Abstract
The inflammatory bowel diseases (IBD) are complex diseases caused by environmental, immunological and genetic factors. Vitamin D status is low in patients with IBD and experimental IBD is more severe in vitamin D deficient or vitamin D receptor knockout animals. Vitamin D is beneficial in IBD because it regulates multiple checkpoints and processes essential for homeostasis in the gut. Vitamin D inhibits IFN-γ and IL-17 production while inducing regulatory T cells. In addition, vitamin D regulates epithelial cell integrity, innate immune responses, and the composition of the gut microbiota. Overall vitamin D regulates multiple pathways that maintain gastrointestinal homeostasis. The data support improving vitamin D status in patients with IBD.
Keywords: vitamin D, inflammatory bowel disease, microbiota
Introduction
Since the discovery of the vitamin D receptor (VDR) in cells of the immune system about 30 years ago, there has been a strong interest in understanding the role of vitamin D in the immune system. While investigators have made some progress there are still many unanswered questions about vitamin D as an immune system regulator. Vitamin D deficiency has been associated with a number of chronic diseases including inflammatory bowel diseases (IBD). In addition, experimental IBD is more severe in VDR KO and vitamin D deficient models and vitamin D/1,25(OH)2D3 treatments suppress experimental IBD. Here we will summarize some of the more recent work that describes the mechanisms by which vitamin D regulates IBD development and pathogenesis.
Vitamin D
Vitamin D primarily functions as a regulator of calcium homeostasis and thus bone formation and resorption. The diet is only a poor source of vitamin D since most foods are naturally low in vitamin D. In addition to the diet, vitamin D is also manufactured in the skin via a photolysis reaction. However, the vitamin D available from sunlight exposure is variable based on season and geography. Vitamin D availability through cutaneous production is significantly less in northern parts of North America and Europe, and especially low during the winter (1, 2). Vitamin D produced in the skin or ingested in the diet is inactive. Hydroxylation of vitamin D occurs, in the liver, resulting in 25(OH)D3 which is the circulating form of vitamin D. Synthesis of active vitamin D requires the renal 1α vitamin D hydroxylase (encoded by Cyp27B1), which catalyzes the conversion of 25(OH)D3 to 1,25(OH)2D3. Production of 1,25(OH)2D3 is tightly regulated in order to maintain serum calcium within a narrow range. 1,25(OH)2D3 and the VDR regulate expression of Cyp27B1 in the kidney. The VDR is a member of the steroid/hormone superfamily of nuclear transcription factors (1). The actions of 1,25(OH)2D3 are mediated by its binding to the VDR, which acts as a transcription factor to modulate the expression of genes in a tissue-specific manner. Vitamin D is a nutrient/hormone that transcriptionally regulates gene expression.
IBD
IBD are immune mediated idiopathic diseases that include ulcerative colitis and Crohn’s disease. Crohn’s disease can involve the entire gastrointestinal tract while ulcerative colitis is limited to the colon and rectum (3). Although Crohn’s disease and ulcerative colitis share several clinical features the patterns of gene expression from punch biopsies demonstrated important differences in the two diseases (4). The IBD are multifactorial diseases and it is clear that both genetic and environmental factors affect the development of IBD.
Genetics are important in the development of IBD. The biological relatives of patients with IBD are 10-30 times more likely to develop IBD than individuals without relatives with IBD (5). Genes important in the development of IBD include genes that regulate the immune system such as major histocompatibility, cytokines, signaling molecules, and cytokine receptors (6-8). There are IBD associated genes that are shared between patients with ulcerative colitis and Crohn’s disease and a few genes that are associated with only one of the two forms of IBD (4). Single nucleotide polymorphisms (SNPs) occurring in regulatory cytokines including interleukin (IL)-10, and intracellular signaling molecules including Toll-like receptors are prevalent in both ulcerative colitis and Crohn’s disease (7, 9). A recent meta-analysis suggested an increased risk of both ulcerative colitis and Crohn’s disease associated with several VDR polymorphisms (10). Therefore there is genetic evidence linking vitamin D and the IBD diseases. Although scientists have identified genes that predispose for IBD development, identical twin studies show concordance rates of only 20% for ulcerative colitis and 50% for Crohn’s disease. It seems likely that there are important interactions between environmental factors and the expression of potentially disease inducing genes.
Environmental contributors to disease in general and IBD in particular have been historically difficult to identify (11). It is known that patients with IBD tend to live in urban areas rather than rural areas and northern versus southern parts of North America and Europe (12). For example, Finland and Canada have high incidences of IBD (13, 14). In addition, the incidence of IBD in the US was lower among women living at southern latitudes compared to those living in northern latitudes (15, 16). A protective role for ultraviolet light exposure has been suggested for Crohn’s disease in two different studies and for ulcerative colitis in one study with the other study not finding a significant relationship (15, 17). The environmental factors that might explain these geographical patterns include exposure to pollutants, sunlight, vitamin D and commensal or pathogenic microorganisms.
IBD and the microbiota
A population of nearly 100 trillion dynamic and diverse microbiota—between 500 and 1,000 different species—inhabit the human gut (18). In comparison with all germ and somatic cells in the human body, the gut houses nearly 10 times the number of resident bacteria (19). Additionally, the gut microbiota as a whole contains nearly 100 times more genes than the human genome—some of which are used to tightly control and regulate the host environment (18). The gut microbiota is essential for normal immune system development, displacement of pathogens, and extraction of additional energy (e.g., short chain fatty acids) from otherwise non-digestible dietary substrates (20).
The composition of the intestinal microbiota is one of the environmental factors that have been shown to affect the development of IBD. Patients with IBD had dysbiosis which included changes in the gut microbiota that were associated with disease development (21). In particular, there were decreased numbers from the Bacteroidetes phylum and Lachnospiraceae of the Firmicutes phylum, and increased numbers from the Proteobacteria phylum and Bacillus of the Firmicutes phylum in IBD patients as compared to healthy controls (21). It is not clear whether the changes in the microbiota are contributors to the development of IBD or whether the increased inflammation in the gut alters the mirobiota (11, 22). Disruption of the microbiota using antibiotics or addition of microbiota using probiotics was beneficial in some IBD patients (23). In addition, childhood Helicobacter pylori infection is negatively associated with the development of ulcerative colitis and Crohn’s disease (11, 24). Conversely, some gastrointestinal infections and administration of antibiotics in childhood were associated with an increased risk of IBD (25, 26). The data do suggest differing roles for the microbial flora in childhood that might be critical for the development of mucosal tolerance and later in the adult gastrointestinal tract. There is still no clear relationship between individual microbes or populations of microbes and the development or prevention of IBD.
Animal models of IBD are useful for modeling some aspects of both Crohn’s disease and ulcerative colitis; however, most of the information from mice cannot be directly translated to either Crohn’s disease or ulcerative colitis. Instead, the models are useful for understanding the basic mechanisms following challenge of gastrointestinal homeostasis induced by chemicals, infection, or uncontrolled inflammation.
Clear evidence of the role of the intestinal microbiota in controlling intestinal inflammation has been demonstrated in experimental models of IBD. In dextran sodium sulfate (DSS) induced colitis the microbiota were protective since germ-free mice developed a severe form of the disease (27). In IL-10 KO mice the microbiota were harmful since germfree animals failed to develop disease (28). Disease in IL-10 KO mice was caused by inappropriate immune responses to the commensal microbiota (28). The severity of experimental IBD that developed following a gastrointestinal infection with Citrobacter rodentium depended on the composition of the microbiota since C. rodentium competed for nutrients with the commensal microbiota (29). The intestinal microbiota is an important environmental factor that affects the development of experimental IBD.
Vitamin D and IBD
There is mounting evidence for a link between vitamin D availability either from sunshine or diet and the prevalence of immune mediated diseases including IBD (13). Vitamin D status when it has been measured is low in IBD patients and inversely associated with the risk of developing disease (30, 31). The epidemiological evidence linking lower vitamin D and IBD outcomes was recently reviewed (32). Whether vitamin D deficiency contributes to IBD development or is a result of malabsorption is as yet unclear. As early as 1992 fish oil supplements that contained vitamin D decreased pathology and increased weight gain in IBD patients (33). In a small double blind placebo controlled trial, supplementation with vitamin D improved serum 25(OH)D3 levels of Crohn’s patients and decreased the risk of relapse but only insignificantly (34). In an open label pilot study in Crohn’s patients, vitamin D supplementation increased 25(OH)D3 levels and decreased symptoms (35). Vitamin D status may affect the efficacy of IBD treatments, for example, patients with higher - vitamin D levels before starting anti-TNFα treatments had better outcomes than those with low vitamin D levels (36). Vitamin D insufficiency is associated with IBD and vitamin D supplementation may be helpful in the treatment and prevention of IBD.
Experimentally there is evidence that links the severity of experimental IBD and vitamin D. Vitamin D deficiency increased the symptoms of several experimental models of IBD (37). VDR deficiency increased susceptibility of mice to DSS colitis, T cell transfer induced colitis, and genetic models of experimental IBD (38, 39). In addition, treatments with 1,25(OH)2D3 have been shown to alleviate symptoms of colitis following chemical injury or in IL-10 KO mice (39-41). It should be noted that VDR KO and vitamin D deficient mice do not develop overt symptoms of experimental IBD. Therefore vitamin D deficiency alone does not cause IBD. Instead, vitamin D is one of the many environmental factors that contributes to the development of experimental IBD.
Vitamin D, gut epithelial integrity and IBD
The gastrointestinal tract forms a selectively permeable barrier designed to allow nutrient and water transport while preventing systemic microbial infection. Defective barrier function exists in IBD patients and animal models of IBD. Tight junction proteins including claudin-1, ZO-1, occludin and E-cadherin maintain the integrity of the epithelial barrier in the gut (42). Patients with Crohn’s disease have increased small intestine permeability (43). In IL-10 KO mice and DSS colitis, mice showed increased gastrointestinal permeability to small molecules associated with the development of IBD symptoms (43, 44). In addition, mice with barrier dysfunction developed more severe T cell mediated colitis (45). Anti-TNF α treatments suppressed inflammation and resulted in decreased gut permeability as the symptoms of IBD resolved (46). Compromised gut barrier function is connected to dysbiosis, inflammation and the development of IBD.
There is evidence that vitamin D regulates gut barrier function. VDR KO mice were hyper-responsive to lipopolysaccharide challenge and DSS colitis (38). The injury in VDR KO mice following exposure to DSS included the inability of the mice to maintain the integrity of the epithelial barrier (38, 47). Expression of E-cadherin, claudin-1, ZO-1, and occludin proteins were lower in VDR KO mice treated with DSS than in wild-type (WT) mice (47, 48). 1,25(OH)2D3 induced E-cadherin transcripts in gut epithelial cells (49). As a result of reduced tight junction proteins, vitamin D deficient and VDR KO mice had increased gut permeability compared to vitamin D sufficient WT mice (47). Indirectly the higher levels of TNF-α and other inflammatory cytokines contributed to decreased barrier function found in VDR KO and vitamin D deficient mice (38, 47). Vitamin D is an important regulator of epithelial integrity and barrier function.
Vitamin D, T cells and IBD
Several types of T cells are important for regulation of homeostasis in the gastrointestinal tract and either induce or suppress IBD. In the gut the T cells need to produce IL-17 and IFN-γ to clear infections while not responding to the commensal microbiota. In IBD, Th17 cell functions are unregulated and associated with disease pathology. Highlighting a critical role for Th17 cells in experimental IBD, Th17 deficient mice were resistant to developing IBD (50). FoxP3+ CD4+ regulatory T cells (T reg) are critical regulators of gastrointestinal homeostasis. Mice with defective or absent FoxP3+ T reg cells developed experimental IBD (51, 52). Tregs function by inducing apoptosis of effector cells and producing the inhibitory cytokines IL-10 and TGF- β 1 (53). In addition, the intestine contains other populations of regulatory cells including T cells that express the homodimeric form of CD8, CD8αα (54). These CD8αα T cells have been shown to proliferate slowly and produce IL-10 and TGF-β1 that helps to limit inflammation in the gastrointestinal tract (54, 55). In addition, CD8αα T cells were regulatory in vivo since they prevent T cell induced models of IBD (55). Other regulatory T cells in the gut include invariant NKT (iNKT) cells that were early producers of cytokine that shape the development of the T cell response in the periphery and the gut (56). Stress in the gastrointestinal tract either following infection or chemical injury results in the induction of Th1 and Th17 cells. The regulatory T cells are then critical for turning off the Th1 and Th17 cells and restoring gastrointestinal homeostasis.
The VDR was expressed at low levels in conventional T cells and induced following activation (57). Since the effects of vitamin D as a T cell regulator have been recently reviewed in detail elsewhere (58, 59) here we will only briefly summarize the effects of vitamin D on T cells. CD4 T cells from VDR KO and Cyp27B1 KO mice had a more activated phenotype and overproduced IFN-γ and IL-17 cells compared to WT CD4 cells (60). 1,25(OH)2D3 treatments suppressed the development of experimental IBD and several other models where Th1 and Th17 cells were pathogenic (61). 1,25(OH)2D3 suppressed the proliferation of T cells in vitro (62, 63). VDR KO mice had normal numbers of FoxP3+ T reg cells compared to WT (64). However, 1,25(OH)2D3 treatments in vitro and in vivo induced FoxP3+ T reg cells (41). Cyp27B1 KO and VDR KO mice had fewer iNKT cells and vitamin D regulated iNKT cell maturation and development (65, 66). In addition, vitamin D was a critical regulator of CD8αα T cells in the gastrointestinal tract since VDR KO mice had half as many CD8αα T cells in the gut due to a block in maturation and proliferation of the precursors in the gut (64, 67). Vitamin D is a regulator of T cell development and function.
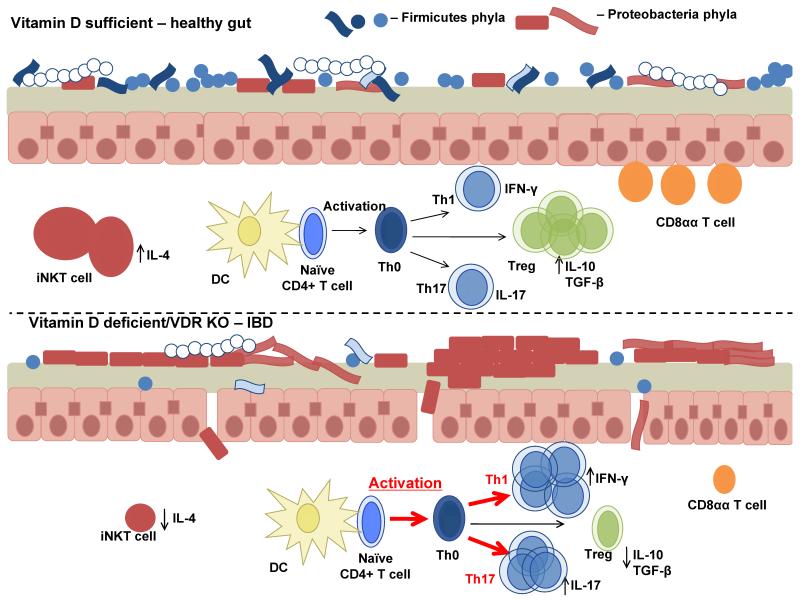
Our current model of the effects of vitamin D on gastrointestinal homeostasis is shown in Fig. 1. In the gastrointestinal tract large numbers of Th1 and Th17 cells producing IL-17 and IFN-γ contribute to inflammation in the vitamin D deficient or VDR KO host (Fig. 1). In addition to inhibiting Th1 and Th17 cells, vitamin D and 1,25(OH)2D3 is important for the development of regulatory cells (T reg, iNKT cells and CD8αα T cells). Vitamin D serves to shut off the Th1 and Th17 cells while boosting iNKT, CD8αα and T reg cell functions (Fig. 1). Vitamin D maintains the balance between effector T cells important for fighting infection and regulatory T cells important for homeostasis of the gastrointestinal tract.
Figure 1.
A model of the effects of vitamin D on gastrointestinal homeostasis. Vitamin D is required to maintain gastrointestinal homeostasis. In T cells vitamin D is important for turning off effector Th1 and Th17 cells, inducing several regulatory cells including Treg, CD8αα expressing T cells and iNKT cells. Vitamin D regulates barrier function in the gut in part by regulating E-cadherin and other tight junction proteins. Changes in the microbiota occur during vitamin D deficiency due to unregulated inflammation and increased gastrointestinal permeability. Dysbiosis of the microbiota during vitamin D deficiency further induces local and systemic inflammation.
Vitamin D and the microbiota
Vitamin D regulates the innate immune response to the microbiota. The expression of several pattern recognition receptors including NOD2, which is genetically linked to the development of Crohn’s disease, is regulated by vitamin D (7, 8, 68). NOD2 recognizes bacterial peptidoglycans and induces bacterial killing both through autophagy of intracellular pathogens, and by promoting antimicrobial peptide production (69). Vitamin D response elements were in the promoter region of NOD2, and 1,25(OH)2D3, induced expression of NOD2 in human monocytes (68). In addition, human macrophages and dendritic cells have been shown to utilize vitamin D to induce the production of several antimicrobial peptides (B-defensin and cathelicidin), suggesting that vitamin D may regulate host responses to bacteria (68). Decreased expression of angiogenin-4 mRNA and protein has been reported in vitamin D-deficient mice (70). Conversely, increased susceptibility of Cyp27B1 KO (1,25(OH)2D3 deficient) mice to DSS colitis was not associated with changes in antibacterial peptides (cathelicidin, angiogenin-4), mucins, or Toll like receptor expression in the colon (47). The discrepancy in results may be because humans and mice do not express the same antimicrobial peptides and the murine cathelicidin gene is not regulated by 1,25(OH)2D3 (71). Interestingly the increased susceptibility of VDR KO and Cyp27B1 KO mice to experimental IBD was associated with changes in the microbiota in the gut and antibiotic disruption of the microbiota protected VDR KO and Cyp27B1 KO mice for IBD symptoms (47).
Prokaryotes do not express the VDR and therefore effects of vitamin D on the microbiota must be indirect effects on the host that change the microbiome. Cyp27B1 KO and VDR KO mice had increased frequencies of Proteobacteria phylum members compared to WT littermates (Fig. 1, (47)). More specifically Cyp27B1 KO mice had higher levels of the Helicobacteraceae family (Proteobacteria phyla) members than WT mice (47). Helicobacter species have been shown to induce IBD symptoms in IL-10 KO mice (72). Other bacteria from the Proteobacteria phylum (Salmonella) have developed strategies that utilize the host inflammatory response to outcompete commensal organisms (73). Our data is consistent with a model where the high levels of inflammation in the gut caused a shift in the microbiota so that more pathogenic organisms (Proteobacteria) out-compete the commensals causing dysbiosis (Fig. 1). The result of the shift in the composition of the bacterial microbiota is an amplified response of the host to injury. The VDR and 1,25(OH)2D3 regulated the microbiome indirectly to maintain tolerance in the gastrointestinal tract. It would be of interest to determine what the effect of vitamin D might be on the human microbiome and whether the effects of vitamin D could be narrowed to specific effects on a microbial family or species.
Conclusions
Vitamin D deficiency exacerbates experimental animal models of IBD. Vitamin D is required for the integrity of the gut epithelium. In addition, vitamin D and 1,25(OH)2D3 are critical regulators of T cell function. In the absence of vitamin D there are many effector T cells that produce IFN-γ and IL-17 in the gut. 1,25(OH)2D3 and vitamin D promote regulatory T cell development and function to turn off the Th1 and Th17 cells and to control inflammation in the gut. Uncontrolled inflammation in the gut leads to dysbiosis in the vitamin D deficient host. The ability of vitamin D and the VDR to inhibit Th1, Th17 cells, induce regulatory T cells and reduce inflammation resulted in a shift in the microbiome and maintenance of tolerance in the gut. Overall the available data support a strong environmental link between vitamin D and experimental IBD; more needs to be done to determine how this might translate to the prevention and maintenance of gastrointestinal homeostasis in human patients with IBD.
Acknowledgments>
This work was supported in part by grants to M.T.C. from the National Institutes of Health/National Institutes of Neurologic and Stroke Grant [NS067563] and National Center for Complementary and Alternative Medicine and the Office of Dietary Supplements [AT005378].
References
- 1.DeLuca HF, Vitamin D. Nutrition Today. 1993;28:6–11. [Google Scholar]
- 2.Clemens TL, Adams JS, Nolan JM, Holick MF. Measurement of circulating vitamin D in man. Clin Chim Acta. 1982;121(3):301–8. doi: 10.1016/0009-8981(82)90239-x. [DOI] [PubMed] [Google Scholar]
- 3.Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448(7152):427–34. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 4.Wu F, Dassopoulos T, Cope L, Maitra A, Brant SR, Harris ML, Bayless TM, Parmigiani G, Chakravarti S. Genome-wide gene expression differences in Crohn’s disease and ulcerative colitis from endoscopic pinch biopsies: insights into distinctive pathogenesis. Inflammatory bowel diseases. 2007;13(7):807–21. doi: 10.1002/ibd.20110. [DOI] [PubMed] [Google Scholar]
- 5.Satsangi J, Jewell DP, Bell JI. The genetics of inflammatory bowel disease. Gut. 1997;40(5):572–4. doi: 10.1136/gut.40.5.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cho JH. The genetics and immunopathogenesis of inflammatory bowel disease. Nat Rev Immunol. 2008;8(6):458–66. doi: 10.1038/nri2340. [DOI] [PubMed] [Google Scholar]
- 7.Verma R, Ahuja V, Paul J. Frequency of single nucleotide polymorphisms in NOD1 gene of ulcerative colitis patients: a case-control study in the Indian population. BMC Med Genet. 2009;10:82. doi: 10.1186/1471-2350-10-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Naser SA, Arce M, Khaja A, Fernandez M, Naser N, Elwasila S, Thanigachalam S. Role of ATG16L, NOD2 and IL23R in Crohn’s disease pathogenesis. World J Gastroenterol. 2012;18(5):412–24. doi: 10.3748/wjg.v18.i5.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moran CJ, Walters TD, Guo CH, Kugathasan S, Klein C, Turner D, Wolters VM, Bandsma RH, Mouzaki M, Zachos M, Langer JC, Cutz E, Benseler SM, Roifman CM, Silverberg MS, Griffiths AM, Snapper SB, Muise AM. IL-10R polymorphisms are associated with very-early-onset ulcerative colitis. Inflamm Bowel Dis. 2013;19(1):115–23. doi: 10.1002/ibd.22974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xue LN, Xu KQ, Zhang W, Wang Q, Wu J, Wang XY. Associations between vitamin D receptor polymorphisms and susceptibility to ulcerative colitis and Crohn’s disease: a meta-analysis. Inflammatory bowel diseases. 2013;19(1):54–60. doi: 10.1002/ibd.22966. [DOI] [PubMed] [Google Scholar]
- 11.Molodecky NA, Kaplan GG. Environmental risk factors for inflammatory bowel disease. Gastroenterology & hepatology. 2010;6(5):339–46. [PMC free article] [PubMed] [Google Scholar]
- 12.Schultz M, Butt AG. Is the north to south gradient in inflammatory bowel disease a global phenomenon? Expert Rev Gastroenterol Hepatol. 2012;6(4):445–7. doi: 10.1586/egh.12.31. [DOI] [PubMed] [Google Scholar]
- 13.Cantorna MT, Mahon BD. Mounting evidence for vitamin D as an environmental factor affecting autoimmune disease prevalence. Exp Biol Med (Maywood) 2004;229(11):1136–42. doi: 10.1177/153537020422901108. [DOI] [PubMed] [Google Scholar]
- 14.Jussila A, Virta LJ, Salomaa V, Maki J, Jula A, Farkkila MA. High and increasing prevalence of inflammatory bowel disease in Finland with a clear North-South difference. J Crohns Colitis. 2013;7(7):e256–62. doi: 10.1016/j.crohns.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 15.Khalili H, Huang ES, Ananthakrishnan AN, Higuchi L, Richter JM, Fuchs CS, Chan AT. Geographical variation and incidence of inflammatory bowel disease among US women. Gut. 2012;61(12):1686–92. doi: 10.1136/gutjnl-2011-301574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sonnenberg A, McCarty DJ, Jacobsen SJ. Geographic variation of inflammatory bowel disease within the United States. Gastroenterology. 1991;100(1):143–9. doi: 10.1016/0016-5085(91)90594-b. [DOI] [PubMed] [Google Scholar]
- 17.Nerich V, Jantchou P, Boutron-Ruault MC, Monnet E, Weill A, Vanbockstael V, Auleley GR, Balaire C, Dubost P, Rican S, Allemand H, Carbonnel F. Low exposure to sunlight is a risk factor for Crohn’s disease. Alimentary pharmacology & therapeutics. 2011;33(8):940–5. doi: 10.1111/j.1365-2036.2011.04601.x. [DOI] [PubMed] [Google Scholar]
- 18.Backhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307(5717):1915–20. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- 19.Xu J, Gordon JI. Inaugural Article: Honor thy symbionts. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(18):10452–9. doi: 10.1073/pnas.1734063100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hooper LV, Midtvedt T, Gordon JI. How host-microbial interactions shape the nutrient environment of the mammalian intestine. Annual review of nutrition. 2002;22:283–307. doi: 10.1146/annurev.nutr.22.011602.092259. [DOI] [PubMed] [Google Scholar]
- 21.Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci U S A. 2007;104(34):13780–5. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guarner F. What is the role of the enteric commensal flora in IBD? Inflammatory bowel diseases. 2008;14(Suppl 2):S83–4. doi: 10.1002/ibd.20548. [DOI] [PubMed] [Google Scholar]
- 23.Sartor RB. Therapeutic manipulation of the enteric microflora in inflammatory bowel diseases: antibiotics, probiotics, and prebiotics. Gastroenterology. 2004;126(6):1620–33. doi: 10.1053/j.gastro.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 24.Luther J, Dave M, Higgins PD, Kao JY. Association between Helicobacter pylori infection and inflammatory bowel disease: a meta-analysis and systematic review of the literature. Inflammatory bowel diseases. 2010;16(6):1077–84. doi: 10.1002/ibd.21116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hviid A, Svanstrom H, Frisch M. Antibiotic use and inflammatory bowel diseases in childhood. Gut. 2011;60(1):49–54. doi: 10.1136/gut.2010.219683. [DOI] [PubMed] [Google Scholar]
- 26.Kronman MP, Zaoutis TE, Haynes K, Feng R, Coffin SE. Antibiotic exposure and IBD development among children: a population-based cohort study. Pediatrics. 2012;130(4):e794–803. doi: 10.1542/peds.2011-3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kitajima S, Morimoto M, Sagara E, Shimizu C, Ikeda Y. Dextran sodium sulfate-induced colitis in germ-free IQI/Jic mice. Exp Anim. 2001;50(5):387–95. doi: 10.1538/expanim.50.387. [DOI] [PubMed] [Google Scholar]
- 28.Sellon RK, Tonkonogy S, Schultz M, Dieleman LA, Grenther W, Balish E, Rennick DM, Sartor RB. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infection and immunity. 1998;66(11):5224–31. doi: 10.1128/iai.66.11.5224-5231.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kamada N, Kim YG, Sham HP, Vallance BA, Puente JL, Martens EC, Nunez G. Regulated virulence controls the ability of a pathogen to compete with the gut microbiota. Science. 2012;336(6086):1325–9. doi: 10.1126/science.1222195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blanck S, Aberra F. Vitamin d deficiency is associated with ulcerative colitis disease activity. Dig Dis Sci. 2013;58(6):1698–702. doi: 10.1007/s10620-012-2531-7. [DOI] [PubMed] [Google Scholar]
- 31.Ananthakrishnan AN, Khalili H, Higuchi LM, Bao Y, Korzenik JR, Giovannucci EL, Richter JM, Fuchs CS, Chan AT. Higher predicted vitamin D status is associated with reduced risk of Crohn’s disease. Gastroenterology. 2012;142(3):482–9. doi: 10.1053/j.gastro.2011.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mouli VP, Ananthakrishnan AN. Review article: vitamin D and inflammatory bowel diseases. Alimentary pharmacology & therapeutics. 2014;39(2):125–36. doi: 10.1111/apt.12553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stenson WF, Cort D, Rodgers J, Burakoff R, DeSchryver-Kecskemeti K, Gramlich TL, Beeken W. Dietary supplementation with fish oil in ulcerative colitis. Annals of internal medicine. 1992;116(8):609–14. doi: 10.7326/0003-4819-116-8-609. [DOI] [PubMed] [Google Scholar]
- 34.Jorgensen SP, Agnholt J, Glerup H, Lyhne S, Villadsen GE, Hvas CL, Bartels LE, Kelsen J, Christensen LA, Dahlerup JF. Clinical trial: vitamin D3 treatment in Crohn’s disease - a randomized double-blind placebo-controlled study. Aliment Pharmacol Ther. 2010;32(3):377–83. doi: 10.1111/j.1365-2036.2010.04355.x. [DOI] [PubMed] [Google Scholar]
- 35.Yang L, Weaver V, Smith JP, Bingaman S, Hartman TJ, Cantorna MT. Therapeutic effect of vitamin d supplementation in a pilot study of Crohn’s patients. Clinical and translational gastroenterology. 2013;4:e33. doi: 10.1038/ctg.2013.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zator ZA, Cantu SM, Konijeti GG, Nguyen DD, Sauk J, Yajnik V, Ananthakrishnan AN. Pretreatment 25-Hydroxyvitamin D Levels and Durability of Anti-Tumor Necrosis Factor-alpha Therapy in Inflammatory Bowel Diseases. JPEN J Parenter Enteral Nutr. 2013 doi: 10.1177/0148607113504002. [DOI] [PubMed] [Google Scholar]
- 37.Cantorna MT. Vitamin D, multiple sclerosis and inflammatory bowel disease. Archives of biochemistry and biophysics. 2012;523(1):103–6. doi: 10.1016/j.abb.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Froicu M, Cantorna MT. Vitamin D and the vitamin D receptor are critical for control of the innate immune response to colonic injury. BMC Immunol. 2007;8:5. doi: 10.1186/1471-2172-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Froicu M, Weaver V, Wynn TA, McDowell MA, Welsh JE, Cantorna MT. A crucial role for the vitamin D receptor in experimental inflammatory bowel diseases. Mol Endocrinol. 2003;17(12):2386–92. doi: 10.1210/me.2003-0281. [DOI] [PubMed] [Google Scholar]
- 40.Cantorna MT, Munsick C, Bemiss C, Mahon BD. 1,25-Dihydroxycholecalciferol prevents and ameliorates symptoms of experimental murine inflammatory bowel disease. J Nutr. 2000;130(11):2648–52. doi: 10.1093/jn/130.11.2648. [DOI] [PubMed] [Google Scholar]
- 41.Daniel C, Sartory NA, Zahn N, Radeke HH, Stein JM. Immune modulatory treatment of trinitrobenzene sulfonic acid colitis with calcitriol is associated with a change of a T helper (Th) 1/Th17 to a Th2 and regulatory T cell profile. J Pharmacol Exp Ther. 2008;324(1):23–33. doi: 10.1124/jpet.107.127209. [DOI] [PubMed] [Google Scholar]
- 42.Zbar AP, Simopoulos C, Karayiannakis AJ. Cadherins: an integral role in inflammatory bowel disease and mucosal restitution. J Gastroenterol. 2004;39(5):413–21. doi: 10.1007/s00535-004-1335-8. [DOI] [PubMed] [Google Scholar]
- 43.Marchiando AM, Graham WV, Turner JR. Epithelial barriers in homeostasis and disease. Annual review of pathology. 2010;5:119–44. doi: 10.1146/annurev.pathol.4.110807.092135. [DOI] [PubMed] [Google Scholar]
- 44.Madsen KL, Malfair D, Gray D, Doyle JS, Jewell LD, Fedorak RN. Interleukin-10 gene-deficient mice develop a primary intestinal permeability defect in response to enteric microflora. Inflammatory bowel diseases. 1999;5(4):262–70. doi: 10.1097/00054725-199911000-00004. [DOI] [PubMed] [Google Scholar]
- 45.Su L, Shen L, Clayburgh DR, Nalle SC, Sullivan EA, Meddings JB, Abraham C, Turner JR. Targeted epithelial tight junction dysfunction causes immune activation and contributes to development of experimental colitis. Gastroenterology. 2009;136(2):551–63. doi: 10.1053/j.gastro.2008.10.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mullin JM, Laughlin KV, Marano CW, Russo LM, Soler AP. Modulation of tumor necrosis factor-induced increase in renal (LLC-PK1) transepithelial permeability. The American journal of physiology. 1992;263(5 Pt 2):F915–24. doi: 10.1152/ajprenal.1992.263.5.F915. [DOI] [PubMed] [Google Scholar]
- 47.Ooi JH, Li Y, Rogers CJ, Cantorna MT. Vitamin D regulates the gut microbiome and protects mice from dextran sodium sulfate-induced colitis. J Nutr. 2013;143(10):1679–86. doi: 10.3945/jn.113.180794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kong J, Zhang Z, Musch MW, Ning G, Sun J, Hart J, Bissonnette M, Li YC. Novel role of the vitamin D receptor in maintaining the integrity of the intestinal mucosal barrier. Am J Physiol Gastrointest Liver Physiol. 2008;294(1):G208–16. doi: 10.1152/ajpgi.00398.2007. [DOI] [PubMed] [Google Scholar]
- 49.Palmer HG, Gonzalez-Sancho JM, Espada J, Berciano MT, Puig I, Baulida J, Quintanilla M, Cano A, de Herreros AG, Lafarga M, Munoz A. Vitamin D(3) promotes the differentiation of colon carcinoma cells by the induction of E-cadherin and the inhibition of beta-catenin signaling. The Journal of cell biology. 2001;154(2):369–87. doi: 10.1083/jcb.200102028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yen D, Cheung J, Scheerens H, Poulet F, McClanahan T, McKenzie B, Kleinschek MA, Owyang A, Mattson J, Blumenschein W, Murphy E, Sathe M, Cua DJ, Kastelein RA, Rennick D. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J Clin Invest. 2006;116(5):1310–6. doi: 10.1172/JCI21404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, Wilkinson JE, Galas D, Ziegler SF, Ramsdell F. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27(1):68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 52.Ochs HD, Gambineri E, Torgerson TR. IPEX, FOXP3 and regulatory T-cells: a model for autoimmunity. Immunol Res. 2007;38(1-3):112–21. doi: 10.1007/s12026-007-0022-2. [DOI] [PubMed] [Google Scholar]
- 53.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8(7):523–32. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cheroutre H, Lambolez F. Doubting the TCR coreceptor function of CD8alphaalpha. Immunity. 2008;28(2):149–59. doi: 10.1016/j.immuni.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 55.Das G, Augustine MM, Das J, Bottomly K, Ray P, Ray A. An important regulatory role for CD4+CD8 alpha alpha T cells in the intestinal epithelial layer in the prevention of inflammatory bowel disease. Proc Natl Acad Sci U S A. 2003;100(9):5324–9. doi: 10.1073/pnas.0831037100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 57.Veldman CM, Cantorna MT, DeLuca HF. Expression of 1,25-dihydroxyvitamin D(3) receptor in the immune system. Arch Biochem Biophys. 2000;374(2):334–8. doi: 10.1006/abbi.1999.1605. [DOI] [PubMed] [Google Scholar]
- 58.Ooi JH, Chen J, Cantorna MT. Vitamin D regulation of immune function in the gut: why do T cells have vitamin D receptors? Mol Aspects Med. 2012;33(1):77–82. doi: 10.1016/j.mam.2011.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cantorna MT. Why do T cells express the vitamin D receptor? Annals of the New York Academy of Sciences. 2011;1217:77–82. doi: 10.1111/j.1749-6632.2010.05823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bruce D, Yu S, Ooi JH, Cantorna MT. Converging pathways lead to overproduction of IL-17 in the absence of vitamin D signaling. Int Immunol. 2011;23(8):519–28. doi: 10.1093/intimm/dxr045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cantorna MT, Yu S, Bruce D. The paradoxical effects of vitamin D on type 1 mediated immunity. Mol Aspects Med. 2008;29(6):369–75. doi: 10.1016/j.mam.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Muller K, Odum N, Bendtzen K. 1,25-dihydroxyvitamin D3 selectively reduces interleukin-2 levels and proliferation of human T cell lines in vitro. Immunol Lett. 1993;35(2):177–82. doi: 10.1016/0165-2478(93)90088-j. [DOI] [PubMed] [Google Scholar]
- 63.Rigby WF, Noelle RJ, Krause K, Fanger MW. The effects of 1,25-dihydroxyvitamin D3 on human T lymphocyte activation and proliferation: a cell cycle analysis. J Immunol. 1985;135(4):2279–86. [PubMed] [Google Scholar]
- 64.Yu S, Bruce D, Froicu M, Weaver V, Cantorna MT. Failure of T cell homing, reduced CD4/CD8alphaalpha intraepithelial lymphocytes, and inflammation in the gut of vitamin D receptor KO mice. Proc Natl Acad Sci U S A. 2008;105(52):20834–9. doi: 10.1073/pnas.0808700106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yu S, Cantorna MT. The vitamin D receptor is required for iNKT cell development. Proc Natl Acad Sci U S A. 2008;105(13):5207–12. doi: 10.1073/pnas.0711558105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yu S, Cantorna MT. Epigenetic reduction in invariant NKT cells following in utero vitamin D deficiency in mice. J Immunol. 2011;186(3):1384–90. doi: 10.4049/jimmunol.1002545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bruce D, Cantorna MT. Intrinsic requirement for the vitamin D receptor in the development of CD8alphaalpha-expressing T cells. J Immunol. 2011;186(5):2819–25. doi: 10.4049/jimmunol.1003444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang TT, Dabbas B, Laperriere D, Bitton AJ, Soualhine H, Tavera-Mendoza LE, Dionne S, Servant MJ, Bitton A, Seidman EG, Mader S, Behr MA, White JH. Direct and indirect induction by 1,25-dihydroxyvitamin D3 of the NOD2/CARD15-defensin beta2 innate immune pathway defective in Crohn disease. The Journal of biological chemistry. 2010;285(4):2227–31. doi: 10.1074/jbc.C109.071225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Homer CR, Richmond AL, Rebert NA, Achkar JP, McDonald C. ATG16L1 and NOD2 interact in an autophagy-dependent antibacterial pathway implicated in Crohn’s disease pathogenesis. Gastroenterology. 2010;139(5):1630–41. 41 e1–2. doi: 10.1053/j.gastro.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lagishetty V, Misharin AV, Liu NQ, Lisse TS, Chun RF, Ouyang Y, McLachlan SM, Adams JS, Hewison M. Vitamin D deficiency in mice impairs colonic antibacterial activity and predisposes to colitis. Endocrinology. 151(6):2423–32. doi: 10.1210/en.2010-0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gombart AF, Borregaard N, Koeffler HP. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2005;19(9):1067–77. doi: 10.1096/fj.04-3284com. [DOI] [PubMed] [Google Scholar]
- 72.Kullberg MC, Ward JM, Gorelick PL, Caspar P, Hieny S, Cheever A, Jankovic D, Sher A. Helicobacter hepaticus triggers colitis in specific-pathogen-free interleukin-10 (IL-10)-deficient mice through an IL-12- and gamma interferon-dependent mechanism. Infection and immunity. 1998;66(11):5157–66. doi: 10.1128/iai.66.11.5157-5166.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Winter SE, Baumler AJ. A breathtaking feat: to compete with the gut microbiota, Salmonella drives its host to provide a respiratory electron acceptor. Gut microbes. 2011;2(1):58–60. doi: 10.4161/gmic.2.1.14911. [DOI] [PMC free article] [PubMed] [Google Scholar]