Abstract
The pig-to-nonhuman primate model is the standard choice for in vivo studies of organ and cell xenotransplantation. In 1998 Lambrigts and his colleagues surveyed the entire world literature and reported all experimental studies in this model. With the increasing number of genetically-engineered pigs that have become available during the past few years, this model is being utilized ever more frequently. We have now reviewed the literature again and have compiled the data we have been able to find for the period January 1st 1998 to December 31st 2013, a period of 16 years. The data are presented for transplants of the heart (heterotopic and orthotopic), kidney, liver, lung, islets, neuronal cells, hepatocytes, corneas, artery patches, and skin. Heart, kidney, and, particularly, islet xenograft survival have increased significantly since 1998, and the reasons for this are briefly discussed. A comment on the limitations of the model has been made, particularly with regard to these will affect progression of xenotransplantation towards the clinic.
Keywords: Baboons, islets, monkeys, nonhuman primates, pigs, xenotransplantation, Cornea, Heart, Kidney, Liver, Lung, Nonhuman primate, Pancreatic islets
Introduction
Because of the immunologic similarities of Old World nonhuman primates (NHP), e.g., baboons, rhesus monkeys, and cynomolgus monkeys, to humans, the NHP represents the preferred surrogate for humans in exploring the response to pig organ or cell transplantation. The pig-to-NHP model was introduced into xenotransplantation research in the mid-1980s (1, 2), and has become the standard model for testing the primate immune response to organs and cells from pigs with genetic manipulations and/or the effect of novel immunosuppressive regimens.
Early experience in this model was comprehensively reviewed by Lambrigts et al., in 1998 (3), but has not been reviewed fully since then. With the aim of assessing progress in the 16 years that have elapsed since 1998, we have therefore attempted to search the literature for experience of pig organ (heart, kidney, liver, lung), islet, neuronal cell, hepatocyte, cornea, and artery patch transplantation. Others have relatively recently reviewed cornea (4–6) and islet (7) xenotransplantation, and their data have been included here. Brief mention has also been made of skin transplantation in the pig-to-NHP model. The early studies on the transfusion of pig red blood cells into nonhuman primates have been reviewed elsewhere (8), but have not been included here.
In 1998, the only genetically-engineered pigs available were those expressing a human complement-regulatory protein, e.g., CD55 (hDAF) (9). Research in the NHP model was greatly facilitated by the generosity of David White and his colleagues at Imutran and Novartis in making these pigs available to the research community.
The major innovations that have been introduced into the field since 1998 include (i) many new genetic modifications in pigs (reviewed by Ekser et al (10), including α1,3-galactosyltransferase gene-knockout (GTKO) pigs (11–15) and (ii) T cell costimulatory blockade agents, first introduced into NHP xenotransplantation models by Buhler et al., in 2000 (16). In 2013, the first pigs that did not express the important N-glycolylneuraminic acid epitopes (NeuGc-KO pigs) became available (17) but, as this oligosaccharide is expressed in all mammals except humans, its relevance cannot be explored in the pig-to-NHP model (discussed in (18)).
The literature has been reviewed from January 1st 1998 to December 31st 2013. On occasion, the same series of experiments has been reported in more than one paper, e.g., one reporting the overall results, one concentrating attention on the histopathology, etc. At times, it has been difficult to determine whether the experiments included in a report are the same as, or overlap with, those reported previously, and so there may be some duplication. If the report is of relevance to the pig-to-NHP model, we have attempted to be comprehensive, but we cannot guarantee we have included all publications.
We have not included reports of studies in NHPs that did not undergo organ or cell transplantation, e.g., immunoadsorption of anti-pig antibodies alone, or reports of ex vivo blood perfusion of pig organs, which has been a relatively common form of experimentation with regard to assessment of pig lungs and livers. Nor have we included bone marrow or hematopoietic cell xenotransplantation between pig and NHP unless it was associated with an organ graft. We have not included papers published in languages other than English, nor abstracts of congresses, and have not always reviewed publications that did not present new data, or presented in vitro data from in vivo studies if the actual results of the transplants were not reported. If a short publication in Transplantation Proceedings was followed by a full publication in another journal, we have not always included reference to the preliminary publication.
Heart xenotransplantation (Table 1)
Table 1A.
Heterotopic transplantation of pig hearts in NHPs (1998–2013)
| FIRST AUTHOR (Year) | DONOR (pig) | RECIPIENT (n) | IMMUNOSUPRESSIVE THERAPY | SURVIVAL-RANGE (MEDIAN) (Days, unless otherwise stated) |
|---|---|---|---|---|
| Simon (1998) | WT | Baboon (n=2) | Intravenous infusion of synthetic Gal oligosaccharides | <1 (4–6 hours) |
| Waterworth (1998) | CD55 | Baboon (n=3) | CyP, CsA, CS | >2, 13, >21 |
| Lin (1998) | CD55/CD59 | Baboon (n=5) Baboon (n=6) |
CyP, CsA, CS CyP, CsA, CS, Ig-depleted (immunoadsorption column) |
<1–5 1–29 |
| Bhatti (1999) | CD55 | Baboon (n=14) | CyP, CsA, CS, MMF | 10–99 (26) |
| Crespo (1999) | CD55 | Baboon (n=13) | Not stated | <3 (n=2) 3–7 (n=11) |
| Kozlowski (1999) | WT (MSw) | Baboon (n=2) | TBI, TI, pig BMTx, splenectomy, immunoadsorption, ATG, CsA, MMF, CS, 15-deoxyspergualin | 8, 15 |
| Romano (1999) | WT | Baboon (n=1) | Intravenous infusion of synthetic Gal ologosaccharide | <1 (<18 hours) |
| Buhler (2000) Alwayn (2000) |
WT (MSw) | Baboon (n=2) | TBI, TI, splenectomy, immunoadsorption, ATG, CVF, CsA or anti-CD154mAb, MMF or 15-deoxyspergualin (not clearly stated) +/− pig hematopoietic stem cells (n=1) | Not applicable (study of hemostasis) |
| Manez (2000) | WT CD55 |
Baboon (n=10) Baboon (n=10) |
None (n=5) Immunoadsorption (n=5) None (n=5) Immunoadsorption (n=5) |
<96 hours 87.6+/−35 hours 89.6+/−42 hours 101.6+/−23 hours |
| Lin (2000) | CD55/CD59 | Baboon (n=5) Baboon (n=4) |
CyP, CsA, CS Immunoadsorption, CyP, CsA CS |
<1–10 (3) 9–39 |
| Brenner (2000) | WT | Cynomolgus (n=1) Rhesus (n=2) Rhesus (n=4) |
Immunoadsorption Immunoadsorption No immunoadsorption |
78+/−28 minutes |
| Lam (2002) | CD55 | Cynomolgus (n=7) | CyP, CsA, CS, MMF | 6–36 (23) |
| Schuurman (2002) (based on previous publications) | WT (n=7) CD55 (n=55) WT (n=5) CD55 (n=28) |
Cynomolgus (n=62) Baboon (n=33) |
CyP, CsA, CS, splenectomy +/− rapa +/− MMF +/−sCR1 CyP, CsA, CS, splenectomy +/− rapa +/− MMF +/−sCR1 |
WT: HAR 57% CD55: HAR 7% WT: HAR 20% CD55: HAR 11%. |
| Ashton-Chess (2003) | CD55 | Baboon (n=2) Baboon (n=2) Baboon (n=9) |
None Immunoadsorption Cyp, CsA, MMF, CS |
4, 5 4, 6 6–29 (14) |
| Domenech (2003a,b) | CD55 | Baboons (n=8) | CyP (high dose), CsA, CS, GAS914 (n=6) CyP (low dose), CsA, CS, GAS914 (n=2) |
6–60 (27) 5, 7 |
| Lam (2003) | CD55 | Rhesus (n=2) | ATG, sCR1, tacrolimus, MMF, CS, GAS914 | <1 |
| Schirmer (2004) | CD46 | Baboon (n=9) Baboon (n=9) |
Anti-CD20mAb, tacrolimus, rapa, CS, TPC, clopidogrel, aspirin Anti-CD20mAb, tacrolimus, rapa, CS, TPC |
15–30 (22) 4–53 (15) |
| Manez (2004a) | CD 55 CD55/CD46 |
Baboon (n=5) Baboon (n=5) |
CsA, GAS914 CsA |
5–8 (6) 4–9 (7) |
| Manez (2004b) | WT CD55 WT CD55 |
Baboon (n=5) Baboon (n=6) Baboon (n=5) Baboon (n=7) |
None None Immunoadsorption Immunoadsorption |
HAR in 3 of 5 3, 4 No HAR <1–<5 No HAR <2–5 No HAR <4–<6 |
| McGregor (2004) | CD46 | Baboon (n=10) | ATG, splenectomy, anti-CD20mAb, tacrolimus, rapa, CS, TPC | 56–113 (76) |
| Houser (2004) Kuwaki (2004) |
CD55 | Baboon (n=10) | ATG, anti-CD2mAb, TI, CVF, anti-CD154mAb, MMF, CS | 4–139 (27) |
| Lam (2004a) Lam (2004b) |
CD55 | Cynomolgus (n=15) | ATG or CyP, CsA or tacrolimus, MMF, CS, immunoadsorption, +/− GAS914 |
No GAS914: HAR in 4 of 6 4, 78 GAS914: HAR in 0 of 9 0–36 (20) |
| Chan (2005) | CD55 | Cynomolgus (n=4) | CyP, CsA, MMF, CS, GAS914 +/− sCR1 | 20, 22, 35, 36 |
| Stalder (2005) | CD55 | Cynomolgus (n=6) | CyP or ATG, CsA or tacrolimus, MMF, CS, GAS914 +/− sCR1 | 2–36 (mean 30.5) |
| Teotia (2005) | CD46 | Baboon (n=16) | Anti-CD20mAb, tacrolimus, rapa, CS, TPC | 6–113 (mean 71) |
| Dor (2005)* | GTKO (MSw) | Baboon (n=8) | ATG, anti-CD154mAb, MMF, CS ATG, anti-CD154mAb, MMF, CS, recombinant human antithrombin III |
16–179 |
| Kuwaki (2005) Tseng (2005) Hisashi (2008) Shimizu (2008) |
GTKO (MSw) | Baboon (n=8) | ATG, Anti-CD2mAb, TI, CVF, anti-CD154mAb, MMF, CS | >16–179 (63) |
| Moscoco (2005) | CD55 CD55/CD46 |
Baboon (n=9) Baboon (n=5) |
CyP, CsA, MMF, CS, GAS914 (n=5) CsA, GAS914 (n=4) CsA, GAS914 |
50+/−19 6+/−1 6+/−2 |
| Weaver (2005) | CD46 | Baboons (n=8) | Anti-CD20mAb, tacrolimus, rapa, CS, TPC | 0–92 (64) |
| Wu (2005) | CD55 CD46 |
Baboon (n=13) Baboon (n=5) |
CyP, CsA, MMF, CS +/− anti-CD20mAb +/− ATG +/− GAS914 or TPC (n=10) ATG, anti-CD154mAb +/− anti-CD20mAb +/− CTLA4-Fc +/− GAS914 0r TPC (n=8) |
2–36 (12) 0–11 (6) |
| McGregor (2005) | CD46 | Baboon (n=7) | Splenectomy, ATG, anti-CD20mAb, tacrolimus, rapa, CS, TPC | 15–137 (96) |
| Byrne (2005) | CD46 | Baboon (n=9) Baboon (n=13) Baboon (n=9) |
Splenectomy, anti-CD20mAb, tacrolimus, rapa, TPC, warfarin + ATG or CyP for rejection episodes Splenectomy, anti-CD20mAb, tacrolimus, rapa, TPC, low molecular weight heparin+ ATG or CyP for rejection episodes Splenectomy, anti-CD20mAb, tacrolimus, rapa, TPC |
3–62 (20) 5–109 (18) 4–53 (15) |
| Brenner (2005) | CD55 | Baboon (n=4) | Immunoadsorption, CyP, CsA, CS, MMF | 2–8 (10) |
| Davila (2006) | CD46 | Baboon (n=1) Baboon (n=2) Baboon (n=1) |
Splenectomy TPC, splenectomy TPC, anti-CD20mAb, splenectomy |
5 6, 7 7 |
| Byrne (2006) | CD46 | Baboon (n=63) | Splenectomy, TPC, anti-CD20mAb, tacrolimus, rapa, CS +/− aspirin/clopidogrel or Lovenox or warfarin | 0–139 (96) |
| Ricci (2007) (based on previous publications) | CD46 | Baboon (n=64) |
Groups 1–4 (n=40): (Low maimtenance IS) Splenectomy, anti-CD20mAb, tacrolimus, rapa, CS, TPC +/− Lovenox or aspirin + clopidogrel or coumadin Group 5 (n=15): (High maintenance IS) Splenectomy, anti-CD20mAb, tacrolimus, rapa, CS, TPC, Lovenox +/− ATG as induction of treatment of rejection |
Groups 1–4: 0–137 (30) Group 5: 0–139 (96) |
| Wu (2007) | CD55 CD46 |
Baboon (n=20) Baboon (n=3) |
CyP, CsA, MMF (n=18); ATG, anti-CD154mAb (n=10); GAS914 (n=8) or TPC (n=3)+/− immunoadsorption; complement inhibitors (n= 12) |
Technical failure (n=1) HAR (n=10) Early graft failure 1–3 (n=4) AHXR 6–36 (15) (n=8) |
| Zahorsky-Reeves (2007) | CD55 | Cynomolgus (n=3) | ATG or CyP, CsA, MMF, CS +/− GAS914 | 36, 39, 78 |
| Byrne (2008) | CD46 GTKO |
Baboon (n=4) Baboon (n=8) |
Splenectomy +/− TPC +/− anti-CD20mAb Splenectomy, ATG, anti-CD20mAb, tacrolimus rapa |
5–7 0–128 (25) |
| Ezzelarab (2009) | GTKO | Baboon (n=9) | ATG, CVF, anti-CD154mAb, MMF, CS | 2–56 |
| Bauer (2010) (Intrathoracic) | WT GTKO/CD46 |
Baboon (n=2) Baboon (n=2) |
None ATG, anti-CD20mAb, tacrolimus, rapa, MMF, CS, bortezomib, immunoadsorption (n=1) |
Euthanized after weaning from cardiopulmonary bypass <1, 50 |
| Tazelaar (2011) (partially based on previous publications) | CD46 CD46 GTKO +/− CD55 |
Baboon (n=11) Baboon (n=8) Baboon (n=5) |
ATG, anti-CD20mAb, tacrolimus, rapa, TPC Immunoadsorption, CyP, CsA ATG, anti-CD20mAb, tacrolimus, rapa |
15–109 (41) 8–42 (13) 18–71 (26) |
| Mohiuddin (2012) Corcoran (2010) Horvath (2010) |
GTKO/CD46 | Baboon (n=2) Baboon (n=2) Baboon (n=9) |
No IS ATG, CVF, anti-CD154mAb, MMF, CS ATG, anti-CD20mAb, CVF, anti-CD154mAb, MMF, CS |
< 1 8, 8 36–236 (71) |
| McGregor (2012) | GTKO GTKO/CD55 |
Baboon (n=6) Baboon (n=5) |
ATG, anti-CD20mAb, tacrolimus, rapa, CS ATG, anti-CD20mAb, tacrolimus, rapa, CS |
<1–128 (21) 15–52 (28) |
| Kim (2013) | GTKO | Cynomolgus (n=4) | ATG, anti-CD20mAb, CVF, anti-CD154mAb, tacrolimus, CS | 11–24 (14) |
| Mohiuddin (2013) | GTKO/CD46 | Baboon (n=9) | ATG, anti-CD20mAb, CVF, anti-CD40mAb (either 3A8 [n=3] or 2C10R4 [n=6]), MMF, CS | 3A8 = 21, 21, 28 (21) 2C10R4 = >30, >40, 60, 107, 146, 149 (84) |
| Mohiuddin (2013/4) | GTKO/CD46/TBM | Baboon (n=5) | ATG, anti-CD20mAb, CVF, anti-CD40mAb, MMF, CS | 0–>380 (4 ongoing at 77–380 days) |
Abbreviations:
ATG = anti-thymocyte globulin; BMTx = bone marrow transplant; CD46 = membrane cofactor protein; CD55 = decay-accelerating factor; CD59 = protectin, membrane inhibitor of reactive lysis; CS = corticosteroids; CsA = cyclosporine; CTLA4-Fc = CTLA4 covalently linked to a human immunoglobulin Fc molecule; CVF = cobra venom factor; CyP = cyclophosphamide; EGF = early graft failure; GAS914 = a soluble glycoconjugate comprising Gal on a poly-L-lysine backbone; GTKO = α1,3-galactosyltransferase gene-knockout; HAR = hyperacute rejection; IS = immunosuppressive therapy; LoCD2b = rat anti-primate CD2b monoclonal antibody; MMF = mycophenolate mofetil (or analog, e.g., mycophenolate sodium); MSw = miniature swine (MGH herd); Rapa = rapamycin (or derivative, e.g., RAD); sCR1 = soluble complement receptor type 1 (in some papers described as TP10); TBI = total body irradiation; TI = thymic irradiation, TPC= an αGal-polyethylene glycol polymer conjugate.
Table 1A: References
Alwayn IP, Buhler L, Basker M, et al. Coagulation disorders associated with organ and cell xenotransplantation. Transplant Proc. 2000;32:1099.
Ashton-Chess J, Roussel J-C, Manez R, et al. Cellular participation in delayed xenograft rejection of hCD55 transgenic pig hearts by baboons. Xenotransplantation 2003;10:446–453.
Bauer A, Postrach J, Thormann M, et al. First experience with heterotopic thoracic pig to baboon cardiac xenotransplanation. Xenotransplantation 2010;17:243–249.
Bhatti FN, Schmoeckel M, Zaidi A, et al. Three month survival of hDAF transgenic pig hearts transplanted into primates. Transplant Proc 1999; 3:958.
Brenner P, Schmoeckel M, Reichenspurner H, et al. Technique of immunoapheresis in heterotopic and orthotopic xenotransplantation of pig hearts into cynomolgus and rhesus monkeys. Transplant Proc. 2000;32:1987–1088.
Brenner P, Schmoeckel M, Wimmer C, et al. Combination of hDAF-transgenic pig hearts and immunoadsorption in heterotopic xenotransplantation of immunosuppressed baboons. Transplant Proc 2005;31:483–486.
Buhler L, Basker M, Alwayn IP, et al. Coagulation and thrombotic disorders associated with pig organ and hematopoietic cell transplantation in nonhuman primates. Transplantation 2000;70:1323–1331.
Byrne GW, Schirmer JM, Fass DN, et al. Warfarin or low-molecular-weight heparin therapy does not prolong pig-to-primate cardiac xenograft function. Am J Transplant 2005; 5:1011–1020.
Byrne GW, Davies WR, Oi K, Rao VP, Teotia SS, Ricci D, et al. Increased immunosuppression, not anticoagulation, extends cardiac xenograft survival. Transplantation 2006;82:1787–91.
Byrne GW, Stalboerger PG, Davila E, et al. Proteomic identification of non-Gal antibody targets after pig-to-primate cardiac xenotransplantation. Xenotransplantation. 2008;15:268–276.
Chan MC, Stalder M, Lam TT, Tye T, Borie DC, Morris RE. Use of echocardiography to assess function of hDAF-transgenic pig cardiac xenografts. Transplant Proc 2005;37:1923–1925.
Corcoran PC, Horvath KA, Singh AK, et al. Surgical and nonsurgical complications of a pig to baboon heterotopic heart transplantation model. Transplant Proc 2010;42:2149–2151.
Crespo FM, Centeno A, Lopez E, Juffe A, Manez R. Abdominal laparoscopy in follow-up of heterotopic pig heart to baboon xenotransplantation. Transplant Proc 1999;31:2619.
Davila E, Byrne GW, LaBreche PT, et al. T-cell responses during pig to primate xenotransplantation. Xenotransplantation 2006;13:31–40.
Domenech N, Diaz T, Moscoso I, Lopez-Pelaez E, Centeno A, Manez R. Elicited non-anti-αGAL antibodies may cause acute humoral rejection of hDAF pig organs transplanted in baboons. Transplant Proc 2003;35:2049–2050. (2003a)
Domenech N, Diaz T, Moscoso I, Centeno A, Lopez-Pelaez E, Manez R. Porcine endothelial cell activation in hDAF pig hearts transplanted in baboons with prolonged survival and lack of rejection. Transplant Proc. 2003;35:2045–2046. (2003b)
Dor FJ, Kuwaki K, Tseng YL. Potential of aspirin to inhibit thrombotic microangiopathy in alpha1,3-galactosyltransferase gene-knockout pig hearts after transplantation in baboons. Transplant Proc. 2005;37:489–490.
Ezzelarab M, Garcia B, Azimzadeh A, et al. The innate immune response and activation of coagulation in alpha1,3-galactosyltransferase gene-knockout xenograft recipients. Transplantation. 2009;87:805–812.
Hisashi Y, Yamada K, Kuwaki K, et al. Rejection of cardiac xenografts transplanted from α1,3-galactosyltransferase gene-knockout (GalT-KO) pigs to baboons. Am J Transplant 2008;8:2516–2526
Horvath KA, Corcoran PC, Singh AK, et al. Left ventricular pressure measurement by telemetry is an effective means to evaluate transplanted heart function in experimental heterotopic cardiac xenotransplantation. Transplant Proc 2010;42:2152–2155.
Houser SL, Kuwaki K, Knosalla C, et al. Thromboticmicroangiopathy and graft arteriopathy in pig hearts following transplantation into baboons. Xenotransplantation 2004;11:416–425.
Kim H, Chee HK, Yang J, et al. Outcomes of alpha 1,3-GT-knockout porcine heart transplants into a preclinical nonhuman primate model. Transplant Proc 2013; 45: 3085–3091.
Kozlowski T, Shimizu A, Lambrigts D, et al. Porcine kidney and heart transplantation in baboons undergoing a tolerance induction regimen and antibody adsorption. Transplantation 1999;67:18–30.
Kuwaki K, Knosalla C, Dor FJMF, et al. Suppression of natural and elicited antibodies in pig-to-baboon heart transplantation using a human anti-CD154 monoclonal antibody-based regimen. Am J Transplant 2004;4:363–372
Kuwaki K, Tseng YL, Dor FJ, et al. Heart transplantation in baboons using α1,3-galactosyltransferase gene-knockout pigsas donors: initial experience. Nat Med 2005; 11:29–31.
Lam TT, Borie D, Masek M, Berry G, Larson M, Morris RE. Graft thrombosis in had-transgenic pig hearts transplanted into rhesus monkeys. Xenotransplantation. 2003;10:185–186.
Lam TT, Hausen B, Squiers E, et al. Cyclophosphamide-induced postoperative anemia in cynomolgus monkey recipients of hDAF-transgenic pig organ xenografts. Transplant Proc 2002;34:1451–1452.
Lam TT, Hauser B, Boeke-Purkis K, et al. Hyperacute rejection of hDAF-transgenic pig organ xenografts in cynomolgus monkeys: influence of pre-existing anti-pig antibodies and prevention by the alpha-Gal glycoconjugate GAS914. Xenotransplantation 2004; 11:517–524. (2004a)
Lam TT, Paniagua R, Shivaram G, et al. Anti-non-Gal porcine endothelial cell antibodies in acute humoral xenograft rejection of hDAF-transgenic porcine hearts in cynomolgus monkeys. Xenotransplantation 2004;11:531–535. (2004b)
Lin SS, Weidner BC, Byrne GW, et al. The role of antibodies in acute vascular rejection of pig-to-baboon cardiac transplants. J Clin Invest 1998;101:1745–1756.
Lin SS, Hanaway MJ, Gonzalez-Stawinski GV, et al. The role of anti-Galalpha1-3Gal antibodies in acute vascular rejection and accommodation of xenografts. Transplantation 2000;70:1667–1674.
Manez R, Crespo F, Gonzalez E, et al. Neutralization of anti-αGalactosyl antibodies without immunosuppression prevets hyperacute rejection but not acute vascular rejection of pig organs transplanted into baboons. Transplant Proc. 2000;32:888–889.
Manez R, Domenech N, Centeno A, et al. Failure to deplete anti-Galalpha1-3Gal antibodies after pig-to-baboon organ xenotransplantation by immunoaffinity columns containg multiple Galalpha1-3Gal oligosaccharides. Xenotransplantation 2004;11:408–415. (2004b)
Manez R, Lopez-Pelaez E, Centeno A, et al. Transgenic expression in pig hearts of both human decay-accelerating factor and human membrane cofactor protein does not provide additional benefit to that of human decay-accelerating factor alone in pig-to-baboon xenotransplantation. Transplantation 2004;78:930933. (2004a)
McGregor CG, Teotia SS, Byrne GW, et al. Cardiac xenotransplation: progress toward the clinic. Transplantation 2004;78:1569–1575.
McGregor CG, Davies WR, Oi K, et al. Cardiac xenotransplantation: recent preclinical progress with 3-month median survival. J Thorac Cardiovasc Surg 2005;130:844–851.
McGregor CGA, Ricci D, Miyagi N, et al. Human CD55 expression blocks hyperacute rejection and restricts complement activation in Gal-knockout cardiac xenografts. Transplantation 2012;93:686–692.
Mohiuddin MM, Corcoran PC, Singh AK, et al. B-cell depletion extends the survival of GTKO.hCD46Tg pig heart xenografts in baboons for up to 8 months. Am J Transplant 2012;12:763–771.
Mohiuddin MM, Singh AK, Corcoran PC, et al. One-year heterotopic cardiac xenograft survival in a pig to baboon model. Am J Transplant. 2014:14:488–489. doi: 10.1111/ajt.12562. Epub 2013 Dec 11.
Mohiuddin MM, Singh AK, Corcoran PC, et al. Role of anti-CD40 antibody-mediated costimulation blockade on non-Gal antibody production and heterotopic cardiac xenograft survival in a GTKO.hCD46Tg pig-to-baboon model. Xenotransplantation. 2013 Oct 29. doi: 10.1111/xen.12066. [Epub ahead of print].
Moscoso I, Hermida-Prieto M, Manez R, et al. lack of cross-species transmission of porcine endogenous retrovirus in pig-to-baboon xenotransplantation with sustained depletion of anti-alphagal antibodies. Transplantation 2005;79:777–782.
Ricci D, Tazelaar HD, Miyagi N. The utility of right ventricular endomyocardial biopsy for the diagnosis of xenograft rejection after CD46 pig-to-baboon cardiac transplantation. J Heart Lung Transplant 2007;26:1025–1032.
Romano E, Neethling FA, Nilsson K, et al. Intravenous synthetic gal saccharides delay hyperacute rejection following pig-to-baboon heart transplantation. Xenotransplantation 1999;6:36–42.
Schirmer JM, Fass DN, Byrne GW, et al. Effective antiplatelet therapy does not prolong transgenic pig to baboon cardiac xenograft survival. Xenotransplantation 2004; 11: 436–443.
Schuurman HJ, Pino-Chavez G, Phillips MJ, et al. Incidence of hyperacute rejection in pig-to-primate transplantation using organs from hDAF-transgenic donors. Transplantation 2002; 73: 1146–1151.
Shimizu A, Hisashi Y, Kuwaki K, et al. Thrombotic microangiopathy associated with humoral rejection of cardiac xenografts from α1,3-galactosyltranserase gene-knockout pigs in baboons. Am J Pathol. 2008;172:1471–1481.
Simon PM, Neethling FA, Taniguchi S, et al. Intravenous infusion of Galα1-3Gal oligosaccharides in baboons delays hyperacute rejection of porcine heart xenografts. Transplantation 1998;65:346–353.
Stalder M, Tye T, Lam TT, et al. Improved assessment of graft function by echocardiography in cynomolgus monkey recipients of hDAF-transgenic pig cardiac xenografts. J Heart Lung Transplant. 2005;24:215–221.
Tazelaar HD, Byrne GW, McGregor CG. Comparison of Gal and non-Gal-mediated cardiac xenograft rejection. Transplantation 2011;91:968–975.
Teotia SS, Walker RC, Schirmer JM, et al. Prevention, detection, and management of early bacterial and fungal infections in a preclinical cardiac xenotransplantation model that achieves prolonged survival. Xenotransplantation 2005;12:127–133.
Tseng Y-L, Kuwaki K, Dor FJMF, et al. α 1,3-galactosyltransferase gene-knockout pig heart transplantation in baboons with survival approaching six months. Transplantation 2005;80:1493–1500.
Waterworth PD, Dunning J, Tolan M, et al. Life-supporting pig-to-baboon heart xenotransplantation. J Heart Lung Transplant 1998; 17:1201–1207.
Weaver JG, McGregor CG, Tazelaar HD, Badley AD. Rejection severity directly correlates with myocyte apoptosis in pig-to-baboon cardiac xenotransplantation. J Heart Lung Transplant. 2005;24:841–847.
Wu G, Pfeiffer S, Schroder C, et al. Costimulation blockade targeting CD154 and CD28/B7 modulates the induced antibody response after a pig-to-baboon cardiac xenograft. Xenotransplantation 2005;12:197–208.
Wu G, Pfeiffer S, Schröder C, et al. Coagulation cascade activation triggers early failure of pig hearts expressing complement regulatory genes. Xenotransplantation 2007;14:34–47.
Zahorsky-Reeves JL, Kearns-Jonker MK, Lam TT, et al. The xenoantibody response and immunoglobulin gene expression profile of cynomolgus monkeys transplanted with hDAF-transgenic porcine hearts. Xenotransplantation 2007;14:135–144.
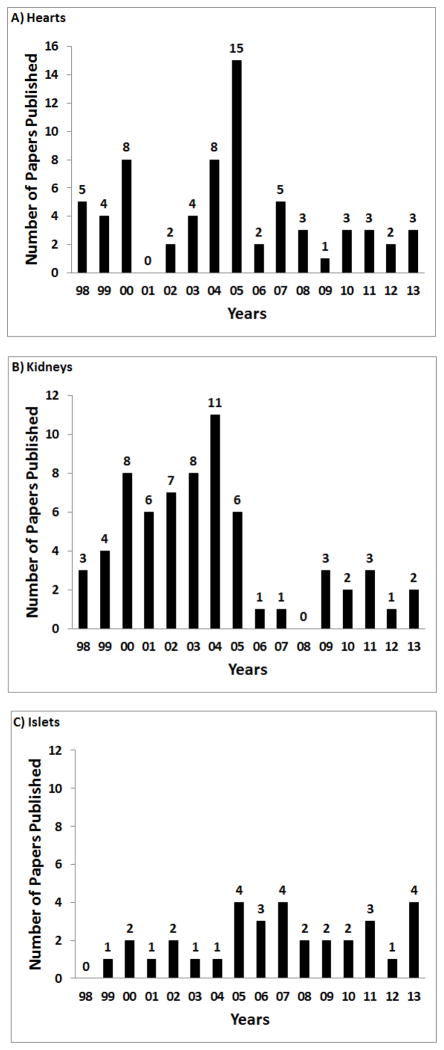
More progress has been made in pig heart transplantation than in the transplantation of other vital organs. The introduction of GTKO pigs was important (reviewed in (19); hyperacute rejection, already minimized by the transplantation of hearts from pigs transgenic for a human complement-regulatory protein, was virtually eliminated, particularly when GTKO pigs expressed a human complement-regulatory protein. With adequate exogenous immunosuppressive therapy, the incidence of delayed xenograft rejection (acute humoral xenograft rejection, acute vascular rejection) was also greatly reduced. However, a new phenomenon, thrombotic microangiography, was reported (20), stimulating the development of pigs transgenic for one or more human coagulation-regulatory proteins, e.g., thrombomodulin, CD39, endothelial cell protein C receptor, which are only now being explored in the pig-to-NHP model.
In 1998, the longest survival of a heterotopically-placed (non-life-supporting) heart was reported to be 31 days (3), whereas by the end of 2013 this has been extended to >12 months (19,21–24) (Table 1A). Survival after othotopic (life-supporting) pig heart transplantation has been extended from a maximum of 19 days (3) to 57 days (Table 1B).
Table 1B.
Orthotopic transplantation of pig hearts in NHPs (1998–2013)
| FIRST AUTHOR (Year) | DONOR (pig) | RECIPIENT (n) | IMMUNOSUPRESSIVE THERAPY | SURVIVAL-RANGE (MEDIAN) (Days unless otherwise stated) |
|---|---|---|---|---|
| Schmoeckel (1998) | CD55 | Baboon (n=10) | CyP, CsA, CS | <1 (x5) 4–9 (x5) |
| Waterworth (1998) | CD55 | Baboon (n=5) | CyP, CsA, CS | >1, >1, 5, 5, 9 |
| Xu (1998) | WT | Baboon (n=2) | Immunoadsorption (through another donor organ), TBI, CsA, methotrexate, | 18, 19 |
| Brenner (2000a) | WT | Cynomolgus (n=2) | Immunoadorption | 130+/−21 minutes |
| Brenner (2000b,c) | WT | Baboon (n=4) | None (n=1) Immunoadsorption (n=3) |
29 minutes <2, 11, 21 hours |
| Vial (2000) | CD55 | Baboon (n=1) | CyP, CsA, MMF, CS | 39 |
| Schuurman (2002) (based on previous publications) | CD55 | Baboon (n=16) | CyP, CsA, CS, splenectomy +/− rapa +/−MMF +/−sCR1 | HAR 1 (6%) |
| Brandl (2005) | CD55 | Baboon (n=4) | ATG, tacrolimus, rapa, CS, GAS914 +/− CyP | 1–25 (9) |
| Brenner (2005) | CD55 | Baboon (n=4) | CyP, CsA, MMF, CS | <1, 11, 13, 20 |
| Bauer (2005) | CD55 | Baboon (n=9) | ATG, tacrolimus or CsA, rapa, CS, GAS914, | Not stated |
| Brandl (2007) | CD46 or CD55 | Baboon (n=2) Baboon (n=2) Baboon (n=4) Baboon (n=5) |
ATG, tacrolimus, rapa, CS, GAS914 ATG, anti-CD20mAb, tacrolimus, rapa, CS, GAS914 ATG, low-dose CyP, tacrolimus, rapa, CS, GAS914 ATG, CyP, tacrolimus, rapa, CS, anti-HLA-DR antibody, +/− GAS914+TPC |
1, 9 <2 (1 technical failure) <1, 14, 25 (1 technical failure) <1–4 |
| Bauer (2007) | CD55 CD46 |
Baboon (n=6) Baboon (n=6) |
Not stated | Not stated |
| Bauer (2011) | CD46 | Baboon (n=6) | ATG +/− CyP, tacrolimus, rapa, CS, GAS914 or TPC, anti-HLA antibody | Not stated |
| Byrne (2011) | CD46 or CD55 or CTKO/CD55 | ATG or CyP, tacrolimus, rapa +/− anti-CD20mAb +/− GAS914 or TPC | 0–57 (6) |
Abbreviations as for Table 1A
Table 1B: References
Bauer A, Baschnegger H, Renz V, et al. Comparison of propofol and isoflurane anesthesia in orthotopic pig-to-baboon cardiac xenotransplantation. Xenotransplantation 2007;14:249–254.
Bauer A, Baschnegger H, Abicht JM, et al. hDAF porcine cardiac xenograft maintains cardiac output after orthotopic transplantation into baboon - a perioperative study. Xenotransplantaion 2005;12:444–449.
Bauer A, Renz V, Baschnegger H, et al. Microcirculatory alterations after orthotopic pig-to-baboon heart transplant. Xenotransplantation 2011;18:232–238.
Brandl U, Michel S, Erhardt M, et al. Administration of GAS914 in an orthotopic pig-to-baboon heart transplantation model. Xenotransplantation 2005;12:134–141.
Brandl U, Michel S, Erhardt M, et al. Transgenic animals in experimental xenotransplantation models: orthotopic heart transplantation in the pig to baboon model. Transplant Proc. 2007;39:577–578
Brenner P, Schmoeckel M, Reichenspurner H, et al. Technique of immunoapheresis in heterotopic and orthotopic xenotransplantation of pig hearts into cynomolgus and rhesus monkeys. Transplant Proc. 2000;32:1987–1088. (2000a)
Brenner P, Schmoeckel M, Wimmer C, et al. Mean xenograft survival of 14.6 days in a small group of hDAF-transgenic pig hearts transplanted orthotopically into baboons. Transplant Proc 2005;37:472–476.
Brenner P, Reichenspurner H, Schmoeckel M, ey al. Prevention of hyperacute xenograft rejection in orthotopic xenotransplantation of pig hearts into baboons using immunoadsorption of antibodies and complement factors. Transpl Int 2000;13 Suppl 1:S508–17. (2000b)
Brenner P, Reichenspurner H, Schmoeckel M, et al. IG-therasorb immunoapheresis in orthotopic xenotransplantation of baboons with landrace pig hearts. Transplantation 2000;69:208–214. (2000c)
Byrne GW, Du Z, Sun Z, Asmann YW, McGregor CG. Changes in cardiac gene expression after pig-to-primate orthotopic xenotransplantation. Xenotransplantation 2011;18:14–27.
Schmoeckel M, Bhatti FN, Zaidi A, et al. Orthotopic heart transplantation in a transgenic pig-to-primate model. Transplantation 1998;65:1570–1577.
Schuurman HJ, Pino-Chavez G, Phillips MJ, et al. Incidence of hyperacute rejection in pig-to-primate transplantation using organs from hDAF-transgenic donors. Transplantation 2002; 73: 1146–1151.
Vial CM, Ostlie DJ, Bhatti FN, et al. Life supporting function over one month of a transgenic porcine heart in a baboon. J Heart Lung Transplant 2000;19:224–229.
Waterworth PD, Dunning J, Tolan M, et al. Life-supporting pig-to-baboon heart xenotransplantation. J Heart Lung Transplant 1998;17:1201–1207
Xu H, Gundry SR, Hancock WW, et al. Prolonged discordant xenograft survival and delayed xenograft rejection in a pig to baboon heart xenograft model. J Thorac Cardiovasc Surg. 1998;115:1342–1349.
Kidney xenotransplantation (Table 2)
Table 2.
Transplantation of pig kidneys into NHPs (1998–2013)
| FIRST AUTHOR (YEAR) |
DONOR (PIG) |
RECIPIENT (n) |
IMMUNOSUPRESSIVE THERAPY |
SURVIVAL-RANGE (MEDIAN) (Days) |
|---|---|---|---|---|
| Ierino (1998) | WT (MSw) | Baboon (n=3) | TBI, BMTx (autologous, transfected with SLA class II [n=2]), splenectomy, apheresis, immunoadsorption, sCR-1, CsA, MMF, CS, 15-deoxyspergualin | 8, 12, 13 |
| Xu (1998) | WT (MSw) | Cynomolgus (n=10) | Immunoadsorption (Gal column n=4; pig liver n=6) | 2–12 (mean 7) |
| Zaidi (1998) | WT | Cynomolgus (n=6) | CyP, CsA, CS | <1–30 (7) |
| CD55 | Cynomolgus (n=7) | 6–35 (13) | ||
| Schmoeckel (1999) | CD55 | Cynomolgus (n=11) | Splenectomy, CyP, CsA, MMF, CS (n= 5) CyP, CsA, MMF, CS (n=6) |
Median 43 Median 15 (but 4 deaths with functioning kidney) |
| Ierino (1999) [Ierino (1998)] |
WT (MSw) | Baboons (n=4; life-supporting in only one) | SLA class II gene transduction of baboon CD34+ and CD34− bone marrow cells (n=3) | Uncertain |
| Kozlowski (1999) | WT (MSw) | Baboon (n=2) | TBI, TI, pig BMTx, splenectomy, Immunoadsorption, ATG, CsA, CS, MMF and/or 15-DSG | 9, 11 |
| Baboon (n=2) | As above (modified) | 3, 6 | ||
| Meyer (1999) | WT | Baboon (n=5) | Plasmapheresis, immunoadsorption | 1–5 (3) |
| vWD | Baboon (n=5) | 1–5 (4) | ||
| Loss (2000) | WT | Cynomolgus (n=7) | (Non-life supporting - study of effect of cold ischemia on HAR) | <1 |
| Cynomolgus (n=8) | Life-supporting - study of effect of cold ischemia on HAR) | <1 | ||
| Cowan (2000) | WT | Baboon (n=4) | None | <1 |
| CD55/H-transferase | Baboon (n=2) | 2 | ||
| CD55/CD59/H-transferase | Baboon (n=2) | 2, 3 | ||
| Baboon (n=4) | Low molecular weight heparin | 3–5 | ||
| Cozzi (2000) [Bhatti (1998)] |
WT | Cynomolgus (n=5) | CyP, CsA, CS, splenectomy | 0–30 (0) |
| CD55 | Cynomolgus (n=9) | CyP, CsA, CS, splenectomy | 5–78 (39) | |
| Cynomolgus (n=7) | CyP, CsA, CS | 6–35 (13) | ||
| Dehoux (2000) | WT | Baboon (n=4) | None (splenectomy in 1) | <1 |
| Baboon (n=5) | anti-IgMmAb | 4–6 (4) | ||
| Buhler (2000) | WT (MSw) | Baboon (n=1) | TBI, TI, ATG, splenectomy, immunoadsorption, CVF, CsA or anti-CD154mAb, MMF or 15-deoxyspergualin (not clearly stated) | Not applicable (study of hemostasis) |
| Baboon (n=3) | As above + pig hematopoietic cells | |||
| Shimizu (2000) | WT (MSw) | Cynomolgus (n=4) | Immunoadsorption, splenectomy, CsA, 15-deoxyspergualin | 0, 1, 7, 8 |
| Cynomolgus (n=10) | As above + TBI, TI, ATG, BMTx (n=1) | 0–15 (9.5) | ||
| Baboon (n=2) | 9, 11 | |||
| Baboon (n=4) | As above + TBI, TI, ATG, MMF or brequinar, BMTx (n=1) | 3, 6, 6, 14 | ||
| Baboon (n=5) | As above + TI, ATG (n=1), MMF, sCR1 or CVF, BMTx | 6–13 (8) | ||
| Kobayashi (2000) | WT | Baboon (n=2) | CyP, CsA or tacrolimus, CS, plasmapheresis | 6, 7 |
| Baboon (n=2) | As above + additional plasmapheresis | <1 | ||
| Loss (2000) | WT | Cynomolgus (n=8) | CyP, CsA, CS | 1–11 (3.5) |
| CD55 | Cynomolgus (n=9) | 1–68 (11) | ||
| Przemeck (2001) | WT | Cynomolgus (n=7) | CyP, CsA, CS | Not stated (study of cardio-circulary parameters) |
| CD55 | Cynomolgus (n=6) | |||
| Bühler (2001) | WT (MSw) | Baboon (n=3) | Splenectomy, TBI, TI, ATG, immunoadsorption, CVF, anti-CD154mAb, MMF, CS | 4, 6, 8 |
| Baboon (n=2) | As above + CyP, but no TBI | 7, 13 | ||
| CD55 | Baboon (n=3) | 28, 29, 29 | ||
| Vangerow (2001) | CD55 | Cynomolgus (n=2) | CyP, CsA, CS | 3, 4 |
| Cynomolgus (n=4) | As above + treatment of rejection with CyP, CS | 9, 11, 11, 15 | ||
| Cynomolgus (n=4) | As above + treatment of rejection with CyP, CS, C1-INH | 18, 21, 28, 68 | ||
| Loss (2001) | WT | Cynomolgus (n=12) (donor kidney <50g) | CsA, CyP, CS: | <1–<15 (3) |
| Cynomolgus (n=3) : (donor kidney >70g) | CsA, CyP, CS | 1, 4, 11 | ||
| Loss (2001) | WT | Cynomolgus (n=7) | CyP, CsA, CS (Non life-supporting graft. Study on transmission of porcine endogenous retrovirus and porcine chimerism) | 4–287 (28) |
| Cynomolgus (n=5) | CyP, CsA, CS (Study on transmission of porcine endogenous retrovirus and porcine chimerism) | 1–11 (3) | ||
| Dean (2001) | CD55 | Baboon (n=5) | CyP, CsA, CS | 3–7 (mean 5+/−1) |
| Baboon (n=6) | Splenectomy, immunoadorption, CyP, CsA, CS | 6–12 (mean 10+/−1) | ||
| Baboon (n=3) | Splenectomy, intensive immunoadorption, CyP, CsA, CS | 13–15 (mean 14+/−0.3) | ||
| McInnes (2002) | WT | Cynomolgus (n=11) | CyP, CsA, CS, splenectomy +/− rapa +/−MMF +/−sCR1 (Study on post-transplant lymphoproliferative disease) | <1–78 (6.5) |
| CD55 | Cynomolgus (n=234) | |||
| Hecker (2002) | WT | Cynomolgus (n=3) | CyP, CsA, MMF, CS, C1-INH | 5, 13, 15 |
| Richards (2002) | CD55 | Cynomolgus (n=20) | CyP, CsA, CS +/−rapa +/−MMF +/−sCR1 | 4–60 (31) |
| Schuurman (2002) | WT | Cynomolgus (n=11) | CyP, CsA, CS, splenectomy +/− rapa +/−MMF +/−sCR1 | HAR: n=3 |
| CD55 | Cynomolgus (n=234) | HAR: n=0 | ||
| Ghanekar (2002) | CD55 | Baboon (n=2) | CsA, GAS914, CS, CyP, rapa | 20, 36 |
| Baboon (n=4) | ATG, CsA, GAS914, CS, rapa | 20, 22, 23, 26 | ||
| Baboon (n=3) | ATG, CsA, GAS914, CS | 18, 21, 22 | ||
| Dehoux (2002) | WT | Baboon (n=5) | Anti-IgM mAb | 4–6 (4) |
| Cowan (2002) | CD55/CD59 | Baboon (n=1) | Low molecular weight heparin | <1–6 |
| Baboon (n=3) | Low molecular weight heparin, recombinant human antithrombin III | |||
| Baboon (n=1) | Recombinant human antithrombin III | |||
| Zhong (2003) | CD55 | Baboon (n=4) | CyP, CsA, CS, rapa | 4, 4, 26, 40 |
| Baboon (n=12) | CyP, CsA, CS, rapa, GAS914 | 7–37 (14) | ||
| Barth (2003) | CD55 | Baboon (n=5) | (Thymokidneys), thymectomy/TI, splenectomy, immunoadsorption, anti-CD2mAb, ATG/anti-CD3IT, anti-CD154mAb, CyP, CVF, MMF, CS | 24–229 (27) |
| Gollackner (2003) | WT (MSw) | Baboons (n=2) | TI, splenectomy, immunoadsorption, ATG, anti-CD154mAb, CyP, CVF, MMF, CS | 7, 13 |
| CD55 | Baboons (n=3) | 28, 29, 29 | ||
| WT (MSw) | Baboons (n=4) | As above + BSA-Gal | 8, 9, 11, 11 | |
| Cozzi (2003) | CD55 | Cynomolgus (n=10) | Splenectomy, CsA, CyP, Cs, MMF | 2–51 (22) |
| Ashton-Chess (2003) | WT | Baboon (n=7) | Immunoadsorption, CyP, CsA, MMF, CS | 4–8 (5) |
| CD55 | Baboon (n=4) | As above | 5, 5, 5, 9 | |
| CD55 | Baboon (n=4) | As above, but no immunoadsorption | 7, 8, 10, 12 | |
| CD55 | Baboon (n=4) | As above, but immunoadsorption x1 | 5, 5, 7, 10 | |
| Cozzi (2003) | CD55 | Cynomolgus (n=5) | Methotrexate (6 doses), CsA, MMF, CS | 6, 9, 9, 10, 39 |
| Cynomolgus (n=3) | Methotrexate (4 doses), CsA, MPS, CS | 0, 16, 34 | ||
| Cynomolgus (n=2) | Methotrexate (5 doses), CsA, CS | 16, 41 | ||
| Gollackner (2003) | WT (MSw) | Baboon (n=3) | Immunoadsorption, splenectomy, TI, ATG, CVF, anti-CD154mAb, MMF, CS, CyP | 6, 7, 13 |
| Baboon (n=2) | As above + BSA-Gal | 8, 11 | ||
| Baboon (n=2) | As above + BSA-Gal, but no CyP | 9, 12 | ||
| Knosalla (2003) | WT (MSw) | Baboon (n=3) | Immunoadsorption, splenectomy, TI, ATG, CVF, CyP, anti-CD154mAb, MMF, CS | 6, 7, 13 |
| CD55 | Baboon (n=3) | 28, 29, 29 | ||
| Ashton-Chess (2004) | CD55/CD59 | Baboon (n=2) | None | 5, 6 |
| Baboon (n=4) | CsA, MMF, CS, mitoxantrone | 6, 7, 8, 10 (8) | ||
| Baboon (n=2) | As above, but CyP not mitoxantrone | 9, 9 | ||
| Loveland (2004) | WT | Baboon (n=7) | (Non-life-supporting) None | Not stated |
| CD46 | Baboon (n=9 | |||
| Key (2004) | CD55 | Cynomolgus (n=52) | Various | 1–53 (17.5) |
| Lam (2004) | CD55 | Cynomolgus (n=4) | CyP, CsA, MMF, CS | <0, <0, <0, 4 |
| Cynomolgus (n=4) | As above + GAS914 | 6, 12, 31, 37 | ||
| Cozzi (2004) | CD55 (Donor pig pretreated with carbon monoxide) | Cynomolgus (n=5) | CyP, CsA, MMF, CS, GAS914 | 2–37 (12) |
| Baldan (2004) | CD55 | Cynomolgus (n=7) | CyP, CsA, MMF, CS | 1–90 (48) |
| Cynomolgus (n=10) | As above + methotrexate | 0–39 (13) | ||
| Cynomolgus (n=5) | As above + GAS914 | 7–38 (21) | ||
| Cynomolgus (n=6) | (Donor pig pretreated with carbon monoxide) As above + GAS914 | 2–37 (8.5)) | ||
| Cynomolgus (n=2) | As above + recombinant human antithrombin III | 23, 23 | ||
| Diaz (2004) | CD55 | Baboon (n=9) | Not stated | Not stated |
| Garcia (2004) | CD55 | Baboons (n=27) | CyP/ATG, CsA/tacrolimus, rapa/MMF, CS, GAS914 | 4–75 (mean 21) |
| Menoret (2004) | WT | Baboon (n=2) | None | <1 |
| CD55/CD59 | Baboon (n=2) | 5, 6 | ||
| Gollackner (2004) | WT (MSw) | Baboon (n=6) | WBI/CyP, IAD, SpX, TI, ATG, CVF, anti-CD154, MMF, CS | 4–13 (6.5) |
| CD55 | Baboon (n=3) | 28, 29, 29 | ||
| WT (MSw) | Baboon (n=4) | Same, in addition BSA-Gal +/− CyP | 8, 9, 11, 11 | |
| Ghanekar (2004) | CD55 | Baboon | Various (based on Ghanekar 2002 and Zhong 2003) | |
| Yamada (2005) | GTKO (MSw) | Baboon (n=6) | (Vascularized thymic lobe) +/−WBI, thymectomy, splenectomy, anti-CD2mAb, anti-CD154mAb, MMF, CS, +/− CVF | 4–68 (32) |
| Baboon (n=5) | (Thymokidney) As above | 16–83 (26) | ||
| Baboon (n=3) | (Kidney) As above | 20–34 (33) | ||
| Chen (2005) | GTKO | Baboon (n=3) | ATG, tacrolimus, CS | 8, 10, 11 |
| Baboon (n=3) | As above + MMF, CVF | 9, 13, 16 | ||
| Moscoso (2005) | CD55 | Baboon (n=8) | GAS914, CyP, CsA, MMF, CS | 11±9 |
| Baboon (n=5) | Anti-CD25mAb, FTY, CsA, CS | 12±10 | ||
| Sun (2005) | CD55 (from supplier A) | Baboon (n=4) | CyP, CsA, rapa | 4, 4, 26, 40 |
| Baboon (n=10) | As above + GAS914 | 9–37 (17) | ||
| Baboon (n=3) | ATG, CsA, rapa, GAS914 | 20, 23, 26 | ||
| Baboon (n=1) | ATG, tacrolimus, MMF, GAS914/TPC | 75 | ||
| CD55 (from supplier B) | Baboon (n=5) | 7–16 (13) | ||
| Shimizu (2005) | CD55 | Baboon (n=6) | (Thymokidney) Splenectomy, thymectomy/TI, immunoadsorption, ATG/anti-CD3IT, CyP, CVF, MMF, anti-CD154mAb | 9–27 (12) |
| Baboon (n=10) | 2–30 (16) | |||
| Lam (2005) | CD55 | Cynomolgus (n=3) | CyP, CsA, MMF, CS, GAS | 6, 12, 31 |
| Cynomolgus (n=2) | As above + sCR-1 | 3, 15 | ||
| Cynomolgus (n=3) | As above + GAS914 + sCR-1 | 10, 20, 32, 37 | ||
| Chen (2006) | CD55 | Baboon (n=5) | GAS914/TPC, ATG, tacrolimus, MMF, CS | 7–75 (13) |
| Baboon (n=2) | GAS914/TPC, ATG, tacrolimus, CVF, anti-CD20mAb | 8,14 | ||
| Cavicchioli (2007) | CD55 | Cynomolgus (n=7) | CyP, CsA, MMF, CS | 1–90 (20) |
| Cynomolgus (n=8) | As above, but methotrexate not CyP | 0–39 (10) | ||
| Yazaki (2009) | CD55/endo-β-galactosidase C | Baboon (n=4) | Splenectomy, CyP, tacrolimus, CS | 2, 8, 9, 11 |
| Knosalla (2009) | WT (MSw) or CD55 | Baboon (n=6) | Not stated | Not stated |
| GTKO (MSw) | Baboon (n=14) | (+/− vascularized thymic lobe or thymokidney) Not stated |
||
| Griesemer (2009) | GTKO (MSw) | Baboon (n=7) | Thymectomy, splenectomy, TBI (n=1), ATG +/− anti-CD2mAb, anti-CD154mAb, tacrolimus, MMF, anti-CD20mAb | 18–83 (49) |
| Lin (2010) | WT | Baboon (n=1) | None | <1 |
| GTKO/CD46 | Baboon (n=1) | 2 | ||
| WT | Baboon (n=1) | ATG, anti-CD154, MMF, CVF, CS | 6 | |
| GTKO | Baboon (n=1) | 7 | ||
| GTKO/CD46 | Baboon (n=1) | As above, but no CVF | 4 | |
| Baboon (n=4) | As above, but no CVF or CS | 9, 10, 10, 16 | ||
| Griesemer (2010) | GTKO (MSw) | Baboon (n=2) | TBI, TI, splenectomy, ATG, anti-CD2mAb, tacrolimus, GTKO BMTx +/− CVF (n=1) | 8, 11 |
| Simioni (2011) | CD55 | Cynomolgus (n=2) | Extracorporeal kidney perfusion, GAS914, CyP, CsA, MMF, CS | 28, 55 |
| Cynomolgus (n=2) | As above + recombinant human antithrombin III + recombinant human activated protein C | 12, 34 | ||
| Cynomolgus (n=9) | As above + recombinant human activated protein C | 8–37 (20) | ||
| Nishimura (2011) | GTKO (MSw) | Baboon (n=2) | (Thymokidney) Thymectomy, splenectomy, anti-CD3IT +/−anti-CD2mAb, ATG, anti-CD20mAb, tacrolimus, MMF, anti-CD154mAb | 15, 15 |
| Baboon (n=2) | (Thymokidney) As above, but no anti-CD3IT |
14, 14 | ||
| Le Bas Bernadet (2011) | GTKO/CD55/CD59/CD39/H-transferase | Baboon (n=2) | None | 3, 4 |
| Baboon (n=4) | Splenectomy (n=2), CyP, tacrolimus, MMF, CS, C1-INH | 4, 12, 13, 15 | ||
| Shimizu (2012) | GTKO (MSw) | Baboon (n=2) | ATG, anti-CD2mAb, anti-CD154, MMF, CS | 20–33 |
| Baboon (n=1) | As above + thymectomy, splenectomy, TBI | 34 | ||
| Baboon (n=4) | (Vascular thymic lobe or thymokidney) Thymectomy, splenectomy, ATG, anti-CD2mAb, anti-CD154mAb, MMF, CS +/− TBI (n=1) | 56, 68, 81, 83 | ||
| Pintore (2013) | GTKO/CD55/CD59/CD39/H-transferase | Cynomolgus (n=5) | CyP, CsA, MMF, CS | 18±3.2 (16) |
| Cynomolgus (n=3) | Anti-CD20mAb, CsA, MMF, CS | 13±2.3 (12) | ||
| Spiezia (2013) | CD55 (n=4) | Cynomolgus (n=8) | CyP or anti-CD20mAb, CsA, MMF, CS | 19, 30, 34, 55 |
| GTKO/CD55 (n=2) | 12, 13 | |||
| GTKO/CD55/CD59/CD39/H-transferase (n=2) | 8, 8 |
Abbreviations (if not used previously):
Anti-CD3IT = anti-CD3 immunotoxin; BSA-Gal = bovine serum albumin conjugated to Gal oligosaccharides; C1-INH = complement component 1 inhibitor; FTY = sphingosine 1-phosphate receptor agonist; vWD = pigs homozygous for von Willebrand disease
Table 2: References
Ashton-Chess J, Muerette G, Karam G et al. the study of mitoxantrone as a potential immunosuppressor in transgenic pig renal xenotransplanation in baboons: comparison with cyclophosphamide. Xenotransplantation 2004; 11:112–122.
Ashton-Chess J, Rousell JC, Bernard P et al. The effect of immunoglobulin immunadsorptions on delayed xenograft rejection of human CD55 transgenic pig kidneys in baboons. Xenotransplantation 2003; 10:552–561.
Baldan N, Rigotti P, Calabrese F et al. Ureteral stenosis in HDAF pig-to-primate renal xenotransplantation: a phenomenon related to immunological events? Am J Transplant 2004; 4: 475–481.
Barth RN, Yamamoto S, Lamattina JC et al. Xenogeneic thymokidney and thymic tissue transplantation in a pig-to-baboon model: I. Evidence for pig-specific T-cell unresponsiveness. Transplantation 2003; 75: 1615–1624.
Bhatti FNK, Zaidi A, Schmoeckel M, et al. Survival of life-supporting hDAF transgenic kidneys in primates is enhanced by splenectomy. Transplant Proc. 1998;30:2467.
Bühler L, Basker M, Alwayn IP et al. Coagulation and thrombotic disorders associated with pig organ and hematopoietic cell transplantation in nonhuman primates. Transplantation 2000; 70: 1323–1331.
Bühler L, Yamada K, Kitamura H et al. Pig kidney transplantation in baboons: anti-Gal (alpha) 1-3Gal IgM alone is associated with acute humoral xenograft rejection and disseminated intravascular coagulation. Transplantation 2001; 72: 1743–1752.
Cavicchioli L, De Zan G, Zappulli V et al. Histopathological findings in the gastrointestinal tract of primate recipients of porcine renal xenografts following different immunosuppressive regimens. Xenotransplantation 2007; 14: 145–156.
Chen G, Qian H, Starzl T et al. Acute rejection is associated with antibodies to non-Gal antigens in baboons using Gal-knockout pig kidneys. Nat Med 2005; 11: 1295–1298.
Chen G, Sun H, Yang H et al. The role of anti-non-gal antibodies in the development of acute humoral xenograft rejection of hDAF transgenic porcine kidneys in baboons receiving anti-Gal antibody neutralizationtherapy. Transplantation 2006; 81: 273–283.
Cowan PJ, Aminian A, Barlow H et al. Protective effects of recombinant human antithrombin III in pig to primate renal xenotransplanatation. Am J Transplant 2002; 2: 520–525.
Cowan PJ, Aminian A, Barlow H et al. Renal xenografts from triple-transgenic pigs are not hyperacutely rejected but cause coagulopathy in non-immunosuppressed baboons. Transplantation 2000; 69: 2504–2515.
Cozzi E, Bhatti F, Schmoeckel M et al. Long-term survival of non-human primates receiving life-supporting transgenic porcine kidney xenografts. Transplantation 2000; 70: 15–21.
Cozzi E, Cadrobbi R, Baldan N et al. Methotrexate for immunosuppression in life-supporting pig-to-cynomolgus monkey renal xenotransplantation. Xenotransplantation 2003; 10: 587–595.
Cozzi E, Simioni P, Boldrin M, et al. Alterations in the coagulation profile in renal pig-to-monkey xenotransplantation. Am J Transplant 2004; 4:335–345.
Cozzi E, Vial C, Ostlie D et al. Maintenance triple immunosuppression with cyclosporin A, mycophenolate sodium and steroids allows prolonged survival of primate recipients of hDAF porcine renal xenografts. Xenotransplantation 2003; 10: 300–310.
Dean PG, Cohen AJ, Dalla Valle H, et al. The effect of anti-alphaGal antibody removal with immunoasbsorption and splenectomy on CD46 transgenic kidney xenograft survival. Transplant Proc 2001;33:721–722.
Dehoux JP, De La Parra B, Latinne D et al. Characterization of baboon anti-porcine IgG antibodies during acute vascular rejection of porcine kidney xenograft. Xenotransplantation 2002; 9: 338–349.
Dehoux JP, Hori S, Talpe S et al. Specific depletion of preformed IgM natural antibodies by administration of anti-mu monoclonal antibody suppresses hyperacute rejection of pig to baboon renal xenografts. Transplantation 2000; 70: 935–946.
Diaz TM, Moscoso I, Centeno A et al. Flow cytometry complement-mediated cytotoxicity assay detects baboon xenoantibodies directed to porcine epitopes undetected by hemolytic assay. Transpl Immunol 2004; 13: 313–317.
Garcia B, Sun HT, Yang HJ, Chen G, Zhong R. Xenotransplantation of human decay accelerating factor transgenic porcine kidney to non-human primates: 4 years experience at a Canadian center. Transplant Proc 2004; 36: 1714–1716.
Ghanekar A, Lajoie G, Luo Y et al. Improvement in rejection of human decay accelerating factor transgenic pig-to-primate renal xenografts with administration of rabbit antithymocyte serum. Transplantation 2002; 74: 28–35.
Ghanekar A, Mendicino M, Liu H et al. Endothelial induction of fgl2 contributes to thrombosis during acute vascular xenograft rejection. J Immunol 2004; 172: 5693–5701.
Gollackner B, Goh SK, Qawi I et al. Acute vascular rejection of xenografts: roles of natural and elicited xenoreactive antibodies in activation of vascular endothelial cells and induction of procoagulant activity. Transplantation 2004; 77: 1735–1741.
Gollackner B, Knosalla C, Houser S et al. Pig kidney transplantation in baboons treated intravenously with a bovine serum albumin-Galα1-3Gal conjugate. Xenotransplantation 2003; 10: 606–614.
Gollackner B, Mueller NJ, Houser S et al. Porcine cytomegalovirus and coagulopathy in pig-to-primate xenotransplantation. Transplantation 2003; 75: 1841–1847.
Griesemer AD, Hirakata A, Shimizu A et al. Results of Gal-Knockout porcine thymokidney xenografts. Am J Transplant 2009; 9: 2669–2678.
Griesemer AD, Liang F, Hirataka A et al. Occurrence of specific humoral non-responsiveness toswine antigens following administration of GalT-KO bone marrow to baboons. Xenotransplantation 2010; 17: 300–312.
Hecker JM, Lorenz R, Appiah R et al. C1-inhibitor for prophylaxis of xenograft rejection after pig to cynomolgus monkey kidney transplantation. Transplantation 2002; 73: 688–694.
Ierino FL, Kozlowski T, Seigel JB et al. Disseminated intravascular coagulation in association with the delayed rejection of pig-to-baboon renal xenografts. Transplantation 1998; 66: 1439–1450.
Ierino FL, Gojo S, Banerjee PT, et al. Transfer of swine major histocompatibility complex class II genes into autologous bone marrow cells of baboons for the induction of tolerance across xenogeneic barriers. Transplantation 1999;67:1119–1128.
Key T, Schuurman HJ, Taylor CJ. Does exposure to swine leukocyte antigens after pig-to-nonhuman primate xenotransplantation provoke antibodies that cross-react with human leukocyte antigens? Xenotransplantation 2004; 11: 452–456.
Knosalla C, Gollackner B, Bühler L et al. Correlation of biochemical and hematological changes with graft failure following pig heart and kidney transplantation in baboons. Am J Transplant 2003; 3: 1510–1519.
Knosalla C, Yazawa K, Behdad A et al. Renal and cardiac endothelial heterogeneity impact acute vascular rejection in pig-to-baboon xenotransplantation. Am J Transplant 2009; 9:1006–1016.
Kobayashi T, Yokoyama I, Morozumi K et al. Comparative study of the efficacy of removal of anti-ABO and anti-gal antibodies by double filtration plasmapheresis. Xenotransplantation 2000; 7: 101–108.
Kozlowski T, Shimizu A, Lambrigts D et al. Porcine kidney and heart transplantation in baboons undergoing a tolerance induction regimen and antibody adsorption. Transplantation 1999; 67: 18–30.
Lam TT, Hausen B, Hook L et al. The effect of soluble complement receptor type 1 on acute humoral xenograft rejection in hDAF-transgenic pig-to-primate life-supporting kidney xenografts. Xenotransplantation 2005; 12: 20–29.
Lam TT, Hauser B, Boeke-Purkis K et al. Hyperacute rejection of hDAF-transgenic pig organ xenografts in cynomolgus monkeys: influence of pre-existing anti-pig antibodies and prevention by the alpha-Gal glycoconjugate GAS914. Xenotransplantation 2004; 11: 517–524.
Le Bas-Bernardet S, Tillou X, Poirier N et al. Xenotransplantation of galactosyl transferase knockout, CD55, CD59, CD39, and fucosyl-transferase transgenic pig kidneys into baboons. Transplant Proc 2011; 43: 3426–3430.
Lin CC, Ezzelarab E, Shapiro R et al. Recipient tissue factor expression is associated with consumptive coagulopathy in pig-to-primate kidney xenotransplantation. Am J Transplant 2010; 7: 1556–1568.
Loss M, Arends H, Winkler M et al. Analysis of potential porcine endogenous retrovirus (PERV) transmission in a whole-organ xenotransplantation model without interfering microchimerism. Transpl Int 2001; 14: 31–37.
Loss M, Kunz R, Przemeck M et al. Influence of cold ischemia time, pretransplant anti-porcine antibodies, and donor/recipient size matching on hyperacute graft rejection after discordant porcine to cynomolgus kidney transplantation. Transplantation 2000; 69: 1155–1159.
Loss M, Schmidtko J, Przemeck M et al. A primate model for discordant pig to primate kidney xenotransplantation without hyperacute graft rejection. J Invest Surg 2001; 14: 21–29.
Loss M, Vangerow B, Schmidtko J et al. Acute vascular rejection is associated with systemic complement activation in a pig-to-primate kidney xenograft model. Xenotransplantation 2000; 7: 186–196.
Loveland BE, Milland J, Kyriakou P et al. Characterization of a CD46 transgenic pig and protection of transgenic kidneys against hyperacute rejection in non-immunosuppressed baboons. Xenotransplantation 2004; 11: 171–83.
Mcinnes EF, Jarrett RF, Langford G et al. Posttransplant lymphoproliferative disorder associated with primate gamma-herpesvirus in cynomolgus monkeys used in pig-to-primate renal xenotransplantation and primate renal allotransplantation. Transplantation 2002; 73: 44–52.
Ménoret S, Plat M, Blancho G et al. Characterization of human CD55 and CD59 transgenic pigs and kidney xenotransplantation in the pig-to-baboon combination. Transplantation 2004; 77: 1468–1471.
Meyer C, Wolf P, Romain N et al. Use of von Willebrand diseased kidney as donor in a pig-to-primate model of xenotransplantation. Transplantation 1999; 67: 38–45.
Moscoso I, Hermida-Prieto M, Mañez R et al. Lack of cross-species transmission of porcine endogenous retrovirus in pig-to-baboon xenotransplantation with sustained depletion of anti-alphagal antibodies. Transplantation 2005; 79: 777–782.
Nishimura H, Scalea J, Wang Z et al. First experience with the use of recombinant CD3 immunotoxin as induction therapy in pig to primate xenotransplantation: the effect of T-cell depletion on outcome. Transplanation 2011; 92: 641–647.
Pintore L, Paltrinieri S, Vadori M, et al. Clinicopathological findings in non-human primate recipients of porcine renal xenografts: quantitative and qualitative evaluation of proteinuria. Xenotransplantation 2013; 20:449–457.
Przemeck M, Vangerow B, Loss M et al. Hemodynamic consequences of porcine kidney xenograft reperfusion in cynomolgus monkeys. Transplantation 2001; 71: 1512–1514.
Richards AC, Davies HF, Mclaughlin Ml et al. Serum anti-pig antibodies as potential indicators of acute humoral xenograft rejection in pig-to-cynomolgus monkey kidney transplantation. Transplantation 2002; 73: 881–889.
Schmoeckel M, Bhatti FN, Zaidi A, et al. Splenectomy improves survival of hDAF transgenic pig kidneys in primates. Transplant Proc. 1999;31:961.
Schuurman HJ, Pino-Chavez G, Phillips MJ et al. Incidence of hyperacute rejection in pig-to-primate transplantation using organs from hDAF-transgenic donors. Transplantation 2002; 73: 1146–1151.
Shimizu A, Meehan SM, Kozlowski T et al. Acute humoral xenograft rejection: destruction of the microvascular capillary endothelium in pig-to-nonhuman primate renal grafts. Lab Invest 2000; 80: 815–830.
Shimizu A, Yamada K, Robson SC, Sachs DH, Colvin RB. Pathologic characteristics of transplanted kidney xenografts. J Am Soc Nephrol 2012; 23: 225–235.
Shimizu A, Yamada K, Yamamoto S et al. Thrombotic microangiopathic glomerolopathy in human decay accelerating factor-transgenic swine-to-baboon kidney xenografts. J Am Soc Nephrol 2005; 16: 2745–3732.
Simioni P, Boldrin M, Gavasso S et al. Effects of long-term administration of recombinant human protein C in xenografted primates. Transplantation 2011; 91: 161–168.
Spiezia L, Boldrin M, Radu C, et al. Thromboelastographic evaluation of coagulative profiles in pig-to-monkey kidney xenotransplantation. Xenotransplantation 2013; 20:89–99.
Sun H, Chen G, Liu W et al. The influence of baseline expression of human decay accelerating factor transgene on graft survival and acute humoral xenograft rejection. Transplantation 2005; 80: 1331–1339.
Vangerow B, Hecker JM, Lorenz R et al. C1-inhibitor for treatment of acute vascular xenograft rejection in cynomolgus recipients of hDAF transgenic porcine kidneys. Xenotransplantation 2001; 8: 266–272.
Xu Y, Lorf T, Sablinski T, et al. Removal of anti-porcine natural antibodies from human and nonhuman primate plasma in vitro and in vivo by a Galalpha1-3Galbeta1-4betaGlc-X immunoaffinity column. Transplantation 1998;65:172–179.
Yamada K, Yazawa K, Shimizu A et al. Marked prolongation of porcine renal xenograft survival in baboon through the use of alpha 1,3-galactosyltransferase gene-knockout donors and the cotransplantation of thymic tissue. Nat Med 2005; 11: 32–34.
Yazaki S, Iwamoto M, Onishi A et al. Successful cross-breeding of cloned pigs expressing endo-beta-galactosidase C and human decay accelerating factor. Xenotransplantation 2009; 16: 511–521.
Zaidi A, Schmoeckel M, Bhatti F et al. Life-supporting pig-to-primate renal xenotransplantation using genetically modified donors. Transplantation 1998; 65: 1584–1590.
Zhong R, Luo Y, Yang H et al. Improvement in human decay accelerating factor transgenic porcine kidney xenograft rejection with intravenous administration of gas914, a polymeric form of alphaGAL. Transplantation 2003; 75: 10–19.
Progress in the pig kidney-to-NHP model has been slower than in the pig heart-to-NHP model, though this conclusion may be misleading since the kidney is transplanted as a life-supporting organ whereas in the majority of cases the heart is transplanted as a heterotopic, non-life-supporting organ. However, the complications of consumptive coagulopathy appear to develop more rapidly when the kidney is transplanted (25). For reasons not fully understood, this model may therefore be a more difficult one than when the heart is transplanted. The longest life-supporting kidney graft survival in 1998 was reported to be 23 days (3), but this has been extended to 90 days (26).
With regard to co-transplantation of pig kidney and thymic tissue, which has resulted in a maximum kidney graft survival of 83 days (27), there have been studies of pig thymic grafts in NHPs in the absence of kidney transplants (28–31). Six baboons underwent a regimen aimed towards inducing tolerance, three of which received fetal or neonatal pig thymic tissue transplants (31). There was some in vitro evidence that the thymic tissue induced xenogeneic hyporesponsiveness.
Liver xenotransplantation (Table 3)
Table 3.
Transplantation of pig livers into NHPs (1998–2013)
| FIRST AUTHOR (Year) | DONOR (pig) | RECIPIENT (n) | IMMUNOSUPRESSIVE THERAPY | SURVIVAL – RANGE (MEDIAN) (Hours) |
|---|---|---|---|---|
| Luo (1998) | WT | Baboons (n=2) Rhesus (n=6) |
CyP, CsA, CS None (n=3) Cyp, CsA, CS, Dashen (traditional Chinese medicine) (n=3) |
<2 <6 |
| Ramirez (2000) | WT CD55 |
Baboon (n=3) Baboon (n=2) |
CyP, CsA, CS | <12 96,192 |
| Ramirez (2005) | WT CD46/CD59/FT |
Baboon (n=4) Baboon (n=5) |
Anti-CD25mAb, CyP, anti-CD20mAb, CsA, MMF, CS | <16 13–24 (20) |
| Ekser (2010) | WT GTKO GTKO/CD46 |
Baboon (n=1) Baboon (n=2) Baboon (n=8) |
ATG, CyP, CVF, tacrolimus, MMF, CS | <24 <24,144 <24–168 (144) |
| Kim (2012) | GTKO | Baboon (n=3) | ATG, LoCd2b, CVF, anti-CD154mAb, azathioprine, tacrolimus, CS | 72–216 |
Abbreviations as used previously
Table 3: References
Ekser B, Long C, Echeverri GJ et al. Impact of thrombocytopenia on survival of baboons with genetically modified pig liver transplants: clinical relevance. Am J Transplant 2010; 10:273–285.
Kim K, Scheutz C, Elias N, et al. Up to 9-day survival and control of thrombocytopenia following alpha1,3-galactosyl transferase knockout swine liver xenotransplantation in baboons. Xenotransplantation 2012; 19:256–264.
Ramirez P, Chavez R, Majado M et al. Life-supportinghuman complement regulator decay accelerating factor transgenic pig liver xenograft maintains the metabolic function and coagulation in the nonhuman primate for upto 8 days. Transplantation 2000; 70:989–998.
Ramírez P, Montoya MJ, Ríos A, García Palenciano C, Majado M, Chávez R, et al. Prevention of hyperacute rejection in a model of orthotopic liver xenotransplantation from pig to baboon using polytransgenic pig livers (CD55, CD59, and H-transferase). Transplant Proc 2005; 37:4103–6.
Special problems relating to pig liver transplantation have proved a major barrier to progress, largely because the genetic manipulations of the organ-source pigs have to date largely been directed towards extending survival of heart and kidney grafts. The rapid development of thrombocytopenia in the recipient NHP following pig liver transplantation remains unresolved. Nevertheless, graft survival has been extended from <3 days in 1998 (3) to 10 days today (32).
Lung xenotransplantation (Table 4)
Table 4.
Transplantation of pig lungs into NHPs (1998–2013)
| FIRST AUTHOR (Year) | DONOR (pig) | RECIPIENT (n) | IMMUNOSUPRESSIVE THERAPY | SURVIVAL (Hours) |
|---|---|---|---|---|
| Daggett (1998) | WT | Baboon (n=7) | Pre-Tx perfusion with pig lungs (n=4), CsA, azathioprine, CS (n=7) | <11 |
| Yeatman (1998) | WT or CD55/CD59 | Baboon (n=10) | CsA, azathioprine, CS +/− CVF | <3 |
| Lau (2000) | CD55/CD59 | Baboon (n=9) | Immunodepletion (3 different methods), CsA, CS, CyP or azathioprine | <24 (details not stated) |
| Gaca (2002) | CD46 | Baboon (n=7) | Immunodepletion, dexamethasone, CsA, CyP, indomethacin, azathioprine +/− anti-human GPIb mAb | <9 |
| Gonzalez-Stawinski (2002) | CD46 | Baboon (n=8; control 4, experimental 4) | Anti-Gal antibody depletion, CsA, CS, CyP or azathioprine, gamma globulin, anti-CD20 mAb, splenectomy | <16 |
| Lau (2003) | vWF-deficient | Baboon (n=5) | CsA, CS, azathioprine | <5 |
| Gaca (2006) | CD46 | Baboon (n=5) | Immunodepletion +/− anti-human C5a mAb (n=3) | <12 |
| Cantu (2007) | WT/macrophage and vWF-deficient | Baboon (n=15) | Immunodepletion | 19–109 |
| Nguyen (2007) | CD46 or GTKO | Baboon (n=6) | CS (details not stated) | <4 |
| Bush (2011) | GTKO/CD55, PIM-depleted (Clodronate liposomes to donor pig) | Baboon (n=2) | CsA, azathioprine, CS | 3–48 |
Abbreviations (if not used previously)
PIM = pulmonary intravascular macrophages
Table 4: References
Bush EL, Barbas AS, Holzknecht ZE, et al. Coagulopathy in α-galactosyl transferase knockout pulmonary xenotransplants. Xenotransplantation. 2011;18:6–13.
Cantu E, Balsara KR, Li B, Lau C, et al. Prolonged function of macrophage, von Willebrand factor-deficient porcine pulmonary xenografts. Am J Transplant 2007;7:66–75.
Daggett CW, Yeatman M, Lodge AJ, et al. Total respiratory support from swine lungs in primate recipients. J Thorac Cardiovasc Surg 1998;115:19–27.
Gaca JG, Lesher A, Aksoy O, Ruggeri ZM, Parker W, Davis RD. The role of the porcine von Willebrand factor: baboon platelet interactions in pulmonary xenotransplantation. Transplantation. 2002;74:1596–1603.
Gaca JG, Appel JZ 3rd, Lukes JG, et al. Effect of an anti-C5a monoclonal antibody indicates a prominent role for anaphylatoxin in pulmonary xenograft dysfunction. Transplantation. 2006;81:1686–1694.
Gonzalez-Stawinski GV, Daggett CW, Lau CL, et al. Non-anti-Gal alpha 1-3 Gal antibody mechanisms are sufficient to cause hyperacute lung dysfunction in pulmonary xenotransplantation. J Am Coll Surg 2002;194:765–773.
Lau CL, Daggett WC, Yeatman MF, et al. The role of antibodies in dysfunction of pig-to-baboon pulmonary transplants. J Thorac Cardiovasc Surg 2000;120:29–38.
Lau CL, Cantu E 3rd, Gonzalez-Stawinski GV, et al. The role of antibodies and von Willebrand factor in discordant pulmonary xenotransplantation. Am J Transplant. 2003;3:1065–1075.
Nguyen BN, Azimzadeh AM, Zhang T, et al. Life-supporting function of genetically modified swine lungs in baboons. J Thorac Cardiovasc Surg. 2007;133:1354–1363.
Yeatman M, Daggett CW, Parker WW, et al. Complement-mediated pulmonary injury: studies in swine-to-primate orthotopic single lung transplant models. Transplantation 1998;65:1084–1093.
The lung continues to provide major barriers, and considerable attention is currently being directed towards overcoming them. Most studies have been of ex vivo pig lung perfusion with human blood, which are not reviewed here. Despite major efforts, to date, pig lung graft survival after transplantation into NHPs has been extended only from 9 hours in 1998 (3) to 5 days today.
Pancreatic islet xenotransplantation (Table 5)
Table 5.
Transplantation of pig pancreatic islets in NHPs (1998–2013)
| FIRST AUTHOR (Year) | DONOR (Pig) | RECIPIENT (n) | IMMUNOSUPRESSIVE THERAPY | SURVIVAL – RANGE (MEDIAN) (Days) |
|---|---|---|---|---|
| Söderlund (1999) | WT fetal islet-like cell clusters | Cynomolgus (n=8) Cynomolgus (n=6) |
None CsA, 15-deoxyspergualin |
<6 <12 |
| Rijkelijkhuizen (2000a) | WT adult | Cynomolgus (n=4) | CyP, CsA, CS | 4–11 |
| Rijkelijkhuizen (2000b) | WT adult | Cynomolgus (n=4) | CyP, CsA, CS | 4–11 |
| Jonker (2001) | WT adult | Rhesus (n=4) | ATG, CsA, CS, anti-IL2RmAb | 21–53 |
| Buhler (2002) | WT adult WT adult islets + PBPC infusion |
Baboon (n=3) Baboon (n=2) |
Splenectomy, ATG, CsA, azathioprine Splenectomy, multiple immunoadsorptions, TBI, TI, ATG, CVF, anti-CD154mAb, CsA, MMF, CS |
<2 12, 28 |
| Cantarovich (2002) | WT adult | Baboon (n=4) Cynomolgus (n=1) |
ATG, CsA, MMF, CS ATG, deoxyspergualin, MMF, CS |
<2 <2 |
| Rijkelijkhuizen (2003) | WT adult | Cynomolgus (n=4) Rhesus (n=4) |
CyP, CsA, CS ATG, CsA, CS, anti-IL2RmAb |
4–11 21–53 |
| Kirchhof (2004) | WT adult | Rhesus (n=6) | None | <1–>3 |
| Elliott (2005a) | WT neonatal | Cynomolgus (n=2) | Encapsulation, nicotinamide | 56 |
| Isaac (2005) | WT neonatal + Sertoli cells | Cynomolgus (n=7) | None | <56 |
| Elliott (2005b) | WT neonatal | Cynomolgus (n=8) | Encapsulation, nicotinamide | >252 |
| Komoda (2005) | WT adult GnT-III adult |
Cynomolgus (n=3) Cynomolgus (n=4) |
None None |
1–3 1–5 |
| Hering (2006) | WT adult | Cynomolgus (n=3) Cynomolgus (n=4) Cynomolgus (n= 5) |
Anti-CD25mAb, FTY720, rapamycin Anti-CD25mAb, FTY720, rapamycin, anti-CD154mAb Anti-CD25mAb, FTY720/tacrolimus, rapamycin, anti-CD154mAb, leflunomide |
24–45 47–187 68–>158 |
| Cardona (2006) | WT neonatal | Rhesus (n=9) | Anti-CD25mAb, anti-CD154mAb, CTLA4Ig (belatacept), rapamycin | 4–>260 (140) |
| Dufrane (2006) | WT adult | Cynomolgus (n=2) Cynomolgus (n=12) |
None Encapsulation |
<7 <7–60 (30) |
| Gianello (2007) | WT adult | Cynomolgus (n=4) Cynomolgus (n=4) |
Encapsulation, (renal subcapsular) Encapsulation (subcutaneous with islet mono-layer device) |
Data not available |
| Rood (2007) | WT and GTKO adult | Cynomolgus (n=2) Cynomolgus (n=4) Cynomolgus (n=4) |
ATG, anti-CD20mAb, tacrolimus, rapamycin ATG, CVF, anti-CD154mAb, MMF or rapamycin + tacrolimus ATG, anti-CD154mAb, MMF |
<5 >58 (partial function) 5–7 |
| Cardona (2007) | WT adult | Rhesus (n=5) | Anti-CD25mAb, anti-CD154mAb, CTLA4-Ig (belatacept), rapamycin | 3–76 |
| Rogers (2007) | WT embryonic pancreatic primordia | Rhesus (n=3) | No IS, multiple transplants | 78–409 |
| Casu (2008) | WT adult | Cynomolgus (n=9) | ATG, anti-CD154mAb, MMF | Partial function >60 |
| Garkavenko (2008) | WT neonatal | Cynomolgus (n=12) | No IS | >180 |
| van der Windt (2009) | CD46 adult | Cynomolgus (n=9) | ATG, anti-CD154mAb, MMF | 5–396 (46) |
| Hecht (2009) | Fetal pancreatic fragments | Cynomolgus (n=2) | ATG, anti-CD25mAb, anti-CD20mAb, FTY720, rapamycin, CTLA4-Ig | 280, 380 |
| Igarashi (2010) | WT adult | Cynomolgus (n=4) Cynomolgus (n=5) |
Microencapsulation Macrodevice |
>42 >180 |
| Dufrane (2010) | WT adult | Cynomolgus (n=4) Cynomolgus (n=5) |
Microencapsulation Macrodevice |
14 136–180 |
| Rogers (2011) | WT embryonic pancreatic primordia WT adult |
Rhesus (n=3) Rhesus (n=3) |
None None |
56 (Experiments were electively terminated) |
| Thompson (2011a) | WT neonatal | Rhesus (n=9) | Anti–CD25mAb, anti-CD40mAb, rapamycin, CTLA4-Ig (belatacept) | 47–203 (80) |
| Thompson (2011b) | GTKO neonatal (n=5) WT neonatal (n=5) |
Rhesus | Anti-CD154mAb, anti-LFA1mAb, MMF, CTLA4-Ig (belatacept) | 50–249 (137) |
| Thompson (2012) | WT neonatal | Rhesus (n=3) Rhesus (n=5) Rhesus (n=5) |
MMF, CTLA4-Ig (belatacept), anti-LFA-1mAb, basiliximab MMF, CTLA4-Ig (belatacept), anti-LFA-1mAb, basiliximab, tacrolimus MMF, CTLA4-Ig (belatacept), anti-LFA-1mAb, alefacept, tacrolimus |
<50 (none engrafted) 46–99 92–114 |
| Kim (2013) | WT adult Msw | Rhesus (n=3) | None | <5 |
| Lee (2013) | WT adult | Rhesus (n=2) | Not stated | >120 |
| Vériter (2013) | WT adult | Cynomolgus (n=4) Cynomolgus (n=6) |
Coencapsulation with bone marrow-drived stem cells Coencapsulation with adipose-derived stem cells |
1–217 14–224 |
| Graham (2013) | WT adult | Cynomolgus (n=not stated) Cynomolgus (n=1) |
Various (review of previous data from Hering [2006] and additional data) Anti-CD25mAb, CTLA4-Ig (abatacept), tacrolimus, rapamycin |
Various >180 |
Abbreviations (if not used previously):
GnT-III= N-acetylglucosaminyltransferase III
Table 5: References
Buhler L, Deng S, O’Neil J et al. Adult porcine islet transplantation in baboons treated with conventional immunosuppression or a non-myeloablative regimen and CD154 blockade. Xenotransplantation 2002; 9:3–13.
Cantarovich D, Blancho G, Potiron N et al. Rapid failure of pig islet transplantation in non human primates. Xenotransplantation. 2002; 9:25–35.
Cardona K, Korbutt GS, Milas Z, et al. Long-term survival of neonatal porcine islets in nonhuman primates by targeting costimulation pathways. Nat Med. 2006; 12:304–306
Cardona K, Milas Z, Strobert E, et al. Engraftment of adult porcine isletxenografts in diabetic nonhuman primates through targeting of costimulation pathways. Am J Transplant. 2007; 7:2260–2268
Casu A, Bottino R, Balamurugan AN, et al. Metabolic aspects of pig-to-monkey islet transplantation: implications for translation into clinical practice. Diabetologia 2008; 51:120–129.
Dufrane D, Goebbels RM, Saliez A et al. Six-month survival of microencapsulated pig islets and alginate biocompatibility in primates: proof of concept. Transplantation. 2006; 81:1345–1353.
Dufrane D, Goebbels RM, Gianello P. Alginate macroencapsulation of pig islets allows correction of streptozotocin-induced diabetes in primates up to 6 months without immunosuppression. Transplantation. 2010; 90:1054–1062.
Elliott RB, Escobar L, Calafiore R et al. Transplantation of micro- and macroencapsulated piglet islets into mice and monkeys. Transplant Proc. 2005; 37:466–469. (2005a)
Elliott RB, Escobar L, Tan PL, et al. Intraperitoneal alginate –encapsulated neonatal porcine islets in a placebo-controlled study with 16 diabetic cynomolgus primates. Transplant Proc 2005; 37:3505–3508. (2005b)
Garkavenko O, Dieckhoff B, Wynyard S, et al. Absence of transmission of potentially xenotic viruses in a prospective pig to primate islet xenotransplantation study. J Med Virol. 2008; 80:2046–2052.
Gianello P, Dufrane D. Correction of diabetes mellitus type 1 on primate with encapsulated islet of pig pancreatic transplant. Bull Mem Acad R Med Belg 2007; 162:439–449.
Graham ML, Schuurman H-J. The usefulness and limitations of the diabetic macaque model in evaluating long-term porcine islet xenograft survival. Xenotransplantation 2013;20:5–17.
Hecht G, Eventov-Friedman S, Rosen C, et al. Embryonic pig pancreatictissue for the treatment of diabetes in a nonhuman primate model. Proc Natl Acad Sci USA. 2009; 106:8659–8664.
Hering BJ, Wijkstrom M, Graham ML, et al. Prolonged diabetes reversalafter intraportal xenotransplantation of wild-type porcine islets in immunosuppressed nonhuman primates. Nat Med 2006; 12:301–303
Isaac JR, Skinner S, Elliot R et al. Transplantation of neonatal porcine islets and sertoli cells into nonimmunosuppressed nonhuman primates. Transplant Proc. 2005; 37:487–8.
Igarashi Y, D’hoore W, Goebbels RM. Beta-5 score to evaluate pig islet graft function in a primate pre-clinical model. Xenotransplantation. 2010; 17:449–459.
Jonker M, Rijkelijkhuizen JK, Haanstra KG et al. T-cell-directed immunosuppression allows prolonged survival of xenogeneic pig islets in monkeys. Transplant Proc. 2001; 33:726.
Kim YH, Kim JS, Yoon IH, et al. Application of the multiplex cytokine analysis to monitor xenogeneic immune responses to the porcine islet graft in non-human primate. J Korean Med Sci 2013;28:1729–1733.
Kirchhof N, Shibata S, Wijkstrom M, et al. Reversal of diabetes in non-immunosuppressed rhesus macaques by intraportal porcine islet xenografts precedes acute cellular rejection. Xenotransplantation 2004; 11:396–407.
Komoda H, Miyagawa S, Omori T, et al. survival of adult islet grafts from transgenic pigs with N-acetylglucosaminyltransferase (GnT-III) in cynomolgus monkeys. Xenotransplantation 2005; 12:209–216.
Lee JI, Shin JS, Jung WY, et al. Porcine islet adaptation to metabolic need of monkeys in pig-to-monkey intraportal islet xenotransplantation. Transplant Proc 2013;45:1866–1868.
Rijkelijkhuizen JK, Ossevoort M, Ringers J et al. Xenografting of pig islets in monkeys does not result in hyperacute rejection. Transplant Proc. 2000a; 32:1064.
Rijkelijkhuizen JK, Bouwman E, van der Burg MP et al. Successful suppression of the early rejection of pig islets in monkeys. Cell Transplant. 2000b; 9:909–912.
Rijkelijkhuizen JK, Haanstra KG, Wubben J et al. T-cell-specific immunosuppression results in more than 53 days survival of porcine islets of langerhans in the monkey. Transplantation. 2003; 76:1359–1368.
Rogers SA, Chen F, Talcott MR et. al. Long-term engraftment following transplantation of pig pancreatic primordia into non-immunosuppressed diabetic rhesus macaques. Xenotransplantation. 2007; 14:591–602.
Rogers SA, Tripathi P, Mohanakumar T, et al. Engraftment of cells from porcine islets of Langerhans following transplantation of pig pancreatic primordia in non-immunosuppressed diabetic rhesus macaques. Organogenesis. 2011; 7:154–162.
Rood PP, Bottino R, Balamurugan AN, et al. Reduction of early graft loss afterintraportal porcine islet transplant in monkeys. Transplantation 2007; 83:202–210.
Söderlund J, Wennberg L, Castaños-Velez E et al. Fetal porcine islet-like cell clusters transplanted to cynomolgus monkeys: an immunohistochemical study. Transplantation. 1999; 67:784–791.
Thompson P, Cardona K, Russell M, et al. CD40-specific costimulation blockade enhances neonatal porcine islet survival in nonhuman primates. Am J Transplant. 2011; 11:947–957. (2011a)
Thompson P, Badell IR, Lowe M, et al. Islet xenotransplantation using Gal-deficient neonatal donors improves engraftment and function. Am J Transplant 2011; 11:2593–2602. (2011b)
Thompson P, Badella IR, Lowea M, et al. Alternative immunomodulatory startegies for xenotransplantation: CD40-CD154Pathway sparing regimens promote xenograft survival. Am J Transpl 2012; 12:1765–1775.
van der Windt DJ, Bottino R, Casu A, et al. Long-term controlled normoglycemia in diabetic non-human primates after transplantation with hCD46transgenic porcine islets. Am J Transplant 2009; 9:2716–2726.
Vériter S, Gianello P, Igarashi Y, et al. Improvement of subcutaneous bioartificial pancreas vascularization and function by co-encapsulation of pig islets and mesenchymal stem cells in primates. Cell Transplant 2013; [Epub ahead of print]
More progress has been made in the transplantation of pig cells than pig organs. Although the instant blood-mediated inflammatory reaction remains a major barrier after islet transplantation into the portal vein, pig islet transplantation in NHPs has been successful for >1 year.
These reports and those for neuronal cell transplantation (see below) suggest that, with the possible exception of corneal xenotransplantation (see below), these cellular transplants are likely to be the first introduced into the clinic. Indeed, clinical trials of encapsulated wild-type pig islets have already been undertaken (33–35) but detailed reports have been scarce.
Neuronal cell xenotransplantation
A field in which there were no reports in NHP models in 1998 is pig neuronal cell transplantation. Research has been largely limited to one European consortium, but graft function in monkeys with a Parkinson-like disorder has been documented for periods >1 year (36, 37).
Hepatocyte xenotransplantation
Although the transplantation of pig hepatocytes may have some advantages over liver xenotransplantation, few studies have been undertaken in NHP models to date. However, hepatocyte function has been documented for >80 days after transplantation and for 253 days when a second transplant was performed (38).
Corneal xenotransplantation (Table 6)
Table 6.
Transplantation of pig corneas into NHPs (1998–2013)
| FIRST AUTHOR (Year) | DONOR (Pig) | RECIPIENT (n) | IMMUNOSUPRESSIVE THERAPY | SURVIVAL - RANGE (MEDIAN) (Days) |
|---|---|---|---|---|
| Amano (2003) | WT | Cynomolgus (n=6) | None (Stromal disk into stromal pocket) | 60–180 (165) |
| Pan (2007) | WT | Rhesus (n=6) Rhesus (n=4) Rhesus (n=4) |
None (PKP) Local betamethasone (PKP) None (ALK) |
12–18 (15) 129–276 (183) >90 |
| Li (2011) | WT | Rhesus (n=5) Rhesus (n=5) Rhesus (n=5) |
None (ALK, dehydrated) None (ALK, fresh) Local triamcinolone (ALK, dehydrated) |
>180 >180 >180 |
| Choi (2011) | WT | Rhesus (n=5) Rhesus (n=4) |
Local prednisone + dexamethasone, systemic CS (ALK, decellularized) Local prednisone + dexamethasone, systemic CS (ALK, fresh) |
195–>391 194–>398 |
| Jie (2013) | WT | Rhesus (n=6) Rhesus (n=6) |
CYP +bone marrow Tx (PKP) CYP (PKP) |
30–42 (36) 12–20 (19) |
| Choi (2013) | PKP allograft, following previous decellularized WT pig ALK | Rhesus (n=5) | Local prednisone + dexamethasone, systemic CS | 35, 49, >324, >379, >421. Previous xeno-sensitization did not influence outcome |
Abbreviations (if not used previously):
ALK = anterior lamellar keratoplasty
PKP = penetrating keratoplasty
Table 6: References
Amano S, Shimomura N, Kaji Y, et al. Antigenicity of porcine cornea as xenograft. Curr Eye Res. 2003; 26:313–318.
Choi HJ, Kim MK, Lee HJ et al. Efficacy of pig to rhesus lamellar corneal xenotransplantation. IOVS 2011; 52:6643–6650.
Choi HJ, Lee JJ, Kim MK, Lee HJ, Ko AY, Kang HJ, Park CG, Wee WR. Cross-reactivity between decellulrized porcine corneal lamellae for corneal xenobridging and subsequent corneal allotransplantation. Xenotransplantation 2013 Dec 11. Doi:10.1111/xen.12075. (Epub ahead of print]
Jie Y, Liu L, Pan Z, Wang L. Survival of pig-to-rhesus corneal xenografts prolonged by prior donor bone marrow transplantation. Mol Med Rep. 2013; 7:869–874.
Li A, Pan Z, Jie Y et al. Comparison of immunogenicity and porcine to rhesus lamellar corneal xenografts survival between fresh preserved and dehydrated porcine corneas. Xenotransplantation 2011; 18:46–55.
Pan Z, Sun C, Jie Y, et al. WZS-pig is a potential donor alternative in corneal xenotransplantation. Xenotransplantation 2007; 14:603–611.
To our knowledge, no studies of pig corneal transplantation in NHPs had been reported before 1998, whereas a number of studies have been published since then, with encouraging results. Anterior lamellar keratoplasty using decellularized corneas from wild-type pigs has resulted in graft transparency for >1 year.
Artery patch xenotransplantation (Table 7)
Table 7.
Transplantation of pig artery patches into NHPs (1998–2013)
| FIRST AUTHOR (Year) | DONOR (Pig) | RECIPIENT (n) | IMMUNOSUPRESSIVE THERAPY | SURVIVAL - RANGE (MEDIAN) (Days) |
|---|---|---|---|---|
| Ezzelarab (2012) | GTKO | Baboon (n=3) Baboon (n=5) Baboon (n=1) Baboon (n=1) Baboon (n=4) |
No IS Anti-CD154mAb Anti-CD154 mAb, ATG, MMF CTLA4-Ig ATG, MMF, CTLA4-Ig |
14–>28 2–>28 >28 >28 14–>28 |
Abbreviations as used previously
Table 7: References
Ezzelarab MB, Ekser B, Echeverri G, et al. Costimulation blockade in pig artery patch xenotransplantation - a simple model to monitor the adaptive immune response in nonhuman primates. Xenotransplantation. 2012; 4:221–32.
Because of the development of thrombotic microangiopathy and/or consumptive coagulopathy, the assessment of immunosuppressive regimens or immunomodulatory approaches directed to the T cell response have been difficult to assess following pig-to-NHP organ transplantation. At our own center, Ezzelarab et al. have introduced a simple pig artery patch model which allows the adaptive immune response and, in particular, the production of T cell-dependent elicited anti-pig antibodies to be followed in the absence of the complicating factors of coagulation dysfunction (39). No such model had been reported in 1998, but today artery patch xenotransplantation is proving of value in assessing various immunosuppressive regimens.
Skin xenotransplantation
In very complex experiments involving transduction of baboon bone marrow cells with SLA class II genes, followed by bone marrow transplantation and either pig kidney or skin transplantation, Ierino et al reported pig skin graft survival for 17 (control) or 21 days (40). Wiener et al reported prolonged GTKO pig skin graft survival in baboons (41). The GTKO skin grafts survived for up to 14 days whereas wild-type pig skin grafts were rejected by day 4.
Discussion
In summary, we were unable to identify any reports on experimental studies of pig thymic tissue, neuronal cell, hepatocyte, corneal, skin, or artery patch transplantation in NHPs before 1998. Pig heart, kidney, and, particularly, islet graft survival have increased significantly since 1998. This has been associated largely with the increasing availability of pigs with genetic manipulations aimed at protecting the pig tissues from the primate immune response. Both GTKO and the introduction of human complement- and coagulation-regulatory transgenes have played a role, particularly with regard to heart transplantation. Although encouraging results have been achieved after the transplantation of wild-type pig islets into NHPs, the transplantation of islets from genetically-engineered pigs may allow a reduction in the intensity of the immunosuppressive therapy required to prevent graft loss.
Many successful immunosuppressive regimens in the pig-to-NHP model have been based on costimulation blockade with an anti-CD154 monoclonal antibody (mAb), which is unlikely to be available for clinical use because of its thrombogenic effects. Attention is now being directed towards replacing this agent with others, e.g., an anti-CD40 mAb (+/− an agent that blocks the CD28/B7 costimulation pathway). This problem will have to be resolved if approval for clinical trials is to be obtained by most national regulatory administrations, e.g., the Food and Drug Administration (FDA) in the USA. Recent studies by Mohiuddin and his colleagues (21–24) suggest that an anti-CD40 mAb-based regimen is likely to be successful, but this agent is not yet approved for clinical use. Blockade of the CD28/B7 pathway alone would appear to be inadequate (Iwase H et al, submitted).
A second topic that needs to be addressed before clinical trials are likely to be fully successful is that of expression of NeuGc in pigs (and in all NHPs), but not in humans (18). However, we would suggest that a successful GTKO pig transplant in a NHP provides an indicator of the likelihood of success of a GTKO/NeuGc-KO pig transplant in a human. We therefore continue to believe that the pig-to-NHP model provides very important data that cannot be obtained from in vitro, ex vivo perfusion, or other in vivo models. This particularly applies to the efficacy and safety testing of immunosuppressive protocols.
There are, of course, other differences in the biological (e.g., physiologic, immunologic) responses of NHPs and humans to transplanted pig organs, tissues, or cells, and resolutions to some of these may not be possible until clinical trials are undertaken. Nevertheless, we believe it is important to accumulate as many data from the pig-to-NHP model before proceeding to clinical trials, as this will provide the greatest likelihood of success. Whatever barriers identified in the pig-to-NHP model should be addressed before proceeding to the possibly more complex pig-to-human studies. Even though the management of a patient with a pig graft will undoubtedly be easier in many respects, failure from graft rejection or from the complications of excessive immunosuppressive therapy in the pig-to-NHP model is unlikely to be fully reversed in the pig-to-human model.
It is of interest to note the relative publication rates of the papers surveyed. For example, more papers relating to kidney xenotransplantation were published during the period covered by this survey than those relating to other organs. However, there was a marked reduction in the number of papers published after 2005 (Figure 1B). This may be associated with the increased problems related to pig kidney than to heart transplantation, where consumptive coagulopathy appears to be less problematic. However, after a peak period of publications in 2005, the rate of publication of papers reporting studies of pig heart transplantation also fell (Figure 1A).
Figure 1.
Numbers of publications relating to pig-to-nonhuman primate heart (A, top), kidney (B, middle), and islet (C, bottom) transplantation during the period 1998–2013.
The decline is possibly more likely a consequence of lack of sufficient funding for xenotransplantation research during the past few years, and this may also be a major factor in the reduction in publications relating to kidney transplantation after 2005 (Figure 1B). In turn, this decline in funding was at least in part associated with reluctance on the part of certain commercial/industrial sponsors to continue to participate in the development of xenotransplantation in view of the scares being propagated at that time relating to the transfer of porcine endogenous retroviruses (PERV) with the graft to the recipient. Since the first report of the potential of pig-to-human infection by PERV in 1996, much subsequent research has demonstrated that the risk of cross-species transmission is unlikely and, in any event, manageable (42). Of note, regulatory authorities are largely concentrating their attention on proper patient monitoring and archiving of samples rather than on preventing the introduction of xenotransplantation.
In contrast, pig islet xenotransplantation, which has provided very encouraging results, was reported fairly consistently throughout the period with between 1 and 4 publications per year (Figure 1C).
Acknowledgments
We thank Mariette Lapalud, librarian, Department of Surgery, University Hospital Geneva, for help with the literature search. We thank the following for checking data from their own centers – Agnes Azimzadeh, Leo Buhler, Guerard Byrne, Peter Cowan, Emanuele Cozzi (with Marta Vadori), Mee Kum Kim, Allan Kirk (with Benjamin Martin), Muhammad Mohiuddin, Richard Pierson III, David Sachs, and Kazuhiko Yamada. Studies on xenotransplantation at the Thomas E. Starzl Transplantation Institute are supported in part by NIH grants #1U19AI090959-01, #U01A1066331, and #1PO1 HL107152, and by Sponsored Research Agreements between the University of Pittsburgh and Revivicor, Inc., Blacksburg, VA, USA.
Abbreviations
- Gal
galactose-α1,3-galactose
- GTKO
α1,3-galactosyltransferase gene-knockout
- mAb
monoclonal antibody
- NeuGc
N-glycolylneuraminic acid
- NHP
nonhuman primate
- PERV
porcine endogenous retroviruses
Footnotes
Statement re authors’ contributions
DKCC conceived the study. All authors contributed to the literature search and collection of data. DKCC and VS wrote the original draft, and all authors contributed to the final manuscript.
Statement regarding conflict of interest
The authors report no conflicts of interest.
References
- 1.COOPER DKC, HUMAN PA, LEXER G, et al. Effects of cyclosporine and antibody adsorption on pig cardiac xenograft survival in the baboon. J Heart Transplant. 1988;7:238–246. [PubMed] [Google Scholar]
- 2.LEXER G, COOPER DKC, ROSE AG, et al. Hyperacute rejection in a discordant (pig to baboon) cardiac xenograft model. J Heart Transplant. 1986;5:411–418. [PubMed] [Google Scholar]
- 3.LAMBRIGTS D, SACHS DH, COOPER DKC. Discordant organ xenotransplantation in primates: world experience and current status. Transplantation. 1998;66:547–561. doi: 10.1097/00007890-199809150-00001. [DOI] [PubMed] [Google Scholar]
- 4.HARA H, COOPER DKC. The immunology of corneal xenotransplantation: a review of the literature. Xenotransplantation. 2010;17:338–349. doi: 10.1111/j.1399-3089.2010.00608.x. [DOI] [PubMed] [Google Scholar]
- 5.HARA H, COOPER DKC. Xenotransplantation--the future of corneal transplantation? Cornea. 2011;30:371–378. doi: 10.1097/ICO.0b013e3181f237ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.KIM MK, WEE WR, PARK CG, KIM SJ. Xenocorneal transplantation. Curr Opin Organ Transplant. 2011;16:231–236. doi: 10.1097/MOT.0b013e328344870c. [DOI] [PubMed] [Google Scholar]
- 7.VAN DER WINDT DJ, BOTTINO R, KUMAR G, et al. Clinical islet xenotransplantation: how close are we? Diabetes. 2012;61:3046–3055. doi: 10.2337/db12-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.COOPER DKC, HARA H, YAZER M. Genetically engineered pigs as a source for clinical red blood cell transfusion. Clin Lab Med. 2010;30:365–380. doi: 10.1016/j.cll.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 9.COZZI E, WHITE DJ. The generation of transgenic pigs as potential organ donors for humans. Nat Med. 1995;1:964–966. doi: 10.1038/nm0995-964. [DOI] [PubMed] [Google Scholar]
- 10.EKSER B, EZZELARAB M, HARA H, et al. Clinical xenotransplantation: the next medical revolution? Lancet. 2012;379:672–683. doi: 10.1016/S0140-6736(11)61091-X. [DOI] [PubMed] [Google Scholar]
- 11.COOPER DKC, KOREN E, ORIOL R. Genetically engineered pigs. Lancet. 1993;342:682–683. doi: 10.1016/0140-6736(93)91791-j. [DOI] [PubMed] [Google Scholar]
- 12.DAI Y, VAUGHT TD, BOONE J, et al. Targeted disruption of the alpha1,3-galactosyltransferase gene in cloned pigs. Nat Biotechnol. 2002;20:251–255. doi: 10.1038/nbt0302-251. [DOI] [PubMed] [Google Scholar]
- 13.KOLBER-SIMONDS D, LAI L, WATT SR, et al. Production of alpha-1,3-galactosyltransferase null pigs by means of nuclear transfer with fibroblasts bearing loss of heterozygosity mutations. Proc Natl Acad Sci U S A. 2004;101:7335–7340. doi: 10.1073/pnas.0307819101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.LAI L, KOLBER-SIMONDS D, PARK KW, et al. Production of alpha-1,3-galactosyltransferase knockout pigs by nuclear transfer cloning. Science. 2002;295:1089–1092. doi: 10.1126/science.1068228. [DOI] [PubMed] [Google Scholar]
- 15.PHELPS CJ, KOIKE C, VAUGHT TD, et al. Production of alpha 1,3-galactosyltransferase-deficient pigs. Science. 2003;299:411–414. doi: 10.1126/science.1078942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.BUHLER L, AWWAD M, BASKER M, et al. High-dose porcine hematopoietic cell transplantation combined with CD40 ligand blockade in baboons prevents an induced anti-pig humoral response. Transplantation. 2000;69:2296–2304. doi: 10.1097/00007890-200006150-00013. [DOI] [PubMed] [Google Scholar]
- 17.LUTZ AJ, LI P, ESTRADA JL, et al. Double knockout pigs deficient in N-glycolylneuraminic acid and galactose alpha-1,3-galactose reduce the humoral barrier to xenotransplantation. Xenotransplantation. 2013;20:27–35. doi: 10.1111/xen.12019. [DOI] [PubMed] [Google Scholar]
- 18.PADLER-KARAVANI V, VARKI A. Potential impact of the non-human sialic acid N-glycolylneuraminic acid on transplant rejection risk. Xenotransplantation. 2011;18:1–5. doi: 10.1111/j.1399-3089.2011.00622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.ZHU X, DOR FJ, COOPER DKC. Pig-to-non-human primate heart transplantation: immunologic progress over 20 years. J Heart Lung Transplant. 2007;26:210–218. doi: 10.1016/j.healun.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 20.HOUSER SL, KUWAKI K, KNOSALLA C, et al. Thrombotic microangiopathy and graft arteriopathy in pig hearts following transplantation into baboons. Xenotransplantation. 2004;11:416–425. doi: 10.1111/j.1399-3089.2004.00155.x. [DOI] [PubMed] [Google Scholar]
- 21.COOPER DKC. A milestone in xenotransplantation research. Xenotransplantation. 2013;21:13–15. doi: 10.1111/xen.12079. [DOI] [PubMed] [Google Scholar]
- 22.MOHIUDDIN MM, CORCORAN PC, SINGH AK, et al. B-cell depletion extends the survival of GTKO. hCD46Tg pig heart xenografts in baboons for up to 8 months. Am J Transplant. 2012;12:763–771. doi: 10.1111/j.1600-6143.2011.03846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.MOHIUDDIN MM, SINGH AK, CORCORAN PC, et al. Role of anti-CD40 antibody-mediated costimulation blockade on non-Gal antibody production and heterotopic cardiac xenograft survival in a GTKO. hCD46Tg pig-to-baboon model. Xenotransplantation. 2013;21:35–45. doi: 10.1111/xen.12066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.MOHIUDDIN MM, SINGH AK, CORCORAN PC, et al. One-year heterotopic cardiac xenograft survival in a pig to baboon model. Am J Transplant. 2014;14:488–489. doi: 10.1111/ajt.12562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.LIN CC, EZZELARAB M, SHAPIRO R, et al. Recipient tissue factor expression is associated with consumptive coagulopathy in pig-to-primate kidney xenotransplantation. Am J Transplant. 2010;10:1556–1568. doi: 10.1111/j.1600-6143.2010.03147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.BALDAN N, RIGOTTI P, CALABRESE F, et al. Ureteral stenosis in HDAF pig-to-primate renal xenotransplantation: a phenomenon related to immunological events? Am J Transplant. 2004;4:475–481. doi: 10.1111/j.1600-6143.2004.00407.x. [DOI] [PubMed] [Google Scholar]
- 27.YAMADA K, YAZAWA K, SHIMIZU A, et al. Marked prolongation of porcine renal xenograft survival in baboons through the use of alpha1,3-galactosyltransferase gene-knockout donors and the cotransplantation of vascularized thymic tissue. Nat Med. 2005;11:32–34. doi: 10.1038/nm1172. [DOI] [PubMed] [Google Scholar]
- 28.WU A, ESNAOLA NF, YAMADA K, et al. Xenogeneic thymic transplantation in a pig-to-nonhuman primate model. Transplant Proc. 1999;31:957. doi: 10.1016/s0041-1345(98)01854-5. [DOI] [PubMed] [Google Scholar]
- 29.WU A, YAMADA K, AWWAD M, et al. Effects of xenogeneic thymic transplantation in baboons. Transplant Proc. 2001;33:766. doi: 10.1016/s0041-1345(00)02244-2. [DOI] [PubMed] [Google Scholar]
- 30.WU A, YAMADA K, AWWAD M, et al. Experience with porcine thymic transplantation in baboons. Transplant Proc. 2000;32:1048. doi: 10.1016/s0041-1345(00)01113-1. [DOI] [PubMed] [Google Scholar]
- 31.WU A, YAMADA K, NEVILLE DM, et al. Xenogeneic thymus transplantation in a pig-to-baboon model. Transplantation. 2003;75:282–291. doi: 10.1097/01.TP.0000044137.97841.99. [DOI] [PubMed] [Google Scholar]
- 32.KIM K, SCHUETZ C, ELIAS N, et al. Up to 9-day survival and control of thrombocytopenia following alpha1,3-galactosyl transferase knockout swine liver xenotransplantation in baboons. Xenotransplantation. 2012;19:256–264. doi: 10.1111/j.1399-3089.2012.00717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.VALDES-GONZALEZ RA, DORANTES LM, GARIBAY GN, et al. Xenotransplantation of porcine neonatal islets of Langerhans and Sertoli cells: a 4-year study. Eur J Endocrinol. 2005;153:419–427. doi: 10.1530/eje.1.01982. [DOI] [PubMed] [Google Scholar]
- 34.SGROI A, BUHLER LH, MOREL P, SYKES M, NOEL L. International human xenotransplantation inventory. Transplantation. 2010;90:597–603. doi: 10.1097/TP.0b013e3181eb2e8c. [DOI] [PubMed] [Google Scholar]
- 35.ELLIOTT RB. Towards xenotransplantation of pig islets in the clinic. Curr Opin Organ Transplant. 2011;16:195–200. doi: 10.1097/MOT.0b013e3283449dec. [DOI] [PubMed] [Google Scholar]
- 36.MARTIN C, PLAT M, NERRIERE-DAGUIN V, et al. Transgenic expression of CTLA4-Ig by fetal pig neurons for xenotransplantation. Transgenic Res. 2005;14:373–384. doi: 10.1007/s11248-004-7268-4. [DOI] [PubMed] [Google Scholar]
- 37.LEVEQUE X, COZZI E, NAVEILHAN P, NEVEU I. Intracerebral xenotransplantation: recent findings and perspectives for local immunosuppression. Curr Opin Organ Transplant. 2011;16:190–194. doi: 10.1097/MOT.0b013e32834494b5. [DOI] [PubMed] [Google Scholar]
- 38.NAGATA H, NISHITAI R, SHIROTA C, et al. Prolonged survival of porcine hepatocytes in cynomolgus monkeys. Gastroenterology. 2007;132:321–329. doi: 10.1053/j.gastro.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 39.EZZELARAB MB, EKSER B, ECHEVERRI G, et al. Costimulation blockade in pig artery patch xenotransplantation - a simple model to monitor the adaptive immune response in nonhuman primates. Xenotransplantation. 2012;19:221–232. doi: 10.1111/j.1399-3089.2012.00711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.IERINO FL, GOJO S, BANERJEE PT, et al. Transfer of swine major histocompatibility complex class II genes into autologous bone marrow cells of baboons for the induction of tolerance across xenogeneic barriers. Transplantation. 1999;67:1119–1128. doi: 10.1097/00007890-199904270-00006. [DOI] [PubMed] [Google Scholar]
- 41.WEINER J, YAMADA K, ISHIKAWA Y, et al. Prolonged survival of GalT-KO swine skin on baboons. Xenotransplantation. 2010;17:147–152. doi: 10.1111/j.1399-3089.2010.00576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.DENNER J, TONJES RR. Infection barriers to successful xenotransplantation focusing on porcine endogenous retroviruses. Clin Microbiol Rev. 2012;25:318–343. doi: 10.1128/CMR.05011-11. [DOI] [PMC free article] [PubMed] [Google Scholar]