Abstract
Schistosomes are parasitic worms that have a complex life cycle. The larval stage cercaria, infectious to mammals, is described as highly susceptible to the complement system, largely due to the glycocalyx that covers the cercarial membrane. In an attempt to have a more complete understanding of cercaria reaction to the complement system, three different approaches were used. Live cercariae exposed to normal human serum (NHS) as source of complement factors were assessed for: i) Membrane Attack Complex (MAC) deposition on the parasite surface; ii) cercaria survival rate by Hoechst staining of parasite DNA; and iii) transformation into schistosomula by detection of the glucose transporter protein 4 (SGTP4), a marker for new tegument formation. We found that 82–95% of cercariae directly exposed to NHS for 18 h were viable and retained their ability to shed the glycocalyx, suggesting minimal tegument damage. In contrast, inhibition of glycocalyx shedding using eserine caused significant MAC binding and parasite death. Culturing complement-exposed cercariae to measure long term survival showed that more parasites died over time, reaching a survival rate of 18–31% by day 6 in culture. The reason for this slow death is unknown, but the surviving parasites were able to form a new tegument as shown by detection of SGTP4 on the parasite surface. Furthermore, we found that complement activation significantly damaged the acetabular gland ducts and lysed secretory vesicles released by transforming cercariae. These findings should contribute for future in vivo studies of the effects of the complement system in skin migrating cercariae.
Keywords: Schistosoma mansoni, complement system, cercaria, glycocalyx, tegument, Membrane Attack Complex, Hoechst dye, glucose transporter
Introduction
Shistosomes are parasitic worms with a complex life cycle which requires passage through vertebrate and invertebrate hosts. The infectious larval stage, cercaria, is released in the water from infected snails. Cercaria has the unique ability to actively invade the mammalian skin, migrate through the skin layers (epidermis, basement membrane and dermis) and successfully enter the circulatory system despite the host cellular immune response elicited in the skin (Mountford and Trottein 2004). During invasion, cercariae undergo a series of changes called transformation which is required for their development into schistosomula (the juvenile parasitic stage in the vertebrate host). Once attached to the skin surface, cercariae lose their tails and release secretion vesicles from the acetabular glands which contain proteases and other molecules (Jones et al. 2008; Paveley et al. 2009). Cercarial proteases aid the parasite to migrate through the skin layers and reach small venules in the dermis (Hansell et al. 2008; Jenkins et al. 2005; Salter et al. 2000). A 28-kDa serine protease has been shown to accelerate the release of the glycocalyx and to cleave certain complement proteins (Fishelson et al. 1992; Marikovsky et al. 1988). Other molecules are implicated in the down regulation of the dermal inflammatory response and in the protection from oxidative stress (Brannstrom et al. 2009; Curwen et al. 2006; Hansell et al. 2008). In addition to acetabular gland secretion, cercariae lose their surface glycocalyx after invasion. The glycocalyx is a complex, carbohydrate-rich coat, which protects the underneath single unit membrane against osmotic shock in the aquatic environment before mammalian host entry (Nanduri et al. 1991; Samuelson and Caulfield 1985). Cercaria killing has been associated with the presence of the glycocalyx on the parasite surface, inducing complement activation and facilitating neutrophil adherence (Bicalho et al. 1993; Samuelson and Caulfield 1986). Thus, glycocalyx shedding has been considered a parasite strategy to evade the host immune system and ensure successful infection. Cercaria transformation to schistosomulum includes the replacement of the trilaminate cercarial membrane to a new heptalaminate membrane (Brink et al. 1977; Samuelson and Caulfield 1986). The new tegument consists of two lipid bilayer membranes that cover the entire schistosome body and it has been described as a barrier to protect the parasite against the host immune attack (Dessein et al. 1981; Wilson and Coulson 2009).
Regardless of numerous studies indicating the presence of a skin-derived cellular immune response to cercaria and its secretions, to our knowledge there is no work showing the effects of the host complement system in cercaria while migrating in the skin. Yet, relatively recent proteomic analysis of human skin following invasion by cercariae has shown that migrating cercariae encountered components of the complement system while in the dermis, before reaching the vascular system (Hansell et al. 2008). This finding raises the question of how migrating parasites react to the complement system. The time spent by cercariae to penetrate the skin and find a vascular portal is debatable. Apparently, it can vary considerably among vertebrate hosts, taking minutes to over 48 h (Curwen and Wilson 2003; McKerrow and Salter 2002). In contrast, it has been reported that most of the parasites that migrate through the lower epidermis and dermis are recently transformed schistosomula (McKerrow and Salter 2002) and thus might be resistant to complement attack. Another intriguing fact is that intravenous injection of cercariae in mice and hamsters results in adult worm recovery, despite immediate cercaria contact with blood and thus the host complement system (Holanda et al. 1974; Zelia and Kovalenko 1986).
In the present study, we proposed to evaluate the effects of the human complement system, independent of antibody on cercariae in an attempt to have a more complete understanding of how cercariae react to complement activation. To accomplish that, three different approaches were used. First, cercariae were exposed to normal human serum (NHS) as a source of complement factors and parasites were stained with a mouse monoclonal antibody (mAb) specific to a neoepitope present in the activated human complement component C9 (neoC9), but not in native C9 (Falk et al. 1983; Farkas et al. 2002). The neoepitope becomes exposed once C9 associates with C5b-8 to form the Membrane Attack Complex (MAC) and thus is used to detect the last step of the complement activation. The MAC is a component of the lytic pathway of the complement system and can lyse cells when further polymerization of C9 molecules occurs by generating pores in the cell membrane (Lachmann and Thompson 1970). Second, the viability of complement-exposed cercariae was determined by the DNA binding stain Hoechst 33258 dye, which has been previously used as an indirect evidence for tegument damage in schistosomula and adult worms (Couto et al. 2010; Farias et al. 2013; Jones et al. 1988). Third, cercariae exposed to complement were stained with an antibody that reacts with the glucose transporter protein 4 (SGTP4). This protein is localized in the tegument outer membrane (Skelly and Shoemaker 1996) and has been characterized as a marker for new tegumental membrane formation in transforming cercariae (Skelly and Shoemaker 2000). We provide novel evidence that complement activation does not prevent cercaria from shedding the glycocalyx; the tegument is not significantly damaged, contrary to acetabular gland ducts; and secretory vesicles are lysed by the complement system.
Materials and methods
Parasites
Cercariae were harvested by shedding Biomphalaria glabrata snails infected with a Puerto Rican strain of S. mansoni. Infected snails were supplied by the Schistosome Research Reagent Resource Center for distribution by Biomedical Research Institute, National Institute of Health, National Institutes of Allergy and Infectious Diseases (NIH-NIAID). In some experiments, cercariae were kept in 1 mM of the acetylcholinesterase inhibitor eserine sulfate (ES) (Sigma, MO, USA) in all steps of the protocol to reversibly inhibit swimming, acetabular gland secretion and transformation into shchistosomula as previously reported (Samuelson and Caulfield 1985). To reverse ES effects, parasites were washed three times with DMEM/F12 medium. Mechanically transformed schistosomula were obtained by in vitro transformation of cercariae (Lazdins et al, 1982), followed by parasite culturing for 18 h at 37°C and 5% CO2 in DMEM/F12 medium containing 10% fetal calf serum, 2 mM L-glutamine, 100 units/mL penicillin and 100 μg/mL streptomycin sulfate (complete DMEM/F12).
Detection of MAC in the parasite tegument
A total of 200 cercariae per sample were incubated on ice for 30 minutes, pelleted by centrifugation, washed once with ice cold distilled water containing antibiotics, before exposure to undiluted NHS (Complement Technology, TX, USA) at 37°C for 2 h or longer according to each experiment described in the results section. Cercariae were washed three times with Hanks Balanced Salt Solution containing 0.2% BSA (HBSS-BSA) and incubated at room temperature for 30 min with 2 μg/ml of the mouse mAb anti-neoC9. Parasites were washed three times with HBSS-BSA and incubated for 30 min at room temperature with a goat anti-mouse IgG labeled with Alexa-Fluor 488 (AF 488) at 1:200. After washing three times with HBSS-BSA, cercariae were transferred to a 96-well plate and observed under an inverted fluorescent microscope (TH4–100; Olympus, Tokyo, Japan) equipped with a Retiga 1300 camera (Q Imaging, BC, Canada). Cercariae were also exposed to NHS pre-incubated for 60 min at 56°C to heat-inactivate the complement (iNHS) and were used as negative control for the labeling with anti-neoC9. Mechanically transformed schistosomula cultured for 18 h were washed three times with HBSS-BSA and 200 parasites per sample were exposed to NHS or iNHS following labeling with MAb anti-neoC9 as described for cercariae.
Detection of MAC in acetabular gland vesicles
Freshly transformed schistosomula were cultured in a 96-well plate for 18h to obtain secretory vesicles released by the acetabular glands. Next, parasites were carefully removed from the plate and secretory vesicles were washed three times with HBSS, followed by addition of 50μl of undiluted NHS or diluted 10x and 100x with HBSS. The vesicles were also incubated with iNHS as a negative control. After incubation for 2 h at 37°C, the vesicles were washed with HBSS-BSA and stained with anti-neoC9, following the same procedure described above for cercariae.
Viability of cercariae exposed to complement
Cercariae (200 parasites/sample in triplicates) treated with undiluted NHS or iNHS were stained with 1 μg/ml of Hoechst 33258 dye for 10 min and examined with an inverted fluorescent microscope under ultraviolet light (352 excitation /455 emission). Hoechst 33258 is a hydrophilic dye which becomes fluorescent only when it binds to DNA. When there is substantial tegument damage due to complement activation, Hoechst dye will quickly diffuse into sub-tegumental cells and act as an indicator of membrane integrity. Thus, parasites showing strong fluorescence in the entire body are considered not viable. Percentage of dead cercariae was assessed by counting the fluorescent and non-fluorescent parasites. Background mortality was determined by counting fluorescent parasites exposed to iNHS. In some experiments, cercariae were exposed to complement, washed in complete DMEM/F12 and kept in culture to measure parasite long term survival.
Detection of SGTP4 in transforming cercariae exposed to complement
Cercariae exposed to undiluted NHS or iNHS for 18 h at 37°C were washed twice with complete DMEM/F12 followed by overnight culture in the same medium. Next, parasites were washed three times with HBSS, fixed in ice cold acetone for 5 min, washed twice with HBSS containing 1% fetal calf serum (HBSS-FCS) and blocked in the same buffer for 30 min at room temperature. Parasites were then incubated with rabbit IgG anti-SGTP4 at 1:25 dilution for 1 h at room temperature as previously described (Skelly and Shoemaker 2000). Parasites were washed three times for 5 min each with HBSS-FCS and incubated for 30 min at room temperature with a goat anti-rabbit IgG -AF 488 at 1:200, washed three times with HBSS-FCS and examined by conventional fluorescence microscopy.
Statistical Analysis
One- or two-away ANOVA tests were used to compare the difference between more than two experimental groups when indicated. Posttest analyses were performed following each ANOVA test to compare the difference between two groups. Differences were considered statistically significant at P ≤ 0.05. Statistical analyses were performed using Graph-Pad Prism® version 5.0 (GrapPad Software, CA, USA). Each experiment was repeated two to five times.
Results
Cercaria reaction to human complement
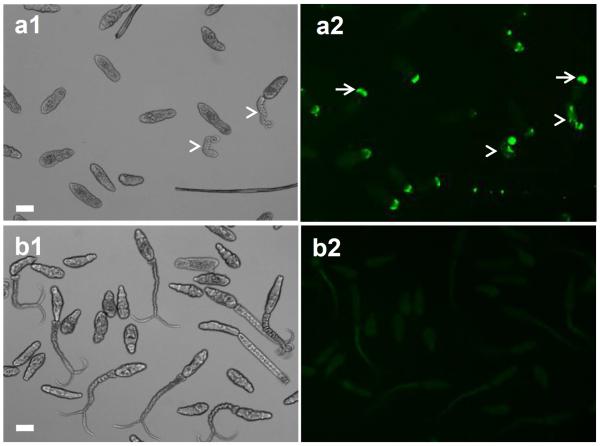
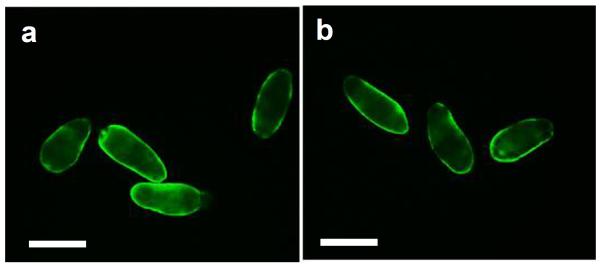
To better understand the mechanism of cercaria killing by the complement system, parasites were exposed to NHS and tested for complement activation, viability, as well as for their ability to transform into schistosomula. Cercariae exposed to NHS for 2h and stained with mAb anti-neoC9 to detect MAC deposition in the tegument had bright fluorescence only at the anterior tip of the body (Fig. 1a2, arrows). As expected, most of the cercariae had lost their tails which were lysed by complement and removed during the washing steps, and the few remaining tails were fluorescent and greatly damaged (Fig. 1a, arrowheads). The cercarial bodies (CB) were intact and viable (Fig. 1a1), but moving slowly. In contrast, no neoC9 labeling was observed in parasites incubated with iNHS; in the absence of complement activation, cercariae were very motile and most of the tails remained intact (Fig. 1b1).
Fig. 1.
Deposition of MAC on the tegument of cercariae exposed to human complement proteins. Cercariae were incubated with normal human serum (a) or heat inactivated normal human serum (b) for 2 h and stained with the antibody anti-neoC9 to detect activated C9 (a1 and b1 are bright fields, a2 and b2 fluorescent fields). Fluorescent signal could be detected only in the anterior tip of bodies (a2, arrows) and in a few remaining tails (a2, arrowheads) of parasites treated with normal human serum. No staining was detected in cercariae treated with inactivated serum (b2). Scale bars are 50 μm in a 10× magnification field
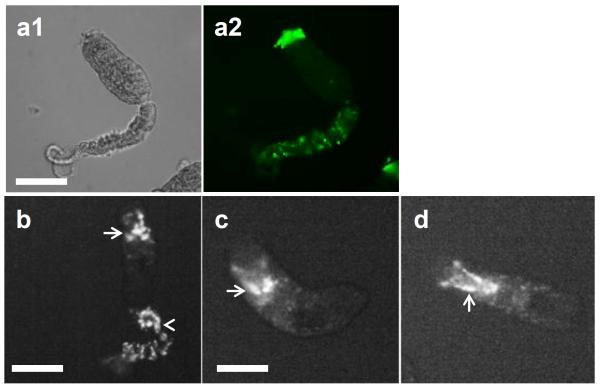
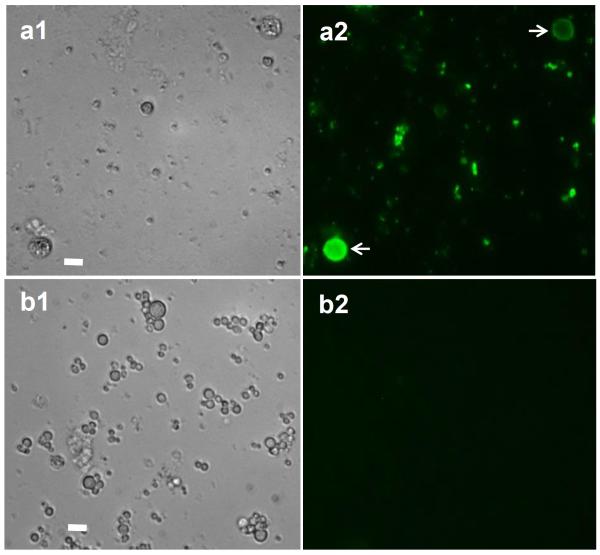
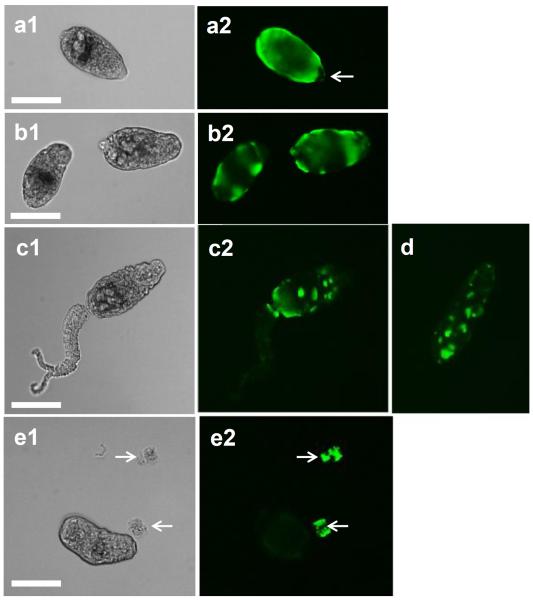
The viability of the complement and control-treated cercariae was analyzed using Hoechst 33258 dye, which fluoresces only when conjugated to DNA. It is interesting to note that labeling with Hoechst to evaluate tegument damage shows that cercariae exposed to NHS were stained only at the anterior region where anti-neoC9 localized, and there was variable Hoechst labeling intensity among the parasites (Fig. 2a, arrows). Remaining tails were damaged and had strong fluorescence (Fig. 2a, arrowheads), also resembling the labeling with anti-neoC9 shown in Fig. 1a. As expected, cercariae treated with iNHS were very motile, had no Hoechst staining in the bodies and minimal fluorescence in the tails which is localized at the junction with the bodies (Fig. 2b, arrowheads) as an indication of tails detaching off the bodies. A closer view of NHS-exposed cercariae shows that most of the staining with anti-neoC9 is in the anterior tip of the CB tegument and entire tail when present (Fig. 3a2). Hoechst dye also stained the whole tail, but in the CB most of the fluorescent signal was found in the acetabular ducts (Fig. 3b–d, arrows). Cercariae treated with complement were still alive and motile as shown by two consecutive pictures of the same CB, with the acetabular ducts clearly visible by Hoechst staining (Fig. 3c and 3d). To determine whether secretory vesicles released by acetabular glands activate the complement system, vesicles obtained from freshly transformed schistosomula were incubated with NHS or iNHS. Indeed, the vesicles were completely lysed by undiluted NHS and after 10x dilution (not shown). In the 100x NHS dilution, a few vesicles were still intact and were labeled with anti-neoC9 (Fig. 4a2, arrows). Significant vesicle rupture is clearly seen by the presence of debris covering the entire bright and fluorescent fields (Fig 4a1 and 4a2). In contrast, the vesicles exposed to iNHS were intact and not labeled by anti-neoC9 (Fig. 4b1 and 4b2 respectively). Curiously, the vesicles were not uniform in size, most likely reflecting the amount of material stored (Fig. 4b1).
Fig. 2.
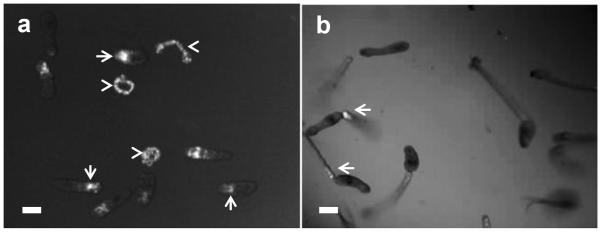
Hoechst dye staining of cercariae exposed to human complement. Cercariae were incubated with normal human serum (a) or heat inactivated normal human serum (b) for 2 h and stained with Hoechst dye to evaluate the level of tegument damage by the complement. Fluorescent signal could be detected only in the anterior region of bodies (arrows) and in a few remaining tails (arrowheads) of parasites treated with normal human serum (a). Cercariae exposed to inactivated serum depicted some fluorescence only in the junction between body and tail (b, arrows). Scale bars are 50 μm in a 10× magnification field
Fig. 3.
Higher magnification of cercariae exposed to human complement. Cercaria incubated with normal human serum for 2 h and stained with anti-neoC9 antibody (a1and a2) shows strong fluorescence in the anterior tip of the parasite (a2). Hoechst dye (b–d) strongly stains the tail when present (b, arrowhead) and the acetabular ducts (arrows in b, c, d). Scale bars are 100 μm in a 20× magnification field
Fig. 4.
Secretory vesicles from acetabular glands were lysed due to complement activation. Vesicles released from freshly transformed schistosomula were incubated for 2 h with normal human serum (a1 and a2) or inactivated serum (b1 and b2) and stained with anti-neoC9 antibody. Note that a few vesicles not ruptured by the complement were stained for MAC (a2, arrows). Vesicles exposed to inactivated serum remained intact and were negative for MAC labeling. Scale bars are 25 μm in a 40× magnification field
Next, we evaluated the impact of long exposure of cercariae to complement. Cercariae were incubated with NHS for 18 h, then analyzed for MAC formation using anti-neoC9 antibody, as well as for viability using Hoechst dye. At this time point, the fluorescent signal due to C9 detection formed a ring around the CB, indicating that MAC is bound to the entire surface of the parasites (Fig. 5a2) and not only in the anterior tip of the tegument as shown for the 2 h-time point. However, despite MAC deposition in the whole surface of CB, Hoechst dye did not significantly stain the majority of the CB, indicating that complement activation did not substantially affect the tegument integrity even after 18 h of complement exposure (Fig. 5b2). Again, only tails showed the expected rapid complement-mediated damage as depicted by arrowheads in the bright field (Fig. 5b1) and positive Hoechst labeling in the fluorescent field (Fig. 5b2). Interestingly, C9 detection on CB was similar to that of 18 h cultured schistosomula (Fig. 5c2) which suggested that cercariae exposed to complement had removed the glycocalyx and formed the double membrane tegument.
Fig. 5.
Complement activation did not substantially affect the tegument integrity after 18 h of complement exposure. Cercaria and 18 h-cultured schistosomula were exposed to normal human serum for 18 h. Cercariae (a) and schistosomula (c) were stained with anti-neoC9 antibody. Cercariae (b) were labeled with Hoechst dye. Note that the tegument of both cercarial bodies and schistosomula is fluorescent after labeling with anti-neoC9. Tails but not bodies of cercariae show fluorescent labeling with Hoechst dye. Scale bars are 50 μm in a 10× magnification field
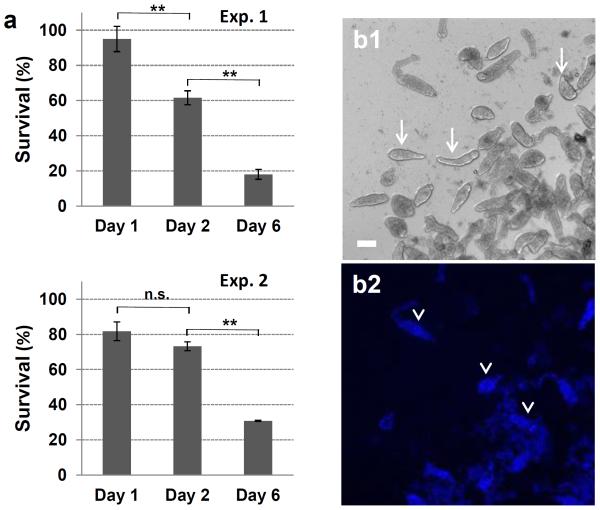
To confirm whether cercariae can fully transform to schistosomula when exposed to the complement system, parasites were stained for the tegumental marker SGTP4 (Fig. 6), which is localized in the tegument outer membrane and has been used as a marker for new tegumental membrane formation in transforming cercariae. Despite complement activation, viable cercariae were capable of transforming into schistosomula, evidenced by positive SGTP4 tegumental staining (Fig. 6a) which was indistinguishable from parasites incubated with iNHS included as positive control for cercarial transformation (Fig. 6b). To measure the long term survival, following 18 h incubation with NHS (day1), parasites were cultured for 1(day 2) and 5 (day 6) additional days before staining with Hoechst dye (Fig. 7). Complement exposure did not substantially affect the percentage of cercaria survival at day 1 (viability was 95% and 82% for experiments 1 and 2, respectively), but more parasites died over time (Fig. 7a). The percentage of survival ranged from 62% to 73% at day 2 and from 18% to 31% at day 6 respectively for both experiments (Fig. 7a). Live parasites (Fig. 7b, arrows in bright field) and dead parasites stained with Hoechst dye (Fig. 7b, arrowheads in fluorescent field) are shown in a representative image of day 6. Viability on day 6 was assessed by counting the remaining live parasites (motile and not stained with Hoechst dye) because many dead parasites were degenerated.
Fig. 6.
Complement activation did not affect cercariae transformation. Staining for the tegumental marker protein SGTP4 following exposure to human complement was used to assess cercarial transformation. Parasites exposed to normal human serum (a) showed surface staining for SGTP4 similar to the parasites exposed to heat-inactivated NHS (b) as the positive control for transformation into schistosomula. Scale bars are 100 μm in a 20× magnification field
Fig. 7.
Viability of cercariae exposed to normal human serum for 18 h as determined by Hoechst dye staining. The percentage of parasite survival was determined on days 1, 2 and 6 after complement exposure; background mortality of parasites exposed to inactivated serum was subtracted from corresponding time points. Two independent experiments are shown (a). Representative images of parasites at day 6 in culture after complement exposure, showing live (b1, arrows) and dead fluorescent (b2, arrowheads) parasites. One-way ANOVA was used to calculate the differences between days 1, 2 and 6. **P ≤ 0. 01; n.s., not significant. Scale bars are 50 μm in a 10× magnification field
Cercaria reaction to human complement in the presence of eserine
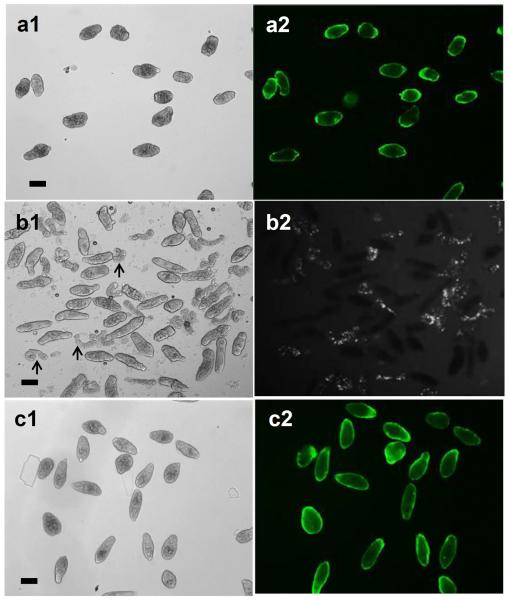
It has been reported that eserine (ES) inhibits cercarial transformation into schistosomula. Therefore, in this study, we evaluated the effect of complement system on cercariae treated with ES. Cercariae pre-treated with ES were exposed to NHS for 2h then stained with anti-neoC9. Unlike parasites not treated with ES which exhibited fluorescence only in the anterior tip of the tegument (Fig. 3a2), ES-treated cercariae had a variable fluorescent pattern on their surface, indicating complement activation due to the presence of the glycocalyx (Fig. 8). CB ranged from being entirely fluorescent (Fig. 8a) to containing large to small patches of fluorescence (Fig. 8b–d), suggesting partial retention of the glycocalyx by ES. Thus, even in the presence of ES, CB were able to initiate shedding of the glycocalyx, albeit much less efficiently. ES inhibition of acetabular gland secretion may be the reason why the anterior tip of ES-treated parasites do not show strong MAC deposition (Fig. 8a2, arrow), in contrast to parasites not exposed to ES (Fig. 3a2). ES removal from the medium followed by parasite culturing for an additional 16 h showed that all CB completely released their glycocalyx and were no longer fluorescent. Released glycocalyx could be seen as fluorescent particles in the medium (Figure 8e, arrows).
Fig. 8.
Inhibition of glycocalyx shedding enhanced MAC formation on the cercarial surface. Cercariae were treated for 2h with normal human serum in the presence of eserine sulfate and stained with anti-neoC9. Parasites had assorted patterns of fluorescence (a–d) due to partial glycocalyx shedding. There is no staining at the anterior tip of the parasites (a2, arrow) due to inhibition of acetabular gland secretion by eserine. Culturing for additional 16 h in medium without eserine promoted complete shedding of the glycocalyx seen as large particles in the bottom of the plate (e1 and e2, arrows). Scale bars are 100 μm in a 20× magnification field
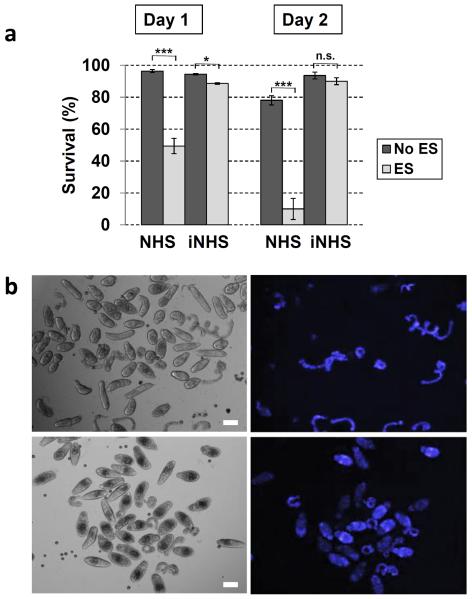
To determine whether the retention of the glycocalyx by ES enhances tegument damage and thus parasite death, cercariae were exposed to NHS for 18 h in the presence or absence of ES, followed by staining with Hoechst dye (Fig. 9). Fluorescent parasites were counted immediately following 18 h of complement exposure (day 1) and 24 h later (day 2). On day 1, approximately 49% of the parasites cultured with ES were viable, compared to 96% in the absence of ES (Fig. 9a). By day 2, the mortality was even more pronounced in the ES group resulting in approximately 10% of parasite survival, compared to 78% in the control group (Fig. 9a). Cercariae exposed to iNHS had approximately 90% survival, regardless ES treatment (Fig. 9a). Representative images at day 1of NHS-exposed cercariae showed that in the absence of ES (Fig. 9b, top panels), only tails but not CB were fluorescent, similar to the results shown on Fig.5b, but when ES was added, a significant number of bodies were also stained by Hoechst (Fig. 9b, bottom panels). Taken together, these results clearly demonstrate that glycocalyx shedding is essential to protect cercariae from complement killing.
Fig. 9.
Viability of cercariae exposed to normal human serum (NHS) for 18 h in the presence or absence of eserine sulfate (ES) as determined by Hoechst staining. Heat-inactivated NHS (iNHS) groups are shown as control for background mortality. The percentage of parasite survival was determined on days 1 and 2 after complement exposure and was significantly higher in samples not containing ES (a). Representative images of parasites at day 1 in culture after complement exposure (b). Note that in the absence of ES (top panels; left is bright field and right is Hoechst staining) almost exclusively tails were fluorescent, an indication that the tegument of cercaria bodies were not compromised. However, when ES is present, a significant number of bodies were labeled with Hoechst (bottom panels; left is bright field and right is Hoechst staining), indicating that inhibition of glycocalyx shedding enhanced cercaria killing by the complement. Two-way ANOVA was used to calculate the differences among groups containing or not ES. *P≤ 0.05; ***P ≤ 0.001; n.s., not significant. Scales are 50 μm in a 10× magnification field”
Discussion
Schistosomes are helminth parasites that cause a chronic and debilitating disease called schistosomiasis which affects over 200 million people worldwide (Gryseels 2012; Steinmann et al. 2006). These parasites have a complex life cycle. Cercariae, the infectious larvae, infect vertebrate hosts by penetrating the skin where they transform into schistosomula. Transformation involves the loss of the cercarial outer membrane, the glycocalyx. Schistosome parasites have evolved to evade the innate and adaptive host immune response. Nonetheless, cercariae from Schistosoma mansoni are considered highly susceptible to complement due to the glycocalyx that covers their external surface. Previous work has shown that cercaria treatment with ES, an acetylcholinesterase inhibitor, inhibited glycocalyx shedding (Samuelson and Caulfield 1985). This resulted in higher levels of complement factor C3 binding to cercariae rendering them more susceptible to complement killing than recently transformed schistosomula, which at this stage of maturation loses about two thirds of the glycocalyx (Samuelson and Caulfield 1986). In this study, we set forth to provide new insights into the cercaria reaction to the complement system. Live parasites were exposed to NHS as source of complement factors and tested for activation of the lytic pathway of the complement (via detection of MAC deposition on the parasite surface), for survival rate (via Hoechst dye staining) and for new tegument formation (via SGTP4 expression).
When cercariae were treated for 2 h with NHS, MAC was detected in the anterior tip of the tegument. This result indicated that cercariae were able to remove most of the glycocalyx during complement activation. Strong MAC deposition in the tip of the head was most likely due to complement activation by secretory vesicles released from the acetabular glands. In a rare occasion, we could observe one cercaria releasing a secretory vesicle which was stained for MAC. This result prompted us to incubate the vesicles with NHS. Indeed, the secretory vesicles were highly susceptible to complement activation and most of the vesicles were lysed; a few remaining were stained for MAC, confirming our expectations. Curiously, previous work has shown that during invasion of human skin by cercariae, groups of intact vesicles were released from the acetabular glands and the vesicles ruptured within the host tissue. Ruptured vesicles were found along the cercarial surface, and the authors suggested that the protease content might help the parasites to release the glycocalyx (Fishelson et al. 1992). Our results showing complement-mediated rupture of the vesicles indicate the presence of a lipid bilayer surrounding the secreted material which promoted MAC insertion and formation of pores. Paveley et al. (2009) have shown that the outer portion of the vesicles surrounding the protein-rich content is not labeled by an amine-reactive fluorescent dye, thus suggesting that the outer surface is formed by non-protein molecules such as lipids and/or glycans. It is worth to speculate that rupture of secretory vesicles due to complement activation could benefit the parasite, by facilitating the release of the vesicle proteases that aid the cercaria to shed the glycocalyx and migrate through the skin layers. Additionally, complement activation may participate in the process of skin inflammation observed during cercaria migration as reviewed by Mountford and Trottein (2004).
Hoechst staining of cercaria incubated for 2 h with NHS showed that the dye strongly stained only the acetabular ducts and not the entire parasite body, suggesting that the tegument was not seriously compromised. No Hoechst staining was detected in acetabular ducts of parasites exposed to iNHS, indicating that complement was indeed damaging the acetabular ducts, allowing intracellular dye penetration in the anterior region of NHS-exposed parasites. In the present study, even 18 h exposure of cercariae to NHS, did not substantially compromise the parasite surface as judged by absence of Hoechst staining in approximately 80–95% of the parasites. However, a time course viability of these parasites showed an increasing mortality rate over time, resulting in 20–30% survival at day 6 in culture, despite the absence of significant tegument damage. Notably, these values were comparable to the percentages of adult worms recovered from mice injected with schistosomula transformed in fresh normal rat serum (Brink et al. 1977) and also in two other studies following intravenous injection of cercariae in mice and hamsters respectively (Holanda et al. 1974; Zelia and Kovalenko 1986).
One possibility to reconcile the apparent low tegumental damage, if any, and slow parasite mortality following complement activation is that death is occurring primarily because of acetabular gland injury, preventing the recovery of most of the parasites. We are currently investigating this hypothesis. Another potential reason is that survival rate of complement-exposed parasites may depend on how fast and/or effective cercariae undergo the transformation process to prevent irreversible complement damage and parasite death. Cercariae exposed for 18 h to complement had MAC deposition forming a ring around the parasite. We found that the pattern of staining in cercariae was similar to that observed in 18 h- cultured schistosomula, suggesting that transforming cercariae were able to form the new double layer tegument and transform into schistosomula. To prove this possibility, cercariae exposed to complement were stained for SGTP4, confirming the presence of the new tegument. Previous work has shown that the formation of the new tegument using SGTP4 as a marker is not a synchronic process and can be initiated in 20% to 30% of cercariae while still in water (Skelly and Shoemaker 2000). Also, tail removal does not accelerate cercaria transformation compared to parasites containing tails (Skelly and Shoemaker, 2000) and that may explain why tail shedding is not an accurate measurement of parasite transformation and resistance to complement. Another study has concluded that cercariae incubated in fresh rat serum which contains active complement require the same period of time as mechanically and ex-vivo skin transformed parasites to develop into schistosomula and form a new tegument (Brink et al. 1977), confirming the present work using human serum. Our finding that NHS-exposed cercariae were able to evade complement killing and transform into schistosomula, despite MAC detection on the surface of surviving parasites, supports previous studies indicating the presence of MAC regulatory proteins in the outer membrane of recently transformed schistosomula (Deng et al. 2003; Parizade et al. 1994). Another possibility relies on the intrinsic properties of the tegument outer membrane, described as a membrane-like secretion (Skelly and Alan Wilson 2006), which might be refractory to C9 polymerization, preventing pore formation.
In order to prove whether glycocalyx shedding plays a direct role on cercaria survival when exposed to complement, the percentage of viable parasites was compared between samples with or without ES. Indeed, parasites treated with ES and thus forbidden to efficiently shed the glycocalyx, were significantly more susceptible to complement killing than controls. ES-treated cercariae were highly fluorescent after MAC staining, but varied from having a fluorescent uniform staining to a patchy appearance. This was probably due to partial glycocalyx shedding. It is important to mention that the glycocalyx is not an amorphous surface coat, but a mesh of fibrils and spherical particles that appeared to aggregate to form dimers, trimers, and tetramers (Nanduri et al. 1991; Samuelson and Caulfield 1985). The aggregation process may be a parasite strategy to facilitate glycocalyx shedding. The observation that parasites became negative for MAC staining after ES removal from the sample, confirmed that remaining cercariae were still viable and able to release the glycocalyx after MAC formation. Nevertheless, we cannot exclude the possibility that glycocalyx shedding was initiated before or even simultaneously with MAC formation. Tails were lysed by the complement, while CB remained intact in ES-treated parasites. This is probably because tail glycocalyx activates more complement than CB glycocalyx. Indeed, it has previously been shown that tails can bind more complement factor C3 than CB (Samuelson and Caulfield 1986). Even though the glycocalyx of both body and tail have a similar ultra-structure (fibrils and spheres), the carbohydrate composition differs, with fucose being the major component in bodies and glucose in tails. Furthermore, the body glycocalyx is also smaller and less negatively charged than tail glycocalyx (Nanduri et al, 1991). These differences may explain why bodies are more resistant to complement damage than tails and deserve further investigation.
In summary, direct exposure of cercariae to NHS induces complement activation, but does not prevent cercariae from shedding the glycocalyx. Transformation into schistosomula occurs in approximately 20–30% of the parasites. During complement activation there is significant damage of the acetabular gland ducts and lysis of the secretion vesicles. Cercarial mortality occurs over time and not immediately after complement exposure, suggesting that the tegument is not seriously compromised. Retention of the glycocalyx increases cercarial killing, most likely due to the higher deposition of complement in the parasite surface. These results, albeit in vitro, provide new information into the reaction of cercaria to the complement system. Future work in vivo would be necessary to further understand the role of the complement system in the process of cercaria migration through the host skin.
Acknowledgments
This work was supported by National Institutes of Health, National Institute of Allergy and Infectious Diseases (NIH-NIAID), Grant AI-095893 to G.K.P. We thank Dr. Patrick Skelly and Dr. Charles Shoemaker from Tufts University, Cumming School of Veterinary Medicine, MA, USA for helpful comments and Dr. Anne Nicholson-Weller from Beth Israel Deaconess Medical Center, MA, USA for advice on the lytic pathway of the complement system. Infected snails were provided by BRI via the NIAID schistosomiasis resource center under NIH-NIAID Contract No. HHSN272201000005I. Parasites were kindly provided by Dr. Skelly through NIHNIAID Grant AI-056273.
Ethical Standards These studies did not use animal or human subjects. All procedures and analyses in support of this original research were performed in accordance with the ethical standards of the institution.
Footnotes
Conflict of Interest The authors declare that they have no conflict of interest.
References
- Bicalho RS, de Melo AL, Pereira LH. Schistosoma mansoni development in the peritoneal cavity of inbred AKR/J mice. Rev Inst Med Trop Sao Paulo. 1993;35(5):411–6. [PubMed] [Google Scholar]
- Brannstrom K, Sellin ME, Holmfeldt P, Brattsand M, Gullberg M. The Schistosoma mansoni protein Sm16/SmSLP/SmSPO-1 assembles into a nine-subunit oligomer with potential To inhibit Toll-like receptor signaling. Infect Immun. 2009;77(3):1144–54. doi: 10.1128/IAI.01126-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brink LH, McLaren DJ, Smithers SR. Schistosoma mansoni: a comparative study of artificially transformed schistosomula and schistosomula recovered after cercarial penetration of isolated skin. Parasitology. 1977;74(1):73–86. doi: 10.1017/s0031182000047545. [DOI] [PubMed] [Google Scholar]
- Couto FF, Coelho PM, Araujo N, Kusel JR, Katz N, Mattos AC. Use of fluorescent probes as a useful tool to identify resistant Schistosoma mansoni isolates to praziquantel. Parasitology. 2010;137(12):1791–7. doi: 10.1017/S003118201000065X. [DOI] [PubMed] [Google Scholar]
- Curwen RS, Ashton PD, Sundaralingam S, Wilson RA. Identification of novel proteases and immunomodulators in the secretions of schistosome cercariae that facilitate host entry. Mol Cell Proteomics. 2006;5(5):835–44. doi: 10.1074/mcp.M500313-MCP200. [DOI] [PubMed] [Google Scholar]
- Curwen RS, Wilson RA. Invasion of skin by schistosome cercariae: some neglected facts. Trends Parasitol. 2003;19(2):63–6. doi: 10.1016/s1471-4922(02)00019-3. [DOI] [PubMed] [Google Scholar]
- Deng J, Gold D, LoVerde PT, Fishelson Z. Inhibition of the complement membrane attack complex by Schistosoma mansoni paramyosin. Infect Immun. 2003;71(11):6402–10. doi: 10.1128/IAI.71.11.6402-6410.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dessein A, Samuelson JC, Butterworth AE, Hogan M, Sherry BA, Vadas MA, David JR. Immune evasion by Schistosoma mansoni: loss of susceptibility to antibody or complement-dependent eosinophil attack by schistosomula cultured in medium free of macromolecules. Parasitology. 1981;82(Pt 3):357–74. doi: 10.1017/s0031182000066890. [DOI] [PubMed] [Google Scholar]
- Falk RJ, Dalmasso AP, Kim Y, Tsai CH, Scheinman JI, Gewurz H, Michael AF. Neoantigen of the polymerized ninth component of complement. Characterization of a monoclonal antibody and immunohistochemical localization in renal disease. J Clin Invest. 1983;72(2):560–73. doi: 10.1172/JCI111004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farias LP, Krautz-Peterson G, Tararam CA, Araujo-Montoya BO, Fraga TR, Rofatto HK, Silva-Jr FP, Isaac L, Da'dara AA, Wilson RA, Shoemaker CB, Leite LC. On the three-finger protein domain fold and CD59-like proteins in Schistosoma mansoni. PLoS Negl Trop Dis. 2013;7(10):e2482. doi: 10.1371/journal.pntd.0002482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas I, Baranyi L, Ishikawa Y, Okada N, Bohata C, Budai D, Fukuda A, Imai M, Okada H. CD59 blocks not only the insertion of C9 into MAC but inhibits ion channel formation by homologous C5b-8 as well as C5b-9. J Physiol. 2002;539(Pt 2):537–45. doi: 10.1113/jphysiol.2001.013381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishelson Z, Amiri P, Friend DS, Marikovsky M, Petitt M, Newport G, McKerrow JH. Schistosoma mansoni: cell-specific expression and secretion of a serine protease during development of cercariae. Exp Parasitol. 1992;75(1):87–98. doi: 10.1016/0014-4894(92)90124-s. [DOI] [PubMed] [Google Scholar]
- Gryseels B. Schistosomiasis. Infectious disease clinics of North America. 2012;26(2):383–97. doi: 10.1016/j.idc.2012.03.004. [DOI] [PubMed] [Google Scholar]
- Hansell E, Braschi S, Medzihradszky KF, Sajid M, Debnath M, Ingram J, Lim KC, McKerrow JH. Proteomic analysis of skin invasion by blood fluke larvae. PLoS Negl Trop Dis. 2008;2(7):e262. doi: 10.1371/journal.pntd.0000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holanda JC, Pellegrino J, Gazzinelli G. Infection of mice with cercariae and schistosomula of Schistosoma mansoni by intravenous and subcutaneous routes. Rev Inst Med Trop Sao Paulo. 1974;16(3):132–4. [PubMed] [Google Scholar]
- Jenkins SJ, Hewitson JP, Jenkins GR, Mountford AP. Modulation of the host's immune response by schistosome larvae. Parasite Immunol. 2005;27(10–11):385–93. doi: 10.1111/j.1365-3024.2005.00789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JT, Helm CN, Kusel JR. Variation in susceptibility of Schistosoma mansoni to damage by polycations. Mol Biochem Parasitol. 1988;30(1):35–44. doi: 10.1016/0166-6851(88)90130-2. [DOI] [PubMed] [Google Scholar]
- Jones MK, Lustigman S, Loukas A. Tracking the odysseys of juvenile schistosomes to understand host interactions. PLoS Negl Trop Dis. 2008;2(7):e257. doi: 10.1371/journal.pntd.0000257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachmann PJ, Thompson RA. Reactive lysis: the complement-mediated lysis of unsensitized cells. II. The characterization of activated reactor as C56 and the participation of C8 and C9. J Exp Med. 1970;131(4):643–57. doi: 10.1084/jem.131.4.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marikovsky M, Arnon R, Fishelson Z. Proteases secreted by transforming schistosomula of Schistosoma mansoni promote resistance to killing by complement. J Immunol. 1988;141(1):273–8. [PubMed] [Google Scholar]
- McKerrow JH, Salter J. Invasion of skin by Schistosoma cercariae. Trends Parasitol. 2002;18(5):193–5. doi: 10.1016/s1471-4922(02)02309-7. [DOI] [PubMed] [Google Scholar]
- Mountford AP, Trottein F. Schistosomes in the skin: a balance between immune priming and regulation. Trends in parasitology. 2004;20(5):221–6. doi: 10.1016/j.pt.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Nanduri J, Dennis JE, Rosenberry TL, Mahmoud AA, Tartakoff AM. Glycocalyx of bodies versus tails of Schistosoma mansoni cercariae. Lectin-binding, size, charge, and electron microscopic characterization. J Biol Chem. 1991;266(2):1341–7. [PubMed] [Google Scholar]
- Parizade M, Arnon R, Lachmann PJ, Fishelson Z. Functional and antigenic similarities between a 94-kD protein of Schistosoma mansoni (SCIP-1) and human CD59. J Exp Med. 1994;179(5):1625–36. doi: 10.1084/jem.179.5.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paveley RA, Aynsley SA, Cook PC, Turner JD, Mountford AP. Fluorescent imaging of antigen released by a skin-invading helminth reveals differential uptake and activation profiles by antigen presenting cells. PLoS Negl Trop Dis. 2009;3(10):e528. doi: 10.1371/journal.pntd.0000528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter JP, Lim KC, Hansell E, Hsieh I, McKerrow JH. Schistosome invasion of human skin and degradation of dermal elastin are mediated by a single serine protease. J Biol Chem. 2000;275(49):38667–73. doi: 10.1074/jbc.M006997200. [DOI] [PubMed] [Google Scholar]
- Samuelson JC, Caulfield JP. The cercarial glycocalyx of Schistosoma mansoni. J Cell Biol. 1985;100(5):1423–34. doi: 10.1083/jcb.100.5.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelson JC, Caulfield JP. Cercarial glycocalyx of Schistosoma mansoni activates human complement. Infect Immun. 1986;51(1):181–6. doi: 10.1128/iai.51.1.181-186.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skelly PJ, Alan Wilson R. Making sense of the schistosome surface. Adv Parasitol. 2006;63:185–284. doi: 10.1016/S0065-308X(06)63003-0. [DOI] [PubMed] [Google Scholar]
- Skelly PJ, Shoemaker CB. Rapid appearance and asymmetric distribution of glucose transporter SGTP4 at the apical surface of intramammalian-stage Schistosoma mansoni. Proc Natl Acad Sci U S A. 1996;93(8):3642–6. doi: 10.1073/pnas.93.8.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skelly PJ, Shoemaker CB. Induction cues for tegument formation during the transformation of Schistosoma mansoni cercariae. Int J Parasitol. 2000;30(5):625–31. doi: 10.1016/s0020-7519(00)00031-x. [DOI] [PubMed] [Google Scholar]
- Steinmann P, Keiser J, Bos R, Tanner M, Utzinger J. Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk. Lancet Infect Dis. 2006;6(7):411–25. doi: 10.1016/S1473-3099(06)70521-7. [DOI] [PubMed] [Google Scholar]
- Wilson RA, Coulson PS. Immune effector mechanisms against schistosomiasis: looking for a chink in the parasite's armour. Trends Parasitol. 2009;25(9):423–31. doi: 10.1016/j.pt.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelia OP, Kovalenko FP. Comparative efficiency of infecting laboratory animals via intravenous and subcutaneous administration of Schistosoma mansoni cercaria. Parazitologiia. 1986;20(6):461–5. [PubMed] [Google Scholar]