Abstract
Rationale
Evidence suggests that glutamate transporter 1 (GLT-1) and cystine/glutamate exchanger transporter (xCT) are critical in maintaining glutamate homeostasis. We have recently demonstrated that ceftriaxone treatment induced up-regulation of GLT1 levels and attenuated ethanol intake; however, less is known about the involvement of xCT on ethanol intake. In this study, we investigated the effects of ceftriaxone on the levels of xCT in both continuous and relapse-like ethanol drinking, as well as GLT-1 isoforms, and glutamate aspartate transporter (GLAST) in relapse-like ethanol intake.
Methods
P rats received free choice of 15 and 30 % ethanol and water for 5 weeks and then deprived of ethanol for 2 weeks. Rats were treated with ceftriaxone (100 mg/kg, i.p.) or saline during the last 5 days of the 2-week deprivation period. After deprivation period, P rats were re-exposed to free choice of 15 and 30 % ethanol and water for nine consecutive days. A second group of P rats was given continuous ethanol access for 5 weeks, then ceftriaxone (100 mg/kg, i.p.) or saline throughout the week 6.
Results
Ceftriaxone significantly attenuated relapse-like ethanol intake. Importantly, this effect of ceftriaxone was associated in part with upregulation of the levels of GLT-1a and GLT-1b isoforms and xCT in the prefrontal cortex (PFC) and the nucleus accumbens (NAc). There were no significant differences in GLAST expression among all groups. We also found that ceftriaxone treatment increased xCT levels in both PFC and NAc in continuous ethanol intake.
Conclusion
These findings suggest that xCT and GLT-1 isoforms might be target proteins for the treatment of alcohol dependence.
Keywords: Relapse, Glutamate, Ethanol intake, GLT-1a, GLT-1b, xCT
Introduction
Studies have shown that addiction to alcohol and other drugs of abuse involves changes in glutamate transmission. Neuroadaptations of the glutamatergic system are critical in ethanol dependence and relapse-like ethanol drinking (Backstrom and Hyytia 2004; Bird et al. 2008; Besheer et al. 2009; Rao and Sari 2012). Glutamate transmission within the prefrontal cortex (PFC) is involved in drug reinforcement (Goldstein and Volkow 2002). Furthermore, the nucleus accumbens (NAc), receiving glutamatergic projections from the PFC, has long been implicated in goal-directed behavior, including drug seeking (Childress et al. 1999). Glutamatergic projections from the PFC to the NAc are the primary driver of drug abuse, including ethanol (Rao and Sari 2012).
Although glutamate accumulation in extracellular fluid is controlled by a family of glutamate transporter proteins (Gegelashvili and Schousboe 1997; Seal and Amara 1999; Anderson and Swanson 2000), glutamate transporter 1 (GLT-1, human homolog is termed excitatory amino acid transporter 2, EAAT2) is found to regulate the majority of extracellular glutamate (Rothstein et al. 1995; Danbolt 2001; Mitani and Tanaka 2003). We have recently reported that ceftriaxone (CEF), a β-lactam antibiotic, attenuated relapse-like ethanol drinking and upregulated GLT-1 levels in the PFC and NAc in male alcohol-preferring (P) rats (Qrunfleh et al. 2013; Sari et al. 2011, 2013a, 2013b).
It is noteworthy that GLT-1 exists in two splice variant isoforms: GLT-1a and GLT-1b (Chen et al. 2002, 2004; Berger et al. 2005). Of these variants, GLT-1a is predominantly expressed in neurons and astrocytes, and GLT-1b is mainly expressed in astrocytes (Berger et al. 2005; Holmseth et al. 2009). Although there is no evidence for any differences in the transport abilities between these two isoforms, differences exist in their localization in the brain (Sullivan et al. 2004). It was shown that GLT-1a is localized to glial processes interposed between multiple synapse types; however, GLT-1b is expressed by astrocytic processes at sites not interposed between synapses (Sullivan et al. 2004). Studies have demonstrated that these isoforms are differentially affected in several disease models. For example, GLT-1b level was found increased in the motor cortex in amyotrophic lateral sclerosis, whereas GLT-1a level was found decreased (Maragakis et al. 2004). Thus, in this study, we investigated the levels of GLT-1 isoforms in the PFC and NAc using a relapse-like ethanol paradigm, and we determined whether CEF has any effects on these isoforms.
In addition to GLT-1 isoforms, cystine/glutamate exchanger transporter (xCT) is a glial protein involved in regulating glutamate homeostasis. This regulatory effect of xCT involves the exchange of extracellular cystine for intracellular glutamate (Bannai and Ishii 1982; Bannai et al. 1984). It is important to note that xCT has been shown to play a key role in relapse to cocaine seeking and self-administered nicotine animal models (Knackstedt et al. 2009, 2010). The rationale for investigating xCT in both continuous and relapse-like ethanol intake paradigms is based on recent studies that demonstrated that CEF upregulated xCT level, restored glutamate reuptake, and attenuated cue-induced reinstatement of cocaine-seeking behavior (Knackstedt et al. 2010; Trantham-Davidson et al. 2012). Thus, we have investigated the levels of xCT in the PFC and NAc using both continuous ethanol drinking and relapse-like ethanol drinking paradigms. We have further determined whether CEF-induced attenuation of ethanol intake and relapse-like ethanol drinking is associated in part with upregulation of xCT levels in the target brain regions.
In addition, we investigated the effects of CEF on glutamate aspartate transporter (GLAST) as a positive glial protein control in a relapse-like ethanol drinking paradigm.
Materials and methods
Animals
P rats were obtained from Indiana University School of Medicine, Indianapolis, IN. Rats were received at the age of 21–30 days, and they were housed in standard plastic tubs with corn-cob bedding in the Department of Laboratory Animal Resources, The University of Toledo, Health Science Campus. Rats were acclimated to a room with 21 °C temperature and 50 % humidity in a 12-h light/dark cycle. Rats had ad libitum access to food (Harlan 2016 Teklad) and water throughout the experimental procedures. All animal procedures were approved by the Institutional Animal Care and Use committee of The University of Toledo in accordance with the guidelines of the Institutional Animal Care and Use Committee of the National Institutes of Health and the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, Commission on Life Sciences 1996). Animal procedures and programs are accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care International.
Ethanol drinking procedures
Animals started experimental procedures at approximately 90 days of age; their body weight averaged 317–395 g. Two drinking paradigms were performed: continuous ethanol-drinking paradigm and relapse-like ethanol-drinking paradigm. Three experimental groups were studied for both drinking paradigms: (1) the ethanol-naïve saline group received water and food only and i.p. injections of saline solution (n=6); (2) the ethanol saline group received i.p. injections of saline (n=6); and (3) the ethanol CEF group received i.p. injections of ceftriaxone (100 mg/kg, n=6). Ceftriaxone (Apotex, USA) was dissolved in saline solution (0.9 % NaCl). The ethanol saline and ethanol CEF groups had free-choice access to water, ethanol (15 and 30 %), and food throughout the experiments. This drinking regimen is known to increase the intake of ethanol in P rats (Rodd-Henricks et al. 2001). For both paradigms, 3-month-old, male P rats drank either ethanol (n=24) or water (n=12) for 5 weeks. Upon week 6, continuous access rats received five daily treatments of ceftriaxone (n=6) or saline (n=6). Animals were euthanized after 24 h of their final injections. Relapse-like drinker (n=12) were ethanol-deprived from week 6 to 7. During week 7, they received five daily treatments of CEF (n=6) or saline (n=6). Relapse-like ethanol drinking was measured during week 8. For the ethanol groups (saline and CEF groups), ethanol intake was measured as grams of ethanol consumed per kilogram of animal body weight per day. The average measurements taken across the last 2 weeks (three times per week) of the 5-week ethanol and water intake, along with body weights, were calculated as baseline. Note that the calculation of the average measurements of ethanol intake was based on cumulative intake of 15 and 30 % ethanol. During relapse-like ethanol drinking, body weights, water and ethanol intake were recorded every other day.
Brain tissue harvesting
After the termination of both continuous and relapse-like ethanol-drinking paradigms, P rats were euthanized and rapidly decapitated with a guillotine. Brains were removed and immediately frozen on dry ice and stored at −70 °C. The PFC and NAc were punched stereotaxically using a cryostat apparatus as described recently (Sari and Sreemantula 2012). These brain regions were then frozen at −70 °C for Western blot assay to determine the levels of proteins, such as xCT, GLT-1a, GLT-1b, GLAST, and β-tubulin.
Western blot
xCT, GLT-1a, GLT-1b, GLAST, and β-tubulin levels were determined in the PFC and the NAc in the ethanol-naïve saline, ethanol saline, and ethanol CEF groups using Western blot assay, as previously described (Sari et al. 2009, 2011; Sari and Sreemantula 2012). In brief, extracted proteins were transferred onto PVDF membranes, and membranes were incubated overnight at 4 °C with one of the following antibodies: rabbit anti-GLT-1a (dilution: 1:10,000; gift from Dr. Jeffery Rothstein at Johns Hopkins University), rabbit anti-GLT-1b (dilution: 1:5,000; gift from Dr. Paul Rosenberg at Harvard Medical School University), rabbit anti-GLAST (dilution: 1:5,000), rabbit anti-xCT (dilution: 1:1,000), and mouse anti-β-tubulin (1:5,000). Membranes were incubated with chemiluminescent kit (SuperSignal West Pico) for protein detection. Membranes were then exposed to Kodak BioMax MR films (Thermo Fisher Scientific), and films were developed using SRX-101A machine. Blots for each detected protein were digitized and quantified using MCID system. Data were calculated as ratios of GLT1a/, GLT1b/, GLAST/, and xCT/β-tubulin.
Statistical analyses
Two-way (mixed) ANOVA followed by Dunnett’s post hoc test was used for statistical analyses between the ethanol saline and ethanol CEF groups for ethanol and water intake as well as body weight data. Immunoblotting data were statistically analyzed using one-way ANOVA and Newman–Keuls’s test for comparison between the ethanol-naïve saline, ethanol saline, and ethanol CEF groups. All statistical tests were based on p<0.05 level of significance.
Results
Effects of CEF treatment on ethanol intake, water intake, and body weight during relapse-like ethanol drinking
In regard to the effect of CEF on the continuous ethanol-drinking paradigm, we have found that CEF treatment significantly decreased ethanol intake, similar to our findings in a recent report (Sari et al. 2011). However, CEF significantly increased water intake. We did not observe any significant difference in body weight with CEF treatment.
Mixed ANOVA revealed a significant main effect of day on ethanol intake [F(1,6)=14.15, p<0.05] and a significant treatment × day interaction [F(1,6) = 4.70, p < 0.05]. Furthermore, statistical analysis revealed a significant reduction in ethanol intake during relapse-like drinking in animals treated with CEF compared to saline-treated animals (p<0.001 for days 1, 5, 7, and 9, and p<0.01 for day 3) (Fig. 1a). Note that there was a significant increase in ethanol intake at day 1 (first day of re-exposure) compared to the baseline level in the ethanol saline group (p<0.05).
Fig. 1.
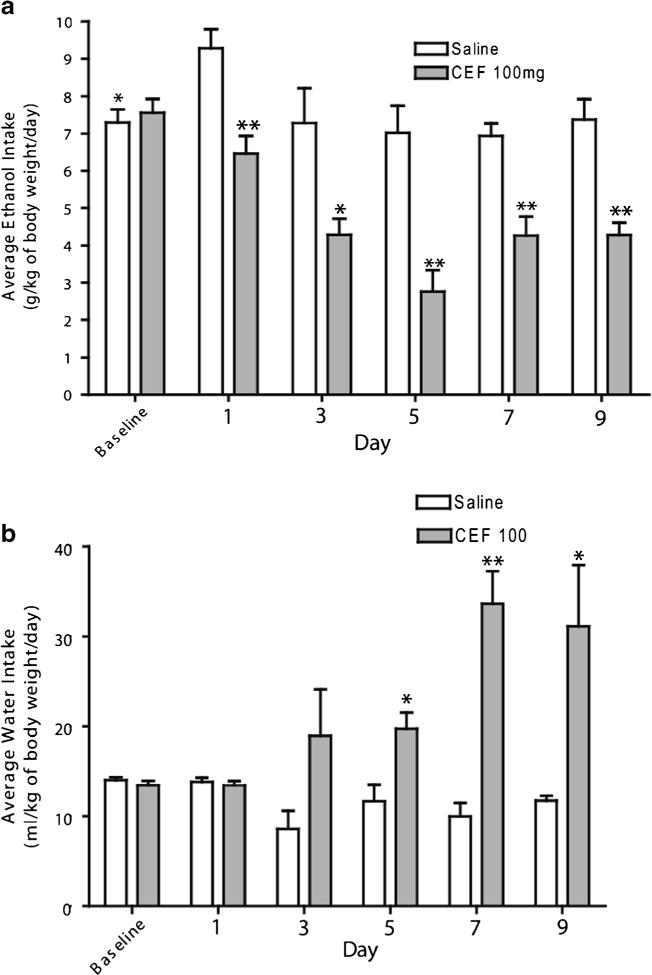
Graph represents average daily ethanol and water intake and body weight during the 9 days of ethanol re-exposure. a Daily ethanol intake of male P rats for 9 days of ethanol re-exposure, following 5 days of treatment with ceftriaxone (CEF) or saline during ethanol deprivation. (*p<0.01; **p<0.001); b daily water intake of male P rats for 9 days, following 5 days of treatment during ethanol deprivation (*p<0.05; **p<0.01). All data are expressed as mean ± SEM. Saline (ethanol saline group, n=6); CEF (ethanol CEF group at 100 mg/kg body weight, n=6)
Furthermore, mixed ANOVA revealed a significant main effect of day on water intake [F(1,6)=5.23, p<0.05] and a significant treatment×day interaction [F(1,6)=9.32, p<0.05]. Moreover, statistical analysis showed a significant increase in water consumption in the ethanol CEF group compared to the ethanol saline group in days 5–9 (Fig. 1b). In addition, mixed ANOVA revealed a significant main effect of day on body weight [F(1,6)=22.18, p<0.05] and a significant treatment×day interaction [F(1,6)=5.14, p<0.05]. Furthermore, statistical analysis did not reveal any significant difference in body weight between the ethanol saline and ethanol CEF groups.
Effect of CEF on GLT-1a expression in relapse-like ethanol drinking
One-way ANOVA analyses revealed a significant main effect among the ethanol-naïve saline, ethanol saline, and ethanol CEF groups on GLT-1a isoform levels in the PFC [F(2, 15)=6.37, p<0.01]. A post hoc test, Newman–Keuls multiple comparison test, demonstrated a significant increase in the percent ratio of GLT-1a/β-tubulin (% of ethanol-naïve saline group) in the ethanol CEF group compared to the ethanol-naïve saline (p<0.05) and ethanol saline (p<0.05) groups (Fig. 2a, b). Furthermore, statistical analyses using one-way ANOVA demonstrated a significant main effect among the ethanol-naïve saline, ethanol saline, and ethanol CEF groups on GLT-1a isoform levels in the NAc [F(2, 15)=6.89, p<0.01]. A significant increase in the percent ratio of GLT-1a/β-tubulin (% of ethanol-naïve saline group) was found in the ethanol CEF group compared to the ethanol-naïve saline (p<0.05) and ethanol saline (p<0.01) groups (Fig. 2c, d).
Fig. 2.
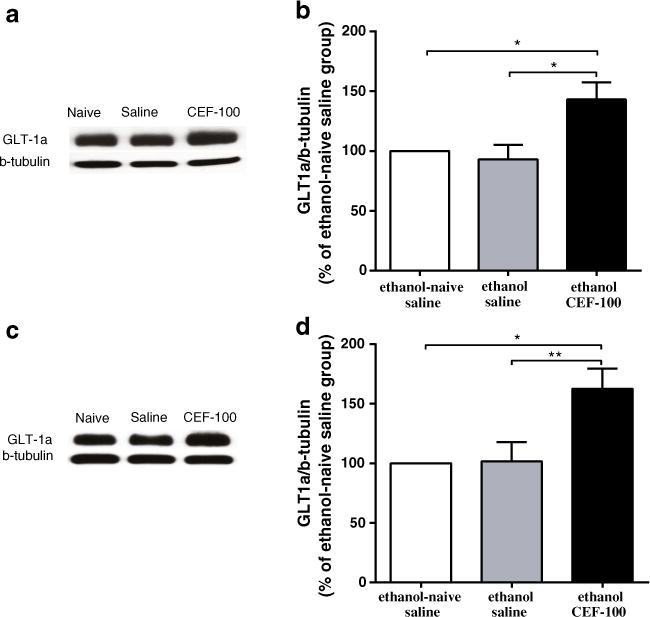
Effects of ceftriaxone (CEF) on GLT-1a expression in the PFC and NAc in relapse-like ethanol-drinking paradigm. a Immunoblots for GLT-1a expression in the PFC and β-tubulin as a control-loading protein. b CEF treatment significantly increased GLT-1a level in the PFC as compared to ethanol-naïve saline and ethanol saline groups. c Immunoblots for GLT-1a expression in the NAc and β-tubulin as a control-loading protein. d CEF treatment significantly increased GLT-1a level in the NAc as compared to ethanol-naïve saline and ethanol saline groups. Data are expressed as mean ± SEM. (*p<0.05, **p<0.01); N=6 for each group
Effect of CEF on GLT-1b expression in relapse-like ethanol drinking
Next, we investigated GLT-1b levels in both the PFC and the NAc in relapse-like ethanol drinking paradigm. One-way ANOVA analyses showed a significant main effect among the ethanol-naïve saline, ethanol saline, and ethanol CEF groups on GLT-1b isoform levels in the PFC [F(2, 15)=6.77, p<0.01]. Newman–Keuls multiple comparison test demonstrated a significant increase in the percent ratio of GLT-1b/β-tubulin (% of ethanol-naïve saline group) in the ethanol CEF group compared to the ethanol-naïve saline (p<0.05) and ethanol saline (p<0.01) groups in the PFC (Fig. 3a, b).
Fig. 3.
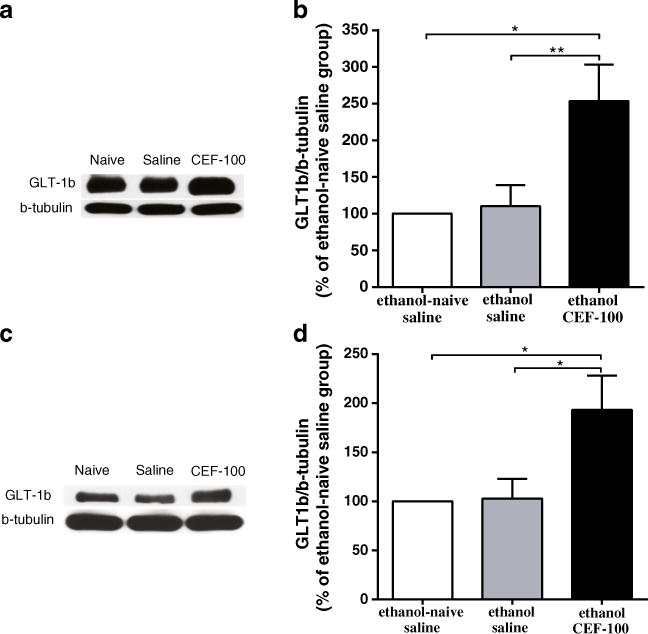
Effects of ceftriaxone (CEF) on GLT-1b expression in the PFC and NAc in relapse-like ethanol-drinking paradigm. a Immunoblots for GLT-1b expression in the PFC and β-tubulin as a control-loading protein. b CEF treatment significantly increased GLT-1b level in the PFC as compared to ethanol-naïve saline and ethanol saline groups. c Immunoblots for GLT-1b expression in the NAc and β-tubulin as a control-loading protein. d CEF treatment significantly increased GLT-1b level in the NAc as compared to ethanol-naïve saline and ethanol saline groups. Data are expressed as mean ± SEM. (*p<0.05, **p<0.01); N=6 for each group
Furthermore, one-way ANOVA demonstrated a significant main effect among the ethanol-naïve saline, ethanol saline, and ethanol CEF groups on GLT-1b level in the NAc [F(2, 15)=5.20, p<0.05]. A significant increase in the percent ratio of GLT-1b/β-tubulin (% of ethanol-naïve saline group) was found in the ethanol CEF group compared to the ethanol-naïve saline and ethanol saline groups (p<0.05, Fig. 3c, d).
Effect of CEF on xCT expression in relapse-like ethanol drinking
One-way ANOVA revealed a significant main effect in the expression of xCT in the PFC among the control and treatment groups [F(2, 15)=7.39, p<0.01]. Newman–Keuls post hoc test demonstrated a significant increase in the percent ratio of xCT/β-tubulin (% of ethanol-naïve saline group) in the ethanol CEF group compared to the ethanol-naïve saline (p<0.05) and ethanol saline (p<0.01) groups (Fig. 4a, b).
Fig. 4.
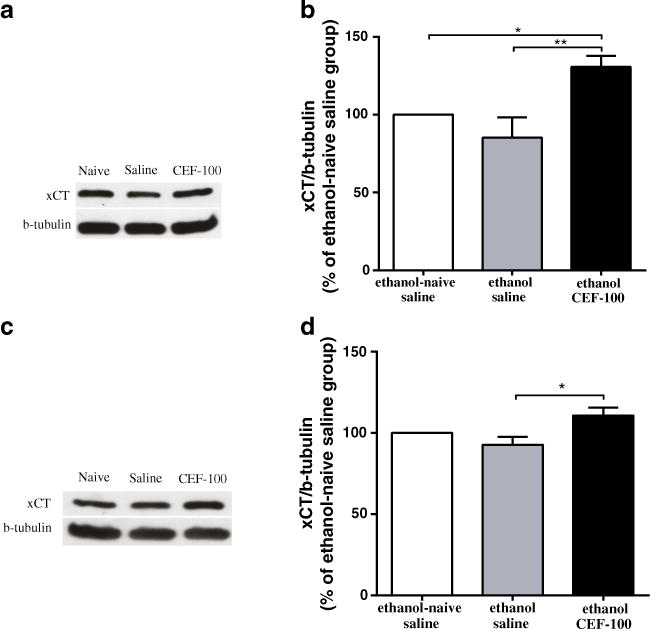
Effects of ceftriaxone (CEF) on xCT expression in the PFC and NAc in relapse-like ethanol-drinking paradigm. a Immunoblots for xCT expression in the PFC and β-tubulin as a control-loading protein. b CEF treatment significantly increased xCT level in the PFC as compared to ethanol-naïve saline and ethanol saline groups. c Immunoblots for xCT expression in the NAc and β-tubulin as a control-loading protein. d CEF treatment significantly increased xCT level in the NAc as compared to ethanol saline group. Data are expressed as mean ± SEM. (*p<0.05, **p<0.01); N=6 for each group
Furthermore, one-way ANOVA revealed a significant main effect in the expression of xCT in the NAc among all groups [F(2, 15)=5.09, p<0.05]. A significant increase in the percent ratio of xCT/β-tubulin (% of ethanol-naïve saline group) was found in the ethanol CEF group compared to the ethanol saline (p<0.05) group (Fig. 4c, d).
Effect of CEF on xCT expression in continuous ethanol drinking
One-way ANOVA revealed a significant main effect in the expression of xCT in the PFC among the control and treatment groups [F(2, 12)=5.51, p<0.05]. Newman–Keuls post hoc test demonstrated a significant decrease in the percent ratio of xCT/β-tubulin (% of ethanol-naïve saline group) in the ethanol saline group compared to the ethanol-naïve saline (p<0.05) group, and a significant increase in the percent ratio of xCT/β-tubulin in the ethanol CEF group compared to ethanol saline (p<0.05) group (Fig. 5a, b).
Fig. 5.
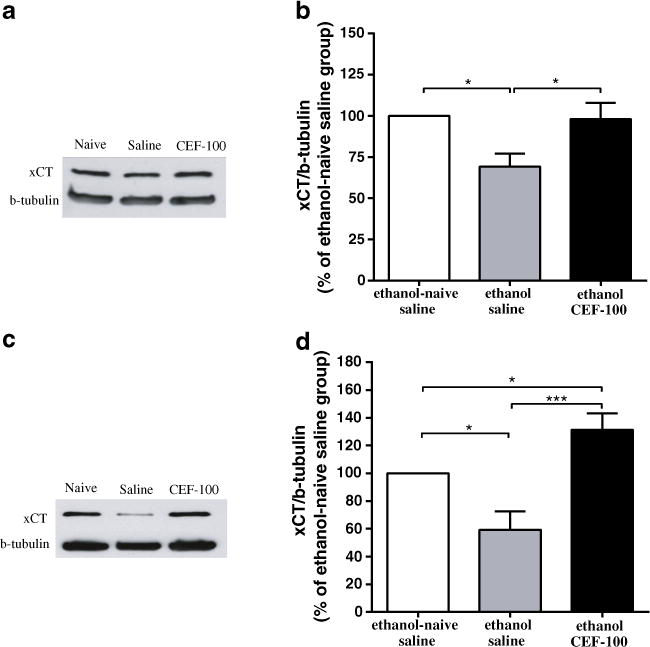
Effects of ceftriaxone (CEF) on xCT expression in the PFC and NAc in continuous ethanol-drinking paradigm. a Immunoblots for xCT expression in the PFC and β-tubulin as a control-loading protein. b There was significant difference in xCT level in the PFC between ethanol-naïve saline and ethanol saline groups. CEF treatment significantly increased xCT level in the PFC as compared to ethanol saline group. c Immunoblots for xCT expression in the NAc and β-tubulin as a control-loading protein. d There was significant difference in the xCT level in the NAc between ethanol-naïve saline and ethanol saline groups. CEF treatment significantly increased the xCT level in the NAc as compared to ethanol-naïve saline and ethanol saline groups. Data are expressed as mean ± SEM. (*p<0.05, ***p<0.001); (N=5–6)
Furthermore, one-way ANOVA revealed a significant main effect among control and treatment groups in NAc [F(2, 15)=12.39, p<0.001]. Newman–Keuls post hoc test demonstrated a significant decrease in the percent ratio of xCT/β-tubulin (% of ethanol-naïve saline group) in the ethanol saline group compared to the ethanol-naïve saline (p<0.05) group, and a significant increase in the percent ratio of xCT/β-tubulin in the ethanol CEF group compared to the ethanol-naïve saline (p<0.05) and ethanol saline (p<0.001) groups (Fig. 5c, d).
Effect of CEF on GLAST expression in relapse-like ethanol drinking
Finally, we determined GLAST levels in both the PFC and NAc in relapse-like ethanol-drinking paradigm. One-way ANOVA did not show any significant main effect among the control and treatment groups in either the PFC [F(2, 15)=0.94, p>0.05] or the NAc [F(2, 15)=0.11, p>0.05]. Newman–Keuls multiple comparison test did not show any significant difference among all groups in GLAST level in either the PFC or the NAc.
Discussion
We previously reported that CEF significantly reduced ethanol consumption in male P rats in the continuous ethanol-drinking paradigm (Sari et al. 2011). We report here that CEF treatment attenuated relapse-like ethanol drinking in male P rats. CEF-treated rats increased their water intake significantly, which is suggested to be a compensatory mechanism for decreasing their ethanol intake; however, there were no meaningful differences in body weight among all groups.
A recent study from our laboratory demonstrated that CEF treatment attenuated relapse-like ethanol drinking in P rats and upregulated GLT-1 levels in the PFC and the NAc core (Qrunfleh et al. 2013). In this study, we tested the effect of CEF on xCT in both continuous and relapse-like ethanol-drinking paradigms. We also tested the effect of CEF on GLT-1 isoforms (GLT-1a and GLT-1b) and GLAST in the relapse-like ethanol-drinking paradigm. Although the two GLT-1 isoforms, GLT-1a and GLT-1b, are differentially regulated (Berger et al. 2005), our results showed that CEF treatment upregulated both GLT-1 isoforms in the PFC and the NAc. CEF treatment may upregulate GLT-1a and GLT-1b through a similar mechanism. It is noteworthy that GLT-1a is predominantly located in astrocytes and neurons, and GLT-1b is mainly expressed in astrocytes (Berger et al. 2005; Holmseth et al. 2009).
These findings suggest that CEF treatment induced upregulation of both neuronal and astrocyte GLT-1 isoforms. Importantly, we also demonstrated that CEF-treatment upregulated another important glial protein, xCT, which plays a role in the exchange of cystine for glutamate. These findings are in accordance with recent findings using a cocaine-relapse model, showing that CEF upregulated xCT in the NAc (Knackstedt et al. 2010). We further tested GLAST, which is mainly found on the membranes of astroglial cells, where it co-expresses with GLT-1 throughout the brain (Berger and Hediger 1998). However, we did not observe any significant changes in the level of GLAST with CEF treatment, which suggests the specific regulatory effect of CEF on GLT-1a, GLT-1b, and xCT expression.
Recent studies from our laboratory showed that GLT-1 level is downregulated in the NAc core but not in the PFC after chronic ethanol consumption for 5 weeks (Sari and Sreemantula 2012). In this study, we did not observe any changes in GLT-1a or GLT-1b levels in the PFC or the NAc in the ethanol saline group compared to the ethanol-naïve saline group. This may be due to the fact that neuroadaptation occurred during the 2-week deprivation period, which might cause GLT-1a and GLT-1b levels to return to their initial levels. Thus, we hypothesized here that the effects of CEF in the upregulation of these GLT-1 isoforms might overcome their dysfunctional levels caused by the 5-week ethanol-drinking paradigm. Pharmacological studies are warranted to determine the dysfunctional levels of these isoforms during these periods with the focus on their internalization in the cell membrane as a consequence of ethanol exposure.
In this study, we also found that chronic ethanol consumption caused downregulation of xCT in both the PFC and NAc, compared to the ethanol-naïve saline group. In addition, CEF treatment resulted in restoration of xCT levels in both the PFC and NAc. We have previously shown that chronic ethanol consumption caused downregulation of GLT-1 in the NAc (Sari and Sreemantula 2012) and CEF induced upregulation of GLT-1 level in both the PFC and NAc (Sari et al. 2011). These findings suggest that glutamate release and glutamate elimination through xCT and GLT-1, respectively, are co-regulated. This effect of CEF in xCT is consistent with a previous report, which demonstrated that CEF restored the xCT level and consequently attenuated the cue to cocaine-seeking behavior (Knackstedt et al. 2010).
It is noteworthy that xCT also plays an important role in glutamate homeostasis. xCT regulates the exchange of extracellular cystine for intracellular glutamate (Baker et al. 2002). A recent study by Knackstedt et al. (2009) demonstrated that xCT was found downregulated in nicotine and cocaine self-administration animal models (Knackstedt et al. 2009, 2010). It is noteworthy that these studies demonstrated that CEF treatment reduced the relapse to cocaine-seeking behavior by normalizing the xCT level in the NAc. In addition, an in vitro study showed that CEF acts as a neuroprotective agent by increasing the levels of xCT and glutathione in a cell line (Lewerenz et al. 2009). The neuroprotective effect was found to be mediated through the nuclear factor erythroid 2-related factor (Nrf2). Nrf2 is an inducer of the xc- system and its catalytic subunit, xCT (Lewerenz et al. 2009). It is noteworthy that the CEF-induced increase in the xCT level and subsequent increases in glutathione levels may be responsible for reversing the glutamate transporter deficits caused by free radical oxidation, and this mechanism might occur independently of GLT-1 upregulation.
Note that we did not observe any significant difference in xCT level between the ethanol-naïve saline and the ethanol saline-treated rats in either the PFC or the NAc in the relapse-like ethanol drinking paradigm. Similar to GLT-1 isoforms, this may be due to the fact that neuroadaptation occurred during the 2-week deprivation period, which might cause xCT levels to return to their initial levels. However, we found downregulation of xCT levels in both the PFC and NAc in continuous ethanol-drinking paradigm in the ethanol saline group as compared to ethanol-naïve saline group. We also hypothesized here that dysfunction may occur with xCT as well; however, CEF treatment may overcome this dysfunctional effect. Studies are warranted to determine the dysfunctional levels of xCT during the deprivation period and relapse-like ethanol drinking.
We conclude that attenuation of relapse-like ethanol drinking in CEF-treated male P rats occurred as a result of upregulation of GLT-1a and GLT-1b isoforms and xCT levels in central reward brain regions such as the PFC and the NAc. The lack of upregulatory effect on the GLAST level provides information about the specific action of CEF in upregulating GLT-1a and GLT-1b isoforms and xCT levels in both the PFC and the NAc for the attenuation of relapse-like ethanol drinking. These findings suggest that the upregulatory effects of these major transporters may restore abnormal glutamate levels to original homeostasis and consequently attenuate relapse-like and continuous ethanol drinking paradigms. Together with recent findings from our laboratory, we propose that CEF may be considered a potential compound for the treatment of alcohol dependence.
Acknowledgments
This work was supported by Award Number R01AA019458 (Y.S.) from the National Institutes on Alcohol Abuse and Alcoholism. The authors would like to thank Dr. Jeffery Rothstein from Johns Hopkins University and Dr. Paul Rosenberg from Harvard Medical School for providing us with GLT-1a and GLT-1b antibodies.
Footnotes
Conflict of interest The authors declare no conflict of interest.
References
- Anderson CM, Swanson RA. Astrocyte glutamate transport: review of properties, regulation, and physiological functions. Glia. 2000;32:1–14. [PubMed] [Google Scholar]
- Backstrom P, Hyytia P. Ionotropic glutamate receptor antagonists modulate cue-induced reinstatement of ethanol-seeking behavior. Alcohol Clin Exp Res. 2004;28:558–565. doi: 10.1097/01.alc.0000122101.13164.21. [DOI] [PubMed] [Google Scholar]
- Baker DA, Xi ZX, Shen H, Swanson CJ, Kalivas PW. The origin and neuronal function of in vivo nonsynaptic glutamate. J Neurosci. 2002;22:9134–9141. doi: 10.1523/JNEUROSCI.22-20-09134.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannai S, Ishii T. Transport of cystine and cysteine and cell growth in cultured human diploid fibroblasts: effect of glutamate and homocysteate. J Cell Physiol. 1982;112:265–272. doi: 10.1002/jcp.1041120216. [DOI] [PubMed] [Google Scholar]
- Bannai S, Christensen HN, Vadgama JV, Ellory JC, Englesberg E, Guidotti GG, Gazzola GC, Kilberg MS, Lajtha A, Sacktor B, et al. Amino acid transport systems. Nature. 1984;311:308. doi: 10.1038/311308b0. [DOI] [PubMed] [Google Scholar]
- Berger UV, Hediger MA. Comparative analysis of glutamate transporter expression in rat brain using differential double in situ hybridization. Anat Embryol. 1998;198:13–30. doi: 10.1007/s004290050161. [DOI] [PubMed] [Google Scholar]
- Berger UV, DeSilva TM, Chen W, Rosenberg PA. Cellular and subcellular mRNA localization of glutamate transporter isoforms GLT1a and GLT1b in rat brain by in situ hybridization. J Comp Neurol. 2005;492:78–89. doi: 10.1002/cne.20737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besheer J, Grondin JJ, Cannady R, Sharko AC, Faccidomo S, Hodge CW. Metabotropic glutamate receptor 5 activity in the nucleus accumbens is required for the maintenance of ethanol self-administration in a rat genetic model of high alcohol intake. Biol Psychiatry. 2009;67:812–822. doi: 10.1016/j.biopsych.2009.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird MK, Kirchhoff J, Djouma E, Lawrence AJ. Metabotropic glutamate 5 receptors regulate sensitivity to ethanol in mice. Int J Neuropsychopharmacol. 2008;11:765–774. doi: 10.1017/S1461145708008572. [DOI] [PubMed] [Google Scholar]
- Chen W, Aoki C, Mahadomrongkul V, Gruber CE, Wang GJ, Blitzblau R, Irwin N, Rosenberg PA. Expression of a variant form of the glutamate transporter GLT1 in neuronal cultures and in neurons and astrocytes in the rat brain. J Neurosci. 2002;22:2142–2152. doi: 10.1523/JNEUROSCI.22-06-02142.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Mahadomrongkul V, Berger UV, Bassan M, DeSilva T, Tanaka K, Irwin N, Aoki C, Rosenberg PA. The glutamate transporter GLT1a is expressed in excitatory axon terminals of mature hippo-campal neurons. J Neurosci. 2004;24:1136–1148. doi: 10.1523/JNEUROSCI.1586-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O’Brien CP. Limbic activation during cue-induced cocaine craving. Am J Psychiatry. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Gegelashvili G, Schousboe A. High affinity glutamate transporters: regulation of expression and activity. Mol Pharmacol. 1997;52:6–15. doi: 10.1124/mol.52.1.6. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmseth S, Scott HA, Real K, Lehre KP, Leergaard TB, Bjaalie JG, Danbolt NC. The concentrations and distributions of three C-terminal variants of the GLT1 (EAAT2; slc1a2) glutamate transporter protein in rat brain tissue suggest differential regulation. Neuroscience. 2009;162:1055–1071. doi: 10.1016/j.neuroscience.2009.03.048. [DOI] [PubMed] [Google Scholar]
- Knackstedt LA, LaRowe S, Mardikian P, Malcolm R, Upadhyaya H, Hedden S, Markou A, Kalivas PW. The role of cystine-glutamate exchange in nicotine dependence in rats and humans. Biol Psychiatry. 2009;65:841–845. doi: 10.1016/j.biopsych.2008.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knackstedt LA, Melendez RI, Kalivas PW. Ceftriaxone restores glutamate homeostasis and prevents relapse to cocaine seeking. Biol Psychiatry. 2010;67:81–84. doi: 10.1016/j.biopsych.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewerenz J, Albrecht P, Tien ML, Henke N, Karumbayaram S, Kornblum HI, Wiedau-Pazos M, Schubert D, Maher P, Methner A. Induction of Nrf2 and xCT are involved in the action of the neuroprotective antibiotic ceftriaxone in vitro. J Neurochem. 2009;111:332–343. doi: 10.1111/j.1471-4159.2009.06347.x. [DOI] [PubMed] [Google Scholar]
- Maragakis NJ, Dykes-Hoberg M, Rothstein JD. Altered expression of the glutamate transporter EAAT2b in neurological disease. Ann Neurol. 2004;55:469–477. doi: 10.1002/ana.20003. [DOI] [PubMed] [Google Scholar]
- Mitani A, Tanaka K. Functional changes of glial glutamate transporter GLT-1 during ischemia: an in vivo study in the hippocampal CA1 of normal mice and mutant mice lacking GLT-1. J Neurosci. 2003;23:7176–7182. doi: 10.1523/JNEUROSCI.23-18-07176.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qrunfleh AM, Alazizi A, Sari Y. Ceftriaxone, a beta-lactam antibiotic, attenuates relapse-like ethanol-drinking behavior in alcohol-preferring rats. J Psychopharmacol. 2013;27:541–549. doi: 10.1177/0269881113482529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao PS, Sari Y. Glutamate transporter 1: target for the treatment of alcohol dependence. Curr Med Chem. 2012;19:5148–5156. doi: 10.2174/092986712803530511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, Bell RL, Kuc KA, Murphy JM, McBride WJ, Lumeng L, Li TK. Effects of concurrent access to multiple ethanol concentrations and repeated deprivations on alcohol intake of alcohol-preferring rats. Alcohol Clin Exp Res. 2001;25:1140–1150. [PubMed] [Google Scholar]
- Rothstein JD, Van Kammen M, Levey AI, Martin LJ, Kuncl RW. Selective loss of glial glutamate transporter GLT-1 in amyotrophic lateral sclerosis. Ann Neurol. 1995;38:73–84. doi: 10.1002/ana.410380114. [DOI] [PubMed] [Google Scholar]
- Sari Y, Sreemantula SN. Neuroimmunophilin GPI-1046 reduces ethanol consumption in part through activation of GLT1 in alcohol-preferring rats. Neuroscience. 2012;227:327–335. doi: 10.1016/j.neuroscience.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sari Y, Smith KD, Ali PK, Rebec GV. Upregulation of GLT1 attenuates cue-induced reinstatement of cocaine-seeking behavior in rats. J Neurosci. 2009;29:9239–9243. doi: 10.1523/JNEUROSCI.1746-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sari Y, Sakai M, Weedman JM, Rebec GV, Bell RL. Ceftriaxone, a beta-lactam antibiotic, reduces ethanol consumption in alcohol-preferring rats. Alcohol Alcohol. 2011;46:239–246. doi: 10.1093/alcalc/agr023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sari Y, Franklin KM, Alazizi A, Rao PS, Bell RL. Effects of ceftriaxone on the acquisition and maintenance of ethanol drinking in peri-adolescent and adult female alcohol-preferring (P) rats. Neuroscience. 2013a;241:229–238. doi: 10.1016/j.neuroscience.2013.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sari Y, Sreemantula SN, Lee MR, Choi DS. Ceftriaxone treatment affects the levels of GLT1 and ENT1 as well as ethanol intake in alcohol-preferring rats. J Molec Neurosci. 2013b;51:779–787. doi: 10.1007/s12031-013-0064-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seal RP, Amara SG. Excitatory amino acid transporters: a family in flux. Annu Rev Pharmacol Toxicol. 1999;39:431–456. doi: 10.1146/annurev.pharmtox.39.1.431. [DOI] [PubMed] [Google Scholar]
- Sullivan R, Rauen T, Fischer F, Wiessner M, Grewer C, Bicho A, Pow DV. Cloning, transport properties, and differential localization of two splice variants of GLT-1 in the rat CNS: implications for CNS glutamate homeostasis. Glia. 2004;45:155–169. doi: 10.1002/glia.10317. [DOI] [PubMed] [Google Scholar]
- Trantham-Davidson H, LaLumiere RT, Reissner KJ, Kalivas PW, Knackstedt LA. Ceftriaxone normalizes nucleus accumbens synaptic transmission, glutamate transport, and export following cocaine self-administration and extinction training. J Neurosci. 2012;32:12406–12410. doi: 10.1523/JNEUROSCI.1976-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]


