Abstract
Purpose
Given the current prevalence of obesity, it is important to identify dietary factors that may aid in disease prevention. The objective of the present study was to evaluate the association between consumption of an energy-dense diet and established markers factors for chronic disease, including body weight and measures of body fatness.
Methods
Data from a nationally representative sample of 9,551 adults ≥18 years who participated in the 2005–2008 National Health and Nutrition Examination Survey were analyzed. The association between dietary energy density (ED, energy per weight of food, kcal/g) and markers for obesity [including body mass index (BMI) and waist circumference (WC)], insulin insensitivity [including fasting glucose, insulin and homeostasis assessment model for insulin resistance (HOMA-IR)], and markers for inflammation was examined.
Results
Dietary ED was positively associated with obesity in both men and women in multivariate models. Overall, obese adults had a significantly higher dietary ED than lean adults (p < 0.0001). Current smokers had significantly higher ED than non-smokers (2.00 vs. 1.75, p < 0.01), and it was determined that smoking status modified the relationship between ED and weight status in women (p interaction 0.03). In both sexes, there was a positive linear relationship between BMI and ED (p trend 0.01 and 0.0002, respectively); a linear trend between WC and ED was also observed in women (p trend<0.001) after adjusting for relevant cofactors. In women, ED was positively associated with HOMA-IR and fasting insulin; though, this relationship was not observed in men. No significant associations between ED and C-reactive protein were observed in either sex.
Conclusion
These findings support recent obesity and disease prevention recommendations to consume a diet low in ED.
Keywords: Energy density, NHANES, Waist circumference, BMI, Obesity
Introduction
Nearly two-thirds of US adults are overweight or obese [body mass index (BMI) ≥25 and ≥30 kg/m2, respectively] [1]. Obesity is a risk factor for insulin resistance, type 2 diabetes, and inflammatory states, all of which have been positively associated with increased risk of chronic disease, including several types of cancer [2]. Several groups that promote public health through diet and diet-related recommendations have recognized energy density (ED) as an important consideration for weight control and disease prevention [2]. ED is a ratio of the amount of energy per weight of food (kcal/g). ED is influenced by various food components, such as the macronutrient and water content. Water has the greatest influence on the ED of a food because it adds substantial weight without adding energy. Of the macronutrients, fat is most influential because of its high energy content (9 kcal/g) relative to either protein or carbohydrate (both 4 kcal/g). Experimental studies have previously demonstrated that lowering the ED of a food or meal can lower energy intake, which, if sustained over time, may result in weight loss [3]. Various population-based studies have examined the association between dietary ED and weight status, including studies evaluating the relationship between ED and markers for metabolic syndrome in a nationally representative sample of adults [4]. Most studies have found a positive relationship between ED and BMI [5–8]. Weight status is a known risk factor for chronic disease and cancer, particularly colon and rectal cancer (CRC) [2]. We have reported that a low-ED, reduced-energy diet led to weight loss with concomitant improvements in biomarkers of inflammation and insulin sensitivity in blood [9]. The present study will examine the association of food-only dietary ED with established markers for chronic disease, including body weight, waist circumference, and markers for insulin sensitivity and inflammation within a recent nationally representative sample of US adults. We hypothesize that dietary ED will be positively associated with BMI, waist circumference, and markers for chronic disease in this population.
Methods
Study design
The National Health and Nutrition Examination Survey (NHANES) is a large, cross-sectional survey conducted by National Center for Health Statistics. NHANES is designed to monitor the health and nutritional status of non-institutionalized civilians in the USA; nationally representative survey and health examination are collected on a continual basis and released in two-year increments. Complete details regarding the NHANES sampling methodology, data collection, and interview process are available on the NHANES website (http://www.cdc.gov/nchs/nhanes.htm). Data from the 2005–2008 survey cycles were combined for this study. The present study was approved by the Institutional Review Board at the Pennsylvania State University.
In both cycles of NHANES, height and weight were measured by trained examiners using standardized protocols and calibrated equipment during the physical examination component of the study. Adults were classified as lean (BMI ≤24.9 kg/m2), overweight (BMI of 25.0–29.9), or obese (BMI ≥30) using Center for Disease Control (CDC) cutoff points (http://www.cdc.gov/obesity/defining.html). For this analysis, underweight (BMI <18.5) participants were included in the lean category. Blood samples were collected on a smaller subset of the population. Depending on the time of day of the exam, either fasting or non-fasting blood samples were collected. All participants, regarding of fasting status, had blood drawn for C-reactive protein (CRP), a marker for inflammation. For participants who were fasting, assessments for serum insulin (mg/L) and serum glucose (mg/L) (as markers for insulin insensitivity) were also collected. We calculated the homeostasis assessment model for insulin resistance (HOMA-IR) [HOMA-IR = fasting insulin (μU/mL) × fasting glucose (mmol/L)/22.5] as an indicator of insulin resistance [10].
Adults who participated in NHANES provided 1 day of dietary recall (24 h) data during their visit to the mobile examination unit as part of the What We Eat in America Study [11]. This 24 h is used to monitor the dietary behaviors of the US population [12] and has been established as a method for assessing mean dietary intake of a population. Data for 24 h were collected in person by trained interviewers using the automated multipass method of 24-h recall. Specific status codes were used to indicate the quality, reliability, and completeness of the dietary data; these status codes accounted for inaccurate data due to substantial over- or underreporting.
The USDA Food and Nutrition Database, version 3.0 was used to process NHANES 24 h. There are several methods to calculate dietary ED [13, 14]. Currently, no standardized method exists for selecting which foods and/ or beverages to include in the calculation, though the majority of studies that have found associations between dietary ED and disease status have done so using the food-only method [4, 5, 13, 15]. Due to their high moisture content, beverages confer a disproportionate contribution to overall dietary ED. Even caloric beverages contain a substantial amount of water, which yields a large amount of weight for a small amount of energy. Previous studies have indicated that this disproportionate influence of beverages on dietary ED may influence true relationships, and as such be excluded from dietary ED calculations, specifically because beverage ED is lower in those who drink more beverages, regardless of caloric intake [4, 13, 16]. In the present analysis, dietary ED was initially calculated both ways: using all foods and beverages, and using foods only. Complete details of this method in a nationally representative sample have been previous described [13]. For each method, ED was calculated dividing the energy content (in kilocalories) by weight of foods (in grams) consumed. USDA food codes were used to identify which items were foods and which were beverages (e.g., differentiating between milk used in cereal vs. milk consumed as a beverage). Due to differences in energy intake between men and women, sex-specific ED quartiles were created in order to analyze ED as both a continuous and categorical variable.
For the present analyses, we initially included all adults aged 18 and older who had complete dietary and anthropometric data. Individuals who were currently following a weight-loss diet, individuals with implausible or very unusual 24 h (e.g., reporting no beverages during the 24-h recall period), and women who were pregnant or lactating were excluded (n = 2,240), resulting in a final analytical dataset of 9,551 adults. Of these 9,551 adults, a smaller subset also underwent additional procedures to collect fasting blood samples (n = 4,039). Age at the time of exam, education level, smoking status (current, former, never smoker), physical activity (measured in MET units), race, and socioeconomic status were all provided in the NHANES dataset. Socioeconomic status was quantified as a continuous variable using poverty-income ratio (PIR) or the ratio of family income to family-size-specific poverty threshold. Total energy intake was evaluated using all foods and beverages reported. Preliminary analysis indicated an effect modification between ED and smoking status (current, past, never smoker), as such all data were analyzed with and without stratifying for smoking status.
Statistical analysis
All data were analyzed using SAS version 9.3 (SAS Institute, Cary, NC). Specific survey procedures were used in the analysis to account for sample weights, unequal selection probability, and clustered design. Analysis of the subset of fasting subjects required use of different survey weights in order to account for the specific characteristics of this subpopulation. Multivariate regression was used to evaluate the association of dietary ED with health outcomes related to chronic disease and cancer (e.g., body mass index, waist circumference), impaired glucose sensitivity (fasting glucose and insulin, available for a small subset), and markers for inflammation (CRP, available for all adults). Sex-specific analysis was conducted to take into account the natural differences in body composition and caloric needs between men and women. Smoking status (never, current, former) was investigated as a modifier for all outcome relationships. A test for linear trend using the Wald statistic was performed by modeling quartiles of ED as a continuous variable. All models were adjusted for age, race, education, socioeconomic status (PIR), physical activity, diabetic status, survey cycle, and body mass index (where appropriate) with significance determined at p < 0.05.
Results
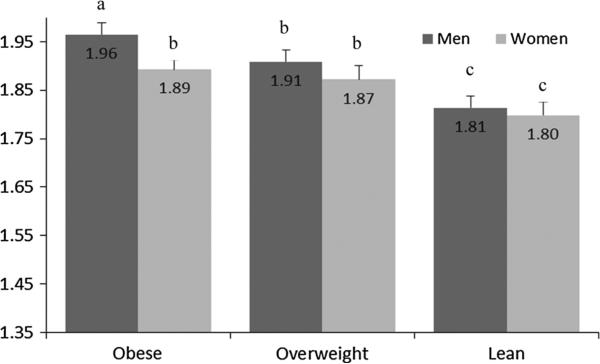
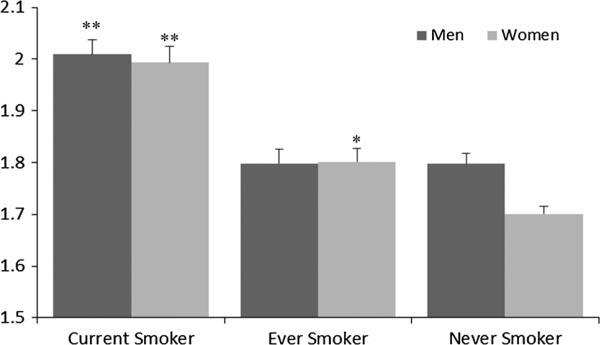
Demographic characteristics are presented in Table 1. Approximately half of the sample had a history of smoking; some differences between the smoking behaviors of men compared with women were noted. Among women, a higher proportion of never smokers (58 %) was observed, compared with the proportion of never smokers in men (44 %). In this representative sample of US adults, a significant positive association was found between dietary ED and body weight status; obese men had a significantly higher ED than over weight or lean men (1.96 vs. 1.91 vs. 1.81 kcal/g, p trend <0.0001); obese women had a significantly higher ED than lean women (1.89 vs. 1.87 vs. 1.80, p trend 0.002) (Fig. 1). In this population, current smokers had a higher dietary ED than non-smokers of either sex (p < 0.0001; Fig. 2), and it is notable that men have a higher mean ED than women. Smoking status was found to modify the association between dietary ED and body mass index (p interaction 0.03) when assessing all adults; in sexstratified analyses, however, smoking was only found to modify the effect of ED on body mass index in women (p interaction 0.008). As such, the interaction effect was included in the model evaluating ED and body mass index for women only.
Table 1.
Demographic characteristics of US adults: NHANES 2005–2008
| Sample na | Percentb | |
|---|---|---|
| Sex | ||
| Female | 4,587 | 50.0 |
| Male | 4,964 | 50.0 |
| Age group | ||
| 18–30 | 2,270 | 23.5 |
| 31–50 | 2,972 | 37.6 |
| 51–70 | 2,712 | 27.7 |
| >70 | 1,597 | 11.2 |
| Racec | ||
| NH-White | 4,502 | 71.0 |
| NH-Black | 2,156 | 11.5 |
| Mex-Amer | 1,802 | 8.3 |
| Other | 1,091 | 9.3 |
| Education | ||
| HS or less | 2,931 | 19.8 |
| High school grad/GED | 2,427 | 26.5 |
| Some college or AA degree | 2,545 | 30.0 |
| College graduate or above | 1,642 | 23.7 |
| Incomed | ||
| PIR < 130 % | 3,356 | 24.9 |
| 130 < PIR < 350 % | 3,483 | 34.4 |
| PIR > 350 % | 2,712 | 40.7 |
| Smoking status | ||
| Never smoker | 4,540 | 50.9 |
| Current smoker | 2,029 | 25.1 |
| Ever smoker (>100 cigarettes) | 2,246 | 23.9 |
| Weight statuse | ||
| Lean (BMI < 25) | 3,259 | 36.2 |
| Overweight (BMI 25–30) | 3,183 | 32.9 |
| Obese (BMI > 30) | 3,109 | 30.9 |
| Survey cycle | ||
| 2005–2006 | 4,296 | 48.2 |
| 2007–2008 | 5,255 | 51.8 |
| Dietary intake | Mean | SE |
|---|---|---|
| Men | ||
| Mean total energy intake (kcal) | 2,594.2 | 30.1 |
| Mean food energy intake (kcal) | 2,068.2 | 25.6 |
| Mean beverage energy intake (kcal) | 526.0 | 22.9 |
| % Energy from carbohydrate | 48.0 | 0.3 |
| % Energy from protein | 15.7 | 0.1 |
| % Energy from fat | 33.5 | 0.2 |
| Mean dietary energy density (kcal/g) | 1.98 | 0.01 |
| Mean BMI (kg/m2) | 28.3 | 0.2 |
| Women | ||
| Mean energy intake (kcal) | 1,791.0 | 19.4 |
| Mean food energy intake (kcal) | 1,488.3 | 18.9 |
| Mean beverage energy intake (kcal) | 302.7 | 16.8 |
| % Energy from carbohydrate | 50.2 | 0.3 |
| % Energy from protein | 15.5 | 0.1 |
| % Energy from fat | 33.6 | 0.2 |
| Mean dietary energy density (kcal/g) | 1.86 | 0.01 |
| Mean BMI (kg/m2) | 27.7 | 0.1 |
Sample n based on cell counts
Population percentages based on NHANES survey weights and represent that population of non-institutionalized US adult residents
Race categories: NH-White, non-Hispanic White; NH-Black, non-Hispanic Black, Mex-Amer, Mexican–American; Others
Adjusted income level based on poverty: income ratio adjusted for household size
Fig. 1.
Dietary Energy density is associated with body weight status in US adults. Results are presented as least-squared means adjusted for age, sex, race/ethnicity, education, family poverty: income ratio, smoking status, physical activity, beverage energy density, self-reported “following a low-fat diet,” and survey cycle. The relationship between energy density and weight status was tested within sexspecific groups. Bars with differing superscripts are significantly different at p < 0.05
Fig. 2.
Smoking status is associated with dietary energy density in US adults. Results are presented as least-squared means adjusted for age, race, education, family poverty: income ratio, physical activity level, and survey cycle. The relationship between energy density and smoking status was tested within sex-specific groups. Never smokers of each sex were the referent group. *p < 0.001. **p < 0.0001
Dietary ED was analyzed both as a continuous and categorical variable to evaluate the relationship between ED and markers for obesity (BMI and WC). ED was initially calculated using two methods: first, with all foods and beverages reported and then using only foods (excluding beverages). For both methods, dietary ED was classified into quartiles. When including beverages, the range of ED was extremely small (Q1–Q4, 0.44–0.80 kcal/ g) compared with the food-only method (Q1–Q4, 1.41–2.31 kcal/g), reflecting the disproportionate contribution of beverages to overall dietary ED. During the analysis, a relationship between weight status and the amount of, but not energy from, beverages consumed was observed. There was no difference in caloric intake from beverages between lean, overweight, and obese individuals after adjusting for relevant cofactors (p = 0.87); however, obese individuals consumed a significantly greater amount (in g) of beverages than lean individuals (2,689 vs. 2,198 g, p < 0.0001), influencing the relationship between beverage ED and weight status. As such, subsequent analysis was conducted using the food-only method, with beverage ED included as a covariate.
When evaluating dietary ED as a continuous variable, a significant linear relationship was found between dietary ED and both body weight and waist circumference in both men and women. In men, a positive linear relationship between dietary ED and both BMI (p trend 0.01) was observed. In women, a stronger positive association between BMI and total ED was observed (p trend 0.0002), as well as a linear relationship between total ED and WC (p trend <0.0001). For categorical analysis, ED was divided into sex-specific quartiles, shown in Table 2. Both men and women in the lowest ED quartile had significantly lower BMI and WC than those in the highest two quartiles of ED (Table 2). Further, the linear trend was significant after controlling for body mass index, indicating that ED is positively associated with waist circumference independent of body mass index.
Table 2.
Anthropometric characteristics of adults by energy density quartile
| Men |
Women |
||||||
|---|---|---|---|---|---|---|---|
| ED quartile (range) | Mean ± SE | p Value | ED quartile (range) | Mean ± SE | p Value | ||
| Body mass index (kg/m2) | |||||||
| Q1 | <1.59 | 27.4 ± 0.2 | Ref | Q1 | <1.43 | 27.1 ± 0.3 | Ref |
| Q2 | 1.59–1.92 | 27.7 ± 0.3 | 0.33 | Q2 | 1.43–1.78 | 27.9 ± 0.3 | 0.016 |
| Q3 | 1.92–2.33 | 28.7 ± 0.3 | 0.006 | Q3 | 1.78–2.21 | 28.7 ± 0.3 | 0.0002 |
| Q4 | >2.33 | 29.7 ± 0.3 | 0.002 | Q4 | >2.21 | 29.2 ± 0.4 | 0.0005 |
| p Trend | 0.01 | 0.0002 | |||||
| Waist circumference (cm)a | |||||||
| Q1 | <1.59 | 98.0 ± 0.6 | Ref | Q1 | <1.43 | 91.5 ± 0.9 | Ref |
| Q2 | 1.59–1.92 | 98.4 ± 0.7 | 0.66 | Q2 | 1.43–1.78 | 93.7 ± 0.7 | 0.03 |
| Q3 | 1.92–2.33 | 100.2 ± 0.9 | 0.04 | Q3 | 1.78–2.21 | 95.7 ± 0.9 | 0.0004 |
| Q4 | >2.33 | 99.7 ± 0.9 | 0.06 | Q4 | >2.21 | 95.9 ± 1.1 | <.0001 |
| p Trend | 0.09 | <.0001 | |||||
Results are presented as least-squared mean ± SE adjusted for age, race, education, family poverty: income ratio, physical activity, smoking status, self-reported following a low-fat diet, beverage energy density, and survey cycle
Models for waist circumference are also controlled for body mass index
Assessing the relationship between CRP and ED, no associations were observed in either sex. When evaluating markers for impaired glucose tolerance and insulin resistance, no associations were observed between fasting glucose and ED in either sex; however, a weak positive association between fasting insulin and ED was observed in women (p trend 0.05) (Table 3). It is notable that mean values for fasting insulin fell within the healthy range for all quartiles among both sexes, with only 3 % of the entire population having a fasting glucose >125 mg/dL. When evaluating HOMA-IR, a marker for insulin insensitivity, women in the highest quartile of ED had significantly higher HOMA-IR than those in the lowest quartile (Table 3), though this trend was not observed in men. However, when insulin insensitivity (HOMA-IR score >2.61) was modeled using logistic regression, the odds of having insulin insensitivity increased as ED quartile increased (OR 1.4, 95 % CI 1.0–1.8) after adjusting for age, sex, race, education, socioeconomic status (PIR), physical activity, and survey cycle. Additionally, in logistic regression models evaluating risk of obesity (BMI > 30 kg/m2), it was determined that the odds of being obese increased for each increase in ED quartile. Those in the highest quartile of ED have a 57 % increase probability of being obese compared with those in the lowest quartile, after adjusting for the same confounders (OR 1.3, 95 % CI 1.0–1.7).
Table 3.
Select biomarkers by energy density quartile
| Men |
Women |
||||||
|---|---|---|---|---|---|---|---|
| ED quartile | Range | Mean ± SE | p Value | ED quartile | Range | Mean ± SE | p Value |
| Fasting glucose mg/dLa | |||||||
| Q1 | <1.59 | 105.8 ± 1.2 | Ref | Q1 | <1.43 | 101.8 ± 1.7 | Ref |
| Q2 | 1.59–1.92 | 106.0 ± 1.9 | 0.83 | Q2 | 1.43–1.78 | 100.4 ± 2.0 | 0.73 |
| Q3 | 1.92–2.33 | 105.9 ± 1.6 | 0.93 | Q3 | 1.78–2.21 | 100.7 ± 1.1 | 0.21 |
| Q4 | >2.33 | 107.2 ± 1.9 | 0.24 | Q4 | >2.21 | 101.8 ± 1.8 | 0.86 |
| p Trend | 0.69 | 0.22 | |||||
| Fasting insulin mg/dLa | |||||||
| Q1 | 12.4 ± 0.7 | Ref | Q1 | 12.0 ± 0.5 | Ref | ||
| Q2 | 12.5 ± 0.7 | 0.51 | Q2 | 11.4 ± 0.7 | 0.15 | ||
| Q3 | 13.2 ± 0.9 | 0.13 | Q3 | 11.7 ± 0.8 | 0.51 | ||
| Q4 | 12.4 ± 0.8 | 0.86 | Q4 | 11.6 ± 0.7 | 0.19 | ||
| p Trend | 0.23 | 0.05 | |||||
| CRP mg/dLb | |||||||
| Q1 | 0.42 ± 0.05 | Ref | Q1 | 0.52 ± 0.04 | Ref | ||
| Q2 | 0.35 ± 0.04 | 0.30 | Q2 | 0.46 ± 0.03 | 0.19 | ||
| Q3 | 0.38 ± 0.04 | 0.53 | Q3 | 0.55 ± 0.04 | 0.51 | ||
| Q4 | 0.41 ± 0.03 | 0.87 | Q4 | 0.51 ± 0.03 | 0.72 | ||
| p Trend | 0.38 | 0.31 | |||||
| HOMA-IRc | |||||||
| Q1 | 3.28 ± 0.30 | Ref | Q1 | 2.87 ± 0.12 | Ref | ||
| Q2 | 2.92 ± 0.23 | 0.40 | Q2 | 2.92 ± 0.13 | 0.75 | ||
| Q3 | 3.42 ± 0.24 | 0.73 | Q3 | 3.13 ± 0.18 | 0.16 | ||
| Q4 | 3.19 ± 0.20 | 0.80 | Q4 | 3.24 ± 0.20 | 0.03 | ||
| p Trend | 0.41 | 0.10 | |||||
Results are presented as least-squared mean ± SE adjusted for age, race, education, family poverty: income ratio, smoking status, physical activity, diabetic status, body mass index, and survey cycle. ED quartile range remains the same in each category
Glucose and insulin assessed in the fasting state: normal glucose range <126 mg/dL and normal insulin range 5–25 μU/mL
CRP, C-reative protein; normal range is <0.8 mg/dL
HOMA-IR is calculated as fasting insulin (μU/mL) × fasting glucose (mmol/L)/22.5
Discussion
In this nationally representative sample of US adults, dietary ED (calculated using foods only) was positively associated with various markers for chronic disease, including BMI and waist circumference, HOMA-IR and fasting insulin. The association between dietary ED and waist circumference was found to be independent of BMI. These results support previous findings in nationally representative samples of children [15], findings reported about the association between ED and metabolic syndrome in adults [4] and analyses of national samples evaluating self-reported BMI and ED [5, 17], as well as markers of insulin insensitivity. The present study initially calculated dietary ED both with and without beverages included. During the initial analyses, the disproportionate contribution of beverages to overall ED was observed, and further analysis was conducted using the food-only method. Unlike previous studies which have used self-reported height and weight to evaluate the relationship between ED and weight status [5], the present study uses measured height and weight to calculate BMI, excludes individuals currently following a weight-loss diet, and also takes into consideration the potential for effect modification by smoking status, a known risk factor for cancer.
The effect of smoking status on the relationship between dietary ED and markers for disease is striking. As a sub-population, current smokers represent a group already at increased risk of cancer; the addition of an increased dietary ED further adds to their risk. The finding that smokers consume a diet higher in ED than non-smokers is not surprising. It has already been demonstrated that smokers have a poorer diet quality than non-smokers [18], yet weigh less than non-smokers. Foods that are relatively high in ED include red and processed meats, as well as fast food and fatty snacks and desserts; these foods contrast from water-rich foods such as vegetables and broth-based soups, which are very low in ED. It is possible that smokers are increasing their risk of cancer by consuming a diet lacking in antioxidant-rich vegetables, and higher in foods thought to increase cancer risk, such as red and processed meats [19]. Perhaps due to the higher dietary ED, the relationship between smoking and body weight status is not the same in smokers as it is in non-smokers, particularly among women. Smokers tend to have higher dietary ED but maintain a lower BMI compared with non-smokers; this effect modification should be considered when evaluating the relationship between dietary ED and disease risk. When evaluating the individual relationships between dietary ED with fasting glucose and insulin, no robust associations were found. There may be several reasons for this finding. When examining the fasting glucose and insulin levels, it is notable that 97 % of the population fell within the healthy range. However, it was observed in logistic regression models that adults in the highest ED quartiles have significantly higher odds of being insulin insensitive than those in the lowest ED quartile.
There are several strengths to the present analysis. First, the results are generalizable to the US population. In contrast to previous studies, this study evaluates the association with markers for chronic disease and dietary ED as opposed to evaluating consumption of specific foods or foods groups. This allows for a broader interpretation of the results and provides a simplified public health message. There are also several possible limitations to this research. The nutritional data within the NHANES study are self-reported and may be subjected to recall bias. The data collection methods in use for the 24-h diet recalls are state of the art with quality control procedures in place during the data collection phase and within our analyses (e.g., excluding implausible data) will help to address this potential concern. Finally, the cross-sectional survey design of NHANES allows for evaluation of population-wide associations, but prevents evaluation of causality.
In conclusion, dietary ED was positively associated with established obesity-related markers for cancer and other chronic diseases in a nationally representative sample of US adults. These findings support the recently released Surgeon General's Vision for a Healthy and Fit Nation 2010 (4), as well as the joint AICR/WCRF cancer prevention recommendation to consume a diet low in ED [20]. (2) It was determined that smoking modified the relationship between ED and obesity. Future research should be conducted to further evaluate the effect modification of smoking on risk factors for obesity and cancer by prospectively assessing the relationship between dietary ED on cancer risk factors and cancer incidence.
Acknowledgments
This study was supported in part by a Grant from American Institute of Cancer Research (10A078). We acknowledge the assistance provided by the Population Research Center at The Pennsylvania State University, which is supported by an infrastructure grant by the National Institutes of Health (2R24HD041025-11).
Contributor Information
Jacqueline A. Vernarelli, Department of Nutritional Sciences, The Pennsylvania State University, 110 Chandlee Laboratory, University Park, PA 16802, USA
Diane C. Mitchell, Department of Nutritional Sciences, The Pennsylvania State University, 110 Chandlee Laboratory, University Park, PA 16802, USA
Barbara J. Rolls, Department of Nutritional Sciences, The Pennsylvania State University, 110 Chandlee Laboratory, University Park, PA 16802, USA
Terryl J. Hartman, Department of Nutritional Sciences, The Pennsylvania State University, 110 Chandlee Laboratory, University Park, PA 16802, USA Department of Epidemiology, Rollins School of Public Health, Emory University, Atlanta, GA 30322, USA.
References
- 1.DiFranza JR, Wellman RJ. A sensitization-homeostasis model of nicotine craving, withdrawal, and tolerance: integrating the clinical and basic science literature. Nicotine Tob Res. 2005;7(1):9–26. doi: 10.1080/14622200412331328538. doi:10.1080/14622200412331328538. [DOI] [PubMed] [Google Scholar]
- 2.Marmot M. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. World Cancer Research Fund/American Institute for Cancer Research; Washington: 2007. [Google Scholar]
- 3.Rolls BJ. The relationship between dietary energy density and energy intake. Physiol Behav. 2009;97(5):609–615. doi: 10.1016/j.physbeh.2009.03.011. doi:10.1016/j.physbeh.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mendoza JA, Drewnowski A, Christakis DA. Dietary energy density is associated with obesity and the metabolic syndrome in U.S. adults. Diabet Care. 2007;30(4):974–979. doi: 10.2337/dc06-2188. doi:10.2337/dc06-2188. [DOI] [PubMed] [Google Scholar]
- 5.Ledikwe JH, Blanck HM, Kettel Khan L, Serdula MK, Seymour JD, Tohill BC, Rolls BJ. Dietary energy density is associated with energy intake and weight status in US adults. Am J Clin Nutr. 2006;83(6):1362–1368. doi: 10.1093/ajcn/83.6.1362. [DOI] [PubMed] [Google Scholar]
- 6.Kant AK, Graubard BI. Energy density of diets reported by American adults: association with food group intake, nutrient intake, and body weight. Int J Obes (Lond) 2005;29(8):950–956. doi: 10.1038/sj.ijo.0802980. doi:10.1038/sj.ijo.0802980. [DOI] [PubMed] [Google Scholar]
- 7.Stookey JD. Energy density, energy intake and weight status in a large free-living sample of Chinese adults: exploring the underlying roles of fat, protein, carbohydrate, fiber and water intakes. Eur J Clin Nutr. 2001;55(5):349–359. doi: 10.1038/sj.ejcn.1601163. doi:10.1038/sj.ejcn.1601163. [DOI] [PubMed] [Google Scholar]
- 8.Howarth NC, Murphy SP, Wilkens LR, Hankin JH, Kolonel LN. Dietary energy density is associated with overweight status among 5 ethnic groups in the multiethnic cohort study. J Nutr. 2006;136(8):2243–2248. doi: 10.1093/jn/136.8.2243. [DOI] [PubMed] [Google Scholar]
- 9.Hartman TJ, Albert PS, Zhang Z, Bagshaw D, Kris-Etherton PM, Ulbrecht J, Miller CK, Bobe G, Colburn NH, Lanza E. Consumption of a legume-enriched, low-glycemic index diet is associated with biomarkers of insulin resistance and inflammation among men at risk for colorectal cancer. J Nutr. 2010;140(1):60–67. doi: 10.3945/jn.109.114249. doi:10.3945/jn.109.114249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Monzillo LU, Hamdy O. Evaluation of insulin sensitivity in clinical practice and in research settings. Nutr Rev. 2003;61(12):397–412. doi: 10.1301/nr.2003.dec.397-412. [DOI] [PubMed] [Google Scholar]
- 11.U.S. Department of Agriculture ARS, Beltsville Human Nutrition Research Center, Food Surveys Research Group (Beltsville, MD) and U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics (Hyattsville, MD) What We Eat in America, NHANES 2005-2008. U.S. Department of Agriculture; 2010. [1 April 2011]. http://www.ars.usda.gov/Services/docs.htm?docid=18354. [Google Scholar]
- 12.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999-2008. JAMA. 2010;303(3):235–241. doi: 10.1001/jama.2009.2014. doi:10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 13.Vernarelli JA, Mitchell DC, Rolls BJ, Hartman TJ. Methods for calculating dietary energy density in a nationally representative sample. Procedia Food Sci. 2013;2:68–74. doi: 10.1016/j.profoo.2013.04.011. doi:10.1016/j.profoo.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ledikwe JH, Blanck HM, Khan LK, Serdula MK, Seymour JD, Tohill BC, Rolls BJ. Dietary energy density determined by eight calculation methods in a nationally representative United States population. J Nutr. 2005;135(2):273–278. doi: 10.1093/jn/135.2.273. [DOI] [PubMed] [Google Scholar]
- 15.Vernarelli JA, Mitchell DC, Hartman TJ, Rolls BJ. Dietary energy density is associated with body weight status and vegetable intake in U.S. Children. J Nutr. 2011;141(12):2204–2210. doi: 10.3945/jn.111.146092. doi:10.3945/jn.111.146092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson L, Wilks DC, Lindroos AK, Jebb SA. Reflections from a systematic review of dietary energy density and weight gain: is the inclusion of drinks valid? Obes Rev. 2009;10(6):681–692. doi: 10.1111/j.1467-789X.2009.00580.x. doi:10.1111/j.1467-789X.2009.00580.x. [DOI] [PubMed] [Google Scholar]
- 17.Ledikwe JH, Blanck HM, Khan LK, Serdula MK, Seymour JD, Tohill BC, Rolls BJ. Low-energy-density diets are associated with high diet quality in adults in the United States. J Am Diet Assoc. 2006;106(8):1172–1180. doi: 10.1016/j.jada.2006.05.013. doi:10.1016/j.jada.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 18.Schroder H, Marrugat J, Elosua R, Covas MI. Tobacco and alcohol consumption: impact on other cardiovascular and cancer risk factors in a southern European Mediterranean population. Br J Nutr. 2002;88(3):273–281. doi: 10.1079/BJN2002655. doi:10.1079/BJN2002655. [DOI] [PubMed] [Google Scholar]
- 19.Miller PE, Lesko SM, Muscat JE, Lazarus P, Hartman TJ. Dietary patterns and colorectal adenoma and cancer risk: a review of the epidemiological evidence. Nutr Cancer. 2010;62(4):413–424. doi: 10.1080/01635580903407114. doi:10.1080/01635580903407114. [DOI] [PubMed] [Google Scholar]
- 20.Perez-Escamilla R, Obbagy JE, Altman JM, Essery EV, McGrane MM, Wong YP, Spahn JM, Williams CL. Dietary energy density and body weight in adults and children: a systematic review. J Acad Nutr Diet. 2012;112(5):671–684. doi: 10.1016/j.jand.2012.01.020. doi:10.1016/j.jand.2012.01.020. [DOI] [PubMed] [Google Scholar]