animals were given ad libitum access to food (Harlan Teklab rat chow) on the days in which they are not tested. Throughout the testing period, rats were food deprived to 87-90% of their free-feeding baseline weight. Free-feeding baseline weight was calculated by averaging their weights from the 3 days prior to testing. Baseline weights were re-calculated before each testing phase (baseline weight range = 280-354 grams). Animals were randomly placed into one of two groups. One group (n=40; mean weight = 335 grams) received a 12-pellet reward for the first half of the experiment (n=20 animals for uncued and 20 animals for cued version of the task), which was then downshifted to a 1-pellet reward for the second half of the experiment. A second group of rats (n=40; mean weight = 342 grams) remained on a 1-pellet reward for the duration of the experiment (n=20 animals for the uncued and n= 20 animals for the cued version of the task). All experiments were approved (protocol 10-016) by the Institutional Animal Care and Use Committee at Bowling Green State University and followed the Guide for Animal Care and Use published by the National Institutes of health (USA).
Apparatus
A black, wooden runway was used for this experiment (244 × 10 × 10cm). During testing there was a clear Plexiglas cover placed on top of the runway. This runway was divided into three sections: a start box (34.5 cm), a run portion (175 cm), and a goal box (34.5 cm). Photocells were attached to the runway. One was located 8.5 cm after the start box and the second one was located 18.5 cm past the goal box opening. This allowed us to acquire both speed from the start of the trial (door opening) to the first photobeam as well as running speed between the initial beam and the second beam in the goal box (goal running speed). The running speed measures used in the present work were similar to measures obtained in previous work on instrumental successive negative contrast (Leszczuk and Flaherty, 2000; Flaherty et al., 1998). The photo beams were connected to MED-PC software (Med. Associates Inc.) for data collection. Two ultrasound detectors were placed on the top of the runway to record ultrasonic vocalizations (Pettersson D230 ultrasonic detector; Uppsala, Sweden). The first detector was placed over the middle of the start box and the second recorder was placed at the end of the run segment immediately prior to the goal box opening.
Procedure
Non-cued runway Task
At the beginning of the first task, rats (n=40) were given a 3-day habituation period to become familiar with the runway and the reward associated with the runway. Each morning one hour prior to the habituation procedure, rats were given access to the sucrose pellets (3-6) in their home cage. This was to acclimate them to the sucrose pellets prior to training. On day 1, rats were placed individually into the start box, with the start box and goal box doors open; they were given free access to the whole runway for two periods of 5 minutes. These open access periods were spaced 7 minutes apart. On the second day of habituation, the rats were given free-exploration of the runway again for two 2-minute periods (spaced 7 minutes apart) followed by three feedings in the goal box. During these feeding periods, the rats were confined to the goal box and were given their designated reward of 1 or 12 sucrose pellets. Half of the animals that are trained on this task were given one pellet and half were given access to 12 pellets. On the final day of habituation (Day 3), rats were given three goal box feedings spaced 7 minutes apart. On this day, rats were given 30 seconds to consume the pellets before they were removed from the goal box and taken back to their home cage. In their home cage, they were given six sucrose pellets, along with their ration of lab chow (30 minutes after the last habituation session for the day).
The training portion of this procedure began on the fourth day, and continued for a total of 6 days. For this training, individual rats were placed into the start box; the start box door was closed at this time. The start box door was opened giving the rat free access to run down the runway to attain a reward (1 or 12 sucrose pellets). Rats were given 40 seconds to complete this task. If the rat did not enter in the goal box by the end of 40 seconds, they were gently nudged to the goal box and 40 seconds was recorded for their time. Once the rat reached the goal box, the experimenter closed the goal box door confining the rat at the goal box. At this time, rats were given 30 seconds to consume their reward before being returned to their cage; any uneaten pellets were noted at this time.
Each day rats completed six runway trials spaced 7 minutes apart. The runway floor was cleaned between trials with a soapy water solution. On the last day of training, half of the group had their reward shifted (the group receiving 12 pellets were shifted to one pellet, and the one pellet group remained the same).
Post shift testing continued for 6 days. Trials were conducted in the same manner as the training trials; the only difference was the available reward. Transport cages were kept in a smaller, separate room outside of the testing area in order to remove any possible contamination of USV recordings from rats other than the rat being tested in the runway. Throughout the training and testing portions of this experiment, bat detectors were used in the start-box and goal-box in order to record any ultrasonic vocalizations that the rats may emit during the procedure. Running speeds were calculated from photobeam breaks taken from the start and goal box locations in the runway and acquired with computer controlled behavioral software for both uncued and cued tasks (Med Associates, Vermont, USA).
Cued-runway Task
Forty animals were tested on this version of the task. This procedure was similar to the procedure above, except for the addition of visual cues associated with the two different reward amounts. The visual cues were on a piece of white unlined printing paper (14 × 14 cm) placed on a stand 6 cm above the goal box for the animal to view. The two visual cues drawn on the paper were a plus (+) symbol for one pellet reward and a shaded square (□) for the 12-pellet reward. At the beginning of each of all trials, a visual cue was placed at the end of the runway to cue the rat into which reward is available. The cue was shifted for the 12 pellet group on postshift day 1 so that the low reward outcome associated cue was not presented along with the 1 pellet outcome. Previous research shows that rats are readily able to learn cues that indicate certain rewards or situations (Lucas and Timberlake, 1992). If they learn the discriminative cues during preshift training, the animals should take run faster to obtain the reward after exposure to the 12-pellet cue, and should run slower to the reward after exposure to the cue associated with the 1-pellet reward.
Ultrasonic vocalization analysis
Ultrasound vocalization data used for analysis in this study were .WAV files obtained from a digital recorder (Marantz PMD660 Digital Solid State Field Recorder) connected by audio cables directly to the output channels of the BAT detector (D-230 Detector, Pettersson Elektronik AB Sweden). The frequency division signal (1/10 division mode) was acquired to enable potential broadband recordings of 22 and 50 kHz USVs. These files were scored for ultrasonic vocalizations produced by rats in the runway apparatus during each trial. USV scoring was completed using spectrographic display of the calls and counted manually (Avisoft Bioacoustics, Berlin, Germany). Detailed spectrographic parameters used for analysis included: 48 kHz sampling rate, 256 FFT length, 2.8 s analytical window and 100% Frame settings and 50% overlap between analytical windows for each file.
Statistical Analysis
Statistical analysis was completed on running speed and ultrasonic vocalization emitted using Statistical Package for the Social Sciences (Version 20; IBM N.Y. USA). A focus on USV and behavioral data obtained from the final day before reward shift and the six days following the reward shift allowed us to investigate the preshift and postshift period effectively. Previous work has shown that contrast effects are similar when using the last preshift day or a longer preshift period and so the use of this single baseline day is a typical procedure (Crespi, 1942; Leszcuk and Flaherty, 2000; Caprio and Flaherty, 1976). The first step for all datasets included tests for normality (Kolmogorov-Smirnov or K-S tests) to determine what type of statistical procedures should be used. These tests were completed for each day of training (preshift) and testing (postshift) on each dependent measure (uncued and cued running speed and USVs). The majority of datasets were non-normally distributed including preshift training period measures (5 out of 6 training days were non-normally distributed; K-S Test statistic range 0.104-0.2; p<0.05), postshift uncued running speeds (K-S test = 0.097 to 0.357; p<0.05), postshift uncued USVs (K-S test statistic = 0.198 to 0.357, p<0.050), postshift cued running speeds (K-S test statistic = 0.195, p<0.05) and postshift cued USVs (K-S test statistic = 0.209 to 0.394, p<0.05). Since the datasets had non-normal distributions then we analyzed the data using non-parametric statistics. First we used a Friedman's analysis of variance (ANOVA) to determine a main effect of time over the testing period and then performed pairwise comparisons between test days using a Wilcoxon Signed-Rank test. Finally we compared between the two groups (shifted versus unshifted) for each test day using the nonparametric Mann-Whitney U test. Differences with the probability α ≤ 0.05 were regarded as significant.
Results
Uncued runway running speed results
We examined the rate and extent of reward discrimination between groups in the training period prior to the reward outcome shift (preshift period). There was a six day preshift period and we found a significant difference between the high (12 pellet) and low (1 pellet) group only for Day 6 (U=42.5, p<0.01; see Figure 1). No other day was significantly different between the groups. We focused only on the goal –speed for this analysis because this was the only measure of running speed that resulted in an incentive contrast effect. This result of finding a difference in speed close to the goalbox is similar to previous findings by Leszczuk and Flaherty (2000) using comparable methods and apparatus. We used the final preshift day for comparison to the postshift in order to maintain the level of similarity to previous work using the runway apparatus (Crespi, 1942; Leszczuk and Flaherty, 2000; Kintsch, 1962). The results of the Friedman's ANOVA tests on the preshift-postshift comparison found a significant difference for running speeds for the both the shifted (see Figure 1; F(6) = 53.01, p<0.01) and unshifted (F(6) = 49.55, p<0.01) groups. The pairwise tests found significant differences between the preshift day and postshift day 1 (W= 2, p<0.001); day 2 (W= 0.001, p<0.001), Day 3 (W= 28, p<0.05), Day 4 (W= 25.5, p<0.05), Day 5 (W= 3, p<0.01), and Day 6 (W= 1, p<0.001). Postshift day differences included: Days 1 and 2 (W= 4, p<0.05), Days 2 and 3 (W= 16, p<0.01), Days 2 and 4 (W= 16, p<0.01), Days 2 and 5 (W= 16, p<0.01), days 4 and 6 (W = 4, p<0.01) and Days 5 and 6 (W= 16, p<0.01). For the unshifted group there were fewer significant differences. These were found on Preshift Day and Postshift Days 1 (W= 2-9, p<0.05) and Day 2 (W=209, p<0.05) and Day 3 (W= 206.5, p<0.01), Day 4 (W= 17, p<0.01) and Day 6 (W=19, p<0.01).
Figure 1.
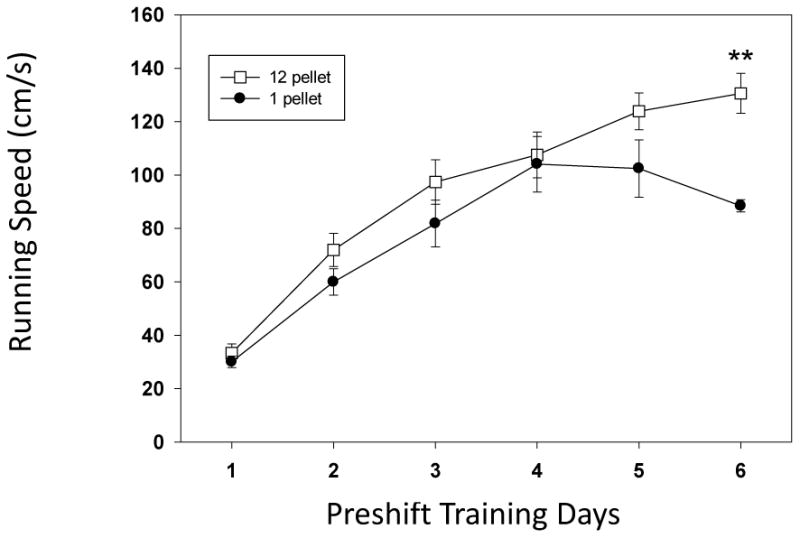
Running speeds (cm/s) for the two groups of animals in the preshift training period (6 days). The days represent the 6 day period during which the animals are exposed to either high reward (12 pellet) or low reward (1 pellet). A significant difference between groups was seen only for day 6 of training (Mann-Whitney U test for between group analysis; **= p<0.01 bars denote standard error of the mean).
The critical comparison to determine iSNC was between the shifted and unshifted groups for the test days. We found significant differences between the groups on days: Preshift (U= 42.5, p<0.001), Postshift Days 1 (U =390.5, p<0.001), Day 2 (U= 397.5, p<0.001), Day 3 (U=295.5, p<0.01), Day 4 (U= 281, p<0.05) and Day 6 (U= 374, p<0.001). The shifted group was significantly slower on each of the postshift days (see Figure 1) with only Day 5 as a timepoint with a no significant postshift slowing of running speed.
Uncued Ultrasonic vocalizations
We detected USV calls only at the initial startbox location in the straight alleyway and not at the later goalbox location. We detected only calls in the range of 50-55 kHz and no calls in the lower frequencies. The analysis for both the uncued and cued versions will be confined to the higher frequency calls (50-55 kHz) obtained from the startbox region of the runway. We found a significant effect for test day only in the shifted group (F(6)= 26.02, p<0.001) and not in the unshifted group. In this shifted group we found significant between day differences for Preshift day and Postshift day 1 (W=20, p<0.01); Day 2(W= 20.5, p<0.01); Day 3( W= 24, p<0.05); Day 4 (W= 45.5, p<0.05); Day 5 (W= 20, p<0.01) and Day 6 (W= 15.5, p<0.05). Shifted group of animals emitted significantly higher calls to the higher magnitude outcome (12 vs. 1 pellet) on the preshift day (see Figure 2; U= 50.5, p<0.001). For the postshift period, only Day 5 varied between the shifted and unshifted groups (U= 105, p<0.01). This supports a dissociation between USVs and reward contrast, because this same day was the lone day that the running speeds did not significantly vary between groups.
Figure 2.
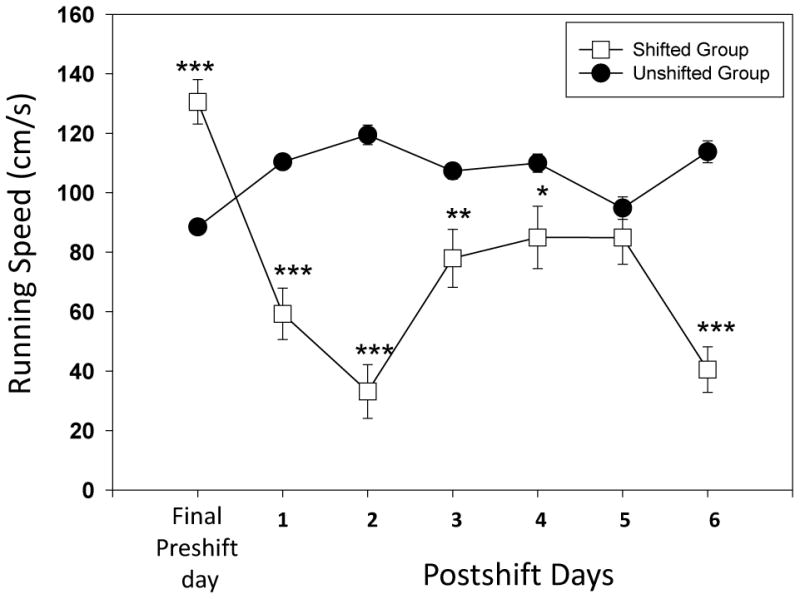
Running speeds (cm/s) for the two groups of animals (shifted versus unshifted) during the uncued version of the runway task. The days represented are the final preshift day as well as six postshift test days (Mann-Whitney U test for between group analysis; *= p<0.05; **= p<0.01 and ***= p<0.001 and bars denote standard error of the mean).
Cued Running Speeds
The cued test with the alleyway allowed us to examine the impact of increasing predictability on the running speeds and USV emission during the task. Previous work has found USV emission is highly sensitive to expectation and emitted prior to reward delivery (Knutson et al., 2001). We examined the impact of cue exposure between the high (12 pellet) and low (1 pellet) groups by analyzing the differences in running speeds during the 6 day training period (preshift). Four out of 6 preshift days were significantly different between the groups. Most impressively, there was a significant difference on day 1 of training (see Figure 4; Day 1 U=128.5, p<0.05). Significant differences were also found on days 2, 3 and 5 (range of Mann-Whitney U values = 90.5 to 131.5; p<0.05 for all tests). For the comparison between the preshift and postshift period, we found both shifted and unshifted groups had significant differences across the testing period (see Figure 3; shifted group, F(6)= 24.84, p<0.001 and unshifted group, F(6) = 38.2, p<0.001). The pairwise tests found significant differences for the shifted group on Preshift day versus Postshift Day 1 (W = 38, p<0.05), (Day 2(W= 35.5, p<0.05) and Day 6 (W= 49.5, p<0.05). Postshift Days 1 and 3 (W=169, p<0.01), 1 and 4 (W= 204, p<0.001), 2 and 3 (W=169, p<0.01), 2 and 4 (W=198, p<0.01), 3 and 6 (W=42, p<0.05) and 4 and 6 (W=30, p<0.05) were all significantly different. For the unshifted group the following comparisons were significantly different: Preshift Day and PostShift Day 1 (W=183, p<0.05), Day 2 (W=187.5, p<0.01) and Postshift Days 1 and 4 (W=45, p<0.05), 1 and 5 (W=8, p<0.001), 1 and 6 (W=3, p<0.001); 2 and 4 (W=42.5, p<0.05); 2 and 5 (W= 5, p<0.001); 2 and 6 (W=5.5, p<0.001); 3 and 5 (W = 10, p<0.01) and 3 and 6 (W = 23, p<0.01). Examining the differences between the two groups, we found that the unshifted group was significantly faster on postshift days 1 (U= 352.5, p<0.001) and Day 2 ( U=352.5, p<0.001) while the shifted group was significantly faster on Day 4 (U=121.5, p<0.05). These mixed results point to a lack of consistent negative contrast for this visual cue version of the task.
Figure 4.
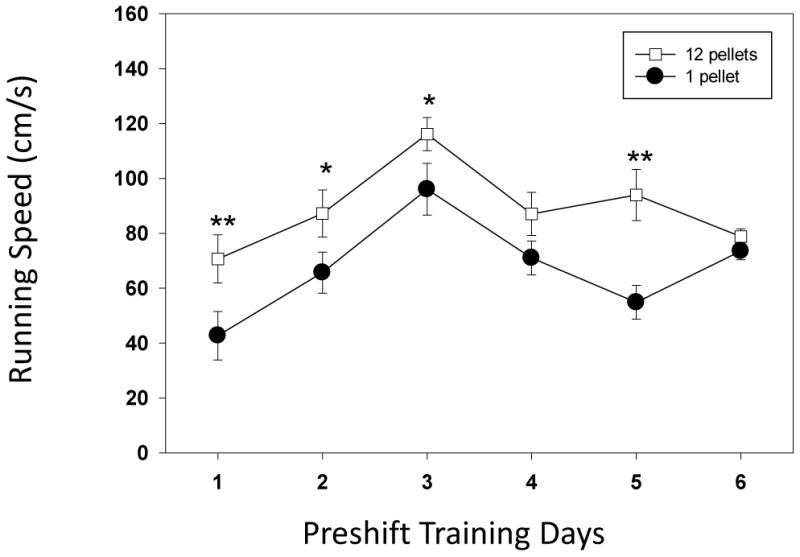
Running speeds (cm/s) for the two groups of animals in the preshift training period (6 days) during exposure to the cues that predict the specific outcome. The days represent the 6 day period during which the animals are exposed to either high reward (12 pellet) or low reward (1 pellet). A significant difference between groups was seen on each except for days 1, 2, 3 and 5 (Mann-Whitney U test for between group analysis; *= p<0.05; **= p<0.01 and *** = p<0.001; bars denote standard error of the mean.
Figure 3.
Average 50 kHz ultrasonic vocalizations observed per trial in the two groups of animals (shifted versus unshifted) during the uncued runway task. The days represented are the final preshift day as well as six postshift test days (Mann-Whitney U test for between group analysis; *= p<0.05; **= p<0.01 and *** = p<0.001; bars denote standard error of the mean).
Cued Ultrasonic Vocalizations
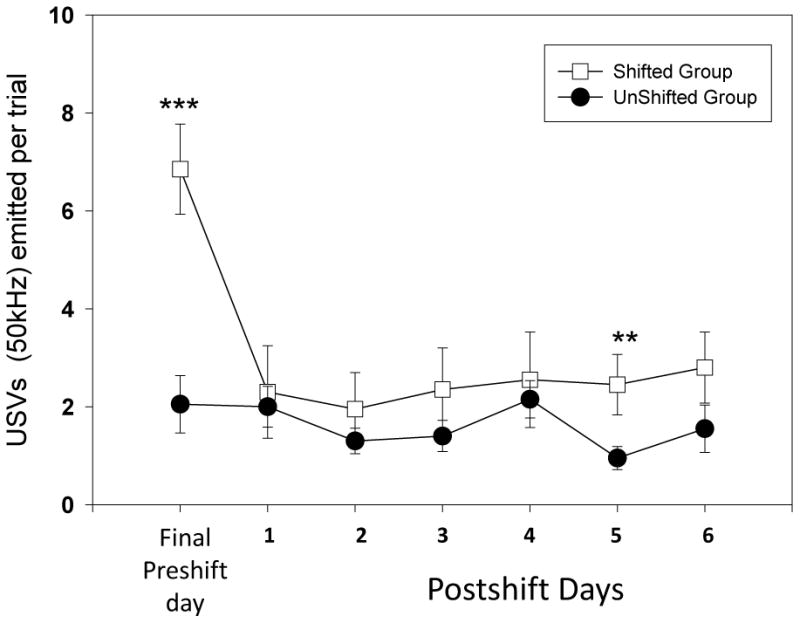
For the ultrasounds during the cued task, we found a significant difference in the shifted group ( see Figure 4; F(6) = 39.4, p<0.001) but not in the unshifted group. We completed the pairwise comparisons between the test days in the shifted group and found differences between the Preshift Day and Postshift Day 2 (W = 23.5, p<0.01); Day 3 (W= 17, p<0.01); Day 4 (W= 16, p<0.01) Day 5 (W=19.5, p<0.01) and Day 6 (W= 9.5, p<0.01). Significant differences between postshift days were found between days 1 and 2 (W=14, p<0.05); 1 and 3 (W=19, p<0.01); 1 and 4 (W=12, p<0.05); 1 and 5 (W= 26, p<0.05); and 1 and 6 (W=10.5, p<0.01). We found higher USV emission in the shifted group on preshift day (see Figure 4; U=79, p<0.01) and lower USV emission on Postshift Day 2 (U=342, p<0.01); Day 3 (U=354, p<0.01); and Day 6 (U= 35.5, p<0.05). This profile reflects negative contrast effect occurring for these USV calls when visual cues are available to the animals.
Comparing results between the cued and uncued tasks
We compared datasets between the uncued and the cued version of the task. To examine a potential difference between having a cue and not having a cue in the initial training period, we completed an analysis between the two conditions using a difference score of the running speeds between the high (12 pellets) and low (1 pellet) groups in both conditions (uncued and cued). We found a significant difference between these difference scores taken from each of the 6 days of preshift training (U=84, p<0.01; uncue X̄ = 13.8±6.9 and cue X̄ =30.1±5.2). The cue group had a larger difference for every preshift test day except for the final day 6 datapoint (see Figures 1 and 4). Next, we compared the data for the 1 pellet trials during the incentive contrast test period (postshift). We found significant differences for the unshifted group for the running speeds between the two task versions. These animals ran faster in the uncued version on the preshift day (U= 328, p<0.01) and postshift day 1 (U367, p<0.001), day 2 (U=392.5, p<0.01), day 3 (U = 368, p<0.001) day 4 (U = 368, p<0.001) day 5 (U = 363, p<0.001) and day 6 (U=392, p<0.001). For the shifted group there were fewer significant differences between the task versions and the differences between the uncued and cued depended upon the reward outcome downshift. Before the shift, the uncued group was significantly faster (U=377, p<0.001). After the shift, the uncued group ran slower relative to the cued group (Postshift day 2, U=117.5, p<0.05 and day 6, U=70, p<0.01). For the USV data, we found that having a cue led to a significant elevation in USV calls in the unshifted group (Postshift day 2, U=86.5, p<0.05 and day 3, U=95, p<0.05). For the shifted group, we found the opposite effect with the uncued animals emitting higher levels of USVs in both the preshift (U= 275.5, p<0.05) and postshift periods (day 3, U=270, p<0.05; day 5, U= 307, p<0.01 and day 6, U=308.5, p<0.01).
Discussion
Overall, the results replicate previous behavioral work on instrumental successive negative contrast using the standard runway paradigm with a dramatic decrease in running speed following the outcome downshift. When exploring a relationship with USVs, the results of the study do not support a clear link and instead, demonstrate a possible, functional dissociation between these measures. The dissociation was found in both tasks and was different depending upon the task. The running speed slowed to levels below the unshifted group in the shifted group consistently in the uncued, traditional version of the task and not as consistently in the modified version with visual cues. The USVs dipped in both tasks but only significantly lower in the postshift period in the visual cue version. There could be several factors involved in producing these results including external reward value properties and internal arousal states that could vary between the two task versions.
An important factor that primarily controls relative reward processes studied in the majority of incentive contrast effects is the quality of reward outcome including hedonic properties (Flaherty et al., 1994; Cromwell et al., 2005; Corbit and Balleine, 2003). The behavioral effects of contrast have been observed using food pellets in diverse studies of the past (Flaherty, 1996) but USV emission is mainly found at high rates in studies with outcomes such as drugs of abuse (Burgdorf et al., 2001; Mu et al., 2009) or electrical stimulation of the reinforcing areas of the brain (Burgdorf et al., 2000). Consistent and strong contrast effects can be obtained using brain stimulation reward as well (Trowill et al., 1969). USVs are also linked most often to social interactions in rats and are seen reliably during social approach and investigation as well as in anticipation of social play or ‘tickling’ experience (Panksepp and Burgdorf, 2000; Willey et al., 2009, 2012). The reason outcome parameters might be important is that USV calling was too close to a ‘floor’ level especially for the low magnitude outcome (1 pellet). This level of reward was not sufficiently high enough to allow for a strong negative contrast effect to be obtained. Ceiling effects are a problem in obtaining positive contrast in behavior (Flaherty, 1996). Action strategies are constrained within a window of physical production limitations as well as guided by incentive value levels. Related to this issue is the idea that outcome differential could play a role in the production of contrast. Behavioral markers of contrast could be sensitive to one level of reward disparity while emotional states could be sensitive to another level of outcome difference. One avenue to explore this factor would be to study different outcome differentials yet the approach is difficult because of satiety effects related to high magnitude outcome levels (Dickinson et al., 1996). Other work has utilized aversive outcomes as alternates to appetitive outcomes to study relative effects (Lovibond, 1969; Grigson, 2008). The results have shown that animals can be significantly altered after the high stress exposure to aversive events in motivated action to goal outcomes (Millan, 2003). Instead of positive contrast for the appetitive outcome, researchers have found enhanced stress, impulsive behavior and conflict within decision-making paradigms making the use of aversive outcomes to study contrast difficult and unclear.
In addition to exploring the role of outcome value on different levels of contrast expression, the present work manipulated emotional state by the extensive training to implement the behavioral indicator of contrast. This training regimen is typical for the runway studies as well as other studies of contrast (Gonzalez et al., 1962; Flaherty et al., 1998). Extensive training could reduce the emotional state involvement during testing and dampen the possible contrast effects elicited by some measures and not others (Flaherty et al., 1977). Habits might still reflect behavioral indicators of contrast without embedding significant emotional properties (Kosaki and Dickinson, 2010). A paradigm that examines contrast effects without the need of extensive training might enable emotional states to influence contrast or be related to the behavior more directly. Another key component examined in this study was predictability. This could be playing a significant role in influencing the emotional states of the animals as they perform the task. When the visual cues were used to signal the impending reward each trial, the behavioral indicators of contrast and reward discrimination disappeared. These cues added to the extensive training may have resulted in a level of habit formation and stimulus-response habit that removes outcome valuation process in the appetitive phase of behavior (deWit and Dickinson, 2009). It is very interesting that the contrast effect was actually triggered by the cues when measured by USV emission in this same paradigm.
This finding might reflect the link between early USV emission and cue salience which basically relies upon the emotional valence of the outcome transferring to the ‘sign’ of the predictive cue (DiFeliceantonio and Berridge, 2012). The USVs emitted while animals were in the start box were triggered by the cue appearance and regulated more tightly by changes in the cue when the downshift occurred. The numbers of USVs were not significantly higher in the cued version for the preshift high magnitude group but relative drop compared to the nonshift group was sufficient to cause to USV negative contrast. In addition to this cue salience effect, the USV contrast could rely on reduced levels of stress in the more predictive cue task compared to the uncued version. Stress and arousal are part of negative contrast effects (Mitchell and Flaherty, 1998; Flaherty and Rowan, 1989; Wood et al., 2005) and reducing these factors might enable positive affect signals to be more highly regulated in relation to context. Our results suggest this type of effect is only apparent on an early emotional indicator (USVs) and not on the appetitive behavior itself. Finally, it is possible that the absence of behavioral contrast in the cued version arises because a certain degree of unpredictability and arousal is necessary for behavioral contrast (Reynolds, 1961). In situations that are highly predictable and less arousing, contrast effects would not be as effective in directing or changing behavior over time (Onishi and Xavier, 2011).
Future work could explore these issues using different outcome properties and systematically varying levels of predictability of the outcome in terms of number/kind of associated links available in the environment. Comparative work using animals to study reward processing has been valuable in the search for factors that mediate decision-making and choice behavior (Watanabe et al., 2001; Wikenheiser et al., 2013). Relative reward effects are pervasive in the animal kingdom (Bentosela et al., 2009; Wiegmann and Smith, 2009; Cromwell et al., 2005; Flaherty, 1996) and can be thought of as fundamental mechanisms that guide action in choice situations. Further analysis of the processes could lead to a better understanding for general mechanisms involved in reward valuation in diverse behavioral paradigms. Animal models of emotion/motivation interaction can open new avenues to allow a rethinking of the study of emotion in animals as it integrates or remains dissociated from motivated behavior as simple or complex goal-directed actions (Cromwell and Panksepp, 2011).
Figure 5.
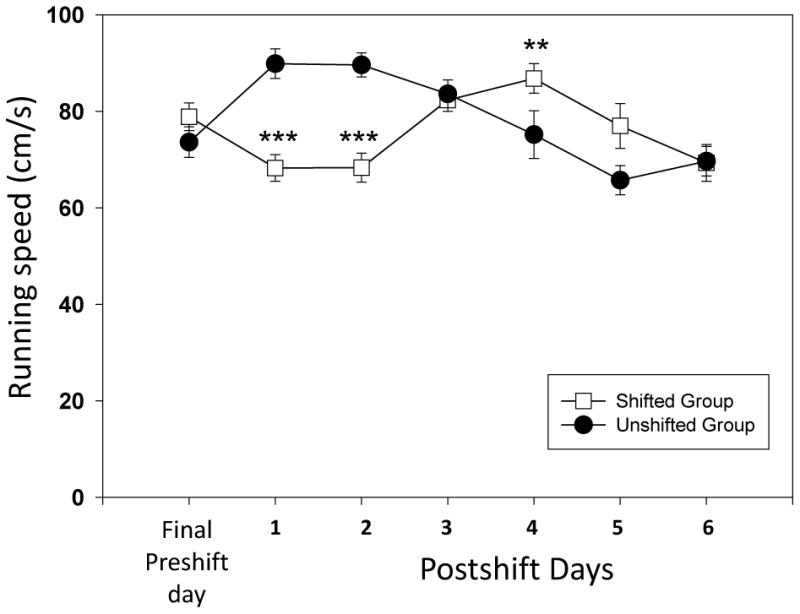
Running speed (cm/s) for the two groups of animals (shifted versus unshifted) during the cued version of the runway task. Cues were placed at the end of the runway as visual predictors for reward magnitude. The days represented are the final preshift day as well as six postshift test days. (Mann-Whitney U test for between group analysis; *= p<0.05; **= p<0.01 and *** = p<0.001; bars denote standard error of the mean).
Figure 6.
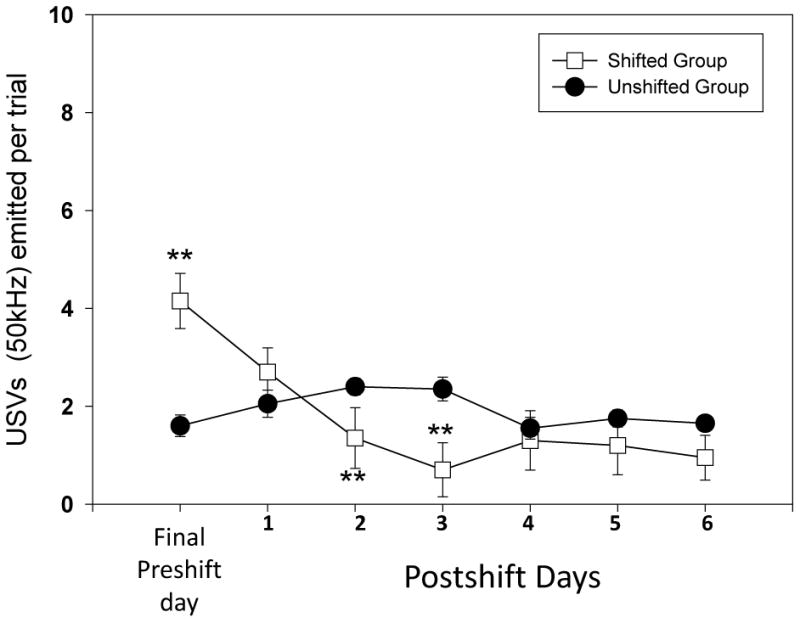
50 kHz ultrasonic vocalizations observed per trial in the two groups of animals shifted versus unshifted) during the cued runway task. The days represented are the final preshift day as well as six postshift test days (Mann-Whitney U test for between group analysis; *= p<0.05; **= p<0.01 and *** = p<0.001; bars denote standard error of the mean).
Highlights.
Running speeds but not ultrasound emission reflect incentive contrast
A non-cue and cue version of the negative anticipatory contrast paradigm was used
Visual cues reduce the incentive contrast effect
Contrast effects for ultrasounds were found in the cue but not the uncued version
The results suggest a dissociation between these measures of motivation and emotion
Acknowledgments
Preliminary results from this study were presented at the annual meeting of the Society for Neuroscience (Binkley et al., 2011). We would like to think Andy Wickiser for his help in building the alleyway apparatus. We would like to thank Drs. Jaak Panksepp and Jeffrey Burgdorf for their help in the experimental design and in the recording and analysis of the rodent ultrasounds.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahrens AM, Nobile CW, Page LE, Maier EY, Duvauchelle CL, Schallert T. Individual differences in the conditioned and unconditioned rat 50-kHz ultrasonic vocalizations elicited by repeated amphetamine exposure. Psychopharmacology (Berl) 2013 doi: 10.1007/s00213-013-3130-9. EPub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amsel A, Radek CC, Graham M, Letz R. Ultrasound emission in infant rats as an indicant of arousal during appetitive learning and extinction. Science. 1977;197(4305):786–8. doi: 10.1126/science.560717. [DOI] [PubMed] [Google Scholar]
- Amsel A. Arousal, Suppression, and Persistence - Frustration Theory, Attention, and Its Disorders. Cognition & Emotion. 1990;4(3):239–268. [Google Scholar]
- Bentosela M, Jakovcevic A, Elgier AM, Mustaca AE, Papini MR. Incentive contrast in domestic dogs (Canis familiaris) J Comp Psychol. 2009;123(2):125–130. doi: 10.1037/a0013340. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Cromwell HC. Motivational-sensorimotor interaction controls aphagia and exaggerated treading after striatopallidal lesions. Behav Neurosci. 1990;104(5):778–795. doi: 10.1037//0735-7044.104.5.778. [DOI] [PubMed] [Google Scholar]
- Binkley K, Webber ES, Cromwell HC. Ultrasonic vocalizations during incentive contrast. Society for Neuroscience Abstracts. 2011;42:s581. [Google Scholar]
- Blanchard RJ, Blanchard DC, Agullana R, Weiss SM. Twenty-two kHz alarm cries to presentation of a predator, by laboratory rats living in visible burrow systems. Physiol Behav. 1991;50(5):967–972. doi: 10.1016/0031-9384(91)90423-l. [DOI] [PubMed] [Google Scholar]
- Brudzynski SM. Pharmacological and behavioral characteristics of 22 kHz alarm calls in rats. Neurosci Biobehav Rev. 2001;25(7-8):611–617. doi: 10.1016/s0149-7634(01)00058-6. [DOI] [PubMed] [Google Scholar]
- Brudzynski SM, Pniak A. Social contacts and production of 50-kHz short ultrasonic calls in adult rats. J Comp Psychol. 2002;116(1):73–82. doi: 10.1037/0735-7036.116.1.73. [DOI] [PubMed] [Google Scholar]
- Burgdorf J, Knutson B, Panksepp J. Anticipation of rewarding electrical brain stimulation evokes ultrasonic vocalization in rats. Behav Neurosci. 2000;114(2):320–327. [PubMed] [Google Scholar]
- Burgdorf J, Panksepp J, Brudzynski SM, Beinfeld MC, Cromwell HC, Kroes RA, et al. The effects of selective breeding for differential rates of 50-kHz ultrasonic vocalizations on emotional behavior in rats. Dev Psychobiol. 2009;51(1):34–46. doi: 10.1002/dev.20343. [DOI] [PubMed] [Google Scholar]
- Corbit LH, Balleine BW. Instrumental and Pavlovian incentive processes have dissociable effects on components of a heterogeneous instrumental chain. J Exp Psychol Anim Behav Process. 2003;29(2):99–106. doi: 10.1037/0097-7403.29.2.99. [DOI] [PubMed] [Google Scholar]
- Crespi LP. Quantitative Variation of Incentive and Performance in the White-Rat. Am J Psychology. 1942;55:467–517. [Google Scholar]
- Cromwell HC, Hassani OK, Schultz W. Relative reward processing in primate striatum. Exp Brain Res. 2005;162(4):520–525. doi: 10.1007/s00221-005-2223-z. [DOI] [PubMed] [Google Scholar]
- Cromwell HC, Panksepp J. Rethinking the cognitive revolution from a neural perspective: how overuse/misuse of the term ‘cognition’ and the neglect of affective controls in behavioral neuroscience could be delaying progress in understanding the BrainMind. Neurosci Biobehav Rev. 2011;35(9):2026–2035. doi: 10.1016/j.neubiorev.2011.02.008. [DOI] [PubMed] [Google Scholar]
- Daly HB. Is Instrumental Responding Necessary for Nonreward Following Reward to Be Frustrating. Journal of Experimental Psychology. 1969;80(1):186. doi: 10.1037/h0027149. -&. [DOI] [PubMed] [Google Scholar]
- de Wit S, Dickinson A. Associative theories of goal-directed behaviour: a case for animal-human translational models. Psychological Research-Psychologische Forschung. 2009;73(4):463–476. doi: 10.1007/s00426-009-0230-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson A, Campos J, Varga ZI, Balleine B. Bidirectional instrumental conditioning. Quarterly Journal of Experimental Psychology Section B-Comparative and Physiological Psychology. 1996;49(4):289–306. doi: 10.1080/713932637. [DOI] [PubMed] [Google Scholar]
- DiFeliceantonio AG, Berridge KC. Which cue to ‘want’? Opioid stimulation of central amygdala makes goal-trackers show stronger goal-tracking, just as sign-trackers show stronger sign-tracking. Behav Brain Res. 2012;230(2):399–408. doi: 10.1016/j.bbr.2012.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunham PJ. Contrasted conditions of reinforcement:A selective critique. Psychol Bull. 1968;69:295–315. doi: 10.1037/h0025690. [DOI] [PubMed] [Google Scholar]
- Flaherty C. Incentive relativity. Cambridge University Press; 1996. [Google Scholar]
- Flaherty C, Checke S. Anticipatory Contrast in Rats. Bulletin of the Psychonomic Society. 1980;16(3):175–175. [Google Scholar]
- Flaherty CF, Checke S. Anticipation of Incentive Gain. Animal Learning & Behavior. 1982;10(2):177–182. [Google Scholar]
- Flaherty CF, Coppotelli C, Grigson PS, Mitchell C, Flaherty JE. Investigation of the devaluation interpretation of anticipatory negative contrast. J Exp Psychol Anim Behav Process. 1995;21(3):229–247. doi: 10.1037//0097-7403.21.3.229. [DOI] [PubMed] [Google Scholar]
- Flaherty CF, Coppotelli C, Hsu D, Otto T. Excitotoxic lesions of the hippocampus disrupt runway but not consummatory contrast. Behav Brain Res. 1998;93(1-2):1–9. doi: 10.1016/s0166-4328(97)00138-1. [DOI] [PubMed] [Google Scholar]
- Flaherty CF, Largen J. Within-subjects positive and negative contrast effects in rats. J Comp Physiol Psychol. 1975;88(2):653–664. doi: 10.1037/h0076416. [DOI] [PubMed] [Google Scholar]
- Flaherty CF, Lombardi BR, Kapust J, d'Amato MR. Incentive contrast undiminished by extended testing, imipramine, or chlordiazepoxide. Pharmacol Biochem Behav. 1977;7(4):315–322. doi: 10.1016/0091-3057(77)90227-1. [DOI] [PubMed] [Google Scholar]
- Flaherty CF, Rowan GA. Successive, simultaneous, and anticipatory contrast in the consumption of saccharin solutions. J Exp Psychol Anim Behav Process. 1986;12(4):381–393. [PubMed] [Google Scholar]
- Flaherty CF, Rowan GA. Successive, simultaneous, and anticipatory contrast in the consumption of saccharin solutions. J Exp Psychol Anim Behav Process. 1986;12(4):381–393. [PubMed] [Google Scholar]
- Flaherty CF, Rowan GA. Rats (Rattus norvegicus) selectively bred to differ in avoidance behavior also differ in response to novelty stress, in glycemic conditioning, and in reward contrast. Behav Neural Biol. 1989;51(2):145–164. doi: 10.1016/s0163-1047(89)90782-6. [DOI] [PubMed] [Google Scholar]
- Flaherty CF, Turovsky J, Krauss KL. Relative hedonic value modulates anticipatory contrast. Physiol Behav. 1994;55(6):1047–1054. doi: 10.1016/0031-9384(94)90386-7. [DOI] [PubMed] [Google Scholar]
- Gold JM, Waltz JA, Prentice KJ, Morris SE, Heerey EA. Reward processing in schizophrenia: a deficit in the representation of value. Schizophr Bull. 2008;34(5):835–47. doi: 10.1093/schbul/sbn068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez RC, Gleitman H, Bitterman ME. Some observations on the depresion effect. J Comp Physiol Psych. 1962;55(578-581) doi: 10.1037/h0041030. [DOI] [PubMed] [Google Scholar]
- Harmon KM, Cromwell HC, Burgdorf J, Moskal JR, Brudzynski SM, Kroes RA, et al. Rats selectively bred for low levels of 50 kHz ultrasonic vocalizations exhibit alterations in early social motivation. Dev Psychobiol. 2008;50(4):322–331. doi: 10.1002/dev.20294. [DOI] [PubMed] [Google Scholar]
- Harmon KM, Greenwald ML, McFarland A, Beckwith T, Cromwell HC. The effects of prenatal stress on motivation in the rat pup. Stress. 2009;12(3):250–258. doi: 10.1080/10253890802367265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jürgens U. Vocalization as an emotional indicator. A neuroethological study in the squirrel monkey. Behaviour. 1979;69(1-2):88–117. doi: 10.1163/156853979x00412. [DOI] [PubMed] [Google Scholar]
- Knutson B, Burgdorf J, Panksepp J. Ultrasonic vocalizations as indices of affective states in rats. Psychol Bull. 2002;128(6):961–977. doi: 10.1037/0033-2909.128.6.961. [DOI] [PubMed] [Google Scholar]
- Kosaki Y, Dickinson A. Choice and Contingency in the Development of Behavioral Autonomy During Instrumental Conditioning. Journal of Experimental Psychology-Animal Behavior Processes. 2010;36(3):334–342. doi: 10.1037/a0016887. [DOI] [PubMed] [Google Scholar]
- Leszczuk MH, Flaherty CF. Lesions of nucleus accumbens reduce instrumental but not consummatory negative contrast in rats. Behav Brain Res. 2000;116(1):61–79. doi: 10.1016/s0166-4328(00)00265-5. [DOI] [PubMed] [Google Scholar]
- Lucas GA, Timberlake W. Negative Anticipatory Contrast and Preference Conditioning - Flavor Cues Support Preference Conditioning, and Environmental Cues Support Contrast. Journal of Experimental Psychology-Animal Behavior Processes. 1992;18(1):34–40. doi: 10.1037//0097-7403.18.1.34. [DOI] [PubMed] [Google Scholar]
- Mellgren RL. Positive Contrast in Rat as a Function of Number of Preshift Trials in Runway. Journal of Comparative and Physiological Psychology. 1971;77(2):329. -&. [Google Scholar]
- Millan MJ. The neurobiology and control of anxious states. Prog Neurobiol. 2003;70(2):83–244. doi: 10.1016/s0301-0082(03)00087-x. [DOI] [PubMed] [Google Scholar]
- Mitchell C, Flaherty C. Temporal dynamics of corticosterone elevation in successive negative contrast. Physiol Behav. 1998;64(3):287–292. doi: 10.1016/s0031-9384(98)00072-9. [DOI] [PubMed] [Google Scholar]
- Mitchell CP, Flaherty CF. Differential effects of removing the glucose or saccharin components of a glucose-saccharin mixture in a successive negative contrast paradigm. Physiol Behav. 2005;84(4):579–583. doi: 10.1016/j.physbeh.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Mitchell EN, Marston HM, Nutt DJ, Robinson ES. Evaluation of an operant successive negative contrast task as a method to study affective state in rodents. Behav Brain Res. 2012;234(2):155–160. doi: 10.1016/j.bbr.2012.06.016. [DOI] [PubMed] [Google Scholar]
- Mogenson GJ, Yang CR. The contribution of basal forebrain to limbic-motor integration and the mediation of motivation to action. Adv Exp Med Biol. 1991;295:267–290. doi: 10.1007/978-1-4757-0145-6_14. [DOI] [PubMed] [Google Scholar]
- Mu P, Fuchs T, Saal DB, Sorg BA, Dong Y, Panksepp J. Repeated cocaine exposure induces sensitization of ultrasonic vocalization in rats. Neurosci Lett. 2009;453(1):31–35. doi: 10.1016/j.neulet.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onishi BK, Xavier GF. Negative anticipatory contrast: does it involve anticipation of an impending reward? Behav Processes. 2011;86(2):263–271. doi: 10.1016/j.beproc.2010.12.008. [DOI] [PubMed] [Google Scholar]
- Panksepp J. Affective Neuroscience. Cambridge: Cambridge University Press; 1998. [Google Scholar]
- Panksepp J. Psychology. Beyond a joke: from animal laughter to human joy? Science. 2005;308(5718):62–63. doi: 10.1126/science.1112066. [DOI] [PubMed] [Google Scholar]
- Panksepp J, Burgdorf J. 50-kHz chirping (laughter?) in response to conditioned and unconditioned tickle-induced reward in rats: effects of social housing and genetic variables. Behav Brain Res. 2000;115(1):25–38. doi: 10.1016/s0166-4328(00)00238-2. [DOI] [PubMed] [Google Scholar]
- Panksepp J, Burgdorf J. “Laughing” rats and the evolutionary antecedents of human joy? Physiol Behav. 2003;79(3):533–547. doi: 10.1016/s0031-9384(03)00159-8. [DOI] [PubMed] [Google Scholar]
- Portfors CV. Types and functions of ultrasonic vocalizations in laboratory rats and mice. J Am Assoc Lab Anim Sci. 2007;46(1):28–34. [PubMed] [Google Scholar]
- Reynolds GS. Behavioral Contrast. J Experimental Analysis of Behavior. 1961;4:57–71. doi: 10.1901/jeab.1961.4-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sales G, Pye D. Ultrasonic communication by animals. New York: John Wiley and Sons; 1974. [Google Scholar]
- Scull J, Davies K, Amsel A. Behavioral Contrast and Frustration Effect in Multiple and Mixed Fixed-Interval Schedules in Rat. Journal of Comparative and Physiological Psychology. 1970;71(3):478. doi: 10.1037/h0029160. -&. [DOI] [PubMed] [Google Scholar]
- Seal MF, Cuenya L, Suarez AB, Mustaca AE. Consummatory suppression due to incentive downshift is not a consequence of enhanced search behavior. Behav Processes. 2013 doi: 10.1016/j.beproc.2013.05.004. [DOI] [PubMed] [Google Scholar]
- Spence KW. Behavior theory and conditioning. New Haven: Yale University Press; 1956. [Google Scholar]
- Trowill JA, Panksepp J, Gandelman R. An incentive model of rewarding brain stimulation. Psychol Rev. 1969;76(3):264–281. doi: 10.1037/h0027295. [DOI] [PubMed] [Google Scholar]
- Tunstall B, Beckett S, Mason R. Ultrasonic vocalisations explain unexpected effects on pre-pulse inhibition responses in rats chronically pre-treated with phencyclidine. Behav Brain Res. 2009;202(2):184–191. doi: 10.1016/j.bbr.2009.03.035. [DOI] [PubMed] [Google Scholar]
- Ursu S, Clark KA, Stenger VA, Carter CS. Distinguishing expected negative outcomes from preparatory control in the human orbitofrontal cortex. Brain Res. 2008;1227:110–119. doi: 10.1016/j.brainres.2008.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Cromwell HC, Tremblay L, Hollerman JR, Hikosaka K, Schultz W. Behavioral reactions reflecting differential reward expectations in monkeys. Exp Brain Res. 2001;140(4):511–518. doi: 10.1007/s002210100856. [DOI] [PubMed] [Google Scholar]
- Webber ES, Harmon KM, Beckwith TJ, Pena S, Burgdorf J, Panksepp J, et al. Selective breeding for 50 kHz ultrasonic vocalization emission produces alterations in the ontogeny and regulation of rough-and-tumble play. Behav Brain Res. 2012;229(1):138–144. doi: 10.1016/j.bbr.2012.01.012. [DOI] [PubMed] [Google Scholar]
- Wiegmann DD, Smith BH. Incentive relativity and the specificity of reward expectations in honey bees. International Journal of Comparative Psychology. 2009;22:141–152. 22, 141-152. [Google Scholar]
- Wikenheiser AM, Stephens DW, Redish AD. Subjective costs drive overly patient foraging strategies in rats on an intertemporal foraging task. Proc Natl Acad Sci U S A. 2013;110(20):8308–8313. doi: 10.1073/pnas.1220738110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willey AR, Spear LP. Development of anticipatory 50 kHz USV production to a social stimuli in adolescent and adult male Sprague-Dawley rats. Behav Brain Res. 2012;226(2):613–618. doi: 10.1016/j.bbr.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willey AR, Varlinskaya EI, Spear LP. Social interactions and 50 kHz ultrasonic vocalizations in adolescent and adult rats. Behav Brain Res. 2009;202(1):122–129. doi: 10.1016/j.bbr.2009.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams BA. The Effects of Stimulus Similarity on Different Types of Behavioral-Contrast. Animal Learning & Behavior. 1988;16(2):206–216. [Google Scholar]
- Williams BA. Behavioral-Contrast and Reinforcement Value. Animal Learning & Behavior. 1991;19(4):337–344. [Google Scholar]
- Williams BA. Behavioral contrast redux. Animal Learning & Behavior. 2002;30(1):1–20. doi: 10.3758/bf03192905. [DOI] [PubMed] [Google Scholar]
- Wood M, Daniel AM, Papini MR. Selective effects of the delta-opioid receptor agonist DPDPE on consummatory successive negative contrast. Behav Neurosci. 2005;119(2):446–454. doi: 10.1037/0735-7044.119.2.446. [DOI] [PubMed] [Google Scholar]


