Abstract
Objectives To review the terminology, clinical features, and management of temporal bone osteomyelitis.
Design and Setting Prospective study in a tertiary care center from 2001 to 2008.
Participants Twenty patients visiting the outpatient department diagnosed with osteomyelitis of the temporal bone.
Main Outcome Measures The age, sex, clinical features, cultured organisms, surgical interventions, and classification were analyzed.
Results Of the 20 cases, 2 (10%) were diagnosed as acute otitis media. Eighteen (90%) had chronic otitis media. Nineteen (95%) were classified as medial temporal bone osteomyelitis and one (5%) as lateral temporal osteomyelitis. The most common clinical features were ear discharge (100%), pain (83%), and granulations (100%). Facial nerve palsy was seen in seven cases (35%) and parotid involvement in one case. Ten patients (56%) had diabetes mellitus. The organisms isolated were Pseudomonas aeruginosa (80%) and Staphylococcus aureus (13.33%). Histopathology revealed chronic inflammation in 20 patients (100%) and osteomyelitic bony changes in 14 (70%). Surgical debridement was the most preferred modality of treatment (87%).
Conclusion A new classification of temporal bone osteomyelitis has been proposed. Bacterial cultures must be performed in all patients. Antibiotic therapy is the treatment of choice. Surgical intervention is necessary in the presence of severe pain, complications, refractory cases, or the presence of bony sequestra on radiology.
Keywords: osteomyelitis, temporal bone, diabetes mellitus, malignant external otitis, skull base osteomyelitis
Introduction
Osteomyelitis of the temporal bone is not a common entity. Osteomyelitis can be defined as an inflammatory condition of the bone that begins as an infection of the medullary cavity, rapidly involves the haversian systems, and extends to involve the periosteum of the affected area. 1 Osteomyelitis commonly occurs in long bones in other parts of the body; temporal bone is not commonly involved. Osteitis of the temporal and adjacent bones was initially described in 1959. 2 Because of the high mortality rate (46%) in early series, this condition is often referred to as malignant otitis externa . 3 Conditions altering the vascularity of the bone like radiation, malignancy, osteoporosis, osteopetrosis, and Paget disease predispose to osteomyelitis. Systemic diseases like diabetes, anemia, and malnutrition that cause concomitant alteration in host defenses profoundly influences the course of osteomyelitis.
Osteomyelitis of the temporal bone is discussed in otology under various names like malignant otitis externa, necrotizing otitis externa, osteomyelitis of the mastoid, skull base osteomyelitis, and osteitis of the base of the skull, but there is a certain lack of clarity in presenting this condition. We present a case series of 20 cases of osteomyelitis of the temporal bone during the last 7 years and discuss the disease pattern.
Materials and Methods
This is a prospective study of 20 cases of osteomyelitis in head and neck over a period of 7 years from 2001 to 2008. This study was conducted at the Department of Otolaryngology - Head and Neck Surgery, Kasturba Medical College, Mangalore, a tertiary health center in South India. This study was cleared by the Manipal University Ethics Committee for Research and Publication. The age, gender, and medical history of these patients were recorded. All patients underwent a thorough clinical examination. Clinical features like deep pain (temporal, parietal, postauricular, retroorbital), intermittent foul otorrhea and spiking fever, preauricular cellulitis, woody induration of pinna, chronic mastoid cutaneous fistula, mastoid granulation tissue, and facial nerve symptoms were used to diagnose osteomyelitis. Clinically osteomyelitis was diagnosed as acute or chronic. Cases were also divided into medial temporal bone osteomyelitis and lateral temporal bone osteomyelitis, avoiding the previously discussed terms like malignant otitis externa and skull base osteomyelitis. Osteomyelitis of the lateral temporal bone includes bone involvement in the mastoid and middle ear cleft, and osteomyelitis of the medial temporal bone includes bone involvement of the otic capsule, petrous apex, and clivus.
Once clinically diagnosed as osteomyelitis, the following relevant investigations were performed to confirm osteomyelitis:
High-resolution computed tomography (HRCT) of the temporal bone (axial and coronal sections)
Pus from the discharging sinus for culture and sensitivity
Biopsies from the granulation tissues for histopathologic examination (HPE)
Routine blood examination, blood sugar analysis, and enzyme-linked immunosorbent assay for human immunodeficiency virus (HIV)
Once the diagnosis and the extent of disease were confirmed, patients were treated medically for acute osteomyelitis and surgically for chronic osteomyelitis. Acute osteomyelitis was the diagnosis if the onset of symptoms (characteristic of osteomyelitis) were abrupt and severe. Chronic osteomyelitis was the diagnosis when the symptoms were long standing and the patients had already received prior courses of relevant antibiotics. In cases of acute osteomyelitis, depending on the culture and sensitivity report, all the patients were administered a high dose of broad-spectrum antibiotics. The patients were first treated with a 6-week course of oral ciprofloxacin, and if there was no resolution, this was followed by intravenous ciprofloxacin or ceftazidime. Surgical intervention was preferred in the presence of severe pain, detection of sequestra in HRCT, or in the presence of complications. Surgery was followed by parenteral antibiotics for 15 days and then oral antibiotics for 4 to 6 weeks. In all cases, the pus was sent for microbiological study and the granulation tissues, if any, were sent for HPE. All patients were supplemented with a high-protein multivitamin diet and general nursing care. The diabetic status was brought under control using a combination of dietary modifications and insulin injections. Antituberculous therapy was initiated in cases of tuberculosis.
Surgeries on the Temporal Bone
Three procedures were undertaken on the temporal bone:
Wound debridement: This included repeated aural toilet, pus drainage, and localized cartilage debridement with skin preservation. After the procedure the external auditory canal (EAC) was packed with steroid antibiotic ointment or 10% ichthammol glycerine packs for 4 to 8 days; packs were changed every 2 days.
Mastoidectomy: A postaural incision was made under general anesthesia. The skin with the periosteum was elevated over the mastoid and along the EAC. The necrosed cartilage was excised and the bony sequestrum when found in the EAC and the mastoid bone were excised. Depending on the position of the sequestrum and the extent of granulations, a canal wall-up or canal wall-down mastoidectomy was performed. In cases of canal wall-down mastoidectomy, a wide meatoplasty was done. Antibiotic pack was placed and changed every 2 days until the wound healed.
Subtotal petrosectomy: This was done in cases of extensive granulations and sequestrations. It included removal of all air cells of the temporal bone leaving behind the otic capsule and the petrous apex. The EAC was closed as a blind sac and the cavity obliterated with temporalis muscle flap.
Only one patient received hyperbaric oxygen therapy. All patients were kept on regular follow-up for at least 1 year.
Results and Observations
Age and Sex Incidence
The most common age group affected was the 40- to 60-year age group accounting for 65% of cases. The age range was 2 to 68 years. We had two sets of identical twin girls, 2 and 3 years of age, respectively, who developed osteomyelitis secondary to acute mastoiditis. The male-to-female ratio was equal (1:1), although the affliction was more in the 40- to 60-year age group ( Table 1 ).
Table 1. Predisposing factors versus age and sex incidence.
| Predisposing factors | 0–20 y | 20–40 y | 40–60 y | > 60 y | Total no. (%) | ||||
|---|---|---|---|---|---|---|---|---|---|
| M | F | M | F | M | F | M | F | ||
| Otitis externa | 1 | 1 | 4 | 6 | 12 (60) | ||||
| Otitis media | 2 | 1 | 1 | 1 | 5 (25) | ||||
| Tuberculosis | 1 | 1 (5) | |||||||
| Trauma | 1 | 1 (5) | |||||||
| Malignancy | 1 | 1 (5) | |||||||
| Total | 1 | 2 | 2 | 2 | 5 | 6 | 2 | 0 | 20 (100) |
Predisposing Factors
Twelve (60%) of the patients had a history of otitis externa prior to development of osteomyelitis of the temporal bone. Five (25%) had a history of otitis media and one each (5%) had tuberculous otitis media and trauma. One patient was diagnosed with a coexisting malignancy ( Table 1 ).
Clinical Features
Two of the patients (10%) were diagnosed with acute osteomyelitis. Sixteen (80%) had chronic osteomyelitis. Of the 20 cases, 19 (95%) were classified as medial temporal bone osteomyelitis and one (5%) as lateral temporal osteomyelitis ( Table 2 ). The most common clinical features were continuous ear discharge (100%), pain (85%) ( Figs. 1 and 2 ), granulations in the EAC (100%) ( Fig. 3 ), and local tenderness (90%). Facial nerve palsy was seen in seven cases (35%) and parotid involvement in one case. Ten patients (50%) had diabetes mellitus ( Table 3 ). One patient developed meningitis.
Table 2. Clinical diagnosis of osteomyelitis.
| Diagnosis | Acute OM No (%) |
Chronic OM No (%) |
Total No (%) |
|---|---|---|---|
| Lateral temporal bone OM | 2 (10) | 17 (85) | 19 (95) |
| Medial temporal bone OM | 0 | 1 (5) | 1 (5) |
| Total; No (%) | 2 (10) | 18 (90) | 20 (100) |
Abbreviation: OM, osteomyelitis.
Fig. 1.
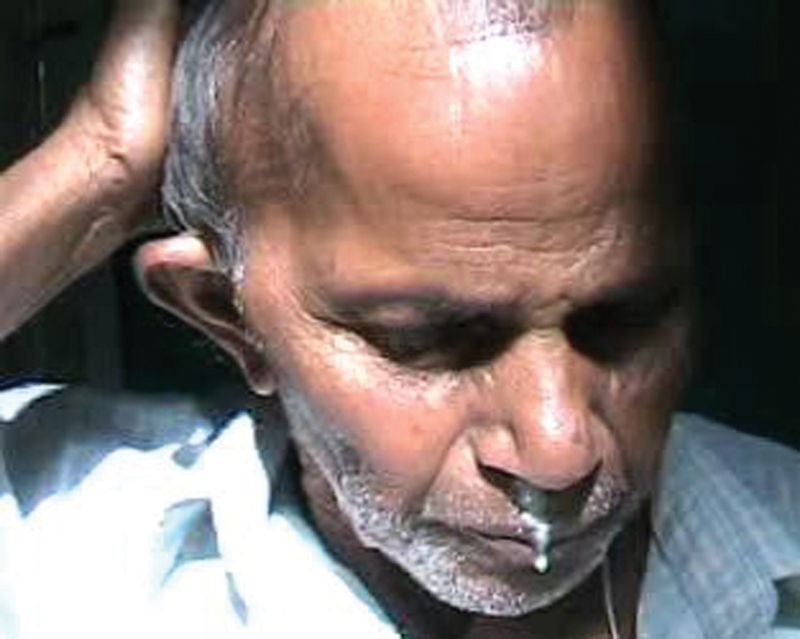
Osteomyelitis of the temporal bone leading to postaural swelling with ear discharge pouring out of the nostril via the eustachian tube.
Fig. 2.
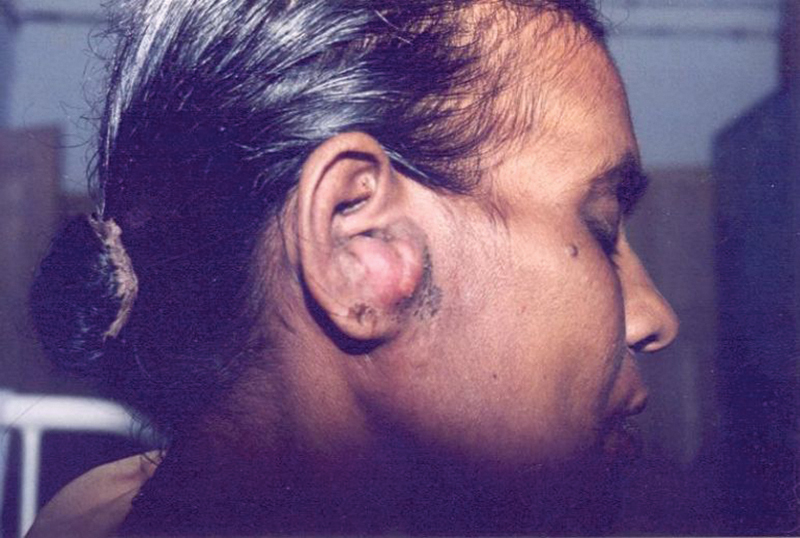
Osteomyelitis of the temporal bone causing swelling of the auricle and perichondritis.
Fig. 3.
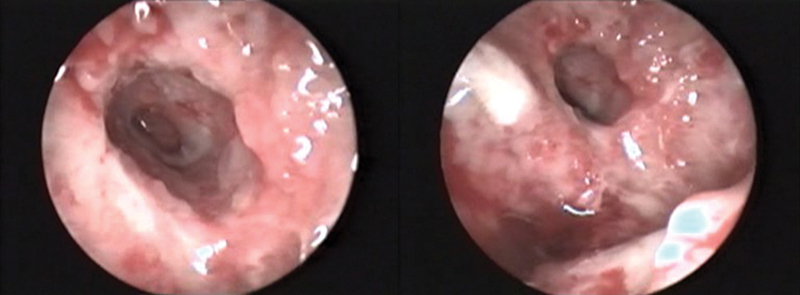
Osteomyelitis of the temporal bone showing granulations in the external auditory canal.
Table 3. Clinical features.
| Clinical features | No. (%) |
|---|---|
| Pain | 17 (85) |
| Ear discharge | 20 (100) |
| Tenderness | 18 (90) |
| Granulations | 20 (100) |
| Facial nerve palsy | 7 (35) |
| Diabetes | 10 (50) |
| Meningitis | 1 (5) |
Investigations
All s uspected cases of osteomyelitis were investigated by HPE of granulations and by sending pus for culture and sensitivity. HRCT of the temporal bone was done in all patients. The most common HRCT finding was a soft tissue density in the EAC and middle ear, opacification of the mastoid air cells along with the cortical bone erosion (100%), and bony sequestra (76%) ( Figs. 4 and 5 ). HRCT in one patient revealed erosion of the floor of the EAC extending anteriorly into the temporomandibular joint and the parotid. The organisms isolated were Pseudomonas aeruginosa (80%) and Staphylococcus aureus (13%). HPE revealed chronic inflammation in all 18 patients (100%) and osteomyelitic bony changes in 12 (67%) of the cases. One case each of tuberculous granulation tissue (6%) and squamous cell carcinoma of the EAC (6%) were also reported in parts of the specimen ( Table 4 ).
Fig. 4.
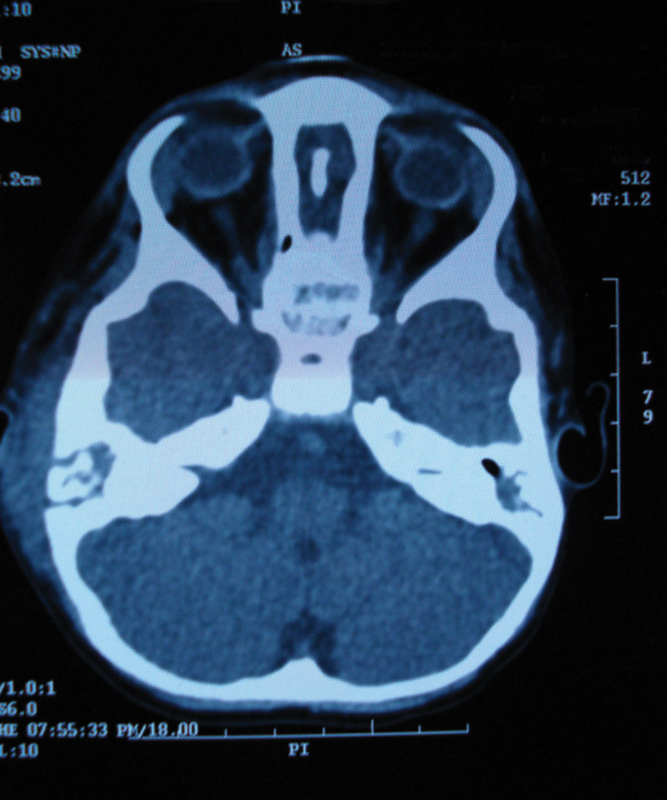
Axial computed tomography scan showing a bony sequestrum in the right mastoid.
Fig. 5.
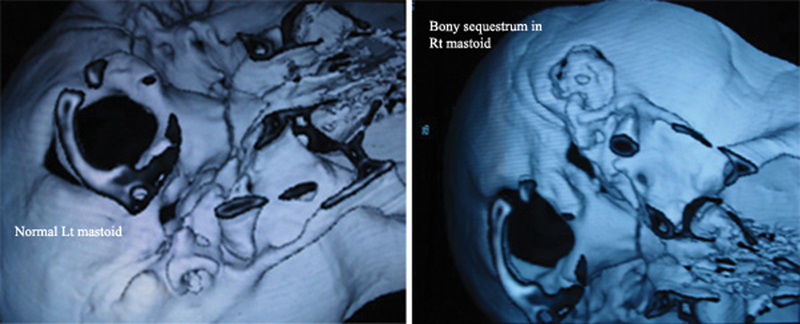
Computed tomography reconstruction comparing normal mastoid with an osteomyelitis mastoid showing a sequestrum.
Table 4. Histopathologic examination findings.
| Histopathology | No. (%) |
|---|---|
| Chronic inflammatory tissue | 20 (100) |
| Osteomyelitic bony changes | 14 (70) |
| Tubercular granulations | 1 (5) |
| Squamous cell carcinoma | 1 (5) |
Surgical Treatment
Surgical debridement by a postaural approach was the most preferred modality of treatment in 18 patients (90%) ( Fig. 6 ). Of them, 16 (88.9%) had canal wall-down procedures. Two patients (10%), one with medial and one with lateral temporal osteomyelitis, underwent subtotal petrosectomy. One of the patients who underwent subtotal petrosectomy had undergone mastoidectomy followed by hyperbaric oxygen therapy. All patients were followed up for at least 1 year. Four cases required multiple surgeries in the form of debridement before the disease process was controlled. All patients were completely cured of the disease.
Fig. 6.
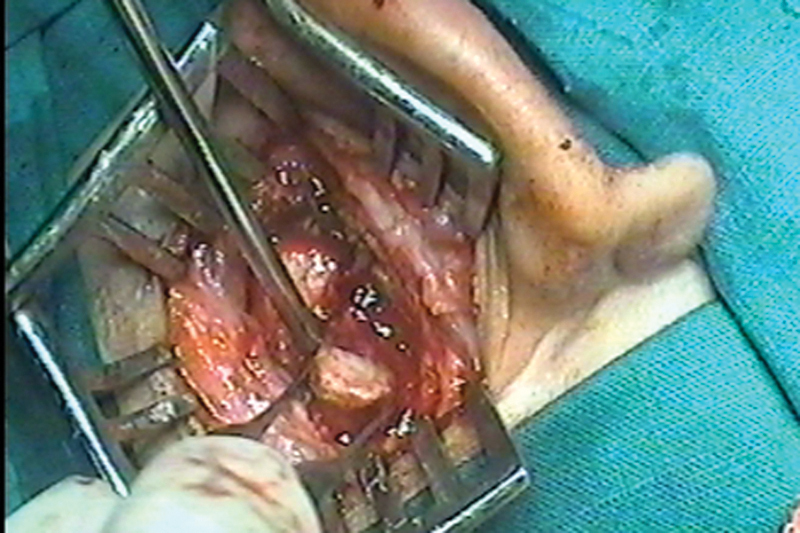
Postauricular exploration showing bony sequestrum in the temporal bone.
Discussion
Osteomyelitis of the temporal bone is not a common entity. The disease process was first cited by Toulmouche in 1883. In 1959, Meltzer and Kelemen were the first to describe Pseudomonas chondritis and osteomyelitis of the EAC and temporal bone. In 1968, Chandler 3 coined the term malignant otitis externa, and the term malignant was used to emphasize the serious nature of this infection. In the original historical report, 6 of the 13 patients died. In osteomyelitis, infection involves the bone's blood vessels, impairs the flow, and causes necrotic or infected bone, known as sequestra, as well as new bone formation around the area of necrosis. Osteomyelitis of the temporal bone is a rare disease that commonly occurs secondary to otitis externa in immunocompromised patients or suppurative otitis media. Abrupt onset of symptoms and signs during the initial stage of infection indicates an acute osteomyelitis. If this phase passes without complete elimination of infection, subacute or chronic osteomyelitis can become apparent. 4
There has been considerable confusion in defining osteomyelitis in the temporal bone. Osteomyelitis of the mastoid and middle ear cleft, malignant external otitis, osteitis of the base of the skull, and skull base osteomyelitis have been used to describe osteomyelitis arising in the temporal bone. Malignant external otitis refers to an infection involving both the EAC and the surrounding bone. Given the fact that it is not a neoplastic process, malignant external otitis is being replaced by the term skull base osteomyelitis in most of the literature. This is also inappropriate because skull base osteomyelitis, as the name suggests, points toward a serious course where the petrous bone, cranial nerves, and adjacent structures are involved.
Although we need clarity of terminologies, we must essentially remember that osteomyelitis is considered when there is pathologically a necrotic bone and sequestrum. Whatever the cause of bony infection (otitis externa or suppurative otitis media), the direction of spread of infection and the part of temporal bone involved decides the course of the disease. Thus, characteristically, two types of osteomyelitis can be recognized in the temporal bone: one developing into the mastoid and the middle ear cleft (lateral temporal bone) and the other developing into the petrous apex (medial temporal bone). Although lateral temporal bone osteomyelitis is predominantly caused by malignant external otitis, acute mastoiditis, and suppurative otitis media, medial temporal bone osteomyelitis (i.e., skull base osteomyelitis) is caused predominantly by malignant external otitis and also by sphenoid sinusitis and skull base infection. This differentiation becomes important because the clinical manifestation of both types of osteomyelitis differ considerably. Thus we can divide temporal bone osteomyelitis into these three categories:
Osteomyelitis of the lateral temporal bone (bone involvement of the mastoid and middle ear cleft)
Osteomyelitis of the medial temporal bone (bone involvement of the otic capsule, petrous apex, and clivus)
Pantemporal osteomyelitis (showing features of both mastoid-middle ear cleft and petrous involvement)
Skull base osteomyelitis originates from a chronic infection that has been inadequately treated. It is important to note, however, that this infection can come from anywhere including chronic otitis media, chronic otitis externa, sphenoid sinusitis, or any inadequately treated infection near the skull base. The infection may spread anteriorly to involve the parotid gland, temporomandibular joint, or cranial nerve VII at the exit of the stylomastoid foramen. It may also spread posteriorly to the mastoid and vertical portion of cranial nerve VII or inferomedially to the skull base including the carotid artery, the jugular bulb, and the sigmoid sinus. Infection typically spreads through the Haversian system of compact bone with progressive replacement of compact bone with granulation tissue. Bone destruction is both osteoplastic and osteoclastic. Multiple abscess formations may occur, and involvement of pneumatized trabeculated bone usually occurs late. Facial nerve paralysis results typically when the extratemporal segment becomes surrounded by infection at the styloid and mastoid foramen. Cranial nerves IX, X, or XI palsies can occur when the jugular foramen becomes involved. The pathophysiology, which is true for skull base osteomyelitis originating in the EAC, can be reflected in any osteomyelitis. There is a chronic infection, which inadequately treated leads to a cellulitis, a chondritis, an osteitis, and finally osteomyelitis. 5
Another ignored fact is that osteomyelitis of the temporal bone can also be an uncommon complication of acute mastoiditis, which in turn is a complication of acute otitis media. This was the cause of osteomyelitis in both our twin infants. The evolution of acute mastoiditis begins when the mucosal lining of the pneumatized cells becomes inflamed and produces an exudate. Serosanguineous fluid eventually becomes mucopurulent. Spontaneous perforation of the tympanic membrane or myringotomy would halt the process at this point. However, 1 to 5% of these cases go on to the next phase. The cellular walls of the pneumatized cells then become demineralized due to increased osteoclastic activity, pressure of the purulent exudate on the thin bony septae, and ischemia of the septae secondary to reduced blood flow (osteitis). As the bony septae break down, small abscess cavities form and lead to coalescence. Finally, the coalescent cells form an empyema, or pus under pressure, which then escapes to surrounding areas. Acute mastoiditis becomes clinically significant when infection spreads through the periosteum (periosteitis). When the blood vessels get involved, there is bony destruction and a sequestrum is formed in the mastoid leading to osteomyelitis. In the pre-antibiotic era, acute mastoiditis was seen more frequently than it is today, but it was rare in infants. However, in the last 2 decades, an increase in the incidence of acute mastoiditis in infancy has been reported in the literature.
There is a thin line of demarcation between osteitis (mastoiditis) and osteomyelitis. Yet the differentiation should be sought for three important reasons. First, while options for treatment of acute mastoiditis are antibiotics alone, antibiotics plus myringotomy, incision and drainage of abscess, and mastoidectomy, osteomyelitis must be treated surgically with debridement and sequestrectomy. Second, the microbiology of osteitis and osteomyelitis are seemingly different. Commonly seen organisms in mastoiditis are Streptococcus pneumoniae, Haemophilus influenzae, Moraxella (Branhamella) catarrhalis, Streptococcus pyogenes, P. aeruginosa, and Staphylococcus ssp. Although osteomyelitis of the temporal bone in adults is predominantly caused by P. aeruginosa , it is not certain what the organisms are commonly involved in children. One of our patients with osteomyelitis of the temporal bone cultured P. aeruginosa ; the other showed Streptococcus pneumoniae, Bacteroides species, Haemophilus influenzae, and Escherichia coli . Dudkiewicz et al, 6 in their series of acute mastoiditis and temporal bone osteomyelitis, cultured Staphylococcus, Bacteroides, and Streptococcus pneumoniae . Third, osteomyelitis takes a protracted course due to bone necrosis with or without infection, whereas osteitis can be controlled if treated adequately. Hence prognosis is good with acute mastoiditis. Although many series deal with acute mastoiditis, very few identify and detail osteomyelitis as a complication of acute mastoiditis. Dudkiewicz et al 6 studied patients who had symptoms, signs, and imaging findings of osteomyelitis of the temporal bone beyond the mastoid area. He found that 5% of patients developing acute mastoiditis developed osteomyelitis of the temporal bone as a complication. Ostfeld and Rubenstein 7 separated mastoiditis and osteomyelitis of the base of the skull while mentioning complications of mastoiditis. The other predisposing factors for osteomyelitis of the temporal bone include an immunocompromised individual, radiotherapy, malignancy, and trauma.
The most common predisposing factor in the development of osteomyelitis of the temporal bone is diabetes mellitus. 8 9 10 Driscoll et al 11 observed that the cerumen from diabetic ears had a higher average pH as compared with the nondiabetic population, and he postulated that the alkaline pH could provide a beneficial environment for bacterial overgrowth. The other reasons for an increased predisposition in diabetics are increased susceptibility to microangiopathic changes facilitating tissue necrosis and reducing antibiotic uptake and their altered immune response compromised by poor migration, reduced chemotaxis, and the defective phagocytosis of polymorphonuclear leukocytes. Other causes of osteomyelitis can be infections of the paranasal sinuses or of the mandible or maxilla due to odontic caries. The other causes in our series were malignant external otitis, mastoiditis, tuberculosis, malignancy of the middle ear, and fracture of the temporal bone. Although classically described in elderly diabetic patients, isolated cases have been reported in a small number of nondiabetic patients, 12 particularly in children who are immunocompromised due to malignancy, malnutrition, and severe anemia. 9 In the recent past HIV and acquired immunodeficiency syndrome were frequently reported as a predisposing factors for osteomyelitis of the temporal bone. 9 13 Dudkiewicz et al 6 observed that ∼ 3% of children treated for acute mastoiditis showed progression of disease to develop osteomyelitis of the temporal bone.
Clinical Features
Schweitzer 14 listed warning signs of temporal bone osteomyelitis such as (1) deep pain (temporal, parietal, postauricular, retroorbital); (2) intermittent foul otorrhea and spiking fever; (3) preauricular cellulites; (4) woody induration of pinna; (5) chronic mastoid cutaneous fistula; (6) fibrotic mastoid granulation tissue; (7) intermittent facial twitching suggestive of facial canal dehiscence, and (8) persistent leukocytosis and an elevated sedimentation rate. Anderhuber et al 12 suggested the following diagnostic criteria: pain, edema, exudate, granulations, microabscess, diabetes, old age, identification of P. aeruginosa, and even cranial nerve involvement. The most common symptoms in our series were ear discharge and pain. The most common sign was granulations in the EAC. Chandler 3 reported a 46% incidence of facial nerve paralysis.
Investigations
Culture and sensitivity is almost exclusively caused by infection with P. aeruginosa . 9 12 This is consistent with our series where we isolated P. aeruginosa in 80% of cases. Other organisms reported include coagulase-positive Staphylococcus , Bacteroides spp., Streptococcus pneumoniae , 6 Klebsiella pneumoniae , 15 Aspergillus 16 spp., and Mycobacterium tuberculosis . 17 In our series, histopathologic examination of biopsy from the ear revealed chronic inflammation, squamous cell carcinoma, and tuberculous granuloma. We recommend performing biopsy and histopathology because a routine culture of pus may fail to isolate tubercle bacilli and other fastidious organisms with exacting growth demands. This also helps to diagnose early malignant change.
Imaging to prove the extension of infection to bony structures is generally necessary to establish the diagnosis of necrotizing external otitis. 18 Imaging modalities include HRCT, technetium 99m medronate methylene diphosphonate bone scanning, and gallium citrate Ga 67 scintigraphy. Computed tomography (CT) defines the location and the extent of the disease at initial evaluation. It is definitive in determining osteitis when positive for bony destruction, but scans only show abnormalities when demineralization of ≥ 30% occurs. Once demineralization occurs, a CT scan rarely returns to normal, and thus it is not a reliable modality in assessing response to therapy. 19 Imaging of the skull base in the setting of cranial neuropathy and probable infection is best accomplished with magnetic resonance imaging (MRI). MRI has the advantage of superior soft tissue discrimination versus CT and is particularly useful for assessing soft tissue planes around the skull base and abnormalities of the medullary cavity of bone. MRI is also important for assessing dural enhancement and impending intracranial complications. Highly sensitive but nonspecific MRI findings of osteomyelitis include marrow T1 hypointensity and T2 hyperintensity. 20 Gallium scans are also very effective. Gallium is absorbed by macrophages and reticular endothelial cells over 48 to 72 hours. They are a sensitive indicator of infection, which quickly returns to normal after an infection has cleared. Benecke 21 in 1989 proposed the following staging system for osteomyelitis of the temporal bone originating from the EAC: Stage I disease has a positive gallium scan and a negative technetium scan, and the extent of the disease is only to the soft tissue. Stage II is a true osteomyelitis with a positive gallium and technetium scan, but the disease is limited to the mastoid. Stage III disease is also true osteomyelitis, and it has a positive gallium and technetium scan and defined as extensive skull-based osteomyelitis. He further went on to state that stage III disease could spread in three distinct patterns, posteriorly to involve the occipital bone, anteriorly to involve the facial bones, or medially across the clivus to involve the contralateral temporal bone.
Treatment
Treatment consists of broad-spectrum antibiotics for not less than 3 months along with surgical debridement and a wide meatoplasty. Local treatment of the auditory canal includes meticulous cleaning and debridement. It is well accepted in the literature that daily debridement of the EAC is performed until any granulation tissue resolves. Use of topical antimicrobial agents is controversial because these drops will affect future culture results if there is no response to therapy. Use of an acidifying agent in the EAC will be helpful. Once the diagnosis of skull base osteomyelitis has been confirmed, long-term antimicrobial therapy remains the mainstay of treatment with three general protocols: aminoglycoside and a β-lactamase antibiotic, a third-generation cephalosporin, Ceftazidime, or an oral quinolone ciprofloxacin. Antipseudomonal penicillins are bacteriocidal and work synergistically with aminoglycosides against Pseudomonas . This helps prevent resistance, but the penicillin may inactivate the aminoglycoside when given simultaneously, and thus they must be given at separate times. 22 23 Lang et al 24 reported successful treatment of 21 of 23 patients with minimal side effects using ciprofloxacin. Ciprofloxacin has since been widely used in the treatment of osteomyelitis of the temporal bone. 25 26 Antibiotic therapy can last several months. However, there have been recent reports of resistance of Pseudomonas to ciprofloxacin therapy in temporal bone osteomyelitis. 27 Despite the reported efficacy of prolonged systemic antibiotic therapy, treatment failures do occur due to tissue hypoperfusion and hypoxia. In such cases the use of hyperbaric oxygen increases wound partial pressure of oxygen levels, enhances phagocytic oxidative killing of aerobic microorganisms, and promotes angioneogenesis and osteoneogenesis. Treatment consists of 100% oxygen given for 90 minutes at 2.5 atm absolute pressure 5 days a week, 20 times as an adjuvant therapy. 28 Strict diabetic control is vital in the treatment of temporal bone osteomyelitis.
Acute osteomyelitis responds well to antibiotic therapy. The first line of treatment of chronic osteomyelitis is also antibiotic therapy that can be continued for 4 to 6 months. However, in the presence of severe pain, complications, refractory cases, or bony sequestra on HRCT, surgical intervention is necessary. In our series, surgery was the treatment of choice for most of the cases of chronic osteomyelitis due to the fact that the presenting symptoms of pain (85%) and ear discharge (100%) were seen in the majority of the patients. Also, most of them had received prior antibiotic therapy that failed to resolve the condition. A bony sequestra was seen on HRCT in 76% of our cases that also merited surgery. One patient had coexisting malignancy, and another was diagnosed to have tuberculous granulation tissue. Further, complications of facial palsy or meningitis were seen in 40% of the patients, two of them in infants, which required immediate intervention. This trend could largely be attributed to the fact that ours is a tertiary care university medical center.
Conclusion
Osteomyelitis of the temporal bone is extremely rare. The treating otolaryngologist must harbor a high index of suspicion in patients who complain of otalgia, particularly if they have diabetes mellitus and external otitis that has been refractory to standard therapy. There is a need to revise the current terminology for temporal bone osteomyelitis to standardize reports and research. We have discussed the terminology of osteomyelitis in the temporal bone and prefer using the terms medial and lateral temporal bone osteomyelitis. There are definitive pointers to osteomyelitis of the temporal bone both clinically and by means of investigations. Bacterial cultures must be performed in all patients with acute mastoiditis. Long-term antibiotics are the treatment of choice, but in more aggressive osteomyelitis, surgical intervention is necessary.
Conflict of Interest The authors have nothing to disclose.
Notes
This paper was presented as a paper at the 28th Politzer Society Meeting, September 28 to October 1, 2011, Athens, Greece, and as a poster at the 14th British Academic Conference in Otolaryngology, July 4–6, 2012, Glasgow, UK.
References
- 1.Fitzgerald R H, Jr, Brewer N S, Dahlin D C. Squamous-cell carcinoma complicating chronic osteomyelitis. J Bone Joint Surg Am. 1976;58(08):1146–1148. [PubMed] [Google Scholar]
- 2.Meltzer P E, Kelemen G. Pyocyaneous osteomyelitis of the temporal bone, mandible and zygoma. Laryngoscope. 1959;169:1300–1316. [Google Scholar]
- 3.Chandler J R.Malignant external otitis Laryngoscope 196878081257–1294.4970362 [Google Scholar]
- 4.Prasad K C, Prasad S C, Mouli N, Agarwal S. Osteomyelitis in the head and neck. Acta Otolaryngol. 2007;127(02):194–205. doi: 10.1080/00016480600818054. [DOI] [PubMed] [Google Scholar]
- 5.Chandler J R, Grobman L, Quencer R, Serafini A. Osteomyelitis of the base of the skull. Laryngoscope. 1986;96(03):245–251. doi: 10.1288/00005537-198603000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Dudkiewicz M, Livni G, Kornreich L, Nageris B, Ulanovski D, Raveh E. Acute mastoiditis and osteomyelitis of the temporal bone. Int J Pediatr Otorhinolaryngol. 2005;69(10):1399–1405. doi: 10.1016/j.ijporl.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 7.Ostfeld E, Rubinstein E. Acute gram-negative bacillary infections of middle ear and mastoid. Ann Otol Rhinol Laryngol. 1980;89(01):33–36. doi: 10.1177/000348948008900109. [DOI] [PubMed] [Google Scholar]
- 8.Chandler J R. Malignant external otitis and osteomyelitis of the base of the skull. Am J Otol. 1989;10(02):108–110. [PubMed] [Google Scholar]
- 9.Hern J D, Almeyda J, Thomas D M, Main J, Patel K S. Malignant otitis externa in HIV and AIDS. J Laryngol Otol. 1996;110(08):770–775. doi: 10.1017/s0022215100134929. [DOI] [PubMed] [Google Scholar]
- 10.Walshe P, Cleary M, McConn W R, Walsh M. Malignant otitis externa—a high index of suspicion is still needed for diagnosis. Ir Med J. 2002;95(01):14–16. [PubMed] [Google Scholar]
- 11.Driscoll P V, Ramachandrula A, Drezner D A, Hicks T A, Schaffer S R. Characteristics of cerumen in diabetic patients: a key to understanding malignant external otitis? Otolaryngol Head Neck Surg. 1993;109(04):676–679. doi: 10.1177/019459989310900407. [DOI] [PubMed] [Google Scholar]
- 12.Anderhuber W, Walch C, Köle W.A rare case of malignant otitis external in a non-diabetic patient[in German].Laryngorhinootologie 19957407456–459. [DOI] [PubMed] [Google Scholar]
- 13.Weinroth S E, Schessel D, Tuazon C U.Malignant otitis externa in AIDS patients: case report and review of the literature Ear Nose Throat J 19947310772–774., 777–778 [PubMed] [Google Scholar]
- 14.Schweitzer V G. Hyperbaric oxygen management of chronic staphylococcal osteomyelitis of the temporal bone. Am J Otol. 1990;11(05):347–353. [PubMed] [Google Scholar]
- 15.Yang T H, Kuo S T, Young Y H. Necrotizing external otitis in a patient caused by Klebsiella pneumoniae. Eur Arch Otorhinolaryngol. 2006;263(04):344–346. doi: 10.1007/s00405-005-0998-y. [DOI] [PubMed] [Google Scholar]
- 16.Kountakis S E, Kemper J V, Jr, Chang C Y, DiMaio D J, Stiernberg C M. Osteomyelitis of the base of the skull secondary to Aspergillus. Am J Otolaryngol. 1997;18(01):19–22. doi: 10.1016/s0196-0709(97)90043-0. [DOI] [PubMed] [Google Scholar]
- 17.Sethi A, Sabherwal A, Gulati A, Sareen D. Primary tuberculous petrositis. Acta Otolaryngol. 2005;125(11):1236–1239. doi: 10.1080/00016480510038158. [DOI] [PubMed] [Google Scholar]
- 18.Amorosa L, Modugno G C, Pirodda A. Malignant external otitis: review and personal experience. Acta Otolaryngol Suppl. 1996;521:3–16. [PubMed] [Google Scholar]
- 19.Gherini S G, Brackmann D E, Bradley W G. Magnetic resonance imaging and computerized tomography in malignant external otitis. Laryngoscope. 1986;96(05):542–548. doi: 10.1288/00005537-198605000-00014. [DOI] [PubMed] [Google Scholar]
- 20.Chang P C, Fischbein N J, Holliday R A. Central skull base osteomyelitis in patients without otitis externa: imaging findings. AJNR Am J Neuroradiol. 2003;24(07):1310–1316. [PMC free article] [PubMed] [Google Scholar]
- 21.Benecke J E., Jr Management of osteomyelitis of the skull base. Laryngoscope. 1989;99(12):1220–1223. doi: 10.1288/00005537-198912000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Kimmelman C P, Lucente F E. Use of ceftazidime for malignant external otitis. Ann Otol Rhinol Laryngol. 1989;98(09):721–725. doi: 10.1177/000348948909800912. [DOI] [PubMed] [Google Scholar]
- 23.Pedersen H B, Rosborg J. Necrotizing external otitis: aminoglycoside and β-lactam antibiotic treatment combined with surgical treatment. Clin Otolaryngol Allied Sci. 1997;22(03):271–274. doi: 10.1046/j.1365-2273.1997.00032.x. [DOI] [PubMed] [Google Scholar]
- 24.Lang R, Goshen S, Kitzes-Cohen R, Sadé J. Successful treatment of malignant external otitis with oral ciprofloxacin: report of experience with 23 patients. J Infect Dis. 1990;161(03):537–540. doi: 10.1093/infdis/161.3.537. [DOI] [PubMed] [Google Scholar]
- 25.Spielmann P M, Yu R, Neeff M. Skull base osteomyelitis: current microbiology and management. J Laryngol Otol. 2013;127 01:S8–S12. doi: 10.1017/S0022215112002356. [DOI] [PubMed] [Google Scholar]
- 26.Cavel O, Fliss D M, Segev Y, Zik D, Khafif A, Landsberg R. The role of the otorhinolaryngologist in the management of central skull base osteomyelitis. Am J Rhinol. 2007;21(03):281–285. doi: 10.2500/ajr.2007.21.3033. [DOI] [PubMed] [Google Scholar]
- 27.Bernstein J M, Holland N J, Porter G C, Maw A R. Resistance of Pseudomonas to ciprofloxacin: implications for the treatment of malignant otitis externa. J Laryngol Otol. 2007;121(02):118–123. doi: 10.1017/S0022215106002775. [DOI] [PubMed] [Google Scholar]
- 28.Mader J T, Love J T. MEO cure with HBO as adjuvant therapy. Arch Otorhinolaryngol. 1982;108:38–40. [Google Scholar]


