Abstract
Epidermal growth factor receptor (EGFR) interacting with Stat3 is considered to be an attractive therapeutic target. In the current study, we investigated the effect of resveratrol and its two 4′-methylthio-trans-stilbene derivatives (3-M-4′-MTS; S2) (3,5-DM-4″-MTS; S5) on EGFR and Stat3 activation in human immortalized HaCaT keratinocytes and epidermoid carcinoma A431 cells. In the HaCaT cells both derivatives, similarly as resveratrol, decreased the total level of the EGFR receptor. In the A431 cells, resveratrol in the higher dose significantly (p < 0.05) reduced Y1173 and Y1068 EGFR residue phosphorylation, while S2 affected only the phosphorylation of the Y1068 residue. In this cell line, resveratrol in both tested doses and the S2 derivative in the lower concentration significantly diminished Stat3 binding capacity to the DNA consensus site. The effect of the tested compounds on Stat3 activation in HaCaT cells was only slightly affected. These results indicate that methylthiostilbenes are not more potent modulators of the EGFR/Stat3 complex than resveratrol and that introducing an additional methoxy group makes them less effective.
Keywords: Resveratrol, Methylthiostilbenes, HaCaT, A431, EGFR, Stat3
Introduction
Keratinocytes are not only primary sensors of stressful conditions, but also major players of the extremely complex response in the skin when conducting the orchestrated recruitment and functions of the immune cells, fibroblasts, and vascular cells which are involved in inflammatory responses and wound healing. The epidermal growth factor receptor (EGFR), located on the cellular membrane of the keratinocytes, is widely recognized as a key regulator of numerous essential processes underlying skin development, homeostasis, and repair [1]. EGFR is a 170-kDa glycoprotein that consists of an extracellular receptor domain, a trans-membrane region, and an intracellular domain with tyrosine kinase function [2]. EGFR is expressed through all layers of the human epidermis, with the strongest presence in the basal layer of the epidermal keratinocytes. Several lines of evidence indicate the existence of two modes of EGFR signaling. The traditional cytoplasmic EGFR route involves transduction of mitogenic signals through the activation of numerous signaling cascades, signal transducers, and activators of transcription (Stats). In the nuclear pathway, activated EGFR undergoes direct nuclear translocation, where it interacts with other transcription factors possessing DNA-binding activity, including (Stat3), which leads to up-regulation of distinct genes controlling cell proliferation and DNA repair [3]. Stat3 is a cytoplasmic protein that is activated in response to cytokines and growth factors and is considered as molecular machinery that regulates cell fate determination, renewal, differentiation, and apoptosis of various cell types, especially that ones at the embryonic developing stages [4, 5]. On activation, Stat3 molecules are translocated to nucleus, where they activate transcription of a series of target genes including c-Myc, survivin, and cyclin D1 that are closely associated with the growth, survival, and progression of cancer cells [5]. Indeed, in cancerous cells Stat3 constitutive activation is common, which is likely due to the aberrant activity of Stat3’s upstream signaling pathways, such as, EGFR, HER2, Src, and JAK2. Tumorigenic Stat3 activation has been frequently linked to more malignant cancer behaviors, including growth, epithelial-mesenchymal transition, migration, invasion, and metastasis. Stat3 activation is also associated with tumor survival and therapeutic resistance [6]. Many studies also have confirmed, the importance of Stat3 in skin carcinogenesis, skin cancer prevention, and treatment [4].
There is steadily growing interest in skin protection by plant polyphenols, although the mechanisms by which these natural compounds exert their beneficial effects are not fully understood [7]. One such polyphenol is resveratrol (3,5,4′-trihydroxy-trans-stilbene), a naturally occurring phytoalexin and the most extensively studied stilbene derivative. This compound has been shown to exert several beneficial effects, including cancer chemopreventive activity, in several preclinical studies [8, 9].
However, an important issue connected with the future application of resveratrol in disease management is its low bioavailability due to its rapid metabolism in mammals [10]. A strategy targeted at discovering and defining novel analogs of resveratrol has been assumed. These analogs should have the same structural backbone of resveratrol, with chemical modifications resulting in superior efficacy [11].
Our earlier studies showed that introduction of the methylthio-group into the stilbene core may influence the efficacy and selectivity of the inhibitory potency of these compounds toward the P450 isozymes, CYP1A1 and 1B1 [12, 13]. Other authors showed that substitution of the 4′ oxygen atom with a less electronegative sulfur atom also reduced toxicity toward the HEK 293 cells and enhanced the compound’s ability to activate human SIRT1 [14].
The aim of the present study was to further explore the activity of resveratrol and its methylthio-derivatives by evaluating their effect on EGFR and Stat3 activation. Two 4′-methylthio-trans-stilbene derivatives possessing one (3-M-4′-MTS; S2) and two (3,5-DM-4′-MTS; S5) methoxy groups were assessed, and immortalized human HaCaT keratinocytes and human epidermoid carcinoma A431 cells differing in EGFR constitutive expression were applied as an experimental model.
Materials and methods
Chemicals
Resveratrol (purity 99 %), dithiothreitol, antibiotic solution (104 U penicillin, 10 mg streptomycin, 25 μg amphotericin B), bovine serum albumin (BSA), dimethyl sulfoxide (DMSO), fetal bovine serum (FBS), Dulbecco’s Modified Eagle’s Medium (DMEM), and Tris were purchased from Sigma Chemicals (St. Louis, MO, USA). Primary and secondary antibodies against EGFR, Y1173-EGFR, Y1068-EGFR, Stat3, c-Myc, Bcl-xL, and β-actin were supplied by Santa Cruz Biotechnology (Santa Cruz, CA, USA). SDS-PAGE Gels (7.5 %, 10 %, 12 %) and the Western blotting detection system were purchased from Bio-Rad Laboratories (Hercules, CA, USA). All other compounds were readily available commercial products.
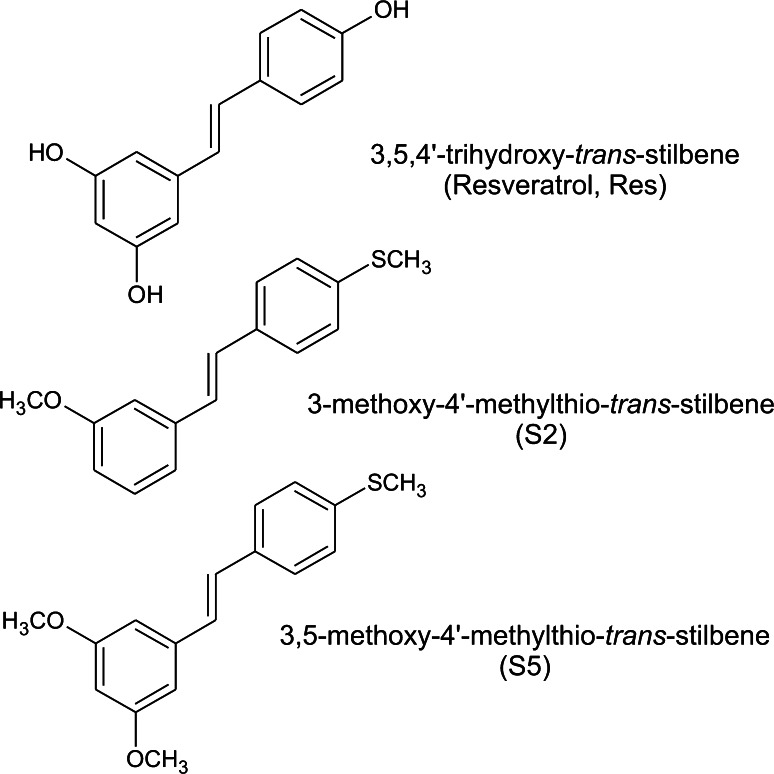
3-methoxy-4′-methylthio-trans-stilbene (S2) and 3,5-dimethoxy-4′-methylthio-trans-stilbene (S5) were synthesized as described previously [12]. Their structures are shown in Fig. 1.
Fig. 1.
Structures of resveratrol and methylthiostilbenes
Cell culture and treatment
Spontaneously immortalized human keratinocyte HaCaT cells, purchased from Cell Lines Service (CLS, Germany), and human epidermoid carcinoma A431 cells, obtained from Deutsche Sammlung von Mikroorganismen und Zellkulturen (DSMZ, Germany), were grown in DMEM containing 10 % FBS supplemented with antibiotics (penicillin, streptomycin, and amphotericin B). The cells were incubated at 37 °C in an atmosphere consisting of 95 % air and 5 % CO2 in a humidified incubator until they reached 80 % confluency. 1 × 106 cells were seeded in 100 mm ø culture dishes. After 24 h of preincubation in DMEM containing 5 % of FBS, the cells were treated with either 20 and 60 μM resveratrol, 20 and 60 μM S2 derivative, 5 and 20 μM S5 compound, or 0.1 % DMSO (control cells). Incubation was continued for a subsequent 24 h, then the cells were harvested. The preliminary experiments were performed in which the effect of resveratrol and its derivatives on EGFR at different time points (2, 4, 12, and 24 h) was estimated. No significant differences in time-course response were observed. For this reason, only one time point of 24 h was chosen for further studies.
Cell viability assay
The effects of resveratrol and methylthiostilbenes on cell viability were assessed with MTT assay according to standard protocols. Since the data on cytotoxicity of these compounds in HaCaT cells were presented previously [15], the MTT test was performed only in A431 cells. Briefly, 104 cells were seeded per well in a 96-well plate. After 24 h of preincubation in DMEM containing 5 % FBS, the tested compounds were added to the culture medium in various concentrations (0–200 μM), and the cells were incubated for a subsequent 72 h. The DMSO concentration did not exceed 0.1 %. After 72 h, the cells were washed twice with PBS buffer, and a fresh medium containing MTT salt (0.5 mg/ml) was added. After 4 h of incubation, formazan crystals were dissolved in acidic isopropanol and absorbance was measured at 540 and 690 nm. All of the experiments were repeated three times, with at least three measurements per assay.
In all of the subsequent experiments, non-toxic concentrations of methylthiostilbenes and resveratrol (viability level above 70 %) were used ranging from 5 to 60 µM, depending on compound.
Cell fractionation
Cytosol and nuclear extracts from HaCaT and A431 cells were prepared using the Nuclear/Cytosol Fractionation Kit (Active Motif, Carlsbad CA, USA). Whole cell lysates were prepared from HaCaT and A431 cells using the standard RIPA buffer.
Western blot analysis
Immunoblot assay was used to determine the level of EGFR, Y-1173-EGFR, Y-1068-EGFR Stat3, c-Myc, and Bcl-xL proteins. The protein content in the samples was determined with the Lowry method. Whole cell lysates (EGFR, Y-1173-EGFR, Y-1068-EGFR, c-Myc, Bcl-xL), nuclear and cytosolic fraction (Stat3) (50–100 μg) were separated on 10 % or 7.5 % or 12 % SDS-PAGE gels, and the proteins were transferred to nitrocellulose membranes [16, 17]. After blocking with 10 % skimmed milk, the proteins were probed with rabbit polyclonal EGFR, rabbit polyclonal Y-1173-EGFR, goat polyclonal Y-1068-EGFR, rabbit polyclonal Stat3, mouse monoclonal c-Myc, mouse monoclonal Bcl-xL, and rabbit polyclonal β-actin antibodies. The β-actin protein was used as an internal control. Alkaline phosphatase-labeled anti-rabbit IgG, anti-mouse IgG, or anti-goat IgG were used as the secondary antibodies in the staining reaction. The amount of immunoreactive product in each lane was determined using Quantity One software (BioRad Laboratories, Hercules, CA, USA). Values were calculated as relative absorbance units (RQ) per mg of protein.
Stat3—DNA-binding assay
Stat3 activation was assessed by estimating Stat3—DNA-binding capacity assay by the means of enzymatic immunoassay according to Jin et al. [18] using commercial kits (TransAM Stat Family; Active Motif, Carlsbad CA, USA) and following the manufacturer’s instructions. The amounts of Stat3 protein contained in DNA-binding complex were measured, and the Stat consensus binding site (5′-TTCCCGGAA-3′) was immobilized on ELISA microplates as a bait. The results were expressed as absorbance (OD450nm) per mg of protein.
Statistical analysis
The statistical analysis was performed by one-way ANOVA. The statistical significance between the experimental groups and their respective controls was assessed by Tukey’s post hoc test, with p < 0.05 being considered significant.
Results
The effect of resveratrol and methylthiostilbenes on cell viability in A431 cells
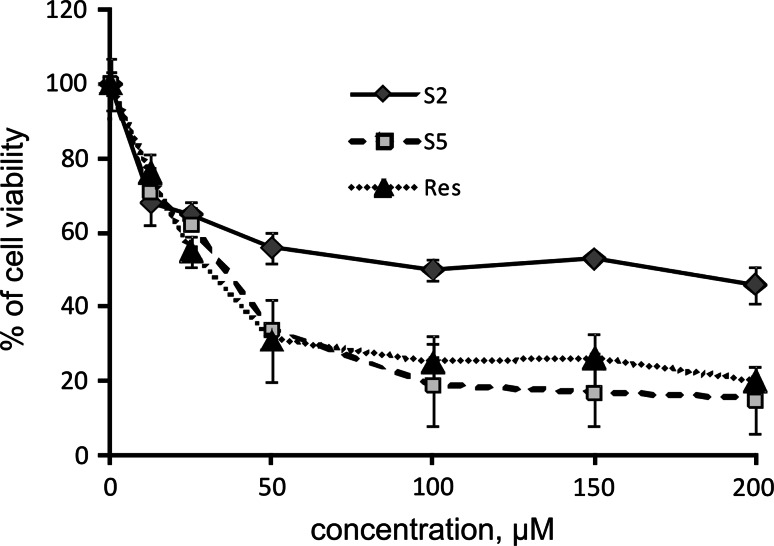
Figure 2 presents data on the effect of stilbenes on A431 viability. Within the concentration range of 2–200 μM, the tested compounds reduced the viability of the A431 cells in a dose-dependent manner. In this cell line, cytotoxicity of S5 was similar to the effect of resveratrol and compound S2 was less toxic. However, compound S2 exhibited higher toxicity toward A431 cells in comparison with HaCaT cells (IC50 182.66 ± 38.71 vs 141.28 ± 2.80 µM). Resveratrol also displayed higher toxicity toward A431 cells than to HaCaT cells (IC50 85.23 ± 29.17 vs 49.44 ± 3.76 µM), while the toxicity of S5 was similar in both cell lines [15].
Fig. 2.
Effect of resveratrol (RES) and methylthiostilbenes (S2, S5) on the viability of A431 cells. The mean values ± SEM from three independent experiments run in triplicate are shown. The viability of vehicle-treated cells was considered 100 %
The effect of resveratrol and methylthiostilbenes on EGFR activation in HaCaT and A431 cells
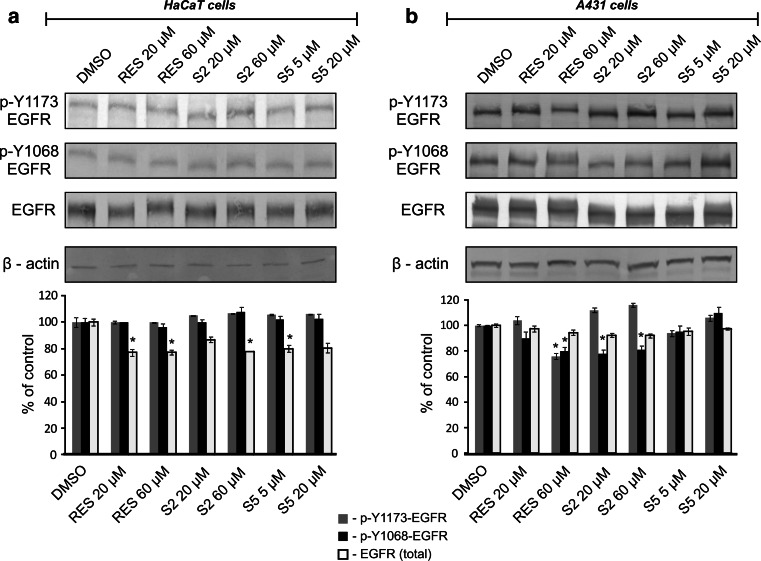
The effects of resveratrol and methylthiostilbenes on both the EGFR protein level and activation are shown in Fig. 3. In the HaCaT cells both derivatives, similarly to resveratrol, decreased the total level of the EGFR protein but did not affect the level of the phosphorylated Y1173 or Y1068 forms of this receptor (Fig. 3a). A different effect was observed in the A431 cells (Fig. 3b), where none of the compounds affected the total level of the EGFR protein, but the level of EGFR phosphorylation of the Y1068 residue was significantly (p < 0.05) reduced by resveratrol in the higher dose and by S2 in both doses. Additionally, resveratrol in the higher dose (60 µM) significantly diminished the level of Y1173 phosphorylation.
Fig. 3.
Effect of resveratrol and methylthiostilbenes on EGFR activation in HaCaT (a) and A431 (b) cells. Levels of the protein were assessed by Western blot analysis. Representative immunoblots and data (mean ± SEM) from three independent experiments run in duplicate are presented. β-actin was used as an internal control. The asterisk above the bar denotes statistically significant differences from the control cells (DMSO), p < 0.05
The effect of resveratrol and methylthiostilbenes on Stat3 activation in HaCaT and A431 cells
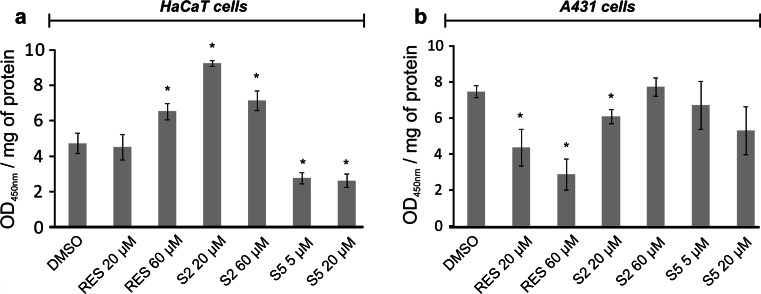
Figures 4 and 5 show the effect of resveratrol and methylthiostilbenes on Stat3 activation, estimated in terms of translocation of the Stat3 protein from the cytosol to the nuclear fraction (Fig. 4), and measured by Western blot and the binding capacity to DNA consensus sequences (Fig. 5). None of the tested compounds changed the level of Stat3 in the cytosolic or nuclear fractions of the HaCaT cells (Fig. 4a). However, resveratrol in the higher dose, and S2 in both doses, significantly increased Stat3 binding capacity to the DNA consensus site (Fig. 5a) in these cells. The S5 derivative had just the opposite effect, as in both of the tested doses this compound significantly diminished Stat3-DNA binding by approximately 50 %.
Fig. 4.
Effect of resveratrol and methylthiostilbenes on Stat3 translocation in HaCaT (a) and A431 (b) cells assayed by Western blot analysis. Representative immunoblots and data (mean ± SEM) from three independent experiments run in duplicate are presented. β-actin was used as an internal control. The asterisk above the bar denotes statistically significant differences from the control cells (DMSO), p < 0.05
Fig. 5.
Effect of resveratrol and methylthiostilbenes on Stat3 activation in HaCaT (a) and A431 (b) cells. Activated Stat3 was assessed in terms of the amount of Stat3 contained in the DNA-binding complexes extracted from the nuclei that were isolated from the cells. The Stat consensus site (5′-TTCCCGGAA-3′) was immobilized on ELISA plates as bait. Bars represent mean ± SEM from three independent experiments run in duplicate. The asterisk above the bar denotes statistically significant differences from the control cells (DMSO), p < 0.05
In human epidermoid carcinoma A431 cells, resveratrol was the only compound affecting the total level of the Stat3 protein, as it significantly diminished this transcription factor content in the nuclear fraction by 20 % (Fig. 4b). Also, resveratrol significantly diminished the Stat3 binding capacity to the Stat consensus sequence in a dose-dependent manner (Fig. 5b), while S2 was active to a much lesser extent and only in the lower concentration. In the case of S5, which significantly affected Stat3 binding in the HaCaT cells, no statistically significant effect was observed in human skin carcinoma A431 cells.
The effect of resveratrol and methylthiostilbenes on Stat3 downstream targeted genes in HaCaT and A431 cells
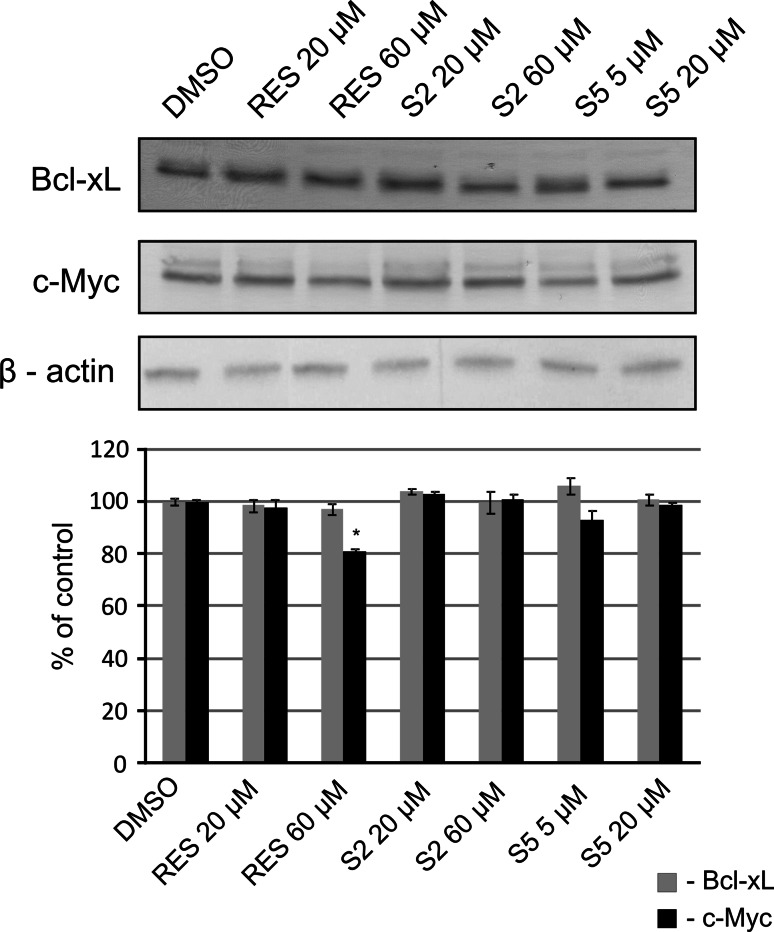
Figure 6 shows the effect of resveratrol and methylthiostilbenes on c-Myc and Bcl-xL protein level in A431 cells. In these cells, only resveratrol in the higher dose (60 µM) significantly diminished the level of c-Myc. The level of these proteins in HaCaT cells was too low for quantitative statistical evaluation.
Fig. 6.
Effect of resveratrol and methylthiostilbenes on Stat3 downstream targeted genes in A431 cells assayed by Western blot analysis. Representative immunoblots and data (mean ± SEM) from three independent experiments run in duplicate are presented. β-actin was used as an internal control. The asterisk above the bar denotes statistically significant differences from the control cells (DMSO), p < 0.05
Discussion
Overexpression and/or increased activity of EGFR are the key characteristics of human tumors and are frequently linked to more aggressive tumor behavior, including increased proliferation, metastasis, and therapeutic resistance [19].
Several reports described the nuclear localization of EGFR which resulted from translocation directly into the nucleus and which omitted the traditional pathway that requires the activation of signaling cascades [20, 21]. Although EGFR possesses transactivational activity, it lacks a DNA-binding domain and requires a DNA-binding transcription cofactor for its transcriptional function. In this regard, it was shown that EGFR physically and functionally interacts with Stat3 in the nucleus, leading to transcriptional activation of the inducible nitric oxide synthase (iNOS) gene. Thus, combining agents targeting both EGRF and Stat3 may improve the chemopreventive and/or therapeutic effect. Indeed, the combination of AG490 and AG1478 was more potent than the inhibitor alone in killing EGFR-overexpressing cells, i.e., A431 and MDA-MB-468 (human breast carcinoma) [22]. Single agents affecting both of these targets are equally desired.
Resveratrol has been demonstrated to affect a multitude of signal transduction pathways associated with tumorigenesis [23]. In mouse skin, reduction of the iNOS protein level and activity elevated by the tumor promoter, TPA, were observed as a result of pretreatment with resveratrol, and even more by its naturally occurring analog, pterostilbene.
This observation may imply the involvement of altered EGFR/Stat3 activation in TPA induction of iNOS in mouse skin. Moreover, it suggests that resveratrol analogs might be more efficient in the modulation of this pathway.
In this study, we compared the effect of resveratrol and its methylthio-derivatives on the expression and activation of EGFR and Stat3 in two cell lines which differed in EGFR expression. While the A431 cells were characterized by EGFR-overexpression, spontaneously immortalized keratinocytes, HaCaT cells, have low constitutive EGFR activity. Moreover, EGFR activation in HaCaT cells had no effect on Stat3 Y705 phosphorylation [24] .
Activation of EGFR results in autophosphorylation of the C-terminal region. The major sites of phosphorylation are the Y1068 and Y1173 tyrosine residues [25].
Treatment of the HaCaT cells with either resveratrol or methylthiostilbenes did not affect the phosphorylation of these residues, although the total level of the EGFR protein was significantly reduced. This may suggest that stilbenes might reduce the constitutive expression of EGFR or stimulate its degradation. The latter may occur e.g., through activating of ubiquitination and sorting machinery such as ESCRT [26]. It also cannot be excluded that other EGFR residues which were not evaluated in this study might be affected and contributed to overall EGFR protein reduction. The possible candidates are 1045 and 1173 tyrosine residues which were found to be phosphorylated in A431 cells, but were not evaluated yet in HaCaT cells [27].
In EGFR-overexpressing A431 cells, a significant reduction of EGFR with both phosphorylated residues was observed as a result of resveratrol at higher dose treatment. Compound S2, 3-methoxy-4′-methylthio-trans-stilbene, significantly reduced the level of EGFR with the phosphorylated Y1068 tyrosine residues. Introducing an additional methoxy group to the stilbene ring made this compound ineffective as an EGFR activation modulator.
Reducing the phosphorylation of the Y1068 residue by resveratrol and compound S2 may have further consequences, as we found in our previous studies that phosphorylation of this tyrosine correlated with AP-1 activation in mouse skin. This transcription factor is involved in COX-2 and iNOS induction, which is reduced by resveratrol in this model [28, 29].
Reduced activation of EGFR by resveratrol and its S2 analog was connected with reduced activation of Stat3. Resveratrol treatment reduced the binding level of this transcription factor to its consensus oligonucleotide measured in terms of its amount in the DNA-binding complex extracted from the nuclear fraction of the A431 cells. Thus this observation indirectly confirmed the interaction of EGFR and Stat3 in these cells, as was described by Lo et al. [30].
On the other hand, in the HaCaT cells the S5 stilbenoid reduced the binding of Stat3 to its consensus sequence, while resveratrol and the S2 compound increased Stat3 activation. The above-mentioned studies by Quadros et al. [24] demonstrated, however, that DNA binding of activated Stat3 is restricted to malignant cells (SCC) and strictly dependent on EGFR activation. Thus the results of our current experiments may suggest that activation of Stat3 in nonmalignant cells, such as HaCaT, in response to stilbene treatment occurs in an EGFR-independent way. Further studies are required to confirm this suggestion. Collectively, the results of our present study indicate that stilbenoids may affect both EGFR and Stat3 in malignant, EGFR-overexpressing tumor cells.
However, modification of the stilbene ring does not affect the EGFR/Stat3 system to a greater extent than resveratrol.
Acknowledgments
This work was supported by the Ministry of Science and Higher Education of Poland, Grant N N 405 209737.
Conflicts of interest
All authors declare no conflicts of interest.
Abbreviations
- A431
Human epidermoid carcinoma cells
- DMEM
Dulbecco’s Modified Eagle’s Medium
- DMSO
Dimethyl sulfoxide
- EGFR
Epidermal growth factor receptor
- FBS
Fetal bovine serum
- HaCaT
Spontaneously immortalized human keratinocytes
- MAPKs
Mitogen-activated protein kinases
- Res
Resveratrol
- S2
3-Methoxy-4′-methylthio-trans-stilbene (3-M-4′-MTS)
- S5
3,5-Dimethoxy-4′-methylthio-trans-stilbene (3,5-DM-4″-MTS)
- Stat
Signal transducer and activator of transcription
Footnotes
Michał Cichocki and Hanna Szaefer have equally contributed to this paper.
References
- 1.Pastore S, Mascia F, Mariani V, Girolomoni G. The epidermal growth factor receptor system in skin repair and inflammation. Invest Dermatol. 2008;128(6):1365–1374. doi: 10.1038/sj.jid.5701184. [DOI] [PubMed] [Google Scholar]
- 2.Herbst RS. Review of epidermal growth factor receptor biology. Int J Radiat Oncol Biol Phys. 2004;59(2 Suppl):21–26. doi: 10.1016/j.ijrobp.2003.11.041. [DOI] [PubMed] [Google Scholar]
- 3.Pastore S, Lulli D, Fidanza P, Potapovich AI, Kostyuk VA, De Luca C, Mikhal’chik E, Korkina LG. Plant polyphenols regulate chemokine expression and tissue repair in human keratinocytes through interaction with cytoplasmic and nuclear components of epidermal growth factor receptor system. Antioxid Redox Signal. 2012;16(4):314–328. doi: 10.1089/ars.2011.4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Macias E, Rao D, DiGiovanni J. Role of stat3 in skin carcinogenesis: insights gained from relevant mouse models. J Skin Cancer. 2013;2013:684050. doi: 10.1155/2013/684050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu LJ, Wu ML, Li H, Chen XY, Wang Q, Sun Y, Kong QY, Liu J. Inhibition of STAT3 expression and signaling in resveratrol-differentiated medulloblastoma cells. Neoplasia. 2008;10(7):736–744. doi: 10.1593/neo.08304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carpenter RL, Lo HW. STAT3 target genes relevant to human cancers. Cancers (Basel) 2014;6(2):897–925. doi: 10.3390/cancers6020897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Korkina LG, Pastore S, De Luca C, Kostyuk VA. Metabolism of plant polyphenols in the skin: beneficial versus deleterious effects. Curr Drug Metab. 2008;9(8):710–729. doi: 10.2174/138920008786049267. [DOI] [PubMed] [Google Scholar]
- 8.Nakata R, Takahashi S, Inoue H. Recent advances in the study on resveratrol. Biol Pharm Bull. 2012;35(3):273–279. doi: 10.1248/bpb.35.273. [DOI] [PubMed] [Google Scholar]
- 9.Aluyen JK, Ton QN, Tran T, Yang AE, Gottlieb HB, Bellanger RA. Resveratrol: potential as anticancer agent. J Diet Suppl. 2012;9(1):45–56. doi: 10.3109/19390211.2011.650842. [DOI] [PubMed] [Google Scholar]
- 10.Ndiaye M, Kumar R, Ahmad N. Resveratrol in cancer management: where are we and where we go from here? Ann N Y Acad Sci. 2011;1215:144–149. doi: 10.1111/j.1749-6632.2010.05851.x. [DOI] [PubMed] [Google Scholar]
- 11.Szekeres T, Fritzer-Szekeres M, Saiko P, Jäger W. Resveratrol and resveratrol analogues-structure-activity relationship. Pharm Res. 2010;27(6):1042–1048. doi: 10.1007/s11095-010-0090-1. [DOI] [PubMed] [Google Scholar]
- 12.Mikstacka R, Baer-Dubowska W, Wieczorek M, Sobiak S. Thiomethylstilbenes as inhibitors of CYP1A1, CYP1A2 and CYP1B1 activities. Mol Nutr Food Res. 2008;52(Suppl 1):S77–S83. doi: 10.1002/mnfr.200700202. [DOI] [PubMed] [Google Scholar]
- 13.Mikstacka R, Rimando AM, Dutkiewicz Z, Stefański T, Sobiak S. Design, synthesis and evaluation of the inhibitory selectivity of novel trans-resveratrol analogues on human recombinant CYP1A1, CYP1A2 and CYP1B1. Bioorg Med Chem. 2012;20(17):5117–5126. doi: 10.1016/j.bmc.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 14.Yang H, Baur JA, Chen A, Miller C, Sinclair DA. Design and synthesis of compounds that extend yeast replicative lifespan. Aging Cell. 2007;6(1):35–43. doi: 10.1111/j.1474-9726.2006.00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Szaefer H, Cichocki M, Krajka-Kuźniak V, Stefański T, Sobiak S, Licznerska B, Baer-Dubowska W. The effect of resveratrol and its methylthio-derivatives on NF-κB and AP-1 signaling pathways in HaCaT keratinocytes. Pharmacol Rep. 2014;66(5):732–740. doi: 10.1016/j.pharep.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 16.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 17.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyarylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jin S, Lu D, Ye S, Ye H, Zhu L, Feng Z, Liu S, Wang D, Hu Q. A simplified probe preparation for ELISA-based NF-kappaB activity assay. J Biochem Biophys Methods. 2005;65(1):20–29. doi: 10.1016/j.jbbm.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 19.Craven RJ, Lightfoot H, Cance WG. A decade of tyrosine kinases: from gene discovery to therapeutics. Surg Oncol. 2003;12(1):39–49. doi: 10.1016/S0960-7404(03)00004-5. [DOI] [PubMed] [Google Scholar]
- 20.Lin SY, Makino K, Xia W, Matin A, Wen Y, Kwong KY, Bourguignon L, Hung MC. Nuclear localization of EGF receptor and its potential new role as a transcription factor. Nat Cell Biol. 2001;3(9):802–808. doi: 10.1038/ncb0901-802. [DOI] [PubMed] [Google Scholar]
- 21.Offterdinger M, Schöfer C, Weipoltshammer K, Grunt TW. c-erbB-3: a nuclear protein in mammary epithelial cells. J Cell Biol. 2002;157(6):929–939. doi: 10.1083/jcb.200109033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dowlati A, Nethery D, Kern JA. Combined inhibition of epidermal growth factor receptor and JAK/STAT pathways results in greater growth inhibition in vitro than single agent therapy. Mol Cancer Ther. 2004;3(4):459–463. [PubMed] [Google Scholar]
- 23.Whitlock NC, Baek SJ. The anticancer effects of resveratrol: modulation of transcription factors. Nutr Cancer. 2012;64(4):493–502. doi: 10.1080/01635581.2012.667862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quadros MR, Peruzzi F, Kari C, Rodeck U. Complex regulation of signal transducers and activators of transcription 3 activation in normal and malignant keratinocytes. Cancer Res. 2004;64(11):3934–3939. doi: 10.1158/0008-5472.CAN-04-0214. [DOI] [PubMed] [Google Scholar]
- 25.Abe M, Kuroda Y, Hirose M, Watanabe Y, Nakano M, Handa T. Inhibition of autophosphorylation of epidermal growth factor receptor by small peptides in vitro. Br J Pharmacol. 2006;147(4):402–411. doi: 10.1038/sj.bjp.0706634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eden ER, Huang F, Sorkin A, Futter CE. The role of EGF receptor ubiquitination in regulating its intracellular traffic. Traffic. 2012;13(2):329–337. doi: 10.1111/j.1600-0854.2011.01305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kondratov KA, Chernorudskiy AL, Amosova AP, Kornilova ES. Termination of tyrphostin AG1478 application results in different recovery of EGF receptor tyrosine residues 1045 and 1173 phosphorylation in A431 cells. Cell Biol Int. 2009;34(1):81–87. doi: 10.1042/CBI20090159. [DOI] [PubMed] [Google Scholar]
- 28.Kundu JK, Shin YK, Kim SH, Surh YJ. Resveratrol inhibits phorbol ester-induced expression of COX-2 and activation of NF-kappaB in mouse skin by blocking IkappaB kinase activity. Carcinogenesis. 2006;27(7):1465–1474. doi: 10.1093/carcin/bgi349. [DOI] [PubMed] [Google Scholar]
- 29.Cichocki M, Szamałek M, Baer-Dubowska W. Correlation between EGFR Y1068 tyrosine phosphorylation and AP-1 activation by tumor promoter 12-O-tetradecanoylphorbol-13-acetate in mouse skin. Environ Toxicol Pharmacol. 2012;33(1):92–97. doi: 10.1016/j.etap.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 30.Lo HW, Hsu SC, Ali-Seyed M, Gunduz M, Xia W, Wei Y, Bartholomeusz G, Shih JY, Hung MC. Nuclear interaction of EGFR and STAT3 in the activation of the iNOS/NO pathway. Cancer Cell. 2005;7(6):575–589. doi: 10.1016/j.ccr.2005.05.007. [DOI] [PubMed] [Google Scholar]