Abstract
Mucopolysaccharidosis (MPS) Type II is caused by mutations in the gene encoding the lysosomal enzyme, iduronate 2-sulfatase (IDS). The majority of MPSII cases affect the brain. However, enzyme replacement therapy with recombinant IDS does not treat the brain, because IDS is a large molecule drug that does not cross the blood-brain barrier (BBB). To enable BBB penetration, IDS has been re-engineered as an IgG-IDS fusion protein, where the IgG domain is a monoclonal antibody (MAb) against the human insulin receptor (HIR). The HIRMAb crosses the BBB via receptor-mediated transport on the endogenous BBB insulin receptor, and the HIRMAb domain of the fusion protein acts as a molecular Trojan horse to ferry the fused IDS into brain from blood. The present study reports on the first safety pharmacology and pharmacokinetics study of the HIRMAb-IDS fusion protein. Juvenile male Rhesus monkeys were infused intravenously (IV) weekly for 26 weeks with 0, 3, 10, or 30 mg/kg of the HIRMAb-IDS fusion protein. The plasma clearance of the fusion protein followed a linear pharmacokinetics profile, which was equivalent either with measurements of the plasma concentration of immunoreactive HIRMAb-IDS fusion protein, or with assays of plasma IDS enzyme activity. Anti-drug antibody (ADA) titers were monitored monthly, and the ADA response was primarily directed against the variable region of the HIRMAb domain of the fusion protein. No infusion related reactions or clinical signs of immune response were observed during the course of the study. A battery of safety pharmacology, clinical chemistry, and tissue histopathology showed no signs of adverse events, and demonstrate the safety profile of chronic treatment of primates with 3–30 mg/kg weekly IV infusion doses of the HIRMAb-IDS fusion protein.
Keywords: monoclonal antibody, blood-brain barrier, lysosomal enzyme, drug delivery
Introduction
Mucopolysaccharidosis (MPS) Type II, or MPSII, also called Hunter syndrome, is a genetic lysosomal storage disease caused by mutations in the gene encoding iduronate 2-sulfatase (IDS) (Wilson et al, 1990). MPSII is currently treated with enzyme replacement therapy (ERT) and recombinant IDS by weekly intravenous (IV) infusion (Muenzer et al, 2006). However, about 70% of children with MPSII have involvement of the central nervous system (CNS) (Al Sawaf et al, 2008), and ERT does not treat the CNS (Wraith et al, 2008), because IDS is a large molecule drug that does not cross the blood-brain barrier (BBB) (Boado et al, 2013a). So as to enable BBB penetration, human IDS was re-engineered as an IgG-IDS fusion protein, wherein the IgG domain was a genetically engineered monoclonal antibody (MAb) against the human insulin receptor (HIR) (Lu et al, 2010). The HIRMAb domain of the fusion protein acts as a molecular Trojan horse to ferry the fused IDS across the BBB, via receptor-mediated transport on the endogenous BBB insulin receptor, and across the target cell plasma membrane, via receptor-mediated endocytosis (Lu et al, 2011). The human BBB expresses an endogenous insulin receptor (Pardridge et al, 1985), which mediates the brain uptake of circulating insulin (Duffy and Pardridge, 1987). The HIRMAb does not react with the insulin receptor in the mouse (Zhou et al, 2012), but does cross react with the insulin receptor of Old World primates, such as the Rhesus monkey (Pardridge et al, 1995).
The HIRMAb-IDS fusion protein is rapidly transported across the BBB in rhesus monkeys, whereas IDS alone does not penetrate the brain (Boado et al, 2013a). The brain uptake of the HIRMAb-IDS fusion protein in the Rhesus monkey is 1% of injected dose (ID), which is a level of brain uptake comparable to the brain uptake of small molecules (Boado et al, 2013a). Chronic weekly IV infusion of the HIRMAb-IDS fusion protein is a potential new treatment of the CNS in MPSII. However, the safety profile, plasma pharmacokinetics, and immune response following chronic treatment with this fusion protein have not been previously investigated. Therefore, the present study was designed to examine the effects of chronic weekly IV infusions of 3, 10, and 30 mg/kg doses of the HIRMAb-IDS fusion protein in juvenile male Rhesus monkeys over a 26 week period. In addition to the safety pharmacology, the pharmacokinetics (PK) of plasma clearance of the HIRMAb-IDS fusion protein was assessed with 2 independent methods, a fluorometric assay of plasma IDS enzyme activity, and a sandwich ELISA that detects both the IgG and the IDS domains of the fusion protein. The anti-drug antibody (ADA) response over 6 months of treatment was measured with a sandwich ELISA. At the end of the study, animals were euthanized for a histopathologic examination of the brain and multiple peripheral tissues.
Materials and Methods
Production and analysis of HIRMAb-IDS fusion protein
A stably transfected Chinese hamster ovary cell line producing the HIRMAb-IDS fusion protein was generated as described previously (Lu et al, 2011). The CHO cells were cultivated in a 50L bioreactor under perfusion-mode conditions in serum free medium. The HIRMAb-IDS fusion protein, also designated AGT-182, was purified by protein A affinity chromatography, cation exchange chromatography, anion exchange chromatography, and nano-filtration, and multiple bio-analytical tests showed the drug product passed all specifications with respect to purity, identity, potency, quality, and safety. Stability studies showed the HIRMAb-IDS fusion protein was stable as a sterile liquid stored at 4°C for up to 2 years. Over 60 grams of HIRMAb-IDS fusion protein was manufactured for this study. The HIRMAb-IDS fusion protein is a hetero-tetrameric molecule comprised of 2 heavy chains and 2 light chains formed by fusion of the mature human IDS to the heavy chain of the genetically engineered chimeric HIRMAb (Lu et al, 2010, 2011).
Study design
Male Rhesus monkeys (Macaca mulatta) were used for all studies, and were housed at MPI Research, Inc. (Mattawan, MI), as described previously (Boado et al, 2013b). All aspects of the primate study performed at MPI Research was conducted in strict compliance with the United States Food and Drug Administration Good Laboratory Practice (GLP) Regulations, 21 CFR Part 58. All procedures were in compliance with the Animal Welfare Act Regulations, and were approved by the Institutional Animal Care and Use Committee.
Juvenile Rhesus monkeys, with starting body weight of 3.5–4.8 kg (mean 4.4 kg) were divided into 4 treatment groups: (a) 9 monkeys were treated with vehicle [0.01 M sodium acetate/0.14 M NaCl/0.001% Tween-80/pH=5.5]; (b) 6 monkeys were treated with low dose drug (3 mg/kg); (c) 6 monkeys were treated with mid dose drug (10 mg/kg); (d) 9 monkeys were treated with high dose drug (30 mg/kg). Drug was infused IV over 60 minutes in 50 mL of normal saline with 5% dextrose via a syringe pump in line with a 0.22 micron filter (1C8363, Baxter, Deerfield, IL), and was administered weekly for 26 consecutive weeks. The IV infusion caused no local inflammation or irritation. To minimize immune reactions, all animals were pretreated with 2 mg/kg diphenhydramine intra-muscular starting at week 4 of the study. Animals were euthanized 24 hours after the last dose at week 26, except for 3 monkeys in the vehicle group and 3 monkeys in the high dose group, which constituted a recovery group, and were euthanized at 30 weeks, or 4 weeks following the last drug dosing. Clinical findings, food consumption, and body weight were recorded weekly. Electrocardiogram (ECG) and ophthalmoscopic exams were performed at the start and end of the study. Clinical chemistry was measured on plasma obtained at the start of the study, at 13 weeks, and at termination, and included hematologic, coagulation, metabolic, and urinalysis tests. Animals were observed for cardiovascular and pulmonary function with surgically implanted telemetry transmitter devices for recording body temperature, blood pressure, heart rate, and ECG for 20 hours post-dosing, as described previously (Pardridge et al, 2009). Neurobehavioral functional observational battery measurements, which included assessment of mental status, gross motor movements, cranial nerve function, and motor strength, was performed as described previously (Pardridge et al, 2009).
At the end of the study, animals were euthanized, and the brain (cerebrum, midbrain, cerebellum, medulla/pons), spinal cord, and 30 peripheral organs were blocked and fixed in formalin, and examined with hematoxylin and eosin by a certified pathologist at MPI Research, as described previously (Pardridge et al, 2009). Bone marrow, large intestine, small intestine, stomach, lymph node, spinal cord, and pancreas were all tested at 3 locations of the organ. The brain blocks (cerebrum, midbrain, cerebellum, medulla/pons) were prepared according to Garman (2003). The brain was examined for astrogliosis with glial fibrillary acidic protein (GFAP) immunohistochemistry, and for neurodegeneration with fluoro Jade B fluorescence microscopy.
Blood was removed for PK analysis at multiple time (T) points (−30, 0, 15, 30, 60, 90, 120, 210, 390, and 1320 min) following the start of the 60 min drug infusion, which corresponds to T = −60 min. PK studies were performed both at the start (week 1) and end (week 25) of the study. Plasma samples removed at the start of the study were analyzed with both the HIRMAb-IDS ELISA and the IDS enzyme activity assay. The HIRMAb-IDS ELISA could not be used on plasma samples removed at the end of the study, owing to interference by ADAs in primate plasma. Therefore, the PK analysis at the end of the study was performed with the plasma IDS enzyme activity measurements. Blood was removed for plasma collection at monthly intervals for immune response ELISA and measurement of ADA.
Immunoreactive HIRMAb-IDS fusion protein measurement by ELISA
The plasma concentration of the HIRMAb-IDS fusion protein was determined with a sandwich ELISA described previously (Lu et al, 2011). The capture reagent is a complex of the lectin-purified recombinant HIR extracellular domain (ECD) and a murine MAb against the HIR ECD. The detector reagent is a complex of a goat polyclonal antiserum against human recombinant IDS and a conjugate of alkaline phosphatase and a rabbit anti-goat IgG secondary antibody. Following the addition of the 4-nitrophenylphosphate substrate, absorbance at 405 nm was measured. A standard curve of 0–600 ng/mL HIRMAb-IDS fusion protein was included in each assay. Each standard curve was fit to a curvilinear function by non-linear regression analysis, as described previously (Boado et al, 2013b). Both domains of the fusion protein, the HIRMAb domain and the IDS domain, must be intact to enable detection of the fusion protein with the ELISA.
IDS enzyme activity
The IDS enzyme specific activity the HIRMAb-IDS fusion protein, or the IDS enzyme activity in primate plasma, was measured with a 2-step fluorometric enzyme assay with 4-methylumbelliferyl α-L-iduronide-2-sulphate (4-MUS) (Voznyi et al, 2001), as previously described (Boado et al, 2013a). In the first step of the assay, which is a 1 hour incubation at pH=4.5 at 37°C, the 4-MUS is converted by IDS to 4-methylumbelliferyl α-L-iduronide (MUBI). In the second step of the assay, the MUBI is converted to 4-methylumbelliferone (4-MU) by iduronidase, and the 4-MU product is detected fluorometrically (Lu et al, 2010). One unit is defined as the nmol per hour formed during the 1 hour IDS incubation step. The mean IDS specific activity of the lots of HIRMAb-IDS fusion protein used in this study was 257 ± 51 units/ug protein (mean ± SD, n=5).
Pharmacokinetics
A mono-exponential decay function was fit to the plasma concentration of immunoreactive HIRMAb-IDS fusion protein, or the plasma IDS enzyme activity, as described previously (Boado et al, 2013b). For plasma IDS enzyme activity analyses, the endogenous plasma IDS enzyme activity in the Rhesus monkey was subtracted from the IDS enzyme activity in plasma from fusion protein treated monkeys. The maximal fusion protein concentration in plasma following the 60 min infusion, the Cmax, was determined directly with either the ELISA or the IDS enzyme activity. The pharmacokinetic parameters were determined from the following relationships:
half-time of clearance (T1/2) = ln 2/k
volume of distribution (Vss) = dose/A
area under the concentration curve (AUC) = A/k
clearance (CL) = dose/AUC
where A=the y-intercept, and k=the slope of the mono-exponential function. The PK data fits and parameter estimates were made by non-linear regression, with a case weight of 1/mean, using the PAR subroutine of the BMDP2007 Statistical Software. Data from all monkeys in a given dose group were meaned for a given time point, and mono-exponential function was fit to the mean time points.
Biotinylation of the HIRMAb-IDS fusion protein
Biotinylated HIRMAb-IDS fusion protein was used in both the immune response sandwich ELISA and in the Tissue Cross-Reactivity (TCR) study. The HIRMAb-IDS fusion protein, or the human IgG1k isotype control, was biotinylated with sulfo biotin-LC-LC-N-hydroxysuccinimide, where LC=long chain (#21338, Pierce Chemical Co., Rockford, IL), as described previously (Boado et al, 2013b).
Immune response ELISA
Plasma was obtained monthly for measurement of ADA titers and 7 days after the most recent infusion of HIRMAb-IDS fusion protein, when plasma concentrations of the fusion protein were undetectable. The level of ADA against the HIRMAb-IDS fusion protein was measured with a bridging ELISA in 96-well plates as described previously (Boado et al, 2013b). The capture reagent is the HIRMAb-IDS fusion protein (250 ng/well) and the detection reagent is a complex of biotinylated HIRMAb-IDS fusion protein (25 ng/well) and a conjugate of streptavidin and horseradish peroxidase (500 ng/well). Owing to the bivalency of ADA binding, the HIRMAb-IDS fusion protein serves as both the capture and detector reagent. Each well contained 100 uL monkey plasma diluted in PBS. Following addition of the o-phenylenediamine substrate, absorbance was measured at 490 nm. Plasma from the treated monkeys was removed at 0, 4, 8, 12, 16, 20, and 24 weeks of the study, and the ADA titer was measured for each animal at each time point using dilutions of monkey plasma prepared in 0.01 M Na2HPO4/0.15 M NaCl/pH=7.4 (PBS). For the time course of ADA formation over 24 weeks, plasma from individual monkeys was diluted 1:50 in PBS. For the dilution curve of ADA formation, an aliquot of plasma from each monkey (10 uL) in each dosing group, all at week 24 of the study, was pooled and the pool was diluted 1:50, 1:100, 1:300, 1:1000, 1:3000, and 1:10,000 in PBS. These dilutions were tested against one of 3 different capture reagents: (a) the HIRMAb-IDS fusion protein, (b) the HIRMAb alone without the enzyme, and (c) the isotype IgG control, human IgG1κ. The ADA titer was expressed as optical density (OD) per uL undiluted plasma, and was determined as described previously (Boado et al, 2013b). The positive control in the ADA ELISA was a rabbit antiserum prepared against the HIRMAb-IDS fusion protein (Prosci, Inc., Poway, CA).
Tissue cross-reactivity
A Tissue-Cross Reactivity (TCR) study of the HIRMAb-IDS fusion protein was performed with autopsy, archival tissues from 3 healthy humans and 2 healthy Rhesus monkeys using the avidin-biotin immunoperoxidase method under GLP conditions by Charles River Laboratories Pathology Associates (Frederick, MD), as described previously (Boado et al, 2013b). A total of 35 human and 34 Rhesus monkey organs were examined, and the primary antibody was either the biotinylated HIRMAb-IDS fusion protein, or biotinylated human IgG1κ, used at concentrations of 2 and 15 ug/mL. Positive tissue staining was verified with an anti-α2-microglobulin antibody. Specificity for the HIR was examined with spot slides using recombinant HIR (R&D Systems, Minneapolis, MN) as a positive control, and human parathyroid hormone-related protein (PTHrP)-1-34 (Sigma Chemical Co., St. Louis, MO) as a negative control.
Results and Discussion
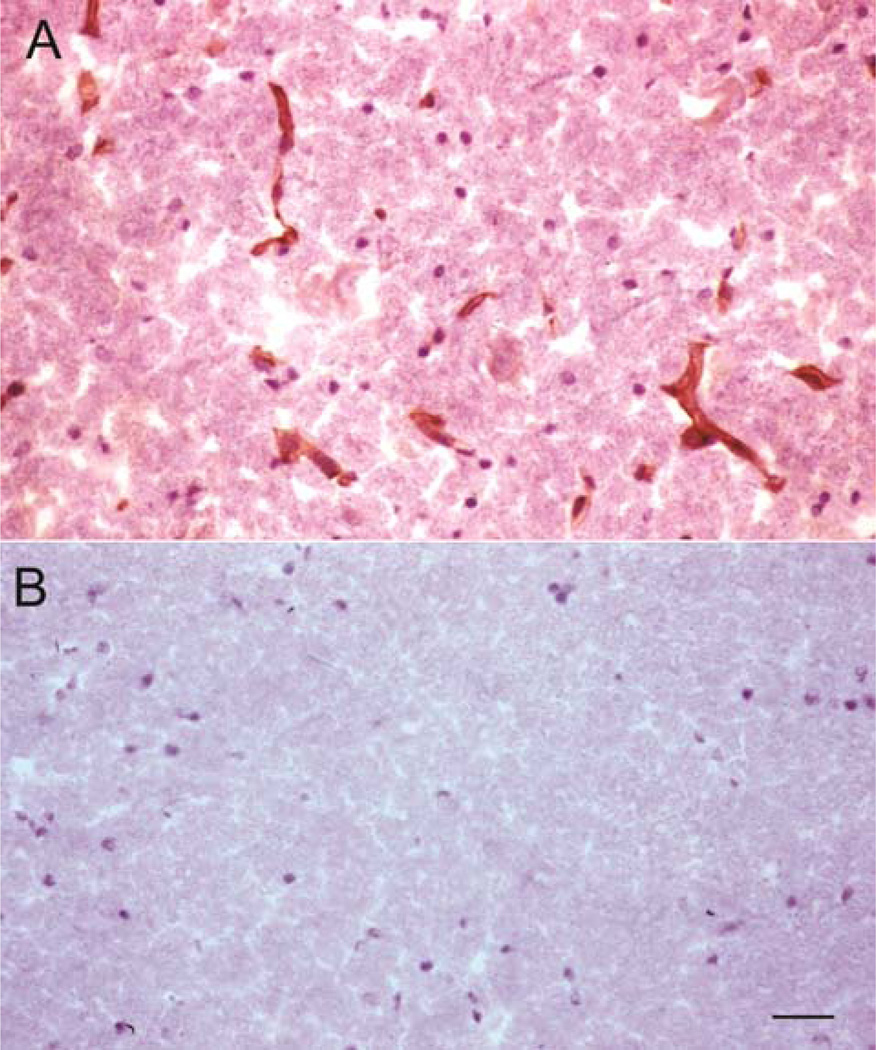
The TCR study showed comparable binding of the HIRMAb-IDS fusion protein to human and Rhesus monkey tissues at both the 2 and 15 ug/mL concentrations of the biotinylated fusion protein. Biotinylated human IgG1κ yielded no tissue reactivity at either concentration. Biotinylated HIRMAb-IDS gave strong staining on spot slides of HIR, but did not react with spot slides of human PTHrP. There was detectable immune staining in virtually all human and primate tissues. In brain, the HIRMAb-IDS fusion protein bound to both the neuropil and the endothelium in the cerebrum, cerebellum, and spinal cord, and a representative micrograph is shown in Figure 1A. No tissue staining in brain was observed with the biotinylated human IgG1κ (Figure 1B). The TCR study, as well as prior in vivo brain uptake studies (Lu et al, 2011; Boado et al, 2013a) support the use of the Rhesus monkey as a test species in which the HIRMAb-IDS fusion protein is biologically active.
Figure 1.
Immunocytochemistry of Rhesus monkey brain stained with 15 ug/mL of either the biotinylated HIRMAb-IDS fusion protein (A) or the biotinylated human IgG1k isotype control antibody (B). The slides are counter-stained with hematoxylin. Both sections were developed under identical conditions. Magnification bar in panel B is 22 microns.
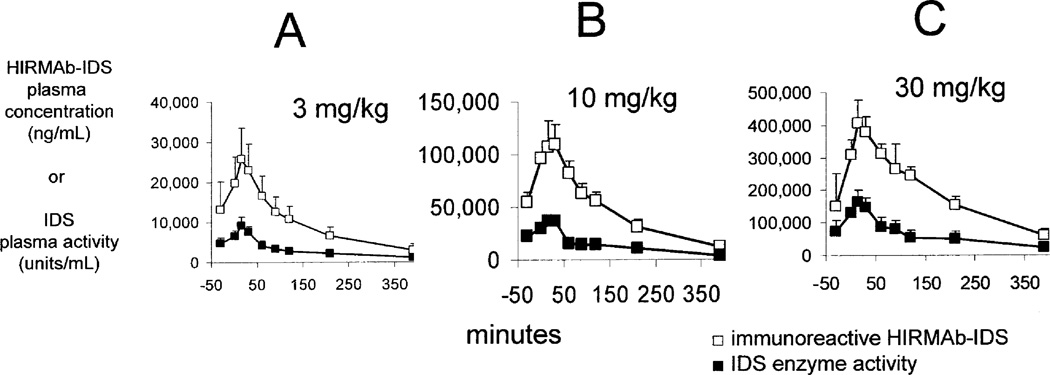
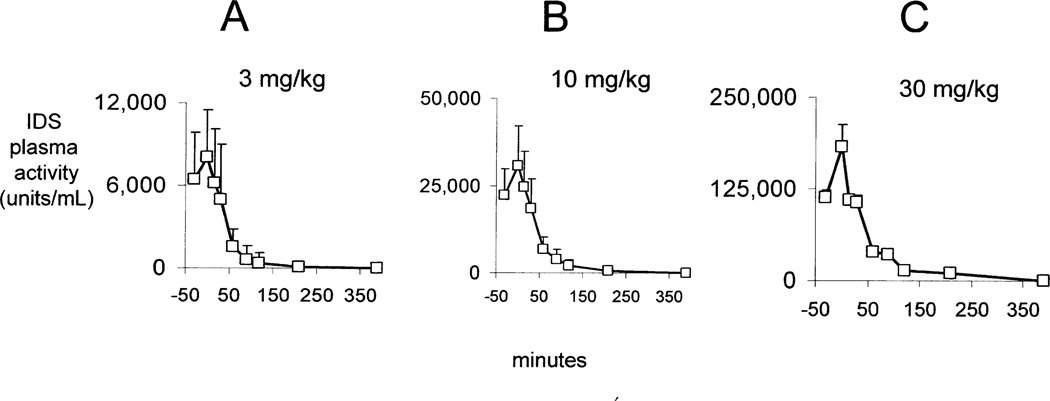
The plasma concentration profile of the immunoreactive HIRMAb-IDS fusion protein at the start of the study is shown in Figure 2 for the low dose (3 mg/kg), mid dose (10 mg/kg), and high dose (30 mg/kg) treatment groups. These plasma concentration profiles generated the PK parameters of the plasma clearance of the immunoreactive HIRMAb-IDS fusion protein (Table I). The Cmax and plasma AUC were directly proportional to the infusion dose (ID), whereas the Vss, and the systemic clearance (CL), were inversely related to the ID (Table I). The Cmax of the immunoreactive HIRMAb-IDS fusion protein ranges from 26 ± 8 ug/mL (mean ± SD), at 3 mg/kg, to 420 ± 55 ug/mL, at 30 mg/kg (Table I). The concentration of the HIRMAb-IDS fusion protein that reduces glycosoaminoglycans in Hunter fibroblasts is 0.3 ug/mL (Lu et al, 2010). Therefore, the Cmax in the high dose group is 1,400-fold above the therapeutic concentration of the HIRMAb-IDS fusion protein. The concentration of the HIRMAb-IDS fusion protein that causes 50% binding to the human insulin receptor (EC50) is 119 ± 16 ng/mL (Lu et al, 2010). Therefore, the Cmax in the high dose group is >3,000-fold higher than the EC50 of HIRMAb-IDS fusion protein binding to the human insulin receptor.
Figure 2.
Plasma profile of immunoreactive HIRMAb-IDS fusion protein (open squares) and IDS enzyme activity (closed squares) at week 1 for 3 mg/kg (A), 10 mg/kg (B), and 30 mg/kg (C) infusion doses of the HIRMAb-IDS fusion protein. Mean ± SD (N=6–9).
Table I.
Pharmacokinetic parameters of plasma clearance of immunoreactive HIRMAb-IDS fusion protein at week 1
| parameter | units | Infusion dose (mg/kg) | ||
|---|---|---|---|---|
| 3 | 10 | 30 | ||
| Cmax | ug/mL | 26.3 ± 8.1 | 120.1 ± 16.9 | 419.6 ± 55.5 |
| T1/2 | min | 120.3 ± 15.4 | 119.0 ± 11.0 | 159.9 ± 18.6 |
| MRT | min | 173.6 ± 22.3 | 171.7 ± 15.9 | 230.7 ± 26.8 |
| AUC | ug•min/ml | 4,048 ± 401 | 19,140 ± 1,387 | 89,010 ± 8,263 |
| Vss | mL/kg | 128.6 ± 8.8 | 89.6 ± 4.6 | 77.7 ± 4.4 |
| CL | mL/min/kg | 0.740 ± 0.073 | 0.521 ± 0.037 | 0.336 ± 0.031 |
| ID | mg | 13.16 ± 1.47 | 46.15 ± 5.25 | 131.57 ± 13.82 |
| BW | kg | 4.39 ± 0.49 | 4.62 ± 0.53 | 4.39 ± 0.46 |
Mean ± SD. Parameters determined by fitting mono-exponential function to plasma concentrations of immunoreactive HIRMAb-IDS fusion protein given in Figure 2.
The plasma IDS enzyme activity profile is plotted in Figure 2 following IV infusion of the HIRMAb-IDS fusion protein at the start (week 1) of the study. The Cmax of IDS enzyme activity in plasma ranges from 9,133 ± 2187 units/mL (mean ± SD), at 3 mg/kg, to 166,893 ± 32,629 units/mL, at 30 mg/kg (Table II). The IDS enzyme activity in human plasma is 80 units/mL (Voznyi et al, 2001). Therefore, the plasma Cmax of IDS enzyme activity in the high dose group is >2,000-fold higher than the endogenous IDS enzyme activity in human plasma.
Table II.
Pharmacokinetic parameters of plasma clearance of IDS enzyme activity at week 1
| parameter | units | Infusion dose (mg/kg) | ||
|---|---|---|---|---|
| 3 | 10 | 30 | ||
| Cmax | units/mL | 9,133 ± 2,187 | 38,350 ± 3,019 | 166,893 ± 32,629 |
| T1/2 | min | 106.0 ± 22.4 | 102.7 ± 24.0 | 121.9 ± 24.2 |
| MRT | min | 152.9 ± 32.3 | 148.1 ± 34.6 | 175.9 ± 35.0 |
| AUC | kunits•min/ml | 1,137 ± 179 | 4,789 ± 845 | 25,061 ± 3,833 |
| Vss | mL/kg | 83.0 ± 9.5 | 63.6 ± 8.3 | 43.3 ± 4.4 |
| CL | mL/min/kg | 0.543 ± 0.085 | 0.429 ± 0.075 | 0.246 ± 0.036 |
| ID | kunits | 2,712 ± 305 | 9,507 ± 1,083 | 27,103 ± 2,847 |
| BW | kg | 4.39 ± 0.49 | 4.62 ± 0.53 | 4.39 ± 0.46 |
Mean ± SD. Parameters determined by fitting mono-exponential function to plasma IDS enzyme activity given in Figure 2; kunits = kilounits.
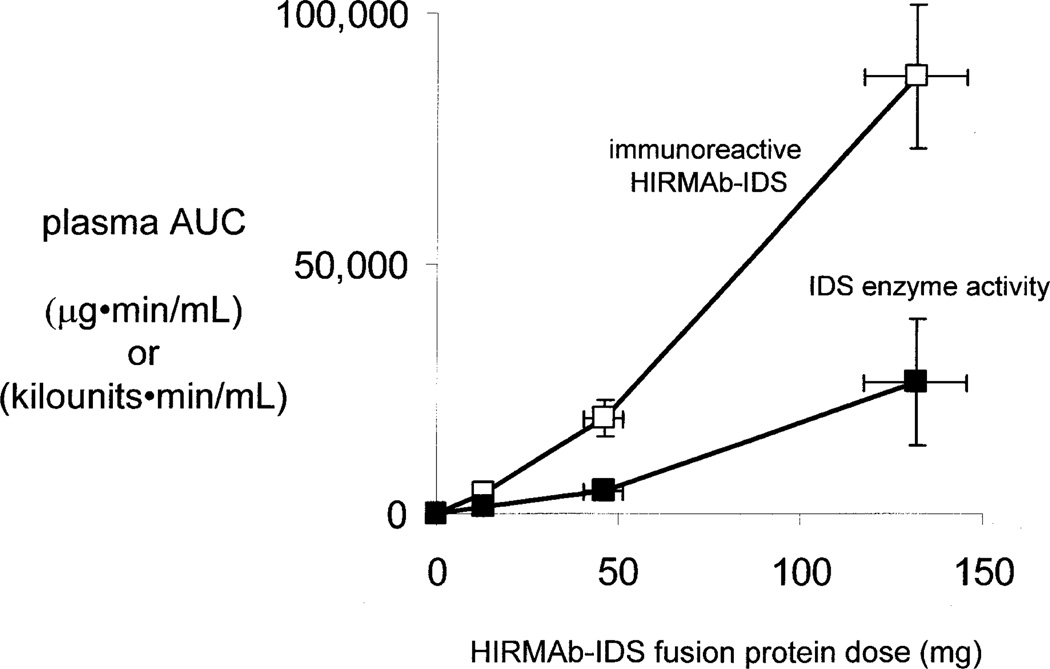
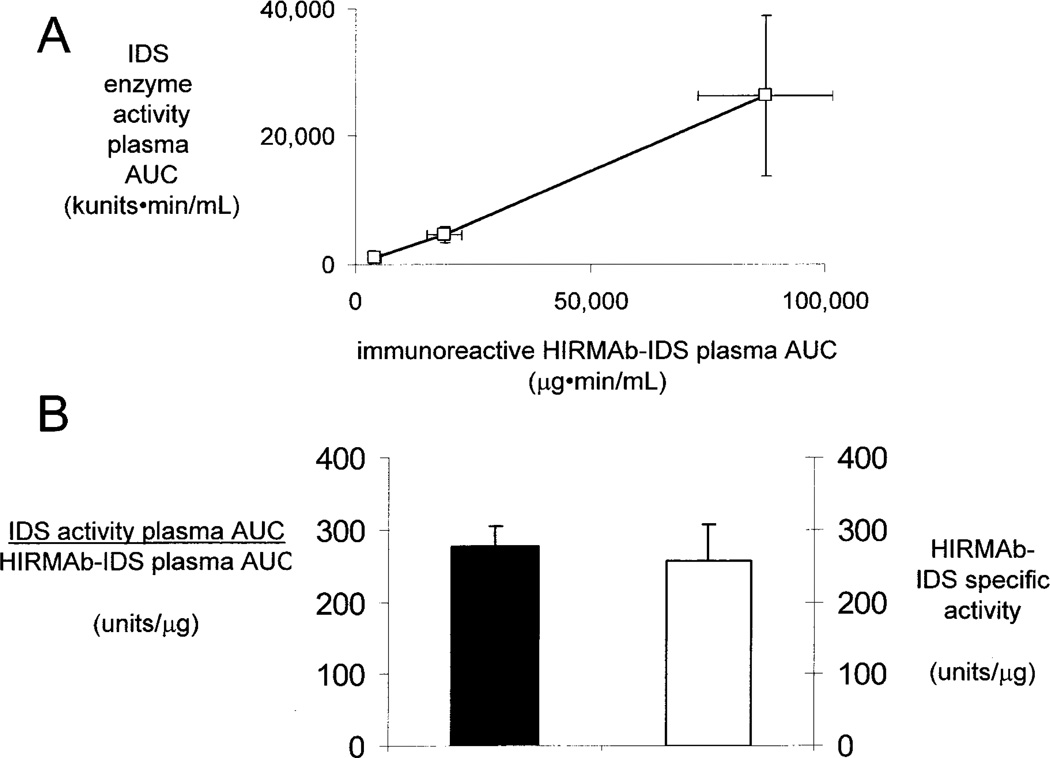
The plasma IDS enzyme activity profiles in Figure 2 generated the PK parameters of plasma clearance of IDS enzyme activity (Table II). Similar to the PK of plasma clearance of the immunoreactive HIRMAb-IDS fusion protein, the Cmax and AUC of plasma IDS enzyme activity were proportional to the infusion dose, whereas the Vss and clearance are inversely related to infusion dose (Table II). The parallel proportionality between the plasma AUC, of either the immunoreactive HIRMAb-IDS fusion protein, or the plasma IDS enzyme activity, vs. the infusion dose is shown by the plot in Figure 3, which indicates the PK of plasma clearance is generally linear. There is a 1:1 correlation between the PK of plasma clearance of the immunoreactive HIRMAb-IDS fusion protein, and the plasma IDS enzyme activity, as illustrated in Figure 4. The plasma AUC of the IDS enzyme activity is linearly related to the plasma AUC of the immunoreactive HIRMAb-IDS fusion protein at the 3 doses (Figure 4A). The slope of the plot in Figure 4A is 277 ± 28 units/ug (Figure 4B, left side), and this slope defines the average IDS specific activity of the HIRMAb-IDS fusion protein in the monkey plasma pool in vivo over the 23 hr sampling period following the 1 hr drug infusion. This plasma specific activity of the HIRMAb-IDS fusion protein is not significantly different from the mean IDS specific activity of the manufactured HIRMAb-IDS fusion protein prior to IV infusion (Figure 4B, right side). This equivalence between the in vivo specific activity and the in vitro specific activity suggests the HIRMAb-IDS fusion protein remains intact in the circulation, which was demonstrated previously by Western blotting for a fusion protein of the HIRMAb and iduronidase (Boado et al, 2009).
Figure 3.
Plasma area under the concentration curve (AUC) of immunoreactive HIRMAb-IDS fusion protein (open squares), in µg•min/mL, and AUC of plasma IDS enzyme activity (closed squares), in kilounits•min/mL, is plotted vs dose of HIRMAb-IDS fusion protein at week 1. Mean ± SD.
Figure 4.
(A) Comparison of plasma area under the concentration curve (AUC) for the IDS enzyme activity vs the plasma AUC of immunoreactive HIRMAb-IDS fusion protein at week 1. Mean ± SD. (B) Comparison of the in vivo specific activity (closed bar) of the HIRMAb-IDS fusion protein in plasma in monkeys over 23 hours after infusion vs the in vitro IDS specific activity (open bar) of the infused HIRMAb-IDS fusion protein. Mean ± SD. The in vivo specific activity was determined from the slope of the plot in panel A.
The plasma IDS enzyme activity profile was measured following IV infusion of the HIRMAb-IDS fusion protein at the end (week 25) of the study (Figure 5). These plasma IDS activity profiles generated the PK parameters of plasma clearance of IDS enzyme activity shown in Table III. The clearance of IDS enzyme activity, at the end of the study, was increased about 4-fold, compared to the start of the study, for all 3 infusion doses (Tables II and III). The Cmax of plasma IDS enzyme activity was equal at the start of the study and at the end of the study for all 3 infusion doses (Tables II and III). For the 3 mg/kg dose, at week 1 of the study, the plasma T1/2 of the immunoreactive HIRMAb-IDS fusion protein, 120 ± 15 min (Table I), is comparable to the plasma T1/2 of IDS enzyme activity, 106 ± 22 min (Table II). The plasma T1/2 of IDS enzyme activity decreases 4-fold to 24 ± 14 min, for the 3 mg/kg dose, at week 25 (Table III), which is consistent with the 4-fold increase in metabolic clearance of the HIRMAb-IDS fusion protein at the end of the study (Table III). In humans, the T1/2 of plasma clearance of recombinant IDS is 44 ± 19 min (Scarpa, 2013). Therefore, the plasma T1/2 of the HIRMAb-IDS fusion protein in primates is comparable to the plasma T1/2 of recombinant IDS in humans. In contrast to the relatively short plasma T1/2 of IDS enzyme activity following infusion of either the HIRMAb-IDS fusion protein, or IDS, the tissue T1/2 of IDS enzyme activity is much higher. The tissue T1/2 of intracellular IDS enzyme activity in MPSII fibroblasts is 3 days following a 2 hr exposure to the HIRMAb-IDS fusion protein (Lu et al, 2011).
Figure 5.
Plasma profile of IDS enzyme activity at week 25 for 3 mg/kg (A), 10 mg/kg (B), and 30 mg/kg (C) infusion doses of the HIRMAb-IDS fusion protein. Mean ± SD (N=6–9).
Table III.
Pharmacokinetic parameters of plasma clearance of IDS enzyme activity at week 25
| parameter | units | Infusion dose (mg/kg) | ||
|---|---|---|---|---|
| 3 | 10 | 30 | ||
| Cmax | units/mL | 8,046 ± 3,474 | 30,789 ± 11,370 | 182,077 ± 28,943 |
| T1/2 | min | 25.3 ± 2.4 | 29.8 ± 1.8 | 34.5 ± 4.2 |
| MRT | min | 36.5 ± 3.5 | 43.0 ± 2.7 | 49.8 ± 6.2 |
| AUC | kunits•min/ml | 320 ± 26 | 1,397 ± 74 | 8,374 ± 811 |
| Vss | mL/kg | 84.0 ± 7.2 | 75.8 ± 4.2 | 43.9 ± 4.4 |
| CL | mL/min/kg | 2.301 ± 0.191 | 1.760 ± 0.091 | 0.881 ± 0.085 |
| ID | kunits | 4,043 ± 643 | 14,018 ± 1,776 | 40,393 ± 4,829 |
| BW | kg | 5.48 ± 0.87 | 5.70 ± 0.72 | 5.47 ± 0.65 |
Mean ± SD. Parameters determined by fitting mono-exponential function to plasma IDS enzyme activity given in Figure 5; kunits = kilounits.
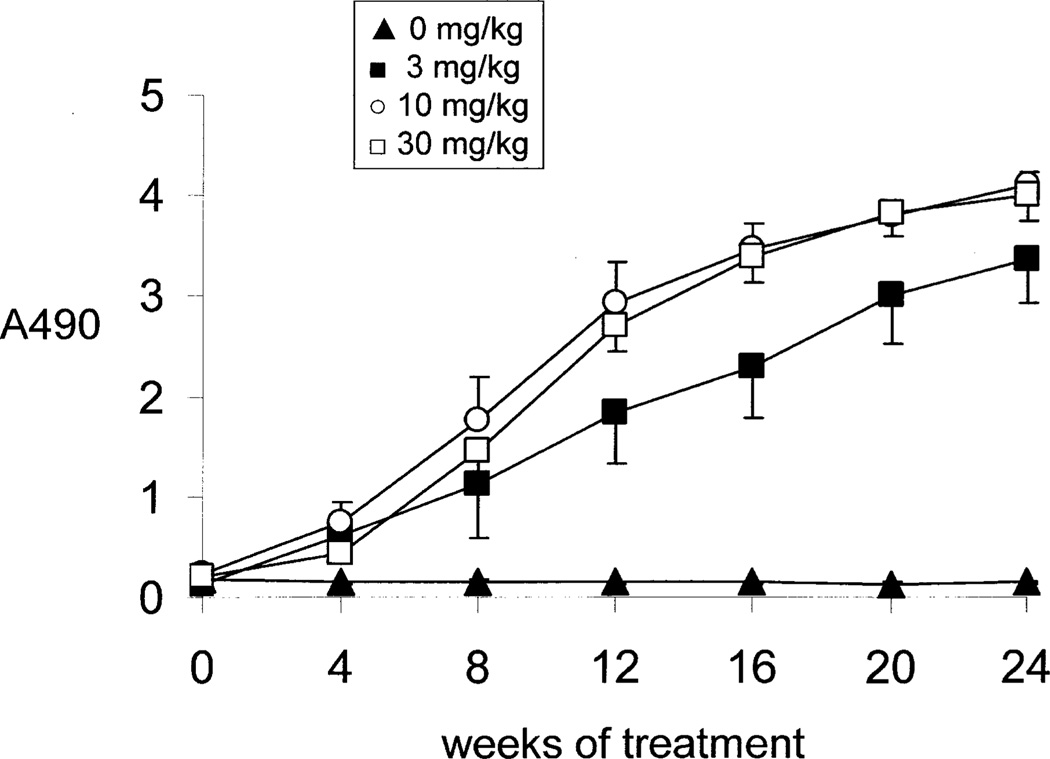
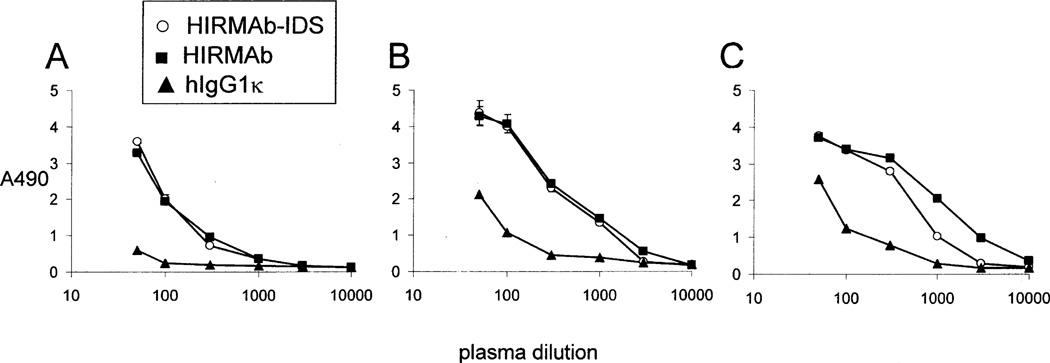
The immune response formed against the HIRMAb-IDS fusion protein over the course of 24 weeks of weekly treatment was assessed with a sandwich ELISA (Methods) and 1:50 dilutions of individual primate plasma. The ADA response increased after 4 weeks of treatment and reached a maximum by 16–20 weeks of treatment (Figure 6). No immune response was detected in the vehicle-treated monkeys (Figure 6). Plasma obtained at 24 weeks for all monkeys in each treatment group were pooled and diluted from 1:50 to 1:10,000, and the titer of ADA was measured with one of 3 capture reagents, including the HIRMAb-IDS fusion protein, the HIRMAb alone, and the human IgG1k isotype control (Figure 7). The ADA titer, or OD/uL plasma (Methods), can be computed from the data in Figure 7. The week 24 ADA titer is proportional to the dose of fusion protein, and is 1.8, 6.5, and 8.1, at 3, 10, and 30 mg/kg, respectively, at a plasma dilution of 1:300 when the capture agent is the HIRMAb-IDS fusion protein. Similarly, the titer is 2.5, 6.9, and 9.1 at 3, 10, and 30 mg/kg doses, respectively, when the capture agent is the HIRMAb alone without the fused IDS (Figure 7). The titer is 0.2, 1.0, and 2.1, at 3, 10, and 30 mg/kg doses, respectively, when the capture agent is the human IgG1κ isotype control antibody (Figure 7), which shares the same IgG1κ heavy chain and light chain constant regions as the HIRMAb-IDS fusion protein. Therefore, the majority of the ADAs against the HIRMAb-IDS fusion protein are captured by the HIRMAb alone, and less than 25% of the ADAs are captured by the IgG1k. These results indicate the principal immune epitope resides is the variable region of the HIRMAb domain of the HIRMAb-IDS fusion protein. In addition, the IDS domain of the fusion protein is immunogenic following chronic administration in monkeys (Felice et al, 2011), and in humans (Scarpa, 2013). The systemic clearance of recombinant IDS in humans is increase 4-fold, from 2.0 to 7.4 mL/min/kg when MPSII patients develop an ADA response against recombinant IDS (Scarpa, 2013), which parallels the 4-fold increase in plasma clearance of the HIRMAb-IDS fusion protein following chronic administration in Rhesus monkeys (Tables II and III).
Figure 6.
Time course of ADA formation against the HIRMAb-IDS fusion protein. Data are mean ± SE (n=6–9 monkeys per time point). All plasma samples were diluted 1:50 in PBS. The dose of each group of monkeys is given in the inset of the figure.
Figure 7.
Absorbance (A490) is plotted against dilution of pools of primate plasma from 1:50 to 1:10,000 in PBS for monkeys administered the HIRMAb-IDS fusion protein at weekly doses of 3 mg/kg (panel A), 10 mg/kg (panel B), and 30 mg/kg (panel C). The pool was produced from the week 24 blood samples. The capture reagent in the sandwich ELISA is the HIRMAb-IDS fusion protein (open circles), the HIRMAb alone (closed squares), or the human IgG1κ isotype control (closed triangles). Data are mean ± SD (N=3 replicates).
No acute or chronic toxicity was observed during this 6-month treatment study. There were no significant changes in physical exam, food intake, cardiac telemetry, pulmonary function, functional observational battery, ophthalmoscopic exam, body weights, or organ weights in any of the treatment groups relative to controls, including monkeys euthanized after a 1 month recovery period. There were no changes in multiple hematologic, coagulation, liver, renal, and metabolic function tests in any treatment group. There was no change in any terminal urinalysis in any of the treatment groups, and glucosuria was not observed. Histological examination was made on brain, spinal cord and 30 peripheral tissues in all monkeys at the end of the study with hematoxylin and eosin staining. All brain sections were also examined for neurodegeneration with fluoro Jade-B fluorescence microscopy and for astrogliosis with GFAP immunocytochemistry. No differences in the fluoro Jade B or GFAP staining were observed between the control and treated monkeys. There were no drug-related microscopic findings in terminal or recovery animals in brain, spinal cord or any of the 30 peripheral organs examined. Clinically significant immune reactions were observed in monkeys in a prior study wherein the HIRMAb-IDUA fusion protein was rapidly infused IV by gravity in less than 30 min (Boado et al, 2013b). However, in the present study, no immune reactions, or other infusion related reactions were observed following constant IV infusion over 60 min of the HIRMAb-IDS fusion protein. The ADA titers in the monkeys administered the HIRMAb-IDUA fusion protein (Boado et al, 2013b) were comparable to the ADA titers measured in the present study with the HIRMAb-IDS fusion protein (Figure 6).
In summary, no adverse events were observed in this study on the chronic weekly IV infusions of the HIRMAb-IDS fusion protein at a dose of 3–30 mg/kg over 26 weeks in male juvenile Rhesus monkeys. This study corroborates prior investigations on the safety of repeat administration of high doses of HIRMAb fusion proteins in Rhesus monkeys (Pardridge et al, 2009; Boado et al, 2009; Boado et al, 2013b). The only adverse event observed previously was hypoglycemia following the rapid IV infusion of the high dose, 30 mg/kg, of the HIRMAb fusion protein in normal saline without glucose (Boado et al, 2012). The hypoglycemia following infusion in saline is not observed at doses lower than 30 mg/kg, and is eliminated by the addition of dextrose to the saline infusion vehicle (Boado et al, 2012). The safety profile of BBB-penetrating IgG fusion proteins is also supported by several studies wherein mice were treated chronically with a rat/mouse chimeric MAb against the mouse transferrin receptor (TfR), designated the cTfRMAb (Pardridge and Boado, 2012). One study engineered a MAb with a low affinity for the mouse TfR, and this antibody, bearing a human constant region, shows acute toxicity in mice, owing to complement fixation (Couch et al, 2013). However, BBB-penetrating TfRMAb’s that do not activate complement have excellent safety profiles. Chronic IV injections of a fusion protein of the cTfRMAb and human glial derived neurotrophic factor (GDNF) over a 12-week period caused no change in serum chemistry, no change in histology of brain or peripheral organs, and no change in either the PK of plasma clearance, or the brain uptake, of the cTfRMAb-GDNF fusion protein (Zhou et al, 2011). The safety profiles of BBB-penetrating IgG fusion proteins in mice and monkeys support future clinical trials of these agents for the treatment of CNS disease with recombinant protein neurotherapeutics.
Acknowledgements
This research was supported by NIH grant R44-NS-067707. The authors are indebted to Winnie Tai, Phuong Tram, and Stephanie Lee for technical support, to Drs. James Callaway, Nancy Wehner and Sheri Barrack for valuable discussions, and to MPI Research, Inc. (Mattawan, MI), and Charles River Laboratories Pathology Associates (Frederick, MD) for collaboration with the primate study. Drs. Boado, Lu, and Hui are employees, and Dr. Pardridge is a consultant, of ArmaGen Technologies.
Abbreviations
- ADA
anti-drug antibody
- AUC
area under the plasma concentration curve
- BBB
blood-brain barrier
- BW
body weight
- CL
plasma clearance
- Cmax
maximal plasma concentration or activity
- CNS
central nervous system
- ECD
extracellular domain
- ECG
electrocardiogram
- GDNF
glial derived neurotrophic factor
- HIR
human insulin receptor
- ID
infusion dose
- IDUA
iduronidase
- IV
intravenous
- MAb
monoclonal antibody
- MPS
mucopolysaccharidosis
- MRT
mean residence time in plasma
- OD
optical density
- PK
pharmacokinetics
- PTHrP
parathyroid hormone related protein
- T1/2
plasma half-time
- TCR
tissue cross-reactivity
- TfR
transferrin receptor
- Vss
systemic volume of distribution
References
- Al Sawaf S, Mayatepek E, Hoffmann B. Neurological findings in Hunter disease: pathology and possible therapeutic effects reviewed. J Inherit Metab Dis. 2008;31:473–480. doi: 10.1007/s10545-008-0878-x. [DOI] [PubMed] [Google Scholar]
- Boado RJ, Hui EK-W, Lu JZ, Pardridge WM. AGT-181: Expression in CHO cells and pharmacokinetics, safety, and plasma iduronidase enzyme activity in Rhesus monkeys. J.. Biotechnol. 2009;144:135–141. doi: 10.1016/j.jbiotec.2009.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boado RJ, Hui EK-W, Lu JZ, Pardridge WM. Glycemic control and chronic dosing of Rhesus monkeys with a fusion protein of iduronidase and a monoclonal antibody against the human insulin receptor. Drug Metab Dispos. 2012;40:2021–2025. doi: 10.1124/dmd.112.046375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boado RJ, Hui EK-W, Lu JZ, Sumbria RK, Pardridge WM. Blood-brain barrier molecular Trojan horse enables brain imaging of radioiodinated recombinant protein in the Rhesus monkey. Bioconj Chem. 2013a;24:1741–1749. doi: 10.1021/bc400319d. [DOI] [PubMed] [Google Scholar]
- Boado RJ, Hui EK-W, Lu JZ, Pardridge WM. IgG-enzyme fusion potein: pharmacokinetics and anti-drug antibody response in Rhesus monkeys. Bioconj Chem. 2013b;24:97–104. doi: 10.1021/bc3005123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couch JA, Yu YJ, Zhang Y, Tarrant JM, Fuji RN, Meilandt WJ, Solanoy H, Tong RK, Hoyte K, Luk W, Lu Y, Gadkar K, Prabhu S, Ordonia BA, Nguyen Q, Lin Y, Lin Z, Balazs M, Scearce-Levie K, Ernst JA, Dennis MS, Watts RJ. Addressing safety liabilities of TfR bispecific antibodies that cross the blood-brain barrier. Science Transl Med. 2013;5:183ra157, 181–112. doi: 10.1126/scitranslmed.3005338. [DOI] [PubMed] [Google Scholar]
- Duffy KR, Pardridge WM. Blood-brain barrier transcytosis of insulin in developing rabbits. Brain Res. 1987;420:32–38. doi: 10.1016/0006-8993(87)90236-8. [DOI] [PubMed] [Google Scholar]
- Felice BR, Wright TL, Boyd RB, Butt MT, Pfeifer RW, Pan J, Ruiz JA, Heartlein MW, Calias P. Safety evaluation of chronic intrathecal administration of idursulfase-IT in cynomolgus monkeys. Toxicol Pathol. 2011;39:879–892. doi: 10.1177/0192623311409595. [DOI] [PubMed] [Google Scholar]
- Garman RH. Evaluation of large-sized brains for neurotoxic endpoints. Toxicol Pathol. 2003;31(Suppl):32–43. doi: 10.1080/01926230390174913. [DOI] [PubMed] [Google Scholar]
- Lu JZ, Hui EK-W, Boado RJ, Pardridge WM. Genetic engineering of a bifunctional IgG fusion protein with iduronate-2-sulfatase. Bioconj Chem. 2010;21:151–156. doi: 10.1021/bc900382q. [DOI] [PubMed] [Google Scholar]
- Lu JZ, Boado RJ, Hui EK-H, Zhou Q-H, Pardridge WM. Expression in CHO cells and pharmacokinetics and brain uptake in the Rhesus monkey of an IgG-iduronate-2-sulfatase fusion protein. Biotechnol Bioeng. 2011;108:1954–1964. doi: 10.1002/bit.23118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muenzer J, Wraith JE, Beck M, Giugliani R, Harmatz P, Eng CM, Vellodi, Martin AR, Ramaswami U, Gucsavas-Calikoglu M, Vijayaraghavan S, Wendt S, Puga AC, Ulbrich B, Shinawi M, Cleary M, Piper D, Conway AM, Kimura A. A phase II/III clinical study of enzyme replacement therapy with idursulfase in mucopolysaccharidosis II (Hunter syndrome) Genet Med. 2006;8:465–473. doi: 10.1097/01.gim.0000232477.37660.fb. [DOI] [PubMed] [Google Scholar]
- Pardridge WM, Eisenberg J, Yang J. Human blood-brain barrier insulin receptor. J Neurochem. 1985;44:1771–1778. doi: 10.1111/j.1471-4159.1985.tb07167.x. [DOI] [PubMed] [Google Scholar]
- Pardridge WM, Kang YS, Buciak JL, Yang J. Human insulin receptor monoclonal antibody undergoes high affinity binding to human brain capillaries in vitro and rapid transcytosis through the blood-brain barrier in vivo in the primate. Pharm Res. 1995;12:807–816. doi: 10.1023/a:1016244500596. [DOI] [PubMed] [Google Scholar]
- Pardridge WM, Boado RJ. Pharmacokinetics and safety in Rhesus monkeys of a monoclonal antibody-GDNF fusion protein for targeted blood-brain barrier delivery. Pharm Res. 2009;26:2227–2236. doi: 10.1007/s11095-009-9939-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardridge WM, Boado RJ. Reengineering biopharmaceuticals for targeted delivery across the blood-brain barrier. Methods Enzymol. 2012;503:269–292. doi: 10.1016/B978-0-12-396962-0.00011-2. [DOI] [PubMed] [Google Scholar]
- Scarpa M. Evaluation of idursulfase for the treatment of mucopolysaccharidosis II (Hunter syndrome) Expert Opin Orphan Drugs. 2013;1:89–98. [Google Scholar]
- Voznyi YV, Keulemans JL, van Diggelen OP. A fluorimetric enzyme assay for the diagnosis of MPS II (Hunter disease) J Inherit Metab Dis. 2001;24:675–680. doi: 10.1023/a:1012763026526. [DOI] [PubMed] [Google Scholar]
- Wilson PJ, Morris CP, Anson DS, Occhiodoro T, Bielicki J, Clements PR, Hopwood JJ. Hunter syndrome: isolation of an iduronate-2-sulfatase cDNA clone and analysis of patient DNA. Proc Natl Acad Sci USA. 1990;87:8531–8535. doi: 10.1073/pnas.87.21.8531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wraith JE, Scarpa M, Beck M, Bodamer OA, De Meirleir L, Guffon L, Meldgaard Lund A, Malm G, Van der Ploeg AT, Zeman J. Mucopolysaccharidosis type II (Hunter syndrome): a clinical review and recommendations for treatment in the era of enzyme replacement therapy. Eur J Pediatr. 2008;167:267–277. doi: 10.1007/s00431-007-0635-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q-H, Boado RJ, Lu JZ, Hui EK-W, Pardridge WM. Chronic dosing of mice with a transferrin receptor monoclonal antibody-GDNF fusion protein. Drug Metab Dispos. 2011;39:1149–1154. doi: 10.1124/dmd.111.038349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou QH, Boado RJ, Pardridge WM. Selective plasma pharmacokinetics and brain uptake in the mouse of enzyme fusion proteins derived from species-specific receptor-targeted antibodies. J Drug Targeting. 2012;20:715–719. doi: 10.3109/1061186X.2012.712132. [DOI] [PubMed] [Google Scholar]