Abstract
Background Many vestibular schwannoma (VS) patients complain of balance dysfunction; however, validated standardized assessments are lacking. The relative contribution of imbalance and factors like anxiety to disability is unknown. Because imbalance significantly affects quality of life in this group and vestibular rehabilitation may improve outcomes, determining the severity of balance dysfunction is important to understand long-term rehabilitation needs.
Aim To assess functional balance (Vertigo Symptom Scale-Vertigo [VSS-VER] and Functional Gait Assessment [FGA]) and the relative contribution of symptom severity (VSS-VER), ambulant posture (FGA), and anxiety symptoms (Vertigo Symptom Scale-Anxiety [VSS-SA]) to disability in untreated patients.
Methods Patients not exposed to surgery completed the VSS, Vertigo Handicap Questionnaire (VHQ), and FGA. VSS scores were compared with migrainous vertigo (MV) patients, a mixed neuro-otological group, and healthy controls.
Results A correlation was found between decreased FGA and increasing age (r = − 0.35; p < 0.01), female sex (r = 0.42; p = 0.001), increasing handicap (r = − 0.55; p < 0.001), and symptom severity (r = − 0.52; p < 0.001). In 12 of 21 patients (57%) > 60 years of age the FGA score was ≤ 22 suggesting increased falls risk.
VSS-VER scores were higher than in healthy controls (p < 0.001) but lower than MV (p < 0.001) and mixed neuro-otology controls (p < 0.001).
VSS-SA scores in VS patients with balance symptoms were higher than normal controls (p < 0.05) and correlated with handicap (r = 0.59; p < 0.001) and symptom severity (r = 0.74; p < 0.001).
After controlling for age and sex, the VSS-VER, VSS-SA, and FGA explained 47% of the variation in VHQ scores.
Conclusion Older VS patients are at significant risk of falls. Balance symptoms are more severe than in healthy controls but less than other neuro-otological patients. Balance symptom severity, anxiety symptoms, and ambulant posture were significant contributors to disability and should be the focus of vestibular rehabilitation strategies.
Keywords: vestibular schwannoma, balance, falls, handicap, anxiety
Introduction
Vestibular schwannoma (VS) is a slow-growing benign tumor arising from the vestibular nerves presenting with unilateral hearing loss, tinnitus, and balance dysfunction, and following diagnostic imaging, management may involve observation with serial magnetic resonance imaging or treatment with microsurgery or radiotherapy.1 In addition, symptomatic rehabilitation may be necessary.
One review2 found that the majority of both pre- and postoperative patients complain of some degree of balance dysfunction. Overall, however, a lack of validated standardized instruments to measure the severity of balance symptoms and functional balance was noted, and this may account in part for the wide range in reported prevalence. In addition, the reviewed studies largely failed to determine the relative contribution of these and other pertinent factors such as anxiety to overall disability.
Vestibular rehabilitation may accelerate recovery in balance function following surgical deafferentation.3 In VS patients planned for surgery, preoperative vestibular rehabilitation, or so-called “prehab”, was shown to improve outcomes postoperatively.4 Vestibular rehabilitation may also be of value in observed VS patients because balance dysfunction has been shown to significantly affect quality of life (QOL) in this group.5 6 In untreated patients, assessment of the severity of subjective balance symptoms and functional balance could thus be useful to identify patients who may benefit from vestibular rehabilitation and offers the potential to tailor vestibular rehabilitation strategies to patient needs.
Neuro-otological (N-O) researchers have developed instruments such as the Vertigo Symptom Scale (VSS),7 Vertigo Handicap Questionnaire (VHQ),8 and Functional Gait Assessment (FGA)9 that are freely available, easy to use, well validated, and add little time to the busy clinic.
In this study we used the VSS to assess the severity of balance symptoms (Vertigo Symptom Scale-Vertigo [VSS-VER]) and the autonomic and somatic symptoms of anxiety (Vertigo Symptom Scale-Anxiety [VSS-SA]), and the FGA to assess functional balance related to ambulant posture. We then attempted to determine the relative contributions of these factors to balance-related disability measured using the VHQ.
The aims of this study were as follows:
To assess the severity of balance symptoms (VSS-VER), balance-related anxiety symptoms (VSS-SA), and functional balance related to ambulant posture (FGA) in untreated vestibular schwannoma patients.
To determine if the severity of balance symptoms (VSS-VER), functional balance related to ambulant posture (FGA), and the autonomic and somatic symptoms of anxiety (VSS-SA) are significant contributors to balance-related disability (VHQ).
Materials and Methods
Study Design and Patient Selection
This cross-sectional study was conducted between December 2010 and December 2012 at the National Hospital for Neurology and Neurosurgery (NHNN), Department of Neuro-otology. Consecutive patients with vestibular schwannoma who had not been exposed to surgery were recruited from the senior author's (M.G.) skull base cohort. All patients were assessed using the VSS, VHQ, and FGA. We used VSS historical control data from other groups of patients attending our department who had been given the VSS during their first visit. These groups included:
Migrainous vertigo (MV) patients who were diagnosed using a structured physician-administered interview/questionnaire to determine history of migraine and vestibular migraine according to standard criteria (International Headache Society Headache Classification Committee 2004)10 11
A mixed neuro-otological (N-O) group presenting with dizziness
In addition, as a further control, a group of healthy individuals were recruited from hospital staff.
Local ethical committee approval (Central London REC 3) was obtained prior to commencement of this study. All statistical analysis was performed using SPSS v.21 software (IBM, Inc., Armonk, New York, United States).
Clinical Tools to Assess Balance Function
Vertigo Symptom Scale
The VSS assesses varying presentations of dizziness and unsteadiness and associated complaints such as nausea and vomiting as well as symptoms and signs of anxiety arousal and somatization. Responses are considered over a year or from the onset of symptoms and scaled from 0 (never) to 4 (very often; on average more than once a week). The questionnaire contains 34 items and produces two subscales reflecting the severity of balance symptoms (VSS-VER) and anxiety symptoms (VSS-SA) as a function of frequency and, when appropriate, duration.7 The VSS-SA yields further subscales describing autonomic symptoms (AUs) and somatization symptoms (SOMs), and convergent validity was verified by the correlation of AU and SOM scales with the HAD anxiety scale (r = 0.43 and 0.46). The Cronbach α is 0.69 to 0.83 according to dimensions, and test-retest reproducibility resulted in an intraclass coefficient of r = 0.89 to 0.98.12 Somatic anxiety and arousal symptoms at baseline were predictive of increased handicap (VHQ) 7 months later.13
Vertigo Handicap Questionnaire
The VHQ assesses the physical, practical, and emotional impact of vertigo with 25 items targeting the disruption and restriction of physical and social activities including relationships and leisure pursuits. Certain items assess perceived social support and stigmatization as well as emotional upset. The questionnaire content was based on statements from open-ended interviews of patients with vertigo and reflects the most frequent psychosocial consequences of balance dysfunction.8 Disability or handicap are rated on a numeric scale from 0 (never or no handicap) to 4 (always or maximum handicap). The VHQ was found to have a high internal consistency (Cronbach α= 0.93) and test-retest reliability (0.97).12 14
Functional Gait Assessment
The FGA, a dynamic balance assessment based on the Dynamic Gait Index (DGI), was developed to evaluate postural stability during ambulation tasks in patients with vestibular disorders and has been found to be a valid and reliable tool.9 Seven of the eight items from the DGI have been incorporated into the FGA. Three new items were added to overcome the ceiling effect when used with vestibular disorder patients. The assessment involves 10 gait-related tasks performed on a marked runway that are scored by the tester. A higher score indicates better ambulant postural mobility. A Cronbach α of 0.79 demonstrated a high internal consistency. The FGA is well correlated with the Dizziness Handicap Index (r = − 0.64) and reported number of falls (r = − 0.66). Interrater and intrarater reliability was demonstrated with intraclass correlation coefficients of 0.84 and 0.83, respectively.9
Results
Subjects
Sixty-three consecutive follow-up cases with untreated VS were recruited, 27 women (43%) and 36 men (57%) with a mean age of 53.4 years (standard deviation [SD]: 11.8). There were 38 MV controls with a mean age of 38.4 years (SD: 12.1). Of the 42 mixed N-O control patients presenting with dizziness, 14 were diagnosed with a peripheral vestibular disorder confirmed on caloric testing, 6 had a condition being managed as a peripheral vestibular disorder although objective tests were normal, 6 had benign paroxysmal positional vertigo, 7 were known to have Meniere disease, 4 had a central vestibular disorder, 1 had idiopathic intracranial hypertension, 2 were found to have postural hypotension, and 2 had other causes of dizziness. The mean age in this group was 45.5 years (SD: 12.4). There were 37 healthy controls recruited from hospital staff with a mean age of 48.35 years (SD: 9). Table 1 describes the sex distribution for the control groups.
Table 1. Sex and age distribution for vestibular schwannoma and control groups.
| Neuro-otology group | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Vestibular schwannoma | Migrainous vertigo | Mixed neuro-otology | Normal | ||||||
| Mean (SD) |
No. (%) |
Mean (SD) |
No. (%) |
Mean (SD) |
No. (%) |
Mean (SD) |
No. (%) |
||
| Age, y | 53 (12) |
38 (12) |
46 (12) |
48 (9) |
|||||
| Sex | Female | 27 (43) |
29 (76) |
27 (64) |
17 (46) |
||||
| Male | 36 (57) |
9 (24) |
15 (35) |
20 (54) |
|||||
| Total | 63 | 38 | 42 | 37 | |||||
Functional Balance
The FGA ranged from 10 to 30 with a median score of 26 and a mean of 24.3 (SD: 5.1). A Spearman rho test found a significant correlation between decreased FGA and increasing age (r = − 0.35; p < 0.01), female sex (r = 0.42; p = 0.001), increasing disability (VHQ) (r = − 0.55; p < 0.001), and symptom severity (VSS-VER) (r = − 0.52; p < 0.001). Further analysis with a Mann-Whitney U test comparing FGA scores in patients younger and older than 60 years found a significant difference between the two groups (p = 0.001). The median score in the ≥60 years of age group was 22 and was 27 in the < 60 years of age group (Table 2).
Table 2. Functional gait assessment according to age distribution.
| Age, y | Functional gait assessment | |||||
|---|---|---|---|---|---|---|
| Count (%) | Median | Mean | SD | Minimum | Maximum | |
| ≥ 60 | 21 (33) | 22 | 21 | 6 | 10 | 29 |
| < 60 | 42 (67) | 27 | 26 | 3 | 15 | 30 |
Abbreviation: SD, standard deviation.
Severity of Balance Symptoms
The mean VSS-VER score for VS patients was 0.46 (SD: 0.51). VSS-VER scores for the MV, mixed N-O and healthy controls are described in Table 3 and Fig. 1. Using a Kruskal-Wallis test, VSS-VER scores were significantly different between groups (p < 0.001). Mann-Whitney U tests were used to follow up the finding, and a Bonferroni correction was applied. VSS-VER scores in VS patients were found to be significantly higher than normal controls (p < 0.001) but significantly lower than MV (p < 0.001) and mixed N-O controls (p < 0.001). Twelve VS patients scored zero on the VSS-VER; the remaining 51 patients (81%) reported some degree of balance dysfunction in the year prior to assessment.
Table 3. Vertigo Symptom Scale balance severity scores in vestibular schwannoma and control groups.
| VSS-VER | ||||||
|---|---|---|---|---|---|---|
| Mean | SD | Median | Minimum | Maximum | ||
| Neuro-otology group | VS | 0.46 | 0.51 | 0.32 | 0.00 | 2.74 |
| MV | 1.61 | 0.86 | 1.55 | 0.16 | 3.63 | |
| Mixed N-O | 1.05 | 0.85 | 0.82 | 0.05 | 3.53 | |
| Normal | 0.07 | 0.09 | 0.00 | 0.00 | 0.32 | |
Abbreviations: MV, migrainous vertigo ; N-O, neuro-otology; SD, standard deviation; VS, vestibular schwannoma; VSS-VER, Vertigo Symptom Scale-Vertigo.
Fig. 1.
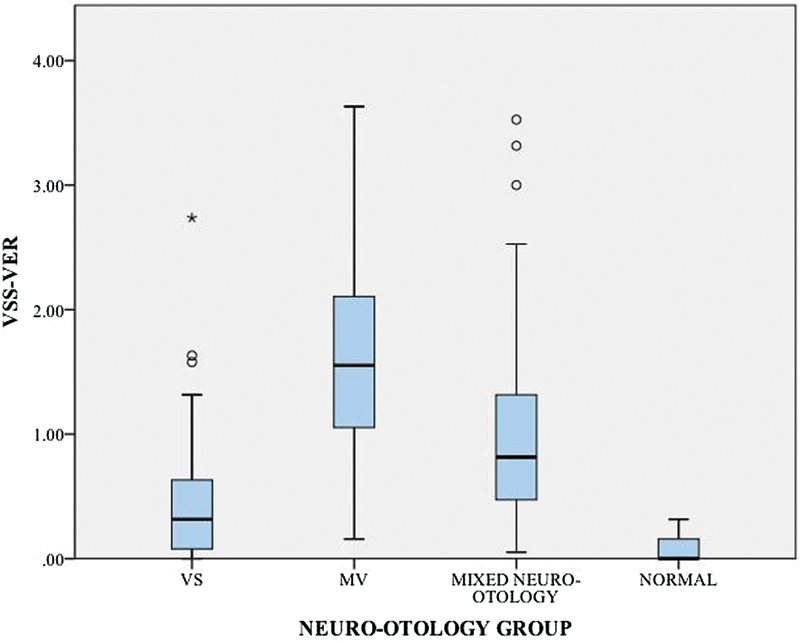
Box plots of Vertigo Symptom Scale balance severity scores in vestibular schwannoma (VS) and control groups. MV, migrainous vertigo; VSS-VER, Vertigo Symptom Scale-Vertigo.
Severity of Balance-Related Anxiety Symptoms
Anxiety symptoms in VS patients were compared with anxiety symptoms in the normal control group using a Mann-Whitney U test. VSS-SA scores in VS patients with balance symptoms were found to be significantly higher than normal controls (p < 0.01). In the untreated VS group, anxiety symptoms were significantly correlated with balance-related disability (VHQ) (r = 0.59; p < 0.001) and balance symptom severity (VSS-VER) (r = 0.74; p < 0.001).
Balance-Related Handicap
The VHQ ranged from 0 to 78 with a median score of 11 and a mean of 16.4 (SD: 18.3). Using Spearman rho, a significant correlation was found between the VHQ and the VSS-VER (r = 0.83; p < 0.001), the VSS-SA (r = 0.59; p < 0.001), female sex (r = 0.44; p < 0.001), and the FGA (r = − 0.55; p < 0.001). Using a Mann-Whitney U test, women were found to have a significantly higher VHQ score compared with men (p < 0.001).
A hierarchical regression model was undertaken to determine whether the relationship between balance-related disability, balance symptom severity, anxiety symptoms, and ambulant posture could be explained by the association between age, sex, and the factors just listed. After controlling for age and sex in the first step, the addition of the VSS-VER, VSS-SA, and FGA in the second step explained a highly significant percentage (47%) of the variation in VHQ scores. The total equation accounted for > 67% of the variance in the VHQ (R2 = 0.67 F(5.57) = 22.9; p < 0.001). In this model, balance symptom severity, autonomic and somatic symptoms of anxiety, and ambulant postural stability were significant predictors of handicap (Table 4).
Table 4. Hierarchical regression controlling for age and sex with the Vertigo Handicap Questionnaire as the dependent factor.
| R2 change | β | ||
|---|---|---|---|
| 1 | 0.20*** | ||
| Sex | − 0.42*** | ||
| Age | .08 | ||
| 2 | 0.47*** | ||
| Sex | − 0.12 | ||
| Age | 0.05 | ||
| VSS-VER | 0.33** | ||
| FGA | − 0.30** | ||
| VSS-SA | 0.27* | ||
Abbreviations: FGA, Functional Gait Assessment, VSS-SA, Vertigo Symptom Scale Anxiety; VSS-VER, Vertigo Symptom Scale-Vertigo.
*p < 0.05.
**p < 0.01.
***p < 0.001.
Discussion
This is the first study to assess ambulant postural stability using the FGA in VS patients. We found that decreased FGA scores correlate with increasing age with patients older than 60 years significantly worse off. Walker et al15 provided reference data in a study of community living adults with subjects' normative data described according to age groups in decades. Table 5 shows the cases falling below the confidence intervals described in Walker et al.16
Table 5. Subjects according to decade falling below the confidence intervalsa .
| Age, y | 95% confidence interval | Cases below confidence interval (%) |
|---|---|---|
| 40–49 | 28.3–29.5 | 9 (70) |
| 50–59 | 27.9–29.0 | 14 (67) |
| 60–69 | 26.5–27.7 | 14 (78) |
| 70–79 | 23.9–26.0 | 1 (33) |
Based on normative data for the Functional Gait Assessment as described by Walker et al.15
According to Wrisley et al17 a cut-off score off 22 has 100% sensitivity and 72% specificity in predicting falls in community-dwelling older adults (> 60 years). In our group of 21 patients ≥ 60 years, the score was ≤ 22 in 12 patients (57%) suggesting a high risk for falls. A recent systematic review found that balance problems confer an increased risk of falls in older community-dwelling adults and this lends support to our findings.18 Falls in the elderly cost the National Health Service and Personal Social Services close to £1 billion in 1999.19 An American study has shown that falls are a leading cause of death and injury in elderly patients.20 Appropriate interventions may be helpful in decreasing the risk of falls.21 We therefore suggest that older patients (> 60 years) be formerly assessed for risk of falls followed by the implementation of a vestibular rehabilitation program. This may have even greater importance when planning for postoperative care when these patients may be more vulnerable.
In addition, we found that ambulant postural stability was a significant predictor of disability (β = − 0.30; p < 0.01) as well as balance symptom severity (β = 0.33; p < 0.01) and symptoms of anxiety (β = 0.27; p < 0.05). Vestibular rehabilitation strategies targeting postural stability may therefore be important in improving QOL. The findings that anxiety symptoms are a significant predictor of balance-related disability and are strongly correlated with balance symptom severity are findings corroborated across the spectrum of N-O disease. Importantly, anxiety symptoms measured using the VSS–SA have been found to be a longitudinal predictor of balance-related disability and vertigo severity, and it has been argued that symptoms of anxiety and stress in response to balance problems reinforce balance symptoms, producing a vicious cycle of increasing balance-related anxiety with increasing disability.13 22 Tackling anxiety symptoms early on as part of a vestibular rehabilitation strategy may thus help to prevent a worsening of vestibular symptoms and balance-related disability.
This is the first study to use a validated standardized questionnaire, the VSS-VER, to assess the severity of balance symptoms in untreated VS patients. Myrseth et al6 compared 199 untreated VS patients with healthy controls. Patients were asked to respond “yes” or “no” to whether dizziness or balance problems were significant complaints, and 40.6% responded affirmatively. In addition, 97 patients completed a vertigo visual analog scale (VAS) with 56.2% reporting vertigo, described as intermittent (42.9%), constant (13%), “nautical” (50%), “rotatory” (25.8%), or “other” (24.2%), and 38% complained of unsteadiness. Mean VAS scores for patients were significantly higher than controls. Lloyd et al5 found that 46.1% of 165 conservatively managed patients reported imbalance. Vogel et al23 report that of 79 VS patients, 38% reported a balance disorder when assessed prior to treatment allocation. Using nonvalidated assessments, 50 to 69% of preoperative patients complained of balance dysfunction.2 In our cohort, 81% of patients reported some degree of balance dysfunction when completing the VSS-VER, and of this group 25 (47%) had scores significantly greater than the normal control group (mean + 2 SD).
The VSS (VER) inquires about these symptoms:
Sensation of spinning or moving around
Unsteadiness: about to lose balance
Unsteadiness severe enough to fall
Feeling lightheaded or feeling giddy
Being unable to stand or walk properly
Nausea or vomiting
It assesses symptoms as a function of their duration and frequency in the year prior to assessment. The use of a validated standardized questionnaire taking into account various vestibular presentations is possibly more sensitive in assessing the true prevalence of balance symptoms. However, although prevalent, in our cohort of VS patients, the severity of balance symptoms tends to feature at the lower end of the spectrum of N-O disease. We compared our untreated VS group with other disorders across the N-O spectrum and found that the severity of balance symptoms was significantly greater than normal controls although not as severe as the MV group or the mixed group of patients presenting with dizziness. Because the tumor is slow growing, its effect on the vestibular system is gradual, allowing compensation to occur. This may explain our results suggesting that most VS patients are possibly better compensated with regard to vestibular symptoms unlike MV, Meniere disease, or benign paroxysmal positional vertigo, which are characterized by repeated, sometimes unpredictable attacks of varying intensity with periods of remission that have significant implications for compensation. In the patients who do present with severe balance symptoms, we suggest that reasons for poor compensation should be investigated as well as the possibility of an alternative vestibular diagnosis.
Myrseth et al6 found that vertigo was significantly associated with a decrease in QOL across all axes on the SF-36 and the general and physical parts of the GBI, whereas vertigo VAS scores were significantly associated with eight axes on the SF-36 and GBI. Lloyd et al5 reported that both the presence of imbalance and increasing Dizziness Handicap Inventory (DHI) scores were significantly associated with decreased QOL (SF-36). Breivik et al24 reported similar findings in 193 observed patients with reduced QOL (SF-36) in the presence of vertigo. Importantly, all three studies reported that balance dysfunction was the most significant negative predictor of general QOL scores when compared with hearing loss or tinnitus.5 6 24 The mean DHI score reported in Lloyd et al5 was 15.1 (SD: 21.5). In 145 preoperative patients, Humphriss et al25 found a median DHI score of 14. Whitney et al26 classified the DHI into mild (0–30), moderate (31–60), and severe (61–100) levels of disability, suggesting that most patients in these studies would be classified as mild. Overall VHQ scores in our series were lower than those reported in a previous NHNN cohort of 127 N-O patients (mean: 44.1; SD: 17.1)22 and thus are in keeping with the low scores in cohorts that have used the DHI. These findings highlight the fact that even a so-called mild balance disability or handicap when compared with other N-O disease, can affect overall QOL more so than tinnitus and hearing impairment, and therefore must not be neglected as part of an holistic approach to rehabilitation in this patient group.
We set out to identify and measure treatable factors that contribute to decreased balance-related QOL in untreated VS patients using tools that are easy to use, standardized, and provide information that would inform vestibular rehabilitation strategies. In this study we draw attention to the relative roles of the subjective severity of balance symptoms, objective severity of functional balance dysfunction related to posture and mobility, and anxiety in terms of overall disability. Understanding the relative contribution of these factors in each patient will allow individualized vestibular rehabilitation programs to be developed.
Funding Source
This study did not depend on any external funding source.
Footnotes
Conflicts of Interest The authors have nothing to disclose.
References
- 1.Gleeson M, Scott-Brown W G. London, UK: Hodder Arnold; 2008. Scott-Brown's Otorhinolaryngology, Head and Neck Surgery. 7th ed. [Google Scholar]
- 2.Saman Y, Bamiou D E, Gleeson M. A contemporary review of balance dysfunction following vestibular schwannoma surgery. Laryngoscope. 2009;119(11):2085–2093. doi: 10.1002/lary.20648. [DOI] [PubMed] [Google Scholar]
- 3.Herdman S J, Clendaniel R A, Mattox D E, Holliday M J, Niparko J K. Vestibular adaptation exercises and recovery: acute stage after acoustic neuroma resection. Otolaryngol Head Neck Surg. 1995;113(1):77–87. doi: 10.1016/s0194-5998(95)70148-6. [DOI] [PubMed] [Google Scholar]
- 4.Magnusson M, Kahlon B, Karlberg M, Lindberg S, Siesjö P. Preoperative vestibular ablation with gentamicin and vestibular 'prehab' enhance postoperative recovery after surgery for pontine angle tumours—first report. Acta Otolaryngol. 2007;127(12):1236–1240. doi: 10.1080/00016480701663433. [DOI] [PubMed] [Google Scholar]
- 5.Lloyd S K, Kasbekar A V, Baguley D M, Moffat D A. Audiovestibular factors influencing quality of life in patients with conservatively managed sporadic vestibular schwannoma. Otol Neurotol. 2010;31(6):968–976. doi: 10.1097/mao.0b013e3181e8c7cb. [DOI] [PubMed] [Google Scholar]
- 6.Myrseth E Møller P Wentzel-Larsen T Goplen F Lund-Johansen M Untreated vestibular schwannomas: vertigo is a powerful predictor for health-related quality of life Neurosurgery 200659167–76.; discussion 67–76 [DOI] [PubMed] [Google Scholar]
- 7.Yardley L, Masson E, Verschuur C, Haacke N, Luxon L. Symptoms, anxiety and handicap in dizzy patients: development of the vertigo symptom scale. J Psychosom Res. 1992;36(8):731–741. doi: 10.1016/0022-3999(92)90131-k. [DOI] [PubMed] [Google Scholar]
- 8.Yardley L, Putman J. Quantitative analysis of factors contributing to handicap and distress in vertiginous patients: a questionnaire study. Clin Otolaryngol Allied Sci. 1992;17(3):231–236. doi: 10.1111/j.1365-2273.1992.tb01833.x. [DOI] [PubMed] [Google Scholar]
- 9.Wrisley D M, Marchetti G F, Kuharsky D K, Whitney S L. Reliability, internal consistency, and validity of data obtained with the functional gait assessment. Phys Ther. 2004;84(10):906–918. [PubMed] [Google Scholar]
- 10.Neuhauser H, Leopold M, von Brevern M, Arnold G, Lempert T. The interrelations of migraine, vertigo, and migrainous vertigo. Neurology. 2001;56(4):436–441. doi: 10.1212/wnl.56.4.436. [DOI] [PubMed] [Google Scholar]
- 11.Headache Classification Subcommittee of the International Headache Society . The International Classification of Headache Disorders. 2nd edition. Cephalalgia. 2004;24 01:9–160. doi: 10.1111/j.1468-2982.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- 12.Duracinsky M Mosnier I Bouccara D Sterkers O Chassany O; Working Group of the Société Française d'Oto-Rhino-Laryngologie (ORL).Literature review of questionnaires assessing vertigo and dizziness, and their impact on patients' quality of life Value Health 2007104273–284. [DOI] [PubMed] [Google Scholar]
- 13.Yardley L, Luxon L M, Haacke N P. A longitudinal study of symptoms, anxiety and subjective well-being in patients with vertigo. Clin Otolaryngol Allied Sci. 1994;19(2):109–116. doi: 10.1111/j.1365-2273.1994.tb01192.x. [DOI] [PubMed] [Google Scholar]
- 14.Yardley L, Verschuur C, Masson E, Luxon L, Haacke N. Somatic and psychological factors contributing to handicap in people with vertigo. Br J Audiol. 1992;26(5):283–290. doi: 10.3109/03005369209076649. [DOI] [PubMed] [Google Scholar]
- 15.Walker M L, Austin A G, Banke G M. et al. Reference group data for the functional gait assessment. Phys Ther. 2007;87(11):1468–1477. doi: 10.2522/ptj.20060344. [DOI] [PubMed] [Google Scholar]
- 16.Rigby P L, Shah S B, Jackler R K, Chung J H, Cooke D D. Acoustic neuroma surgery: outcome analysis of patient-perceived disability. Am J Otol. 1997;18(4):427–435. [PubMed] [Google Scholar]
- 17.Wrisley D M, Kumar N A. Functional gait assessment: concurrent, discriminative, and predictive validity in community-dwelling older adults. Phys Ther. 2010;90(5):761–773. doi: 10.2522/ptj.20090069. [DOI] [PubMed] [Google Scholar]
- 18.Muir S W, Berg K, Chesworth B, Klar N, Speechley M. Quantifying the magnitude of risk for balance impairment on falls in community-dwelling older adults: a systematic review and meta-analysis. J Clin Epidemiol. 2010;63(4):389–406. doi: 10.1016/j.jclinepi.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 19.Scuffham P, Chaplin S, Legood R. Incidence and costs of unintentional falls in older people in the United Kingdom. J Epidemiol Community Health. 2003;57(9):740–744. doi: 10.1136/jech.57.9.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sattin R W, Lambert Huber D A, DeVito C A. et al. The incidence of fall injury events among the elderly in a defined population. Am J Epidemiol. 1990;131(6):1028–1037. doi: 10.1093/oxfordjournals.aje.a115594. [DOI] [PubMed] [Google Scholar]
- 21.Hornbrook M C, Stevens V J, Wingfield D J, Hollis J F, Greenlick M R, Ory M G. Preventing falls among community-dwelling older persons: results from a randomized trial. Gerontologist. 1994;34(1):16–23. doi: 10.1093/geront/34.1.16. [DOI] [PubMed] [Google Scholar]
- 22.Yardley L. Prediction of handicap and emotional distress in patients with recurrent vertigo: symptoms, coping strategies, control beliefs and reciprocal causation. Soc Sci Med. 1994;39(4):573–581. doi: 10.1016/0277-9536(94)90100-7. [DOI] [PubMed] [Google Scholar]
- 23.Vogel J J, Godefroy W P, van der Mey A G, le Cessie S, Kaptein A A. Illness perceptions, coping, and quality of life in vestibular schwannoma patients at diagnosis. Otol Neurotol. 2008;29(6):839–845. doi: 10.1097/MAO.0b013e3181820246. [DOI] [PubMed] [Google Scholar]
- 24.Breivik C N Varughese J K Wentzel-Larsen T Vassbotn F Lund-Johansen M Conservative management of vestibular schwannoma—a prospective cohort study: treatment, symptoms, and quality of life Neurosurgery 20127051072–1080.; discussion 1080 [DOI] [PubMed] [Google Scholar]
- 25.Humphriss R L, Baguley D M, Moffat D A. Change in dizziness handicap after vestibular schwannoma excision. Otol Neurotol. 2003;24(4):661–665. doi: 10.1097/00129492-200307000-00021. [DOI] [PubMed] [Google Scholar]
- 26.Whitney S L, Wrisley D M, Brown K E, Furman J M. Is perception of handicap related to functional performance in persons with vestibular dysfunction? Otol Neurotol. 2004;25(2):139–143. doi: 10.1097/00129492-200403000-00010. [DOI] [PubMed] [Google Scholar]


