Abstract
Objective
To evaluate the association of pretrauma center (PTC) red blood cell (RBC) transfusion with outcomes in severely injured patients.
Background
Hemorrhage remains a major driver of mortality. Little evidence exists supporting PTC interventions to mitigate this.
Methods
Blunt injured patients in shock arriving at a trauma center within 2 hours of injury were included from the Glue Grant database. Subjects were dichotomized by PTC RBC transfusion. Outcomes included 24-hour mortality, 30-day mortality, and trauma-induced coagulopathy [(TIC), admission international normalized ratio >1.5]. Cox regression and logistic regression determined the association of PTC RBC transfusion with outcomes. To address baseline differences, propensity score matching was used.
Results
Of 1415 subjects, 50 received PTC RBC transfusion. Demographics and injury severity score were similar. The PTC RBC group received 1.3 units of RBCs (median), and 52% were scene transports. PTC RBC transfusion was associated with a 95% reduction in odds of 24-hour mortality [odds ratio (OR) = 0.05; 95% confidence interval (CI), 0.01–0.48; P < 0.01], 64% reduction in the risk of 30-day mortality [hazard ratio = 0.36; 95% CI, 0.15–0.83; P = 0.02], and 88% reduction in odds of TIC (OR = 0.12; 95% CI, 0.02–0.79; P = 0.03). The matched cohort included 113 subjects (31% PTC RBC group). Baseline characteristics were similar. PTC RBC transfusion was associated with a 98% reduction in odds of 24-hour mortality (OR = 0.02; 95% CI, 0.01–0.69; P = 0.04), 88% reduction in the risk of 30-day mortality (hazard ratio = 0.12; 95% CI, 0.03–0.61; P = 0.01), and 99% reduction in odds of TIC (OR = 0.01; 95% CI, 0.01–0.95; P = 0.05).
Conclusions
PTC RBC administration was associated with a lower risk of 24-hour mortality, 30-day mortality, and TIC in severely injured patients with blunt trauma, warranting further prospective study.
Keywords: blood, prehospital, resuscitation, trauma, transfusion
Hemorrhage and coagulopathy remain major drivers of early mortality in injured patients.1,2 Recent literature has focused on resuscitation in the trauma center to reduce this burden. Strategies advanced include optimizing crystalloid use,3,4 high ratio of fresh frozen plasma (FFP) and platelets to red blood cell (RBC) transfusion,5–8 and massive transfusion protocols.9 Approaches to early blood transfusion have received significant attention, particularly with the results of the prospective observational PROMMTT study, and now the prospective randomized PROPPR trial, which completed enrollment at the end of 2013.10,11
Prehospital (PH) resuscitation has also received considerable interest. To date, studies in the civilian population have focused on the use of crystalloids for resuscitation in the pretrauma center (PTC) setting.12–14 There is, however, a paucity of evidence examining the use of RBC transfusion in the PTC arena to mitigate early hemorrhage and coagulopathy in injured patients.15 Military data have demonstrated that more than 90% of potentially survivable casualties die from hemorrhage and MEDEVAC platforms from the United States and the United Kingdom have PH RBC transfusion capabilities.16–18 A recent report examining these advanced medical transport platforms demonstrated a 37% reduction in 30-day mortality for casualties with an injury severity score (ISS) of more than 15 when compared with conventional MEDEVAC platforms.19 The authors note that 15% of patients overall received PH RBC transfusion, and among those with ISS of more than 15, a third of casualties received PH RBC transfusion. Although not directly evaluating the effect of PH RBC transfusion, these data suggest that a group of severely injury patients who frequently received PH RBC transfusion in the context of advanced PH capabilities had significantly improved survival. Given the encouraging results from the military and trauma center–based RBC transfusion strategies, it only makes sense to take this concept as far forward as feasible to apply the lessons learned to the civilian PH setting.
The objective of this study was to characterize the association of PTC RBC transfusion with mortality and trauma-induced coagulopathy (TIC) in severely injured patients with blunt trauma. In view of the improvement in outcomes with aggressive blood product resuscitation seen in the hospital setting, we hypothesized that PTC RBC transfusion would similarly be associated with reduced mortality and early TIC.
METHODS
Data were obtained from the Inflammation and the Host Response to Injury Collaborative (www.gluegrant.org), which is a multicenter prospective cohort study of blunt injured adults in hemorrhagic shock.20 Patients admitted to 1 of 9 institutions over an 8-year period (2003–2010) were included. Inclusion criteria for the prospective cohort study included blunt mechanism, presence of PH or emergency department hypotension [systolic blood pressure (SBP) <90 mm Hg] or an elevated base deficit (>6 mEq/L), RBC transfusion within the first 12 hours, any body region exclusive of the brain with an abbreviated injury score (AIS) of 2 or more, allowing exclusion of patients with isolated traumatic brain injury, and arrival at the study trauma center within 6 hours of injury. The time from injury was a required field for all subjects to meet eligibility criteria, and if a reasonable estimate of injury time could not be made, the subject was not enrolled in the prospective cohort. Patients younger than 18 years or older than 90 years and those with cervical spinal cord injury were also excluded. Clinical data were entered and stored in TrialDB, a Web-based data collection platform, by trained research nurses.21 Data integrity was maintained via ongoing curation and external data review by an independent medical record abstractor.
Standard operating procedures were implemented across all centers to minimize variation in care, including early goal-directed resuscitation, strict glycemic control, venous thromboembolism prophylaxis, low tidal volume ventilation, ventilator-associated pneumonia management, and restrictive transfusion guidelines.22–25 Initial laboratory values were either those collected in the field or outside hospital and reported to the study trauma center or those obtained with the first set of blood work on arrival. Admission laboratory values were those collected with the first set of blood work immediately upon arrival.
All subjects who arrived at the study trauma center within 2 hours of injury were included in this analysis. This 2-hour cutoff was chosen in an attempt to reduce confounding from interventions in subjects who spent a prolonged period in the field or outside hospital and minimize survival bias in those patients surviving for a prolonged period of time before arriving at the study center. PTC RBC transfusion was defined as transfusion of RBCs at any time before the subject’s arrival at the study trauma center. For subjects transported from the scene, the PTC RBC transfusion was administered by PH providers en route to the study trauma center. For subjects transferred from another hospital, the PTC RBC transfusion may have occurred at the outside hospital, en route to the study trauma center, or both; however, the data set did not distinguish the timing of PTC RBC transfusion in transfer subjects. All subjects who went to an outside hospital first were transported to the outside hospital and subsequently transported and arrived at the study trauma center within 2 hours from the time of injury. Any PTC RBC transfusion for these subjects occurred within this 2-hour time period. Subjects were then dichotomized into the PTC RBC group, defined as those receiving PTC RBC transfusion, or the no PTC RBC group, defined as those not receiving PTC RBC transfusion. Demographics, transfer status, PTC time (defined as the time from injury to arrival at the study trauma center), injury characteristics, resuscitation requirements, and outcomes were compared between the PTC RBC and no PTC RBC groups, using univariate analysis.
To address missing data, multiple imputation was performed for PH heart rate, PH SBP, PH Glasgow Coma Scale (GCS) score, PH crystalloid volume, initial base deficit, initial hemoglobin, and admission international normalized ratio (INR). Multiple imputation using a fully conditional specification model based on available demographics, PH and admission physiology, and PTC time was performed using 5 imputation steps to develop a complete data set. Analysis was performed on the pooled results. Less than 10% of any imputed variables were missing from the original data set (INR = 6.8%, PH heart rate = 6.6%, PH SBP = 6.5%, PH GCS score = 4.2%, PH crystalloid = 4.0%, hemoglobin = 1.2%, base deficit = 0.8%). No center was found to consistently have higher amounts of missing data than those missing from the overall cohort. Sensitivity analysis was performed with complete cases to assess the success of the multiple imputation procedure.
Outcomes included 24-hour mortality, 30-day mortality, and TIC, defined as admission INR of more than 1.5. Cox proportional hazards regression was used to determine the independent association of PTC RBC transfusion with 30-day mortality after controlling for confounders. Logistic regression was used to determine the independent association of PTC RBC transfusion with 24-hour mortality and TIC. Covariates in the regression models for 24-hour and 30-day mortality included age, sex, year of enrollment, transfer status, PTC time, PH SBP, PH crystalloid volume, admission GCS score, admission INR, initial base deficit, ISS, emergency department hypothermia, vasopressor use, urgent laparotomy or thoracotomy, and in-hospital volume of packed red blood cells (PRBCs), FFP, platelets, and crystalloid at 24 hours (for 24-hour mortality) or 48 hours (for 30-day mortality). Covariates used in the regression model for TIC included age, sex, year of enrollment, transfer status, PTC time, PH SBP, PH crystalloid volume, PH GCS score, initial base deficit, ISS, admission hypothermia, and admission ethanol level. These covariates were selected a priori and all included, as they have known prognostic significance to the outcomes of interest. To adjust for center-level effects, a 2-step cluster analysis was performed and incorporated into all regression models. Interactions between center, 24-hour blood products, and year of enrollment were tested to evaluate potential effects of different transfusion patterns across centers and over time on outcomes. Furthermore, because both scene and transfer patients were included, the interaction between treatment group and transfer status was tested to evaluate potential differential effects of transfer status and PTC RBC transfusion on outcomes. Interactions that were not significant were not included in the final models.
Model discrimination was assessed using the C-statistic for logistic regression models and the Harrell C-statistic for Cox regression models. Goodness of fit was assessed using the Hosmer-Lemeshow test for logistic regression models and the Groennesby and Borgan test for Cox regression models. Cox covariate-adjusted survival curves over the first 30 days were developed for the PTC RBC and no PTC RBC groups to determine the time course of any differences in mortality between the groups.
Because of the imbalance in the number of subjects between groups, propensity matching was performed to examine the outcomes in matched groups. A logistic regression model to predict PTC RBC transfusion was constructed with the following predictors: transfer status, age, sex, PTC time, PH SBP, PH heart rate, PH GCS score, PH crystalloid volume, admission INR, admission hemoglobin, initial base deficit, ISS, and trauma center grouping from cluster analysis.
These factors were selected on the basis of the PH variables in the database that would be available in the PTC period and surrogates of overall injury severity that a provider would evaluate to reasonably guide the decision to administer PTC RBCs. This model designated a propensity score between 0 and 1 for each individual subject corresponding to the likelihood of receiving PTC RBC transfusion based on the aforementioned factors. Propensity model discrimination and goodness of fit were also assessed using the C-statistic and the Hosmer-Lemeshow test, respectively.
Matching was performed using a nearest neighbor algorithm with a 3:1 ratio of control to treatment with replacement. A caliper of 0.2 was specified during the matching procedure. Stratified Cox regression and conditional logistic regression models were constructed using covariates not accounted for in the matching procedures, including year of enrollment, 24-hour blood products, and presence of traumatic brain injury, to determine the association of 24-hour mortality, 30-day mortality, and TIC with PTC RBC transfusion within the matched cohort.
Finally, an exploratory subgroup analysis was undertaken to examine the scene transport group separately to evaluate if similar treatment effects were observed. The models described earlier were used to evaluate the association of PTC RBC transfusion on outcomes of interest in the overall cohort of subjects transported from the scene. There were too few subjects transferred within 2 hours of injury to permit regression analysis as a separate subgroup comparison of PTC RBC transfusion and the outcomes of interest.
Data analysis was conducted using SPSS (version 19; Chicago, IL). For univariate analyses, χ2 tests were used to compare categorical variables and Mann-Whitney tests were used to compare continuous variables. Continuous data are presented as median [interquartile range (IQR)] or mean (standard deviation) unless otherwise indicated. A P value of 0.05 or less was considered significant. The institutional review board of each participating center approved the original prospective study.
RESULTS
Of the 2007 subjects in the prospective cohort study, 1415 subjects arrived at the study trauma center within 2 hours. Mean time from injury to arrival at the study trauma center was 60 (SD = 28) minutes. Fifty subjects (3.5%) received PTC RBC transfusion. Of these, 26 subjects (52%) were transported directly to the enrolling trauma center from the scene of injury.
Six centers had subjects who received PTC RBC transfusion. The centers with no subjects receiving PTC RBC transfusion accounted for only 2% of subjects in the study population. Table 1 demonstrates the distribution of subjects in the study population by year. Subjects receiving PTC RBC transfusion were relatively evenly distributed across study year. Only year 2003 had no subjects in the PTC RBC group; however, that year accounted for only 2% of the subjects in the study.
TABLE 1.
Distribution of Subjects by Study Year Across Overall Cohort and Treatment Groups
| Year | Overall Cohort, n (%) | PTC RBC Group, n (%) | No PTC RBC Group, n (%) |
|---|---|---|---|
| 2003 | 28 (2) | 0 (0) | 28 (2) |
| 2004 | 343 (24) | 6 (12) | 337 (25) |
| 2005 | 228 (16) | 13 (26) | 215 (16) |
| 2006 | 158 (11) | 5 (10) | 153 (11) |
| 2007 | 222 (16) | 8 (16) | 214 (16) |
| 2008 | 228 (16) | 8 (16) | 220 (16) |
| 2009 | 145 (10) | 8 (16) | 137 (10) |
| 2010 | 63 (5) | 2 (4) | 61 (4) |
| Total | 1415 (100) | 50 (100) | 1365 (100) |
There was no difference in age, sex, or ISS between the groups; however, the PTC RBC group has a higher initial base deficit and was more commonly hypotensive in the PTC period, indicating a higher shock severity (Table 2). The PTC RBC group received a median of 1.3 (IQR = 1.0–2.3) units of PRBCs in the PTC period. The PTC RBC group received lower 24-hour crystalloid volume than the no PTC RBC group (10.4 L vs 12.3 L; P = 0.06), although this did not reach statistical significance.
TABLE 2.
Demographics, Injury Characteristics, and Outcomes in the PTC RBC Versus No PTC RBC Groups
| PTC RBC Group (n = 50) | No PTC RBC Group (n = 1365) | P | |
|---|---|---|---|
| Age, yr | 41 (28–52) | 41 (26–54) | 0.77 |
| Male sex, % | 64 | 67 | 0.65 |
| PTC time, min | 93 (78–108) | 54 (36–78) | <0.01* |
| Scene transport, % | 52 | 96 | <0.01* |
| Initial base deficit | 10 (6–15) | 8 (5–11) | <0.01* |
| ISS | 37 (24–43) | 33 (22–41) | 0.18 |
| Initial hemoglobin, g/dL | 11.3 (8.8–13.2) | 11.5 (9.6–13.2) | 0.47 |
| PTC hypotension, % | 79 | 49 | <0.01* |
| Admission hypotension, % | 74 | 73 | 0.86 |
| PTC crystalloids, L | 2.6 (1.9–4.2) | 1.0 (0.4–2.0) | <0.01* |
| PTC RBCs, units | 1.3 (1.0–2.3) | 0 | — |
| 24-h RBC trauma center, units | 15.5 (9.6–26.4) | 6.6 (3.5–12.0) | <0.01* |
| 24-h FFP trauma center, units | 4.4 (0–8.1) | 2.7 (0–7.2) | 0.11 |
| 24-h PLT trauma center (6 pack) | 0.8 (0–2.4) | 0 (0–1.2) | 0.02* |
| 24-h crystalloid trauma center, L | 10.4 (8.0–14.7) | 12.3 (8.8–17.6) | 0.06 |
P < 0.05.
PLT indicates platelets.
Regression analysis revealed that PTC RBC transfusion was independently associated with a 95% reduction in the odds of 24-hour mortality after controlling for confounders [odds ratio (OR) = 0.05; 95% confidence interval (CI), 0.01–0.48; P < 0.01]. Furthermore, PTC RBC transfusion was independently associated with a 64% reduction in the risk of 30-day mortality [hazard ratio (HR) = 0.36; 95% CI, 0.15–0.83; P = 0.02]. Cox covariate-adjusted survival curves demonstrated early separation of the groups at 24 hours, with lower survival of the no PTC RBC group over the first 30 days postinjury (Fig. 1). Finally, PTC RBC transfusion was associated with an 88% reduction in the odds of TIC after adjustment (OR = 0.12; 95% CI, 0.02–0.79; P = 0.03). The interactions of 24-hour PRBC transfusion by year, 24-hour PRBC transfusion by center, and year by center (P = 0.07–0.71) were nonsignificant across all outcome models and excluded from the final models. The interaction between treatment group and transfer status was also nonsignificant across all outcome models (P = 0.31–0.66) and dropped from the final models.
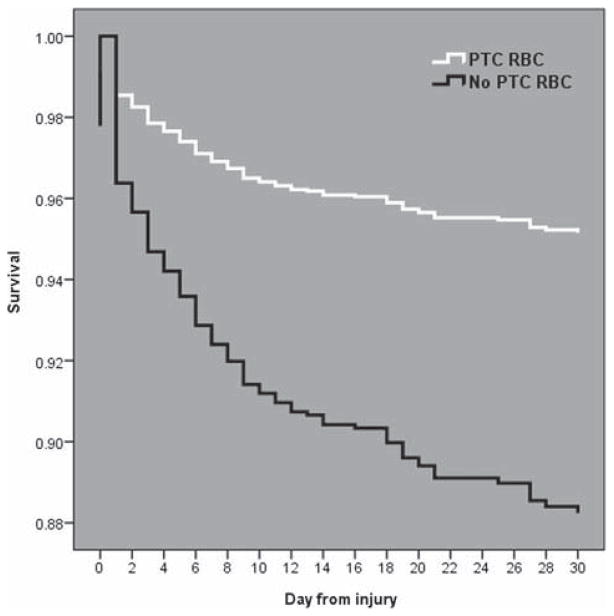
FIGURE 1.
Cox covariate-adjusted survival curves for the PTC RBC (white line) and no PTC RBC (dark line) groups over the first 30 days postinjury. The curves separate early at 24 hours, with lower survival for the no PTC RBC group than for the PTC RBC group over 30 days.
The C-statistic was 0.94 and 0.82 for the 24-hour mortality model and the TIC model, respectively, indicating excellent discrimination of the models. Hosmer-Lemeshow tests were nonsignificant (P = 0.10, P = 0.16, respectively), indicating adequate goodness of fit. The Harrell C-statistic was 0.80 for the 30-day mortality Cox model, indicating excellent discrimination. The Groennesby and Borgan test was nonsignificant (P = 0.07), indicating adequate goodness of fit.
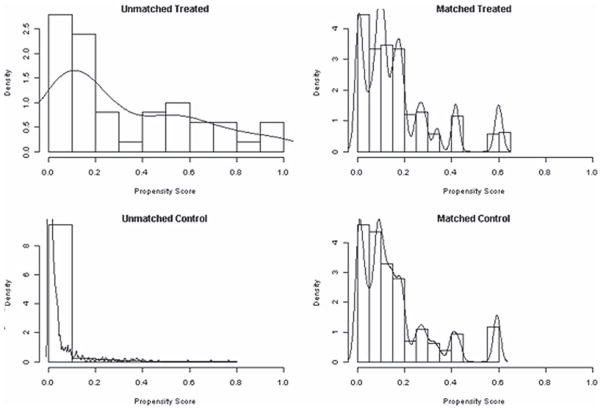
The matched cohort contained 113 subjects. Of these, 35 (31%) were in the PTC RBC group. The C-statistic for the propensity model was 0.90, indicating excellent discrimination. The Hosmer-Lemeshow test was nonsignificant (P = 0.08), indicating adequate goodness of fit. Figure 2 demonstrates the distribution of propensity scores between the unmatched and matched treatment and control groups.
FIGURE 2.
Distribution of propensity scores between unmatched and matched treatment group (PTC RBC) and control group (no PTC RBC) after the matching algorithm was completed.
Table 3 illustrates the balancing of demographics and injury characteristics between matched treatment groups, with no significant baseline differences between the PTC RBC and no PTC RBC groups. Only 24-hour PRBC transfusion remained higher in the PTC RBC group (P = 0.03).
TABLE 3.
Demographics, Injury Characteristics, and Outcomes in the PTC RBC Versus No PTC RBC Groups in the Matched Cohort
| PTC RBC Group (n = 35) | No PTC RBC Group (n = 78) | P | |
|---|---|---|---|
| Age, yr | 36 (28–52) | 37 (24–55) | 0.63 |
| Male sex, % | 60 | 72 | 0.28 |
| PTC time, min | 90 (72–108) | 90 (68–78) | 0.79 |
| Scene transport, % | 71 | 76 | 0.64 |
| Initial base deficit | 10 (5–12) | 9 (7–12) | 0.88 |
| ISS | 34 (18–43) | 30 (23–43) | 0.81 |
| Initial hemoglobin, g/dL | 11 (8.8–13) | 11.1 (9.3–12.6) | 0.90 |
| PTC hypotension, % | 71 | 53 | 0.14 |
| Admission hypotension, % | 60 | 74 | 0.02* |
| PTC crystalloids, L | 2.5 (1.2–3.0) | 1.8 (0.9–3.0) | 0.20 |
| PTC RBCs, units | 1.2 (1.0–2.0) | 0 | — |
| 24-h RBC trauma center, units | 14.0 (7.0–21.7) | 8.3 (3.4–18.5) | 0.03* |
| 24-h FFP trauma center, units | 3.2 (0–8.0) | 3.3 (0–9.2) | 0.80 |
| 24-h PLT trauma center (6 pack) | 0.8 (0–2.0) | 0.4 (0–1.0) | 0.27 |
| 24-h crystalloid trauma center, L | 9.7 (7.6–13.5) | 11.3 (8.4–17.1) | 0.11 |
P < 0.05.
PLT indicates platelets.
In the matched cohort, regression revealed that PTC RBC transfusion remained independently associated with a 98% reduction in the odds of 24-hour mortality (OR = 0.02; 95% CI, 0.01–0.69; P = 0.04). PTC RBC transfusion again was independently associated with an 88% reduction in the risk of 30-day mortality (HR = 0.12; 95% CI, 0.03–0.61; P = 0.01). Furthermore, PTC RBC transfusion was persistently associated with a 99% reduction in the odds of TIC (OR = 0.01; 95% CI, 0.01–0.95; P = 0.05).
The C-statistic was 0.98 and 0.97 for the 24-hour mortality model and the TIC model, respectively, indicating excellent discrimination of the models. The Harrell C-statistic was 0.87 for the 30-day mortality Cox model, indicating excellent discrimination. The Groennesby and Borgan test was nonsignificant (P = 0.40), indicating adequate goodness of fit. Sensitivity analysis using only subjects with complete data in the logistic and Cox regression models revealed similar treatment effects and significance for all outcomes of interest.
In scene subjects, exploratory subgroup analysis revealed that PTC RBC transfusion was associated with a reduced odds of 24-hour mortality (OR = 0.04; 95% CI, 0.01–1.12; P = 0.059), a reduced risk of 30-day mortality (HR = 0.11; 95% CI, 0.02–0.54; P < 0.01), and a reduced odds of TIC (OR = 0.08; 95% CI, 0.01–1.35; P = 0.079). The magnitude of treatment effect of PTC RBC transfusion on the outcomes of interest in subjects transported from the scene of injury was similar to those of the matched cohort. Furthermore, the 30-day mortality reduction remained highly significant, whereas 24-hour mortality and TIC showed a trend toward significance and likely did not reach the 0.05 threshold due to reduced power. Because there were only 71 subjects transferred within 2 hours of injury (PTC RBC, n = 24; no PTC RBC, n = 47), regression models did not converge due to very low power and the transfer subjects could not be evaluated as a separate group.
DISCUSSION
This study demonstrates that PTC RBC transfusion in severely injured patients with blunt trauma arriving at a trauma center within 2 hours of injury was associated with a reduction in the risk of 24-hour mortality, 30-day mortality, and TIC. Previous studies on PH resuscitation have focused on crystalloid resuscitation. Several studies have demonstrated that overzealous PH crystalloid use can have deleterious effects, making crystalloids a less than ideal resuscitation fluid.12,14,26
Blood transfusion remains the mainstay of resuscitation for traumatic shock.27 Retrospective studies in military and civilian populations demonstrate that increasing ratios of FFP and platelets to PRBCs are associated with improved outcomes.5–8 This has been confirmed in the prospective PROMMTT cohort study, where increased ratios of FFP to PRBCs and platelets to PRBCs were associated with increased survival over the first 24 hours.10
Given the impact of PH resuscitation on outcomes and growing evidence supporting current hospital-based transfusion approaches, moving blood product–based resuscitation to the PH arena is compelling. Clearly, there are challenges to this strategy and evidence of outcomes is lacking.15
In this study, 48% of patients in the PTC RBC group underwent interfacility transfer. Nirula et al28 compared patients transported directly to a trauma center with those undergoing transfer, finding that transfer was associated with greater PTC RBC transfusion and mortality. Time from injury to trauma center arrival was associated with mortality, and the authors concluded that delay to definitive care drives worse outcomes. We conversely found that PTC RBC transfusion was a predictor of reduced mortality, and although transfer patients were not examined separately, we limited our PTC time to 2 hours to exclude confounding from delayed transfer.
Current evidence examining PH RBC transfusion occurs exclusively in the setting of helicopter emergency medical services (HEMS). Most studies are small and examine the feasibility and characteristics of patients receiving RBC transfusion. The use of PH RBCs ranged from 1.4% to 4.0%, with trauma patients comprising 48% to 71% of patients and scene transports 9% to 53%.29–31 The proportion of scene transports in these series is consistent with the current analysis demonstrating 52% receiving PTC RBC transfusion were transported from the scene. In our matched cohort, however, three fourths of patients in each group were scene transports. Overall, prior authors concluded that PH RBC transfusion was feasible, safe, and beneficial in selected patients; however, no comparison with nontransfused patients was performed.
Sumida and colleagues32 compared outcomes between patients receiving PTC RBC transfusion versus those receiving only crystalloids in 31 HEMS trauma patients. Seventeen received PTC RBC transfusion with a combination of scene and transfer patients. Groups had similar age and ISS; however, the PTC RBC group had higher base deficit and longer PTC times. The authors found no difference in crude mortality, although they acknowledge that their results were limited by the small sample and lack of adjustment for time and other confounders. The current study conversely found a reduction in the risk of mortality for patients receiving PTC RBC transfusion. This is likely due to the use of regression to control for confounders.
Kim et al33 compared 9 patients receiving FFP and PRBCs, with 50 receiving only PRBCs within their HEMS system. The inhospital FFP to PRBC ratio was closer to 1:1 over the first 24 hours in the plasma group. They noted reduction of INR in both groups after transfusion, similar to the current data demonstrating a reduction in the risk of TIC after PTC RBC transfusion. There was no difference in mortality, and conclusions from the study are limited by the small sample size.
Plasma has garnered significant interest recently for use in PH resuscitation. PH plasma transfusion replaces critical coagulation factor deficiencies at the point of injury, which has been shown to occur as early as 15 minutes from injury.34–36 Plasma also represents an ideal volume expander that remains in the intravascular space. Furthermore, plasma has been shown to prevent endothelial glycocalyx degradation and reduce endothelial permeability leading to an improved inflammatory response to injury compared with conventional isotonic crystalloids.34,36 This has led to the development of remote damage control protocols incorporating plasma transfusion in the PTC setting.37 Zielinski and colleagues38 demonstrated feasible implementation of an HEMS using RBCs and plasma for patients with traumatic brain injury, noting significant improvement in INR from PTC transfusion alone, similar to the results here. Holcomb and Pati34 note that they have equipped their air medical providers with RBCs and plasma, and in preliminary unpublished data report improved outcomes.
This interest in PH plasma has led to 3 large randomized trials funded by the Department of Defense to examine the effects of PH plasma use on outcomes. Our group has undertaken the Prehospital Air Medical Plasma (PAMPer) trial to assess the effects of plasma administration on 30-day mortality, 24-hour blood product requirements, in-hospital mortality, multiple organ failure, and acute lung injury.39 The Denver group has undertaken the Control of Major Bleeding after Trauma (COMBAT) trial with ground ambulance plasma administration, looking at the effect on TIC, transfusion requirements, metabolic recovery, organ failure, and mortality.36 The Prehospital Use of Plasma for Traumatic Hemorrhage (PUPTH) trial by Virginia Commonwealth University will also examine the impact of using thawed PH plasma on mortality and coagulopathy.
With the broadening implementation of PH plasma and the encouraging results of PTC RBC transfusion seen here, there may be additional benefits of a high ratio of plasma to RBC transfusion in the PTC setting. The mounting evidence merits ongoing intensive study of PH blood product transfusion.
This study is the first to our knowledge to demonstrate that PTC RBC transfusion is independently associated with a reduction in the risk of mortality and TIC in a civilian population. These effects were seen in both the overall study population after controlling for confounders and a cohort matched on the propensity to receive PTC RBC transfusion. There may be several reasons for this. It is likely that a reduction in the risk of 24-hour mortality is a reflection of early RBC transfusion in the setting of hemorrhagic shock. RBC transfusion serves as a volume expander and increases oxygen-carrying capacity that is critical to cell metabolism during hypoperfusion. Although we did not see a difference in the hemoglobin at admission, the superior oxygen-carrying capacity of blood likely still plays into attenuating the pathophysiology of shock, as the time from injury is short enough to not yet see a drop in the hemoglobin level in either group. It is less clear why PTC RBC transfusion was associated with a lower risk of 30-day mortality. It may be that the concomitant reduction in the risk of TIC partially mediates this. Patients receiving PTC RBC transfusion and arriving with less TIC may experience fewer downstream physiologic and inflammatory derangements associated with coagulopathy extending through the first 30 days.40 Endothelial activation after trauma is well established, and significant crosstalk between the endothelium, coagulation cascade, and inflammatory pathways occurs after injury.41,42 Transfusion of RBCs is associated with lower endothelial activation after trauma than associated with other resuscitation fluids and blood products and thus may improve coagulopathy and outcomes through this mechanism.43 Although subjects in the PTC RBC group received only a median of 1.3 units of PTBCs in the PTC period, the increased oxygen-carrying capacity and lower endothelial activation and the trend toward lower 24-hour crystalloid volume may have all combined to produce reductions in mortality and coagulopathy seen here. Furthermore, the high proportion of unadjusted outcomes (24-hour mortality = 7%, 30-day mortality = 16%, TIC = 22%) leaves room for a large improvement with a small intervention dose and is likely responsible for the large relative effect sizes seen here. Potential mechanisms advanced here remain hypotheses, requiring further work to explore.
This study has several limitations. This is a secondary analysis of a cohort study not designed to address the specific questions in this analysis. This limits potential confounders and outcomes for evaluation. Missing data were present, but in relatively small proportion, and addressed using multiple imputation.
Although the current data were collected prospectively and standardized protocols were in place, PTC care was not standardized. There are no data on why certain patients received PTC RBC transfusion. There are also no data on other blood products that may have been transfused in the PTC period. Furthermore, this data set does not permit identification of the mode of transport for subjects. As noted earlier, PTC RBC transfusion has generally been limited to use in HEMS. It has been shown that HEMS improves outcomes over ground emergency medical services (GEMS) in both scene and transfer patients.44–46 Thus, this represents a potential confounder on mortality that cannot be accounted for in the current analysis. Despite this, we do not believe that the results seen here are likely explained solely by the effect of HEMS on survival in the PTC RBC group. These studies demonstrated a benefit of HEMS compared with GEMS, ranging from a 9% to 22% increase in the odds of in-hospital survival. The treatment effect of PTC RBC transfusion on 30-day mortality seen in this analysis ranges from a 64% to 88% reduction in the risk of mortality, much larger than that of HEMS alone, and likely is not fully explained by the effect of HEMS. It remains unclear what specific aspects of HEMS drive the mortality benefit,47 and it may be that RBC transfusion underlies some of the benefit of HEMS and certainly deserves further directed study. Moreover, a reduction in the risk of TIC would not be an expected effect of HEMS alone.
As noted, the imbalance between the number of subjects in each group in the overall population led us to perform propensity matching, which we believe adequately addressed this potential bias. In the matched cohort, baseline and PTC characteristics were similar between the groups and only 24-hour PRBC transfusion remained significantly higher in the PTC RBC group. This is due to the propensity model design to predict PTC RBC transfusion, in which we did not include in-hospital data at 24 hours, as predictors such as these would not factor into the decision to give PTC RBC transfusion. Although this indicates some difference between the matched groups, they were matched on the propensity to receive PTC RBC transfusion and not specifically matched to balance all covariates. For this reason, we continued to control for any potential differences between groups across covariates not accounted for in the matching procedure in the matched cohort. We demonstrated similar outcomes in the overall and matched cohorts. Moreover, The PTC RBC group had improved outcomes despite differences that would suggest that this group is sicker.
We included a mix of scene and interfacility transports primarily to increase power; however, there are certainly differences in these populations. For this reason, we limited the PTC time to 2 hours to reduce confounding from resuscitation during delayed transfer. As noted, transfer patients may have received PTC RBC transfusion at the outside hospital and/or en route to the study center; however, the 2-hour window for transport to an outside center and subsequently to the study center from time of injury makes it likely that transfer subjects spent little time at the outside center. Thus, the majority or at least part of any PTC RBC transfusion likely occurred during transport, and these subjects should be more comparable with scene subjects receiving entire PTC RBC transfusion en route to the study trauma center. In addition, our matched cohort had similar proportions of scene and transfer patients between groups, with most being scene transports. The effect of PTC RBC transfusion on outcomes does not seem to be significantly moderated by transfer status, as the interactions between the treatment group and transfer status were strongly nonsignificant in all outcome models. Furthermore, our propensity model includes transfer status and therefore subjects are matched across treatment groups for transfer status in the matched cohort. Our exploratory subgroup analysis of scene subjects did demonstrate similar results to the matched cohort, with PTC RBC transfusion associated with improved outcomes, and scene patients represent the group of most interest for future study. We also used the admission INR as a marker of TIC, which was the only coagulation data available in the current data set and has some precedence in the literature48; however, it has been shown that sophisticated measurements of coagulation status such as thromboelastography are superior to conventional coagulation laboratory values for TIC.49
There is a potential for survival bias in the data. As we evaluated patients enrolled in the prospective cohort, subjects must survive to arrival at the trauma center. Those dying in the field and at nonstudy facilities who may have received PTC RBC transfusion during the study period would not be captured. The study period spans an 8-year period in which transfusion practices have evolved significantly. We did not, however, find a significant imbalance in the distribution of patients receiving PTC RBC transfusion over the study period. There was some variability in the contribution of subjects from centers who received PTC RBC transfusion. However, 6 centers had patients who received PTC RBC transfusion and the relative distribution of subjects treated at each center was not significantly different from the original prospective cohort. Finally, the prospective cohort includes only patients with blunt trauma and no conclusions can be drawn about the potential for use of PTC RBC transfusion in the setting of penetrating injury.
In addition to methodological limitations, PH RBC transfusion carries several logistical concerns. Several authors have shown PH RBC transfusion to be feasible for air medical providers; however, scope of practice and issues of RBC product storage present significant challenges for use in GEMS.50 Use of lyophilized blood products may represent an opportunity in this area.51 Freeze dried plasma has received renewed interest in the military setting and is already undergoing successful PH use in austere conditions by the Israeli armed forces.52 Lessons learned from ongoing work in this area will be vital.36,37,39
CONCLUSIONS
PTC RBC transfusion is independently associated with a lower risk of 24-hour mortality, 30-day mortality, and TIC in severely injured patients with blunt trauma. These early preliminary data are the first to demonstrate these findings in a civilian population and require prospective study and validation. Early resuscitation incorporating RBC transfusion initiated before arrival at the trauma center without delaying transport may be associated with improved outcomes. Further investigation of PH RBC-based resuscitation is compelling, and these data will hopefully serve as an impetus for design of studies to specifically answer the questions raised by the current analysis. Addressing logistical challenges to make RBC products widely available in the PH setting will help us fully realize the potential benefits of PH RBC transfusion in severely injured patients.
Acknowledgments
The participants of the Inflammation and Hospital Response to Injury Large Scale Collaborative Research Program: Ronald G. Tompkins, MD, ScD, Mehmet Toner, PhD, H. Shaw Warren, MD, David A. Schoenfeld, PhD, Laurence Rahme, PhD, Grace P. McDonald-Smith, MEd, Douglas Hayden, MA, Philip Mason, PhD, Shawn Fagan, MD, Yong-Ming Yu, MD, PhD, J. Perren Cobb, MD, Daniel G. Remick, MD, John A. Mannick, MD, James A. Lederer, PhD, Richard L. Gamelli, MD, Michael B. Shapiro, MD, Richard Smith, PhD, David G. Camp, II, PhD, Weijun Qian, PhD, John Storey, PhD, Ronald V. Davis, PhD, Michael Mindrinos, PhD, Wenzhong Xiao, PhD, Wing Wong, PhD, Rob Tibshirani, PhD, Stephen Lowry, MD, Steven Calvano, PhD, Irshad Chaudry, PhD, Michaela West, MD, PhD, Mitchell Cohen, MD, Ernest E. Moore, MD, Jeffrey Johnson, MD, Lyle L. Moldawer, PhD, Henry V. Baker, PhD, Philip A. Efron, MD, Ulysses G. J. Balis, MD, Timothy R. Billiar, MD, Juan B. Ochoa, MD, Jason L. Sperry, MD, MPH, Carol L. Miller-Graziano, PhD, Asit K. De, PhD, Paul E. Bankey, MD, PhD, David N. Herndon, MD, Celeste C. Finnerty, PhD, Marc G. Jeschke, MD, PhD, Joseph P. Minei, MD, Brett D. Arnoldo, MD, John L. Hunt, MD, Jureta Horton, PhD, Ronald V. Maier, MD, Avery B. Nathens, MD, PhD, Joseph Cuschieri, MD, Nicole Gibran, MD, Matthew Klein, MD, Grant O’Keefe, MD, Bernard Brownstein, PhD, and Bradley Freeman, MD.
Footnotes
Author contributions is as follows: Joshua B. Brown, MD: Participation in study concept formulation and design of the study; drafted the study; protocol, performed the literature search, data collection, and data analysis; drafted initial manuscript; contributed to data interpretation and critical revision of the manuscript for important intellectual content; approved current version of manuscript of publication in its current form. Mitchell J. Cohen, MD: Participation in study concept formulation and design of the study; contributed to data interpretation and critical revision of the manuscript for important intellectual content; approved current version of manuscript of publication in its current form. Joseph P. Minei, MD: Participation in study concept formulation and design of the study; contributed to data interpretation and critical revision of the manuscript for important intellectual content; approved current version of manuscript of publication in its current form. Ronald V. Maier, MD: Participation in study concept formulation and design of the study; contributed to data interpretation and critical revision of the manuscript for important intellectual content; approved current version of manuscript of publication in its current form. Michaela West, MD, PhD: Participation in study concept formulation and design of the study; contributed to data interpretation and critical revision of the manuscript for important intellectual content; approved current version of manuscript of publication in its current form. Timothy R. Billiar, MD: Participation in study concept formulation and design of the study; contributed to data interpretation and critical revision of the manuscript for important intellectual content; approved current version of manuscript of publication in its current form. Andrew B. Peitzman, MD: Participation in study concept formulation and design of the study; contributed to data interpretation and critical revision of the manuscript for important intellectual content; approved current version of manuscript of publication in its current form. Ernest E. Moore, MD: Participation in study concept formulation and design of the study; contributed to data interpretation and critical revision of the manuscript for important intellectual content; approved current version of manuscript of publication in its current form. Joseph Cuschieri, MD: Participation in study concept formulation and design of the study; contributed to data interpretation and critical revision of the manuscript for important intellectual content; approved current version of manuscript of publication in its current form. Jason L. Sperry, MD, MPH: Participation in study concept formulation and design of the study; drafted the study protocol, performed the literature search, data collection, and data analysis; drafted initial manuscript; contributed to data interpretation and critical revision of the manuscript for important intellectual content; approved current version of manuscript of publication in its current form.
Disclosure: Supported by NIH NIGMS U54 GM062119-1 and NIH NIGMS K23GM093032-1 to the original Inflammation and the Host Response to Injury study. No funding or support was received for the current study.
The authors declare no conflicts of interest.
References
- 1.MacLeod JB, Lynn M, McKenney MG, et al. Early coagulopathy predicts mortality in trauma. J Trauma. 2003;55:39–44. doi: 10.1097/01.TA.0000075338.21177.EF. [DOI] [PubMed] [Google Scholar]
- 2.Rahbar E, Fox EE, del Junco DJ, et al. Early resuscitation intensity as a surrogate for bleeding severity and early mortality in the PROMMTT study. J Trauma Acute Care Surg. 2013;75:S16–S23. doi: 10.1097/TA.0b013e31828fa535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moore FA. The use of lactated Ringer’s in shock resuscitation: the good, the bad and the ugly. J Trauma. 2011;70(suppl):S15–S16. doi: 10.1097/TA.0b013e31821a4d6e. [DOI] [PubMed] [Google Scholar]
- 4.Schreiber MA. The use of normal saline for resuscitation in trauma. J Trauma. 2011;70(suppl):S13–S14. doi: 10.1097/TA.0b013e31821a4ba5. [DOI] [PubMed] [Google Scholar]
- 5.Borgman MA, Spinella PC, Perkins JG, et al. The ratio of blood products transfused affects mortality in patients receiving massive transfusions at a combat support hospital. J Trauma. 2007;63:805–813. doi: 10.1097/TA.0b013e3181271ba3. [DOI] [PubMed] [Google Scholar]
- 6.Brown JB, Cohen MJ, Minei JP, et al. Debunking the survival bias myth: characterization of mortality during the initial 24 hours for patients requiring massive transfusion. J Trauma Acute Care Surg. 2012;73:358–364. doi: 10.1097/TA.0b013e31825889ba. discussion 364. [DOI] [PubMed] [Google Scholar]
- 7.Holcomb JB, Wade CE, Michalek JE, et al. Increased plasma and platelet to red blood cell ratios improves outcome in 466 massively transfused civilian trauma patients. Ann Surg. 2008;248:447–458. doi: 10.1097/SLA.0b013e318185a9ad. [DOI] [PubMed] [Google Scholar]
- 8.Sperry JL, Ochoa JB, Gunn SR, et al. An FFP:PRBC transfusion ratio >/ = 1:1.5 is associated with a lower risk of mortality after massive transfusion. J Trauma. 2008;65:986–993. doi: 10.1097/TA.0b013e3181878028. [DOI] [PubMed] [Google Scholar]
- 9.Cotton BA, Gunter OL, Isbell J, et al. Damage control hematology: the impact of a trauma exsanguination protocol on survival and blood product utilization. J Trauma. 2008;64:1177–1182. doi: 10.1097/TA.0b013e31816c5c80. discussion 1182–1183. [DOI] [PubMed] [Google Scholar]
- 10.Holcomb JB. The Prospective, Observational, Multicenter, Major Trauma Transfusion (PROMMTT) study: Comparative effectiveness of a time-varying treatment with competing risks. Arch Surg. 2012;148:127–136. doi: 10.1001/2013.jamasurg.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holcomb JB, Fox EE, Wade CE, et al. The Prospective Observational Multi-center Major Trauma Transfusion (PROMMTT) study. J Trauma Acute Care Surg. 2013;75(suppl 1):S1–S12. doi: 10.1097/TA.0b013e3182983876. [DOI] [PubMed] [Google Scholar]
- 12.Brown JB, Cohen MJ, Minei JP, et al. Goal-directed resuscitation in the pre-hospital setting: a propensity-adjusted analysis. J Trauma Acute Care Surg. 2013;74:1207–1212. doi: 10.1097/TA.0b013e31828c44fd. discussion 1212–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hampton DA, Fabricant LJ, Differding J, et al. Prehospital intravenous fluid is associated with increased survival in trauma patients. J Trauma Acute Care Surg. 2013;75:S9–S15. doi: 10.1097/TA.0b013e318290cd52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kasotakis G, Sideris A, Yang Y, et al. Aggressive early crystalloid resuscitation adversely affects outcomes in adult blunt trauma patients: an analysis of the Glue Grant database. J Trauma Acute Care Surg. 2013;74:1215–1221. doi: 10.1097/TA.0b013e3182826e13. discussion 1221–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cotton BA, Jerome R, Collier BR, et al. Guidelines for prehospital fluid resuscitation in the injured patient. J Trauma. 2009;67:389–402. doi: 10.1097/TA.0b013e3181a8b26f. [DOI] [PubMed] [Google Scholar]
- 16.Blackbourne LH, Baer DG, Eastridge BJ, et al. Military medical revolution: prehospital combat casualty care. J Trauma Acute Care Surg. 2012;73(suppl 5):S372–S377. doi: 10.1097/TA.0b013e3182755662. [DOI] [PubMed] [Google Scholar]
- 17.Eastridge BJ, Mabry RL, Seguin P, et al. Death on the battlefield (2001–2011): implications for the future of combat casualty care. J Trauma Acute Care Surg. 2012;73(suppl 5):S431–S437. doi: 10.1097/TA.0b013e3182755dcc. [DOI] [PubMed] [Google Scholar]
- 18.Malsby RF, III, Quesada J, Powell-Dunford N, et al. Prehospital blood product transfusion by U.S. army MEDEVAC during combat operations in Afghanistan: a process improvement initiative. Mil Med. 2013;178:785–791. doi: 10.7205/MILMED-D-13-00047. [DOI] [PubMed] [Google Scholar]
- 19.Morrison JJ, Oh J, DuBose JJ, et al. En-route care capability from point of injury impacts mortality after severe wartime injury. Ann Surg. 2013;257:330–334. doi: 10.1097/SLA.0b013e31827eefcf. [DOI] [PubMed] [Google Scholar]
- 20.Maier RV, Bankey P, McKinley B, et al. Inflammation and the Host Response to Injury, a large-scale collaborative project: patient-oriented research core-standard operating procedures for clinical care [Foreword] J Trauma. 2005;59:762–763. [PubMed] [Google Scholar]
- 21.Brandt CA, Deshpande AM, Lu C, et al. TrialDB: a Web-based clinical study data management system. AMIA Annu Symp Proc. 2003:794. [PMC free article] [PubMed] [Google Scholar]
- 22.Harbrecht BG, Minei JP, Shapiro MB, et al. Inflammation and the Host Response to Injury, a large-scale collaborative project: patient-oriented research core-standard operating procedures for clinical care, part VI: blood glucose control in the critically Ill trauma patient. J Trauma Acute Care Surg. 2007;63:703–708. doi: 10.1097/TA.0b013e31811eadea. [DOI] [PubMed] [Google Scholar]
- 23.Minei JP, Nathens AB, West M, et al. Inflammation and the Host Response to Injury, a large-scale collaborative project: patient-oriented research core-standard operating procedures for clinical care, part II: guidelines for prevention, diagnosis and treatment of ventilator-associated pneumonia (VAP) in the trauma patient. J Trauma Acute Care Surg. 2006;60:1106–1113. doi: 10.1097/01.ta.0000220424.34835.f1. [DOI] [PubMed] [Google Scholar]
- 24.Moore FA, McKinley BA, Moore EE, et al. Inflammation and the Host Response to Injury, a large-scale collaborative project: patient-oriented research core-standard operating procedures for clinical care, part III: guidelines for shock resuscitation. J Trauma Acute Care Surg. 2006;61:82–89. doi: 10.1097/01.ta.0000225933.08478.65. [DOI] [PubMed] [Google Scholar]
- 25.Nathens AB, Johnson JL, Minei JP, et al. Inflammation and the Host Response to Injury, a large-scale collaborative project: patient-oriented research core-standard operating procedures for clinical care, part I: guidelines for mechanical ventilation of the trauma patient. J Trauma Acute Care Surg. 2005;59:764–769. [PubMed] [Google Scholar]
- 26.Cotton BA, Harvin JA, Kostousouv V, et al. Hyperfibrinolysis at admission is an uncommon but highly lethal event associated with shock and prehospital fluid administration. J Trauma Acute Care Surg. 2012;73:365–370. doi: 10.1097/TA.0b013e31825c1234. [DOI] [PubMed] [Google Scholar]
- 27.Advanced Trauma Life Support. 8. Chicago, IL: American College of Surgeons; 2008. [Google Scholar]
- 28.Nirula R, Maier R, Moore E, et al. Scoop and run to the trauma center or stay and play at the local hospital: hospital transfer’s effect on mortality. J Trauma. 2010;69:595–599. doi: 10.1097/TA.0b013e3181ee6e32. discussion 599–601. [DOI] [PubMed] [Google Scholar]
- 29.Barkana Y, Stein M, Maor R, et al. Prehospital blood transfusion in prolonged evacuation. J Trauma. 1999;46:176–180. doi: 10.1097/00005373-199901000-00030. [DOI] [PubMed] [Google Scholar]
- 30.Berns KS, Zietlow SP. Blood usage in rotor-wing transport. Air Med J. 1998;17:105–108. doi: 10.1016/s1067-991x(98)90104-3. [DOI] [PubMed] [Google Scholar]
- 31.Higgins GL, III, Baumann MR, Kendall KM, et al. Red blood cell transfusion: experience in a rural aeromedical transport service. Prehosp Disaster Med. 2012;27:231–234. doi: 10.1017/S1049023X12000659. [DOI] [PubMed] [Google Scholar]
- 32.Sumida MP, Quinn K, Lewis PL, et al. Prehospital blood transfusion versus crystalloid alone in the air medical transport of trauma patients. Air Med J. 2000;19:140–143. doi: 10.1016/s1067-991x(00)90007-5. [DOI] [PubMed] [Google Scholar]
- 33.Kim BD, Zielinski MD, Jenkins DH, et al. The effects of prehospital plasma on patients with injury: a prehospital plasma resuscitation. J Trauma Acute Care Surg. 2012;73(suppl 1):S49–S53. doi: 10.1097/TA.0b013e31826060ff. [DOI] [PubMed] [Google Scholar]
- 34.Holcomb JB, Pati S. Optimal trauma resuscitation with plasma as the primary resuscitative fluid: the surgeon’s perspective. Hematology Am Soc Hematol Educ Program. 2013;2013:656–659. doi: 10.1182/asheducation-2013.1.656. [DOI] [PubMed] [Google Scholar]
- 35.Lee L, Moore EE, Hansen KC, et al. It’s not your grandfather’s field plasma. Surgery. 2013;153:857–860. doi: 10.1016/j.surg.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moore EE, Chin TL, Chapman MC, et al. Plasma first in the field for postinjury hemorrhagic shock [published online ahead of print December 5, 2013] Shock. doi: 10.1097/SHK.0000000000000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jenkins D, Stubbs J, Williams S, et al. Implementation and execution of civilian remote damage control resuscitation programs [published online ahead of print November 27, 2013] Shock. doi: 10.1097/SHK.0000000000000081. [DOI] [PubMed] [Google Scholar]
- 38.Zielinski MD, Smoot DL, Stubbs JR, et al. The development and feasibility of a remote damage control resuscitation prehospital plasma transfusion protocol for warfarin reversal for patients with traumatic brain injury. Transfusion. 2013;53(suppl 1):59S–64S. doi: 10.1111/trf.12037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.ClinicalTrials.gov. [Accessed July 13, 2013];Prehospital Air Medical Plasma Trial (PAMPer) Available at http://clinicaltrials.gov/show/NCT01818427.
- 40.Cohen MJ, West M. Acute traumatic coagulopathy: from endogenous acute coagulopathy to systemic acquired coagulopathy and back. J Trauma. 2011;70(suppl):S47–S49. doi: 10.1097/TA.0b013e31821a5c24. [DOI] [PubMed] [Google Scholar]
- 41.Ganter MT, Cohen MJ, Brohi K, et al. Angiopoietin-2, marker and mediator of endothelial activation with prognostic significance early after trauma? Ann Surg. 2008;247:320–326. doi: 10.1097/SLA.0b013e318162d616. [DOI] [PubMed] [Google Scholar]
- 42.Hess JR, Brohi K, Dutton RP, et al. The coagulopathy of trauma: a review of mechanisms. J Trauma. 2008;65:748–754. doi: 10.1097/TA.0b013e3181877a9c. [DOI] [PubMed] [Google Scholar]
- 43.Urner M, Herrmann IK, Buddeberg F, et al. Effects of blood products on inflammatory response in endothelial cells in vitro. PLoS ONE. 2012;7:e33403. doi: 10.1371/journal.pone.0033403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brown JB, Stassen NA, Bankey PE, et al. Helicopters and the civilian trauma system: national utilization patterns demonstrate improved outcomes after traumatic injury. J Trauma. 2010;69:1030–1034. doi: 10.1097/TA.0b013e3181f6f450. discussion 1034–1036. [DOI] [PubMed] [Google Scholar]
- 45.Brown JB, Stassen NA, Bankey PE, et al. Helicopters improve survival in seriously injured patients requiring interfacility transfer for definitive care. J Trauma. 2011;70:310–314. doi: 10.1097/TA.0b013e3182032b4f. [DOI] [PubMed] [Google Scholar]
- 46.Galvagno SM, Jr, Haut ER, Zafar SN, et al. Association between helicopter vs ground emergency medical services and survival for adults with major trauma. JAMA. 2012;307:1602–1610. doi: 10.1001/jama.2012.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Galvagno SM., Jr Comparative effectiveness of helicopter emergency medical services compared to ground emergency medical services. Crit Care. 2013;17:169. doi: 10.1186/cc12779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kashuk JL, Moore EE, Johnson JL, et al. Postinjury life threatening coagulopathy: is 1:1 fresh frozen plasma:packed red blood cells the answer? J Trauma. 2008;65:261–270. doi: 10.1097/TA.0b013e31817de3e1. discussion 270–271. [DOI] [PubMed] [Google Scholar]
- 49.Holcomb JB, Minei KM, Scerbo ML, et al. Admission rapid thromboelastography can replace conventional coagulation tests in the emergency department: experience with 1974 consecutive trauma patients. Ann Surg. 2012;256:476–486. doi: 10.1097/SLA.0b013e3182658180. [DOI] [PubMed] [Google Scholar]
- 50.Hately T, Ma JO, Weaver N, et al. Flight paramedic scope of practice: current level and breadth. J Emerg Med. 1998;16:731–735. doi: 10.1016/s0736-4679(98)00087-0. [DOI] [PubMed] [Google Scholar]
- 51.Arav A, Natan D. Freeze drying (lyophilization) of red blood cells. J Trauma. 2011;70(suppl):S61–S64. doi: 10.1097/TA.0b013e31821a6083. [DOI] [PubMed] [Google Scholar]
- 52.Glassberg E, Nadler R, Gendler S, et al. Freeze-dried plasma at the point of injury: from concept to doctrine. Shock. 2013;40:444–450. doi: 10.1097/SHK.0000000000000047. [DOI] [PubMed] [Google Scholar]