Abstract
Background
Harmane (1-methyl-9H-pyrido[3,4-b]indole) (HA) is a potent neurotoxin that has been linked to two neurological diseases, essential tremor and Parkinson’s disease. Blood harmane concentrations [HA] are elevated in patients with both diseases. An important question is whether HA is specifically linked with these diseases or alternatively, is a non-specific marker of neurological illness.
Objectives
We assessed whether blood [HA] was elevated in patients with a third neurological disease, dystonia, comparing them to controls.
Methods
Blood [HA] were quantified by high performance liquid chromatography. Subjects comprised 104 dystonia cases and 107 controls.
Results
Mean log blood [HA] in dystonia cases was similar to that of controls (0.41 ± 0.51 g −10/ml vs. 0.38 ± 0.61 g−10/ml, t = 0.42, p = 0.68). In unadjusted and adjusted logistic regression analyses, log blood [HA] was not associated with the outcome (diagnosis of dystonia vs. control): odds ratio (OR) unadjusted = 1.11, 95% confidence interval (CI) = 0.69 – 1.79, p = 0.68; OR adjusted = 1.07, 95% CI = 0.58 – 1.97, p = 0.84.
Conclusions
In contrast to the elevated blood [HA] that has been reported in patients with essential tremor and Parkinson’s disease, our data demonstrate that blood [HA] was similar in patients with dystonia and controls. These findings provide the first support for the notion that an elevated blood [HA] is not a broad feature of neurological disease, and may be a specific feature of certain tremor disorders.
Keywords: harmane, neurotoxin, dystonia, neurological disease, tremor
1. Introduction
β-carboline alkaloids are a group of neurotoxins that have a variety of neurological effects, including tremor (Louis, 2008). Harmane (1-methyl-9H-pyrido[3,4-b]indole) (HA) is among the most potent of the β-carboline alkaloids. Harmane is produced endogenously, but it is also present in the diet, especially in animal tissue, and also to a lesser extent in many vegetables; exogenous exposure is thought to be the main source of bodily harmane (Pfau and Skog, 2004). In several studies, we demonstrated that blood harmane concentration ([HA]) was elevated in patients with neurological diseases characterized by tremor, including essential tremor (ET) patients compared with controls (Louis et al., 2002; Louis et al., 2008; Louis et al., 2013) and Parkinson’s disease (PD) patients compared to controls (Louis et al., 2014), suggesting that harmane is an environmental risk factor for these diseases. These recent studies raise the important but as yet unaddressed question - is elevated blood HA specifically linked with these diseases, or rather, is it merely a global marker of neurological illness?
Dystonia, like ET and PD, is a neurological disease characterized by involuntary movements. Although tremor may occur as a clinical feature of dystonia, the predominant feature is sustained muscle contractions and twisting movements. In 2009, we made the decision to assess blood [HA] in patients with dystonia, with the a priori hypothesis that, in contrast to ET and PD, blood [HA] would not be elevated in patients with dystonia. We now have a sufficient number of subjects to report our results.
2. Materials and Methods
2.1 Participants
Dystonia cases were enrolled in a study of the environmental epidemiology of ET and other neurological disorders at Columbia-University Medical Center (CUMC) (Louis et al., 2008; Louis et al., 2014). Recruitment began in 2009 and continued to present (May 2014). By design, dystonia cases were identified from a computerized billing database at the Center for Parkinson’s Disease and Other Movement Disorders at the Neurological Institute of New York, CUMC; selection was based on date of appointment, with more recent cases being selected first. All cases had received a diagnosis of dystonia from their treating neurologist at the Institute and lived within two hours driving distance of CUMC. One of the authors (E.D.L.) reviewed the office records of all selected dystonia patients; patients with diagnoses or physical signs consistent with PD, ET, spinocerebellar ataxia, or other movement disorders were excluded, and the diagnosis of dystonia was confirmed using published diagnostic criteria (Fahn, 1988). The location of dystonia (writer’s cramp, torticollis, blepharospasm, combination or other locations) was extracted from the chart review.
Control subjects were recruited for the same study during an overlapping time period. Controls were identified using random digit telephone dialing within a defined set of telephone area codes that were represented by neurological cases (e.g., 212, 201, 203, 516, 718, and 914) within the New York Metropolitan area. There was one group of controls for all neurological disease cases (ET, PD, and dystonia). Patients with ET and PD are generally older than those with dystonia. Controls were frequency-matched to ET and PD cases based on current age (5 year intervals); the ratio of controls to dystonia cases was ~1:1.
The CUMC Internal Review Board approved of all study procedures; written informed consent was obtained upon enrollment.
2.2 Clinical Evaluation
All cases and controls were evaluated in person by a trained tester who administered clinical questionnaires. Most evaluations were performed in the late morning or early afternoon, making fasting blood [HA] impractical. Data suggest that plasma [HA] do not change significantly during the day (Rommelspacher et al., 1991). Thus, in one study (Rommelspacher et al., 1991), human subjects ingested food or ethanol, and plasma [HA] were measured hourly for eight hours; the concentration remained stable. The same investigators also demonstrated that variability in concentration was minimal over a longer (i.e., three week) study period (Rommelspacher et al., 1991). Additionally, our data show that log blood [HA] is not correlated with the time latency since last food consumption (Louis et al., 2011).
The tester collected demographic and clinical information, including duration of symptoms. A screening questionnaire included the question, “Do you have shaking or tremor that you can’t control?” Current smoking status was assessed, and all current medications were recorded and then collapsed into 19 medication classes (e.g., neuroleptic medications, cardiac medications, etc).
Weight and height were assessed and body mass index was calculated (weight in kg divided by the square of height in meters).
2.3 Blood [HA]
After phlebotomy, blood [HA] was quantified, blinded to demographic, clinical and diagnostic information, by a well-established high performance liquid chromatography method (Dr. Zheng, Purdue University) used in our previous studies (Zheng et al., 2000; Louis et al., 2002; Louis et al., 2008).
2.4 Statistical Analyses
Chi-square (χ2) tests were used to analyze proportions, and Student’s t tests were used to examine group differences in continuous variables. Pearson’s correlation coefficients were used to assess correlations between continuous variables; when variables were not normally distributed, Spearman’s correlation coefficient was used.
The empirical distribution of harmane was positively skewed. Using a one-sample Kolmogorov-Smirnov test, we tested whether [HA] was normally distributed and it was not (Kolmogorov-Smirnov test, z = 5.56, p < 0.001). Therefore, [HA] were logarithmically transformed, and after this transformation it was normally distributed. Case-control differences in log blood [HA] were assessed using Student’s t tests.
To assess the null hypothesis that blood [HA] was not a predictor of diagnostic group (dystonia vs. control), logistic regression analysis was performed using diagnostic group as the outcome, and log blood [HA] as the primary independent variable. We considered a number of potential confounders (age in years, gender, race, years of education, body mass index, current cigarette smoker, and medications [19 classes]) and included these in the adjusted logistic regression analyses if they were associated with either dystonia or blood [HA] in this dataset or if prior studies suggested a relationship. Analysis of variance (ANOVA) was used to assess whether patients with dystonia in each of the four locations (writer’s cramp, torticollis, blepharospasm, combination or other locations) differed from one another with respect to log blood [HA], and then Tukey’s post hoc tests were used to compare each location to each of the three others. Statistical analyses were performed in SPSS (Version 21.0).
3. Results
The 104 dystonia cases and 107 controls were similar in gender, race, education, body mass index, and current smoking status (Table 1). As expected, the dystonia cases were younger than the controls (Table 1). The median evaluation start time (i.e., time of day that evaluation began) was 11:00 AM both for dystonia cases and controls (Mann Whitney = 1.00, p = 0.31). More than one-half of the dystonia cases had torticollis, and the most of the remainder had blepharospasm or writer’s cramp (Table 1).
Table 1.
Demographic and clinical characteristics of dystonia cases vs. controls
| Characteristic | Dystonia Cases (N = 104) | Controls (N = 107) | Significance |
|---|---|---|---|
|
| |||
| Age in years | 61.7 ± 11.6 | 70.9 ± 8.3 | t = 6.61, p < 0.001 |
|
| |||
| Female gender | 69 (66.3) | 65 (60.7) | χ2 = 0.71, p = 0.40 |
|
| |||
| Non-Hispanic white race | 83 (79.8) | 93 (86.9) | χ2 = 1.93, p = 0.17 |
|
| |||
| Education in years | 15.7 ± 3.2 | 15.9 ± 2.5 | t = 0.57, p = 0.57 |
|
| |||
| Body mass index kg/m2 | 25.5 ± 4.6 | 26.1 ± 5.0 | t = 0.79, p = 0.43 |
|
| |||
| Current cigarette smoker | 9 (8.7) | 5 (4.7) | χ2 = 1.35, p = 0.25 |
|
| |||
| Duration of dystonia symptoms in years | 20.3 ± 15.1 | Not applicable | Not applicable |
|
| |||
| Location of dystonia | Not applicable | Not applicable | |
| Torticollis | 56 (53.9) | ||
| Blepharospasm | 21 (20.2) | ||
| Writer’s cramp | 9 (8.7) | ||
| Combination or Other locations | 18 (17.3) | ||
|
| |||
| Patient complained of tremor | 30 (28.9) | Not applicable | Not applicable |
Values are mean ± standard deviation or numbers (percentages).
Using controls, we examined the correlates of log blood [HA]. Log blood [HA] was not associated with age in years (r = −0.08, p = 0.41), education (r = 0.05, p = 0.64) or body mass index (r = −0.08, p = 0.43). Log blood [HA] did not differ by gender (p = 0.67). Log blood [HA] was similar in current smokers and nonsmokers (p = 0.55). Log blood [HA] was not associated with white vs. non-white race (p = 0.27); and it was not associated with current use of any of the 19 classes of medications (data not shown) or evaluation start time (r = −0.02, p = 0.85).
Mean ± standard deviation log blood [HA] in dystonia cases was similar to that of controls (0.41 ± 0.51 g −10/ml vs. 0.38 ± 0.61 g−10/ml, t = 0.42, p = 0.68) (Figure 1).
Figure 1.
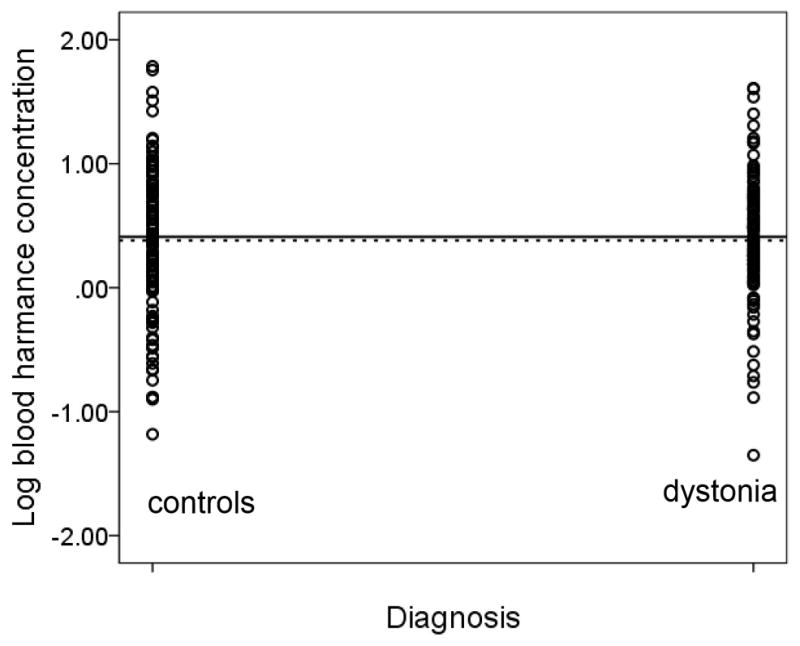
Log blood [HA] in g −10/ml in dystonia cases vs. controls. The solid horizontal line depicts the mean log blood [HA] value in dystonia cases (0.41 g −10/ml) and the dashed horizontal line depicts the mean log blood [HA] value in controls (0.38 g−10/ml).
In an unadjusted logistic regression analysis, log blood [HA] was not associated with the outcome (diagnosis of dystonia vs. control) (odds ratio [OR] = 1.11, 95% confidence interval [CI] = 0.69 – 1.79, p = 0.68). In logistic regression analyses that adjusted for each one of the following variables individually and then in combination, there was no association between log blood [HA] and diagnosis: age in years, gender, body mass index, current smoker, and evaluation start time (e.g., in a model that adjusted for each of these variables in combination, OR = 0.96, 95% CI = 0.55 – 1.65, p = 0.87). In a logistic regression model that used a forward step-wise approach, none of the above-mentioned variables were included in the model; in other words, there was no association between log blood [HA] and the diagnostic outcome, there was no association between any of the covariates and the diagnostic outcome, and none of the covariates confounded the primary association between log blood [HA] and the diagnostic outcome. In another model that adjusted for each of the variables listed above, and which further included a series of 19 variables for use/non-use of each of the 19 classes of medications, there was similarly no association (OR = 1.07, 95% CI = 0.58 – 1.97, p = 0.84). We performed a secondary analysis in which we frequency-matched dystonia cases and controls based on age (85 dystonia cases age 66.0 ± 7.1 years and 72 controls age 67.6 ± 8.1 years, t = 1.27, p = 0.21), and their log blood [HA] were similar (0.40 ± 0.54 g −10/ml [cases] vs. 0.41 ± 0.62 g−10/ml [controls], t = 0.17, p = 0.86).
Among dystonia cases, there was a weak inverse correlation between symptom duration and log blood [HA] (Pearson’s r = −0.31, p = 0.05). The 30 dystonia patients who endorsed having tremor were compared to the 74 who did endorse having tremor, and they had similar log blood [HA]: 0.35 ± 0.42 vs. 0.45 ± 0.54, t = 0.90, p = 0.37. The log blood [HA] was higher, although not to a significant degree, in the patients with writer’s cramp (0.60 ± 0.22) than in those with dystonia in other locations (0.40 ± 0.53 for torticollis, 0.28 ± 0.42 for blepharospasm, 0.51 ± 0.66 for combination or other locations, ANOVA for all 4 locations = 1.07, p = 0.37 and Tukey’s tests for writer’s cramp vs. each of the other locations, p values all > 0.41).
4. Discussion
Dystonia cases and controls were similar with respect to blood [HA]. The blood [HA] was higher, although not to a significant degree, in the dystonia cases with writer’s cramp than in those with dystonia in other locations (e.g., torticollis, blepharospasm). The dystonia cases who endorsed tremor had similar blood [HA] to those who did not endorse tremor. The implication of these results is that they provide the first empiric support for the notion that elevated blood [HA] is not a uniform feature of all neurological disorders, and it may be more a feature of certain disorders characterized by tremor.
There was one group of controls for all neurological disease cases (ET, PD, and dystonia). PD and ET patients are generally considerably older than patients with dystonia. We selected controls whose age overlapped to a greater extent with those of the ET and PD cases. Hence, it is not surprising that our dystonia cases were younger than controls in our sample. This, however, was not a source of confounding because (1) log blood [HA] did not covary with age, (2) in logistic regression models, we adjusted for age, and (3) in a secondary analysis we age-matched a smaller sample of cases and controls and detected no case-control difference.
We reported that the 30 dystonia patients who endorsed having tremor were compared to the 74 who did endorse having tremor. There is debate as to whether the patients with dystonic postures/movements who also have tremor have a single disease characterized by dystonic postures/movements and tremor or two diseases (i.e., dystonia and ET). Our findings support the former.
This study had limitations. The dystonia cases were recruited from a single center, so it would be useful to generalize these results to other centers. The second issue is that we did not conduct fasting blood HA evaluations. Most of the evaluations were performed either in the late morning or early afternoon, making fasting blood [HA]s impractical. Data suggest that plasma [HA] do not change significantly during the day (Rommelspacher et al., 1991). We did not find that log blood [HA] correlated with evaluation start time, and the evaluation start time was similar in dystonia cases and controls; furthermore, when we adjusted for evaluation start time in a multivariate logistic regression model, the results remained the same. Third, we evaluated blood rather than cerebrospinal fluid or brain [HA]; brain tissue is more difficult to obtain, but it might be useful to extend these studies to include brain tissue. At the moment, the determination of blood [HA] is being used as an exploratory research tool rather than clinical laboratory test. Among other things, there are many potential sources of variance in control values, including age, gender and dietary factors, and these remain to be fully elucidated and defined. The study had numerous strengths, including the sample size of more than 200 subjects. Second, all blood [HA] were performed in the same laboratory, which developed the method (Zheng et al., 2000). Third, measurement of blood rather than plasma concentrations allowed for the detection of higher levels of HA. Fourth, in our analyses, we were able to assess the potential confounding effects of multiple relevant covariates. Finally, this is also the first study to attempt to assess [HA] in this neurological disease.
5. Conclusions
In contrast to the elevated blood [HA] that has been reported in patients with ET and PD, blood [HA] was similar in the current study in patients with dystonia and controls. This suggests that elevated blood [HA] is not a uniform feature of all neurological disorders, and it may be more of a feature of certain disorders characterized by tremor.
HIGHLIGHTS.
Harmane (1-methyl-9H-pyrido[3,4-b]indole) is a potent neurotoxin.
Harmane has been linked with several tremor disorders.
Blood harmane levels were quantified by high performance liquid chromatography.
Blood harmane levels were similar in dystonia cases vs. controls.
Elevated blood harmane is not a broad feature of all neurological diseases.
Acknowledgments
Dr. Louis drafted and revised the manuscript for content; designed the study; performed the statistical analyses; analyzed and interpreted the data; obtained funding. Dr. Factor-Litvak drafted and revised the manuscript for content; designed the study, analyzed and interpreted the data; performed the statistical analyses; obtained funding. Monika Michalec drafted and revised the manuscript for content; acquired the data. Dr. Jiang drafted and revised the manuscript for content; acquired the data. Dr. Zheng drafted and revised the manuscript for content; designed the study; analyzed and interpreted the data; contributed vital reagents and tools; and obtained funding.
Footnotes
Conflict of interest statement: The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Pam Factor-Litvak, Email: prf1@cumc.columbia.edu.
Monika Michalec, Email: mg3360@cumc.columbia.edu.
Wendy Jiang, Email: jiangw@purdue.edu.
Wei Zheng, Email: wzheng@purdue.edu.
References
- Fahn S. Concept and classification of dystonia. Adv Neurol. 1988;50:1–8. [PubMed] [Google Scholar]
- Louis ED. Environmental epidemiology of essential tremor. Neuroepidemiology. 2008;31(3):139–49. doi: 10.1159/000151523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis ED, Benito-León J, Moreno-García S, Vega S, Romero JP, Bermejo-Pareja F, et al. Blood harmane (1-methyl-9H-pyrido[3,4-b]indole) concentration in essential tremor cases in Spain. Neurotoxicology. 2013;34:264–8. doi: 10.1016/j.neuro.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis ED, Factor-Litvak P, Gerbin M, Jiang W, Zheng W. Blood harmane concentrations in 497 individuals relative to coffee, cigarettes, and food consumption on the morning of testing. J Toxicol. 2011;2011:628151. doi: 10.1155/2011/628151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis ED, Jiang W, Pellegrino KM, Rios E, Factor-Litvak P, Henchcliffe C, et al. Elevated blood harmane (1-methyl-9H-pyrido[3,4-b]indole) concentrations in essential tremor. Neurotoxicology. 2008;29(2):294–300. doi: 10.1016/j.neuro.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis ED, Michalec M, Jiang W, Factor-Litvak P, Zheng W. Elevated blood harmane (1-methyl-9H-pyrido[3,4-b]indole) concentrations in Parkinson’s disease. Neurotoxicology. 2014;40:52–6. doi: 10.1016/j.neuro.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis ED, Zheng W, Jurewicz EC, Watner D, Chen J, Factor-Litvak P, et al. Elevation of blood beta-carboline alkaloids in essential tremor. Neurology. 2002;59(12):1940–4. doi: 10.1212/01.wnl.0000038385.60538.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfau W, Skog K. Exposure to beta-carbolines norharman and harman. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;802(1):115–26. doi: 10.1016/j.jchromb.2003.10.044. [DOI] [PubMed] [Google Scholar]
- Rommelspacher H, Schmidt LG, May T. Plasma norharman (beta-carboline) levels are elevated in chronic alcoholics. Alcohol Clin Exp Res. 1991;15(3):553–9. doi: 10.1111/j.1530-0277.1991.tb00559.x. [DOI] [PubMed] [Google Scholar]
- Zheng W, Wang S, Barnes LF, Guan Y, Louis ED. Determination of harmane and harmine in human blood using reversed-phased high-performance liquid chromatography and fluorescence detection. Anal Biochem. 2000;279(2):125–9. doi: 10.1006/abio.1999.4456. [DOI] [PMC free article] [PubMed] [Google Scholar]


