Abstract
Spontaneous meningoencephalocele (SME) of the sphenoid wing is a rare cause of cerebrospinal fluid (CSF) leakage. Surgical closure of the fistula is usually required. The approach taken depends on the location of the defect and the extension of the meningoencephalocele. The endoscopic transpterygoid approach may be useful. We prospectively analyzed the three cases of SME of the sphenoid wing with lateral sphenoid sinus extension treated endoscopically at Stanford over the last 3 years with regard to imaging findings, operative technique, and operative morbidity. In our three cases, the extent of pterygopalatine fossa (PPF) exposure undertaken, complete in one and partial in two, depended on the defect site. Follow-up ranged from 17 to 25 months. The fistula was completely closed in all three cases. Extant literature reports a 97% rate of successful closure (N = 65 of 67, with a mean follow-up of 25 months) and no major complications. Endoscopic transpterygoid repair is a useful, safe alternative to traditional approaches for repair of SME of the sphenoid wing. Its feasibility depends on the site of the defect, which can be identified by preoperative imaging. Larger PPF exposure and postoperative lumbar drainage of CSF can be useful and have a low risk of morbidity.
Keywords: endoscopic, transpterygoid, meningoencephalocele, sphenoid wing
Background
Sphenoid wing meningoencephaloceles are a common cause of cerebrospinal fluid (CSF) rhinorrhea.1 2 Some occur spontaneously, but most are secondary,2 3 caused by neoplasms, trauma, inflammation, or surgery. Spontaneous meningoencephalocele (SME) of the sphenoid wing is rarely encountered in a general neurosurgery practice.4 Surgical repair is more often successful for secondary SME leaks than for spontaneous SME leaks.2 To decrease the risk of CSF infection, surgical repair of SME is indicated when CSF leak is documented.2 3 5 The approach to fistula closure depends on the anatomical location of the defect and the direction and magnitude of the extension of meningoencephalocele. These lesions are traditionally repaired through temporal craniotomy.1 2 The endoscopic transpterygoid approach (ETPA) is a relatively new, less invasive approach to these fistulas.6 This report describes its use to close SME of the sphenoid wing with lateral sphenoid sinus extension.
Methods
We prospectively collected data regarding all CSF fistulas and sphenoid sinus meningoencephaloceles over the last 2 years at Stanford University Hospital. We selectively reviewed SME of the sphenoid wing with lateral sphenoid sinus extension. Cases with precipitating injury were excluded. We found three cases treated with ETPA. We catalogued the imaging evaluation, operative technique, and surgical morbidity. We reviewed the literature for SME treated by ETPA through a search from 2000 to 2013 using the words lateral sphenoid, transpterygoid, spontaneous encephalocele, spontaneous meningoencephalocele, and sphenoid wing. We tabulated the surgical approach, repair techniques, use of a lumbar drain (LD), success rate, and complications reported.
Surgical Approach
A thin cut computed tomography (CT) scan through the skull base was obtained for intraoperative guidance in all patients. At the beginning of the procedure, an LD was placed for postoperative CSF diversion. The patient's head was fixed with a Mayfield clamp and rotated 30 degrees to the right. A zero-degree endoscope was used primarily with 30- and 45-degree endoscopes used as needed.
A large contralateral nasoseptal flap based on the posterior nasal artery was raised and placed in the nasopharynx. Posterior septectomy was performed to enable a bilateral nasal approach by two surgeons. Unilateral wide maxillary antrostomy, total ethmoidectomy, and a large sphenoidotomy were performed on the side of lesion. A transpterygoid approach was needed to access the lateral sphenoid recess (Fig. 1A–C). The extent of pterygopalatine fossa exposure sought depended on the location of the bony defect and the direction of the meningoencephalocele sac. For defects lateral to the foramen rotundum with or without extension into the infratemporal fossa, we used the extended endoscopic transpterygoid approach (EETPA). In this approach we exposed the pterygopalatine fossa (PPF) widely, from medial to lateral. If the defect was medial to the foramen rotundum, we exposed the PPF partially utilizing a limited endoscopic transpterygoid approach (LETPA) just to the extent needed to seal the bony defect.
Fig. 1.
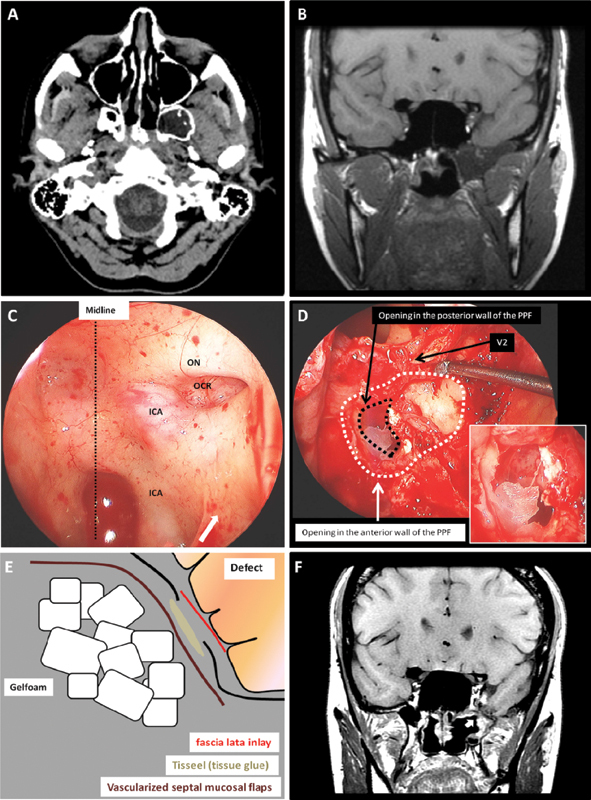
Illustrative case of an extended endoscopic transpterygoid approach (patient 1). (A) Computed tomography scan axial cuts without contrast at the level of the pterygopalatine fossa (PPF) showing the meningocele extending into the lateral recess of the sphenoid sinus and the pneumatized pterygoid plates. (B) T1 sequence magnetic resonance imaging (MRI) coronal cuts without contrast showing the meningoencephalocele extending into the infratemporal fossa. (C) Intraoperative exposure of the sphenoid sinus. This view is obtained after the posterior ethmoidectomy. The arrow indicates the location of the posterior ethmoid sinus. (D) Intraoperative view showing the sphenoid sinus (superiorly) and the exposed PPF. This view is obtained after opening the medial and posterior wall of the maxillary sinus. The encephalocele extending into the meningocele sac can be seen in the magnified view. The black dotted line represents the opening in the posterior wall of the PPF. This is formed by the pneumatized pterygoid plates, which is also part of the middle fossa medial wall. (E) The construction of the defect in multiple layers. (F) T1 sequence MRI coronal cuts with contrast 3 months after the surgery. The enhancing vascularized flap (arrow) is sealing the defect site. ICA, internal carotid artery; OCR, opticocarotid recess; ON, optic nerve.
In both approaches, the PPF dissection began medially and progressed laterally. The sphenopalatine foramen (SPF) and the sphenopalatine artery (SPA), running between the orbital and sphenoidal process of the palatine bone to exit through the SPF, marked the point of medial entry into the PPF. In all three cases the SPA was sacrificed and a contralateral septal flap was used. In LETPA, the vidian nerve (VN) was exposed and the pterygopalatine ganglion was pushed inferolaterally. Bone removed included the perpendicular plate of the palatine bone and part of the medial base of the medial pterygoid process. The V2 branch of the trigeminal nerve was partially visualized. In the EETPA, more of each pterygoid process was removed to allow visualization of more lateral defects. The maxillary artery and the pterygopalatine ganglion were identified and pushed inferiorly. The full course of V2 entering the inferior orbital fissure was seen (Fig. 1D).
Once the defect in the bone was exposed, surrounding soft tissue was removed. Multilayered closure consisted of inlay fascia lata graft, fibrin sealant, and the onlay contralateral nasoseptal flap (Fig. 1E). The construct was supported by Gelfoam and nasal packing. CSF diversion via the LD was continued for at least 2 days postoperatively. The nasal packing was removed 3 days after surgery. Prophylactic antibiotics were discontinued when the LD was removed.
Results
Three cases of SME were treated endoscopically utilizing a transpterygoid approach (ETPA). Two patients, one of whom had meningitis, presented with CSF rhinorrhea. The third patient presented with seizures and blurry vision. Two patients had headaches at presentation (Table 1). The pterygopalatine fossa was fully exposed utilizing EETPA in one case and partially exposed in the two with a tailored LETPA (Fig. 2A–C). Follow-up ranged from 15 to 25 months (average: 19 months). Successful closure of the fistula was achieved on the first attempt in all three cases. One patient experienced transient double vision that fully resolved within 6 months of surgery. The patient with the complete pterygopalatine fossa exposure experienced transient facial numbness and decreased lacrimation on the side of the surgery; both resolved completely within 6 months of surgery. No other intraoperative or postoperative complications were encountered. The two patients presenting with headaches reported significant improvement in their headaches. Postoperative imaging in all three cases showed complete obliteration of the meningoencephalocele sac (Figs. 1F and 2D).
Table 1. Patient characteristics.
| Patient | Age, y/Sex | Clinical evaluation | Radiologic findings | Approach | Follow-up on CSF leak and cranial nerves findings |
|---|---|---|---|---|---|
| Patient 1 (Fig. 1) | 32/Female | CSF leak Headache |
Meningoencephalocele extending into the lateral recess of the sphenoid sinus and the infratemporal fossa | Extended endoscopic transpterygoid approach and resection of gliotic tissue | No leak Transient V2 numbness and decreased lacrimation |
| Patient 2 (Fig. 2) | 27/Female | Seizures Headaches Blurry vision |
Meningocele extending into the lateral recess of the sphenoid sinus | Partial PPF exposure | No leak Intermittent blurry vision |
| Patient 3 | 67/Male | CSF leak Meningitis |
Meningoencephalocele extending into the lateral recess of the sphenoid sinus | Partial PPF exposure | No leak No new cranial nerve deficits |
Abbreviations: CSF, cerebrospinal fluid; PPF, pterygopalatine fossa.
Fig. 2.
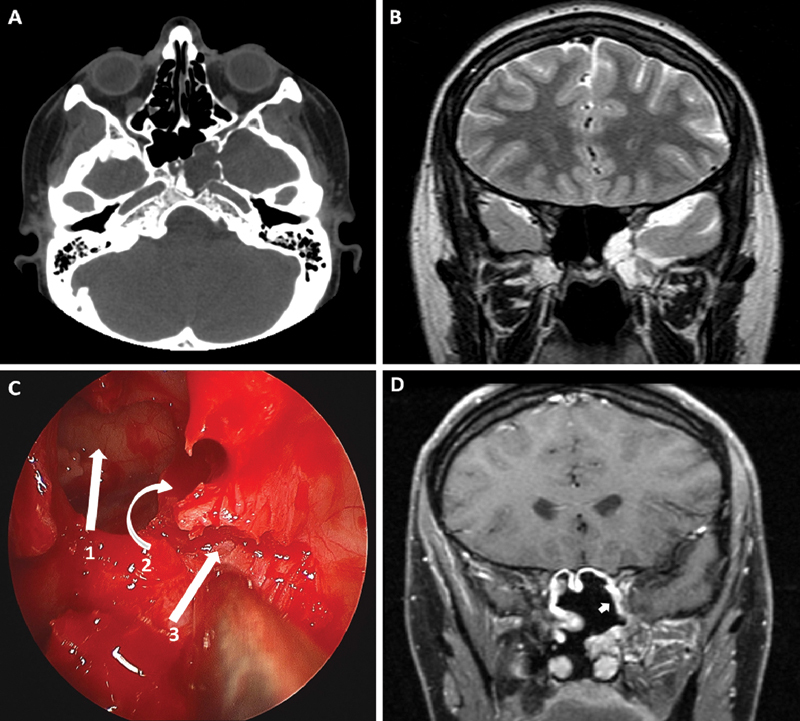
illustrative case of a limited endoscopic transpterygoid approach (patient 2). (A) Computed tomography scan axial cuts without contrast at the level of the pterygopalatine fossa (PPF) showing the meningocele extending into the lateral recess of the sphenoid sinus. (B) T2 sequence magnetic resonance imaging (MRI) coronal cuts showing meningocele extending into the lateral recess of the sphenoid sinus. (C) Intraoperative view showing the sphenoid sinus (1), the posterior ethmoid sinus (2), and the exposed anterior wall of the PPF (3). The medial wall of the maxillary sinus is removed. The next step is to proceed with the removal of the anterior and posterior walls of the PPF from medial to lateral. (D) T1 sequence MRI with contrast 3 months after surgery. The enhancing vascularized flap (arrow) is sealing the defect site.
Twelve reports, reviewing a total of 64 cases of SME with lateral sphenoid sinus extension treated by an EPTA, were found in the English literature between 2000 and 2013.3 4 5 6 7 8 9 10 11 12 13 14 (Table 2). The average follow-up was 25 months. CSF leakage persisted in only two cases, an overall surgical success rate on the first attempt of 97% (n = 62 of 64). Both failures occurred after LEPTA with partial pterygopalatine fossa exposure. An LD was used in 35 patients with no reported complication. Lumbar drainage did not affect the success rate of the procedure. Postoperative facial numbness occurred in 4 of 33 cases, temporary in 2 and persistent in 2 (n = 4 of 33). Occurrence of facial numbness did not correlate with the extent of exposure. Lacrimation was diminished after surgery in two patients (2 of 33), both of whom had the more extensive EETPA. The success rates of different closure constructs were similar.
Table 2. Reported casesa .
| Reported series | No. of SMEsb | No. of ETPAsc | Success rate from first attempt (n) | Follow-up, mo | Complications | The repair and construct | LD | Comments |
|---|---|---|---|---|---|---|---|---|
| Lai et al, 200213 | 8 | 7 EETPAs | 100% (7/7) | 32 | Not reported | Epidural bone graft followed by FL or nasal mucosa | No | |
| Al-Nashar et al, 200412 | 7 (unclear how many SMEs) | 7 tailored ETPA | 100% (7/7) | 38 | Not reported | Variable from only obliteration of the sinus to multilayer inlay pericardium and fat | Yes | |
| Pasquini et al, 200414 | 3 | 3 tailored ETPAs | 100% (3/3) | 15 | Not reported | Fat in the defect, then mucoperiosteum or epidural bone followed mucoperiosteum, or overlay dura, then fat, then mucoperiosteum | No | |
| Bolger, 20056 | 6 (unclear how many SMEs) | 1 EETPA 5 LETPAs |
100% (6/6) | 15 | One V2 numbness One maxillary sinusitis |
Bone flap in epidural space, then FL, then fat supported by Gelfoam to obliterate the sinus | No | Shimmers test done in all, all intact One patient had sphenopalatine ganglia resected and vidian nerve cut. He had no dry eye postoperatively |
| Tami, 200610 | 6 | 6 tailored ETPA | 100% (6/6) | 21 | One transient palatal anesthesia One dry eye in EETPA |
No comment for the reconstruction | Yes | LD for 2–4 days |
| Bachmann-Harilastad, 200611 | 1 | 1 LETPA | 100% (1/1) | 12 | Postoperative headaches and fever (CSF cultures were negative) | Fat in defect, then inlay FL, then outlay FL and mucosal flap, then fibrin glue | Yes | LD for 3.5 days |
| Castelnuovo et al, 20074 | 15 | 9 tailored ETPA | 100% (9/9) | 38 | None | FL inlay followed by epidural bone or cartilage, then mucoperiosteum, then fibrin glue | No | |
| Tomazic and Stammberger, 20093 | 5 | 3 tailored ETPA | 100% (3/3) | 7 | One temporary V2 numbness | Fat in the defect followed by FL overlay, then fibrin glue | Yes | LD only used perioperatively and removed in the operating room |
| Forer et al 2010 | 7 | 7 LETPA | 86% (6/7) | 29 | Not reported | Inlay epidural bone/cartilage, then fat and free mucosa Nasal packing and strict bed rest for 3 d |
No | The failure was due to inadequate exposure |
| Tabaee et al, 20109 | 13 | 3 LETPA | 100% (3/3) | 55 | One facial paresthesia | Fat and Gelfoam in the defect, then FL, then osseous buttress, then tissue sealant Gasket seal for large defects |
Yes | |
| Alexander et al, 20127 | 10 | 10 LETPA | 90% (9/10) | 11 | Not reported | Inlay tissue grafts AlloDerm (LifeCell), Duragen (Integra), or Surgisis (Cook Medical) followed by placement of overlay tissue graft with or without a free fat graft ± pedicle septal flap ± septal bone flap |
Yes | Largest single-institution report |
| Schmidt et al, 20128 | 2 | 2 EETPA | 100% (2/2) | 28 | None | Dermal allograft (Life Cell Corp.) inlay, then a second layer of the same thing, then Gelfoam. For larger defects, FL inlay or thick dermal allograft, then Surgicel, then another layer of FL or allograft, then a septal flap supported by Gelfoam |
Yes | |
| Ajlan et al 2013 | 3 | 2 LETPA 1 EETPA |
100% (3/3) | 19 | One temporary V2 numbness One patient had transient decrease in lacrimation |
Inlay FL, then fibrin sealant, and onlay septal flap. This was supported by Gelfoam and nasal packing | Yes | LD for 3–4 days |
| Total | 85 | 67 | 97% (65/67) | 25 | 2 cases of sinusitis 4 cases of V2 numbness (2 transient) 1 case of transient palatal anesthesia 2 patients had dry eye (one transient) |
Abbreviations: CSF, cerebrospinal fluid; EETPA, extended endoscopic transpterygoid approach; FL, fascia lata; LETPA, limited endoscopic transpterygoid approach; LD, lumbar drain; SME, spontaneous meningoencephalocele.
Total of 67 reported cases: EETPA, LETPA, SME, and LD.
Only SME cases are included.
Only SME cases treated with ETPA are included.
Discussion
Sphenoid wing meningoencephalocele is a common causes of CSF rhinorrhea.1 2 Most are secondary to trauma; SME is rarely reported.6 Spontaneous SMEs are usually attributed to a congenital anomaly at the lateral craniopharyngeal canal, also known as Sternberg canal, caused by the incomplete fusion of deferent sphenoid bone parts. This causes a frank defect or a low-resistance barrier that allows development of a SME.4 15 Elevated intracranial pressure (ICP) and obesity were associated with SME.7 8 9 10 11 12 13 14 15 16 Far lateral pneumatization of the sphenoid bone, a normal variant in 22 to 40%, is commonly present in cases of SME of the sphenoid wing.2 8 17 Surgical obliteration of the fistula is more often successful in secondary than in spontaneous cases.18
CSF rhinorrhea is the most common presentation of SME of the sphenoid wing with lateral sphenoid sinus extension.9 Other presentations include headache, recurrent meningitis, seizures, and cranial nerve impairment. It can also be diagnosed as an incidental finding.1 2 3 4 5 6 Surgical repair of a leaking SME is indicated to decrease the risk of CSF infection.2 3 5 Some authors advocate treating the lesion even without documented CSF leakage or meningitis because of the possibility of an occult fistula.19
Classification of sphenoid wing encephaloceles is based on the location of the bony defect connecting the subarachnoid space to the extracranial compartment and the extension of the meningoencephalocele itself.1 20 These anatomical classification schemes can potentially guide the surgical approach. Traditionally, an open transcranial, an infratemporal, or a transfacial approach has been required to treat these lesions successfully.1 These approaches are associated with significant morbidity mainly caused by brain retraction and the extensive osteotomies needed to directly visualize the defect. The endoscopic approach is a less invasive technique with the advantages of avoiding brain retraction and large skin incisions. Initial reports of treating these lesions transnasally suggested a higher failure rate.2 13 The poor outcomes were due to the inadequate visualization of defects lateral in the lateral recess of the sphenoid sinus. However, more precise appreciation of the surgical anatomy of the nasal sinuses,21 22 23 24 and improved endoscopic illumination and instrumentation, have led to greater use of endoscopic transnasal approaches for SME of the sphenoid wing. Recent reports recommend the transpterygoid route to treat these lateral lesions.6 13 14
A clear understanding of sinonasal and pterygopalatine fossa anatomy is required for ETPA approaches (Fig. 3A, B).22 23 24 25 The posterior wall of the pterygopalatine fossa is formed by the root of the sphenoid bone and the pterygoid plates. The palatine bone forms the anteromedial wall of the pterygopalatine fossa. The posterior wall of the maxillary sinus forms the anterior wall of the pterygopalatine fossa. The pterygopalatine fossa is connected laterally to the masticator space, part of the infratemporal fossa, through the pterygomaxillary fissure. The pterygoid plates' base contains the foramen rotundum superomedially, transmitting the maxillary nerve (V2), and the vidian canal inferomedially, transmitting the VN. The average distance between the foramen rotundum and the vidian canal was 5.6 mm horizontally and 6.22 mm vertically.26 The pterygopalatine fossa connects anteriorly with the inferior orbital fissure that transmits the zygomatic branch of the maxillary nerve (V2), parasympathetic fibers from the pterygopalatine ganglion, and the greater palatine nerve. The palatovaginal canal and the SPF connect the pterygopalatine fossa to the nasal cavity. The sphenopalatine artery runs between the orbital process and sphenoidal process of the palatine bone. The two major components of the pterygopalatine fossa are the pterygopalatine ganglion, located posteromedially, and the maxillary artery located anteriolaterally.27 The lateral recess of the sphenoid sinus is the lateral component of the sinus resulting from the pneumatization of the sphenoid wing. Variable degrees of pneumatization range from a limited lateral recess to complete pneumatization of the pterygoid plates.2 8 17 This extension of the sphenoid sinus is inferior and posterior to the posterior ethmoid air cells. The lateral recess is usually not encountered in midline standard sellar approaches for pituitary lesions.
Fig. 3.
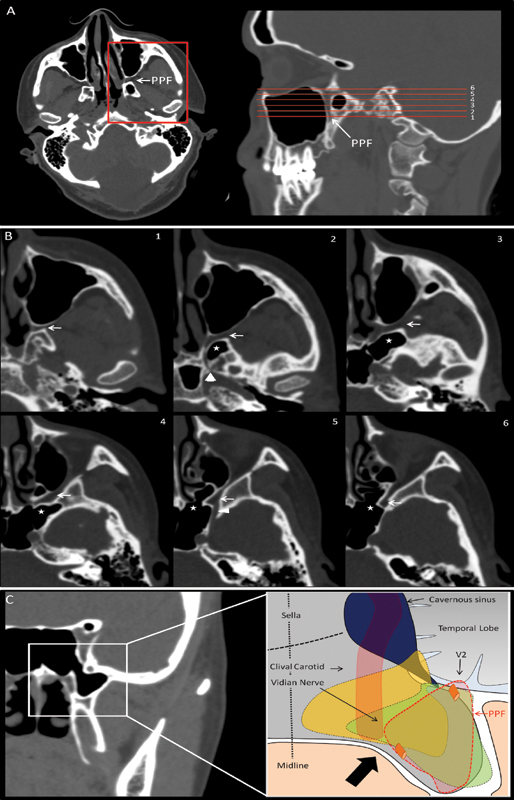
Illustrative anatomy. (A) Axial and sagittal cuts computed tomography (CT) scan at the level of the pterygopalatine fossa (PPF). The red lines correspond to the magnified images in (B). (B) Magnified axial cuts at the level of the PPF. Note the PPF (white arrow), lateral recess of the sphenoid sinus (asterisk), the vidian canal at 2 (arrowhead), and the foramen rotundum at 4 (triangle). (C) Coronal cut CT scan showing the sphenoid sinus and its lateral recess. Note the pterygoid plates just under the lateral recess. The drawing shows the PPF (in red) and a meningoencephalocele extending medially with a vertical bony opening (in yellow) and a meningoencephalocele extending inferiorly with a horizontal bony defect (in green). The approach is lateral to the carotid. The carotid can be localized in a preoperative CT scan or intraoperatively utilizing image guidance and/or Doppler ultrasound.
We report three cases of SME treated with an ETPA. All three cases were treated successfully on the first attempt. We inserted an LD preoperatively to divert CSF for a minimum of 2 days after the reconstruction. The LD also permitted measurement of ICP, whose elevation can contribute to SME formation.16 The LD also allows localization of the defect with intrathecal fluorescein.7 The LD insertion has its own risk and can cause prolonged hospitalization. The average hospital stay was 4 days in our series; this was mandated by the indwelling LD. Insertion of an LD did not affect the overall success rate of surgical treatment in the reviewed reports (n = 64), and it added no morbidity.
We suggest that the extent of pterygopalatine fossa exposure needed depends on the anatomical features of the meningoencephalocele. The location of the bony defect connecting the subarachnoid space to the extracranial compartment determines the extent of pterygopalatine fossa exposure (Fig. 3C). Inferior extension of the meningoencephalocele sac inferiorly into the infratemporal fossa warrants an EETPA (Fig. 1A, B). Tailored exposure sufficient to seal the leak is recommended for medial defects to minimize manipulation of pterygopalatine fossa's neurovascular structures (Fig. 2A, B). The exposure can be started medially and then extended laterally to avoid exposing more critical structures in the pterygopalatine fossa. The location of the defect in relation to the foramen rotundum is a simple guide to tailoring the procedure. Defects lateral to the foramen rotundum usually necessitate an EETPA. The only two reported failures (n = 2/64) of an ETPA occurred when a limited exposure was used. A thin-cut computed tomography (CT) scan can aid preoperative evaluation and help guide the surgical exposure. However, the choice of extent of exposure should be made on the basis of intraoperative findings.
The postoperative morbidity was the same for LETPA and EETPA. Postoperative palatal anesthesia might be avoided by mobilizing the greater palatine nerve larterally.10 26 Kasemsiri et al26 provided a useful 5 grade classification of the transpterygoid approach depending on the targeted area. This classification is useful in guiding and tailoring the exposure. Types A and B approach lesions in the pterygopalatine fossa, and the lateral recess of the sphenoid and are equivalent to our LETPA. Types C and D are extensions of the limited approaches targeting the petrous apex, Meckel cave, and the infratemporal fossa.
We utilized a multilayer closure starting with an inlay fascia lata, followed by tissue glue and a final layer of a vascularized nasoseptal flap. We believe that these defects require multilayer closure for long-lasting results. The use of a nasoseptal flap in high-flow CSF fistulas has been previously reported to decrease the risk of recurrent CSF leak.28 Reconstruction techniques used in the repair of SME have ranged from simple obliteration of the sinus to a multilayered construct supported by a bony buttress and a nasoseptal flap. Obliteration of the sinus alone was associated with a higher rate of failure.4 29 No significant difference in outcomes was seen among the constructs in the cohort reviewed (Table 2). However, adjustment of the reconstruction according to the defect size has been recommended. Many authors reserve use a septal flap for large defects.7 8
In our case of EETPA, the patient experienced transient facial numbness in the V2 distribution and decreased lacrimation; both recovered completely within 3 months. Otherwise, morbidity of these transpterygoid approaches was minimal. In the literature, greater exposure did not increase the risk of postoperative facial paresthesia. The two cases of decreased lacrimation, which occurred after more extensive exposure, caused no significant disability. Only two postoperative infections were reported (2 of 67). These results suggest that enlarging the exposure of an ETPA for better visualization of the defect, more facile closure, and increased likelihood of permanent obliteration of the fistula can be obtained with little risk of additional morbidity.
Our review of the literature noted some limitations. The reviewed reports are all retrospective; our report is the only prospective case series. We had difficulty estimating the extent of exposure in some cases. Many series had brief follow-up; fistulas can occur years after the repair.30 Although the complication rate appears low, only half of the published reports we reviewed reported their complications. The high success rate and reported low morbidity in these series may reflect their origin from institutions with significant experience with endoscopic endonasal treatment of skull base lesions utilizing the endoscopic endonasal route. This may explain the high success rate and the reported low morbidity in these cohorts.
Conclusions
An endoscopic endonasal transpterygoid approach is a useful alternative to craniotomy in the repair of SME of the sphenoid wing extending into the lateral recess of the sphenoid sinus. A success rate approaching 100% is commonly reported from experienced centers. The feasibility of the intervention depends on the site of the defect, which can be determined on preoperative imaging. More extensive pterygopalatine fossa exposure did not add to operative morbidity. In the literature, choice of repair technique varied with the surgeon's preference, but neither this nor use of postoperative LDs for CSF diversion produced significantly different outcomes.
References
- 1.Wind J J, Caputy A J, Roberti F. Spontaneous encephaloceles of the temporal lobe. Neurosurg Focus. 2008;25(6):E11. doi: 10.3171/FOC.2008.25.12.E11. [DOI] [PubMed] [Google Scholar]
- 2.Landreneau F E, Mickey B, Coimbra C. Surgical treatment of cerebrospinal fluid fistulae involving lateral extension of the sphenoid sinus. Neurosurgery. 1998;42(5):1101–1104; discussion 1104–1105. doi: 10.1097/00006123-199805000-00087. [DOI] [PubMed] [Google Scholar]
- 3.Tomazic P V, Stammberger H. Spontaneous CSF-leaks and meningoencephaloceles in sphenoid sinus by persisting Sternberg's canal. Rhinology. 2009;47(4):369–374. doi: 10.4193/Rhin08.236. [DOI] [PubMed] [Google Scholar]
- 4.Castelnuovo P, Dallan I, Pistochini A, Battaglia P, Locatelli D, Bignami M. Endonasal endoscopic repair of Sternberg's canal cerebrospinal fluid leaks. Laryngoscope. 2007;117(2):345–349. doi: 10.1097/01.mlg.0000251452.90657.3a. [DOI] [PubMed] [Google Scholar]
- 5.Lopatin A S, Kapitanov D N, Potapov A A. Endonasal endoscopic repair of spontaneous cerebrospinal fluid leaks. Arch Otolaryngol Head Neck Surg. 2003;129(8):859–863. doi: 10.1001/archotol.129.8.859. [DOI] [PubMed] [Google Scholar]
- 6.Bolger W E. Endoscopic transpterygoid approach to the lateral sphenoid recess: surgical approach and clinical experience. Otolaryngol Head Neck Surg. 2005;133(1):20–26. doi: 10.1016/j.otohns.2005.03.063. [DOI] [PubMed] [Google Scholar]
- 7.Alexander N S, Chaaban M R, Riley K O, Woodworth B A. Treatment strategies for lateral sphenoid sinus recess cerebrospinal fluid leaks. Arch Otolaryngol Head Neck Surg. 2012;138(5):471–478. doi: 10.1001/archoto.2012.614. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt R F, Choudhry O J, Raviv J. et al. Surgical nuances for the endoscopic endonasal transpterygoid approach to lateral sphenoid sinus encephaloceles. Neurosurg Focus. 2012;32(6):E5. doi: 10.3171/2012.3.FOCUS1267. [DOI] [PubMed] [Google Scholar]
- 9.Tabaee A, Anand V K, Cappabianca P, Stamm A, Esposito F, Schwartz T H. Endoscopic management of spontaneous meningoencephalocele of the lateral sphenoid sinus. J Neurosurg. 2010;112(5):1070–1077. doi: 10.3171/2009.7.JNS0842. [DOI] [PubMed] [Google Scholar]
- 10.Tami T A. Surgical management of lesions of the sphenoid lateral recess. Am J Rhinol. 2006;20(4):412–416. doi: 10.2500/ajr.2006.20.2893. [DOI] [PubMed] [Google Scholar]
- 11.Bachmann-Harildstad G, Kloster R, Bajic R. Transpterygoid trans-sphenoid approach to the lateral extension of the sphenoid sinus to repair a spontaneous CSF leak. Skull Base. 2006;16(4):207–212. doi: 10.1055/s-2006-950389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Al-Nashar I S, Carrau R L, Herrera A, Snyderman C H. Endoscopic transnasal transpterygopalatine fossa approach to the lateral recess of the sphenoid sinus. Laryngoscope. 2004;114(3):528–532. doi: 10.1097/00005537-200403000-00026. [DOI] [PubMed] [Google Scholar]
- 13.Lai S Y, Kennedy D W, Bolger W E. Sphenoid encephaloceles: disease management and identification of lesions within the lateral recess of the sphenoid sinus. Laryngoscope. 2002;112(10):1800–1805. doi: 10.1097/00005537-200210000-00018. [DOI] [PubMed] [Google Scholar]
- 14.Pasquini E, Sciarretta V, Farneti G, Mazzatenta D, Modugno G C, Frank G. Endoscopic treatment of encephaloceles of the lateral wall of the sphenoid sinus. Minim Invasive Neurosurg. 2004;47(4):209–213. doi: 10.1055/s-2004-818522. [DOI] [PubMed] [Google Scholar]
- 15.Barañano C F, Curé J, Palmer J N, Woodworth B A. Sternberg's canal: fact or fiction? Am J Rhinol Allergy. 2009;23(2):167–171. doi: 10.2500/ajra.2009.23.3290. [DOI] [PubMed] [Google Scholar]
- 16.Kenning T J, Willcox T O, Artz G J, Schiffmacher P, Farrell C J, Evans J J. Surgical management of temporal meningoencephaloceles, cerebrospinal fluid leaks, and intracranial hypertension: treatment paradigm and outcomes. Neurosurg Focus. 2012;32(6):E6. doi: 10.3171/2012.4.FOCUS1265. [DOI] [PubMed] [Google Scholar]
- 17.Shetty P G, Shroff M M, Fatterpekar G M, Sahani D V, Kirtane M V. A retrospective analysis of spontaneous sphenoid sinus fistula: MR and CT findings. AJNR Am J Neuroradiol. 2000;21(2):337–342. [PMC free article] [PubMed] [Google Scholar]
- 18.Schick B, Ibing R, Brors D, Draf W. Long-term study of endonasal duraplasty and review of the literature. Ann Otol Rhinol Laryngol. 2001;110(2):142–147. doi: 10.1177/000348940111000209. [DOI] [PubMed] [Google Scholar]
- 19.Buchfelder M, Fahlbusch R, Huk W J, Thierauf P. Intrasphenoidal encephaloceles—a clinical entity. Acta Neurochir (Wien) 1987;89(1–2):10–15. doi: 10.1007/BF01406661. [DOI] [PubMed] [Google Scholar]
- 20.Wilkins R H, Radtke R A, Burger P C. Spontaneous temporal encephalocele. Case report. J Neurosurg. 1993;78(3):492–498. doi: 10.3171/jns.1993.78.3.0492. [DOI] [PubMed] [Google Scholar]
- 21.Kassam A B, Gardner P, Snyderman C, Mintz A, Carrau R. Expanded endonasal approach: fully endoscopic, completely transnasal approach to the middle third of the clivus, petrous bone, middle cranial fossa, and infratemporal fossa. Neurosurg Focus. 2005;19(1):E6. [PubMed] [Google Scholar]
- 22.Kassam A B, Vescan A D, Carrau R L. et al. Expanded endonasal approach: vidian canal as a landmark to the petrous internal carotid artery. J Neurosurg. 2008;108(1):177–183. doi: 10.3171/JNS/2008/108/01/0177. [DOI] [PubMed] [Google Scholar]
- 23.Hofstetter C P, Singh A, Anand V K, Kacker A, Schwartz T H. The endoscopic, endonasal, transmaxillary transpterygoid approach to the pterygopalatine fossa, infratemporal fossa, petrous apex, and the Meckel cave. J Neurosurg. 2010;113(5):967–974. doi: 10.3171/2009.10.JNS09157. [DOI] [PubMed] [Google Scholar]
- 24.Hosseini S M, Razfar A, Carrau R L. et al. Endonasal transpterygoid approach to the infratemporal fossa: correlation of endoscopic and multiplanar CT anatomy. Head Neck. 2012;34(3):313–320. doi: 10.1002/hed.21725. [DOI] [PubMed] [Google Scholar]
- 25.Daniels D L, Mark L P, Ulmer J L. et al. Osseous anatomy of the pterygopalatine fossa. AJNR Am J Neuroradiol. 1998;19(8):1423–1432. [PMC free article] [PubMed] [Google Scholar]
- 26.Kasemsiri P, Solares C A, Carrau R L. et al. Endoscopic endonasal transpterygoid approaches: anatomical landmarks for planning the surgical corridor. Laryngoscope. 2013;123(4):811–815. doi: 10.1002/lary.23697. [DOI] [PubMed] [Google Scholar]
- 27.Fortes F S, Sennes L U, Carrau R L. et al. Endoscopic anatomy of the pterygopalatine fossa and the transpterygoid approach: development of a surgical instruction model. Laryngoscope. 2008;118(1):44–49. doi: 10.1097/MLG.0b013e318155a492. [DOI] [PubMed] [Google Scholar]
- 28.Kassam A B, Prevedello D M, Carrau R L. et al. Endoscopic endonasal skull base surgery: analysis of complications in the authors' initial 800 patients. J Neurosurg. 2011;114(6):1544–1568. doi: 10.3171/2010.10.JNS09406. [DOI] [PubMed] [Google Scholar]
- 29.Tosun F, Carrau R L, Snyderman C H, Kassam A, Celin S, Schaitkin B. Endonasal endoscopic repair of cerebrospinal fluid leaks of the sphenoid sinus. Arch Otolaryngol Head Neck Surg. 2003;129(5):576–580. doi: 10.1001/archotol.129.5.576. [DOI] [PubMed] [Google Scholar]
- 30.Bernal-Sprekelsen M, Alobid I, Mullol J, Trobat F, Tomás-Barberán M. Closure of cerebrospinal fluid leaks prevents ascending bacterial meningitis. Rhinology. 2005;43(4):277–281. [PubMed] [Google Scholar]


