Abstract
Background
There is a need to identify novel pharmacological targets to treat alcoholism. Animal and human studies suggest a role of ghrelin in the neurobiology of alcohol dependence and craving. Here, we were the first to test the hypothesis that intravenous administration of exogenous ghrelin acutely increases alcohol craving.
Methods
This was a double-blind placebo-controlled human laboratory proof-of-concept study. Non-treatment seeking alcohol-dependent heavy drinking individuals were randomized to receive intravenous ghrelin 1mcg/kg, 3 mcg/kg or 0 mcg/kg (placebo), followed by a cuereactivity procedure, during which participants were exposed to neutral (juice) and alcohol cues. The primary outcome variable was the increase in alcohol craving (also called “urge”) for alcohol, assessed by the Alcohol Visual Analogue Scale.
Results
Out of 103 screenings, 45 individuals received the study drug. Repeated measures of ANCOVA revealed a group effect across ghrelin doses in increasing alcohol craving (p < .05). A dose-specific examination revealed a significant effect of ghrelin 3 mcg/kg vs. placebo in increasing alcohol craving (p < .05) with a large effect size (d = .94). By contrast, no significant ghrelin effect was found in increasing either urge to drink juice or food craving (p: n.s.). No significant differences in side effects were found (p: n.s.).
Conclusions
Intravenous administration of exogenous ghrelin increased alcohol craving in alcohol-dependent heavy drinking individuals. Although the small sample requires confirmatory studies, these findings provide preliminary evidence that ghrelin may play a role in the neurobiology of alcohol craving, thus demonstrating a novel pharmacological target for treatment.
Keywords: ghrelin, alcoholism, craving, cue-reactivity, neuroendocrinology, feeding peptides
Introduction
Alcoholism is one of the leading causes of mortality and morbidity (1, 2). Therefore, interventions for alcoholism may have important implications. Hence, there is a need to identify new pathways that may serve as pharmacological targets for treatment (3).
Ghrelin is a 28-amino acid peptide acting as the endogenous ligand for the growth hormone secretagogue receptor (GHS-R1a) (4). Ghrelin activates hypothalamic orexigenic neurons and inhibits anorectic neurons to induce hunger and stimulate feeding (5, 6). GHS-R1a’s are highly co-expressed with dopamine receptors, in the midbrain, raphe nuclei and ventral tegmental area (VTA) (7–9), suggesting that ghrelin modulates reward processing. In mice, ghrelin administration intraperitoneally (10), or centrally into the VTA (11, 12) activates measures associated with reward.
Preliminary human studies show differences in endogenous blood ghrelin levels in actively drinking (13–15) and abstinent alcoholics (16, 17) and changes in ghrelin levels over time based on the drinking status (18). Some studies indicated a significant positive correlation between ghrelin levels and alcohol craving [(13, 18, 19) (but see: (17)]. However, while animal studies tested the direct effects of exogenous ghrelin administration, human studies were limited to measuring endogenous blood ghrelin levels and retrospective measures of alcohol craving, thus significantly limiting their bench-to-bedside value (20). There is no human evidence that manipulations of ghrelin signaling via administration of exogenous ghrelin increases alcoholseeking behaviors, such as alcohol craving. This was the first study testing this hypothesis.
Subjects and Methods
Design and Setting
This was a 3-group between-subject double-blind placebo-controlled randomized human laboratory proof-of-concept study, conducted at the Brown University Center for Alcohol and Addiction Studies, Providence (RI).The study was approved by the Brown University Institutional Review Board. The use of synthetic human ghrelin was approved under the Food and Drug Administration (FDA) IND 109,242.
Study drug
Current Good Manufacturing Practice human acetylated ghrelin was purchased from PolyPeptide Laboratories (Torrance, CA). The purity of the peptide was > 95% and its authenticity was confirmed by mass spectrometry, tandem mass spectrometry analysis, and aminoacid analysis. The final ghrelin solution demonstrated excellent stability after 72 hours, was sterile and free of detectable pyrogens. The final solution was always prepared < 48 hours before its administration.
Study population
Non-treatment seeking alcohol-dependent men and women were screened according to inclusion/exclusion criteria (Supplemental Material).
Study overview
Potential participants recruited via mass media were pre-screened by phone. Potentially eligible participants came to our facility. After complete description of the study, written informed consent was obtained and then screening took place (Visit 1). Eligible participants were randomized to ghrelin 1 mcg/kg, 3 mcg/kg or 0 mcg/kg (placebo) and the experimental session (Visit 2) was scheduled. The experimental session consisted of a ~10-minute administration of ghrelin/placebo, followed by a cue-reactivity procedure. A breath alcohol concentration 0.00 was required at each visit. Compensation in the form of cash was provided at each visit.
Experimental session
The session was conducted individually in a one-way mirror room. Subjects came to our lab having fasted. An intravenous cannula was placed, and a fixed light breakfast was served, i.e. ~167 Kcal; approximately 62% carbohydrate, 13% protein, 25% fat (11, 21–23). Consistent with previous cue-reactivity studies (24, 25), participants were exposed to visual, tactile, olfactory, and proprioceptive stimuli associated with neutral and alcohol beverages. As for the neutral condition, a palatable nonalcoholic beverage, rather than water (24, 25), was included. Specifically, consistent with (26), a juice condition was used, i.e. a 295ml bottle of a commercially available fruit punch. Additionally, food craving was assessed via the General Food-Cravings Questionnaire-State (27).
Before intravenous ghrelin/placebo administration, participants underwent a 3-minute relaxation period to collect baseline levels of urge and physiological arousal (Table 1). Then, a staff member entered the room with a tray covered by an inverted pitcher, containing the fruit punch bottle and a glass. The pitcher was removed, the bottle was opened, and the glass was filled. The staff member left the room, and an audiotape instructed the participant to sniff the glass of juice when s/he heard high tones and stop sniffing when s/he heard low tones. This procedure included thirteen 5-second olfactory exposures during each 3-minute trial. Next, participants underwent a 3-minute alcohol cue exposure that was identical to the previous procedure except the juice bottle was replaced with the appropriate commercially-labeled alcohol bottle. After the stimuli were removed, a relaxation period took place, a second juice trial and finally a second alcohol trial were presented (Table 1). For urge, Alcohol Visual Analogue Scale and Juice Visual Analogue Scale were rated on 11-point anchored Likert-type scales (26). The Alcohol Attention Scale was used to assess attention to sight/smell of alcohol cues (24). We also assessed attention to sight/smell of juice cues, adapting the Alcohol Attention Scale (i.e. Juice Attention Scale).
Table 1.
Study activities and timeline during the experimental session.
| Time point (minute) |
Procedures and/or Assessments |
|---|---|
| −40 | Breakfast (~167 Kcal) |
| −15 |
Baseline: Blood Sample #1, Urge to Drink Alcohol, Urge to Drink Juice, Food Craving |
| −13 |
Baseline: Mean Arterial Pressure, Heart Rate |
| −10 |
Study Drug Intravenous (IV) Administration: Ghrelin 1 mcg/kg, Ghrelin 3 mcg/kg or 0 mcg/kg (placebo) |
| +3 |
Juice Trial #1: Mean Arterial Pressure, Heart Rate, Saliva Mass |
| +6 |
Post-Juice Trial #1: Urge to Drink Juice, Juice Attention Scale, Blood Sample #2, Relaxation Period |
| +9 |
Alcohol Trial #1: Mean Arterial Pressure, Heart Rate, Saliva Mass |
| +17 |
Post-Alcohol Trial #1: Urge to Drink Alcohol, Alcohol Attention Scale, Blood Sample #3, Adverse Events, Food Craving, Relaxation Period |
| +20 |
Juice Trial #2: Mean Arterial Pressure, Heart Rate, Saliva Mass |
| +23 |
Post-Juice Trial #2: Urge to Drink Juice, Juice Attention Scale, Blood Sample #4, Relaxation Period |
| +26 |
Alcohol Trial #2: Mean Arterial Pressure, Heart Rate, Saliva Mass |
| +29 |
Post-Alcohol Trial #2: Urge to Drink Alcohol, Alcohol Attention Scale, Blood Sample #5, Adverse Events |
| +46 | Relaxation Period |
| +48 |
Post-Experiment Assessment: Blood Sample #6, Urge to Drink Alcohol, Food Craving |
Previous studies indicate that alcohol cue-elicited craving may be associated with parallel increases in mean arterial pressure (MAP), heart rate (HR) and salivation (28). In this study, we measured HR and salivation, as secondary outcomes, while we monitored MAP only for safety reasons. In fact, a reduction of blood pressure is a possible common transitory side-effect of intravenous ghrelin, therefore, blood pressure might have exhibited low validity in our paradigm. MAP and HR were obtained using using a monitoring machine. As for measuring salivation, participants placed three dental rolls in their mouths, rolls were weighed immediately before and after the cue-reactivity with an analytical scale, so that the net difference indicated the saliva mass produced during cue-reactivity (29). After all procedures were completed, a meal was provided, a post-session debriefing was performed and then participants were released. The Systematic Assessment for Treatment Emergent Events (SAFTEE) was used for adverse events (30, 31). Approximately a week after Visit 2, a brief safety follow-up (Visit 3) took place, during which a brief motivational session to reduce alcohol use was provided, based on the Motivational Enhancement Therapy manual (32).
Blood samples analysis
Blood samples were collected at six timepoints (Table 1), centrifuged and stored at −80 °C. Samples were analyzed together at the end of the study in order to maintain the blind. Total serum ghrelin levels were determined using a fluorescent bead-based Bio-Plex assay (Bio-Rad, Hercules, CA) following the manufacturer’s protocol. Results were expressed as pg/mL.
Statistical analysis
Preliminary analyses included the examination of the distributions of the outcome measures. All outcome measures approximated a normal distribution as indicated by skewness and kurtosis within −2 to +2. The primary statistical method used was repeated measures analysis of covariance (ANCOVA). The number of standard drink units (SDUs) consumed the day before was chosen as a covariate in the model since recent prior alcohol consumption is known to affect craving (33, 34). Furthermore, consistent with previous ghrelinrelated literature [e.g.: (35, 36)], total ghrelin serum level at baseline was also fitted as covariate. Typically, the baseline assessment (when available) for the dependent measure was also entered as a covariate, unless a change from baseline score was used as the dependent measure. This was a proof-of-concept study and the sample size was based on the effects of ghrelin on urge to drink alcohol only. The interaction with non-alcoholic cues (juice) was only an exploratory outcome, given that the study was not powered for a dose×cue type interaction. We also conducted a number of pairwise correlations between total ghrelin serum levels and a number of other variables of interest (alcohol craving, food craving, etc.). All pairwise comparisons were conducted with a Bonferroni correction. SPSS version 21 was used.
Results
Sample description
Forty-five alcohol-dependent heavy drinking individuals received the study drug. Figure S1 outlines the trial flow-chart. Demographics and baseline characteristics are outlined in Table 2.
Table 2.
Demographics and baseline characteristics of the enrolled sample.
| Total sample |
Placebo | Ghrelin 1 mcg/Kg |
Ghrelin 3 mcg/Kg |
P value |
|
|---|---|---|---|---|---|
| Number (n) | 45 | 18 | 13 | 14 | |
| Females(%) | 36 | 39 | 31 | 36 | .90 |
| Age (years) | |||||
| range | 25–62 | 28–62 | 25–57 | 25–58 | |
| M ± SD | 44.7 ± 9.1 | 46.6 ± 9.0 | 42.8 ± 9.8 | 43.9 ± 8.6 | .49 |
| median | 47 | 48 | 43 | 45 | |
| Race/Ethnicity (%) | |||||
| Black | 31.1 | 22.2 | 38.5 | 35.7 | |
| White | 53.3 | 61.1 | 46.2 | 50.0 | |
| Latino | 4.4 | 5.6 | 7.7 | 0 | |
| Others | 11.1 | 11.1 | 7.7 | 14.3 | |
| BMI [M ± SD] | 25.8 ± 3.1 | 26.4 ± 2.5 | 25.2 ± 3.3 | 25.7 ± 3.7 | .56 |
| Age of onset for alcohol problems | |||||
| range | 12–52 | 12–52 | 14–33 | 15–36 | |
| M ± SD | 21.8 ± 8.1 | 22.9 ± 10.9 | 20.3 ± 5.2 | 21.8 ± 6.0 | .68 |
| median | 20 | 20 | 19 | 20 | |
| 90-day baseline drinks/drinking day | 11.8 ± 6.8 | 11.8 ± 7.9 | 11.8 ± 6.8 | 11.8 ± 5.7 | 1.00 |
| Number of drinks the day before the experimental session | 4.5 ± 5.5 | 4.2 ± 5.3 | 4.9 ± 5.7 | 4.5 ± 5.8 | .94 |
| Alcohol Dependence Severity (ADS) | 11.4 ± 7.0 | 12.6 ± 8.4 | 9.0 ± 4.4 | 12.0 ± 6.8 | .35 |
| CIWA-Ar | 1.2 ± 1.6 | 1.7 ± 2.0 | 1.1 ± 1.4 | 0.6 ± 0.8 | .15 |
| 90-day baseline cigarettes/day* | 14.8 ± 10.5 | 16.6 ± 10.8 | 16.4 ± 14.1 | 11.1 ± 5.1 | .42 |
M: Median; SD: Standard Deviation; BMI: Body Mass Index; drink = Standard Drinking Unit (SDU); CIWA-Ar: Clinical Institute Withdrawal Assessment of Alcohol Scale, Revised
76% of the sample were smokers.
Urge to drink alcohol (primary endpoint)
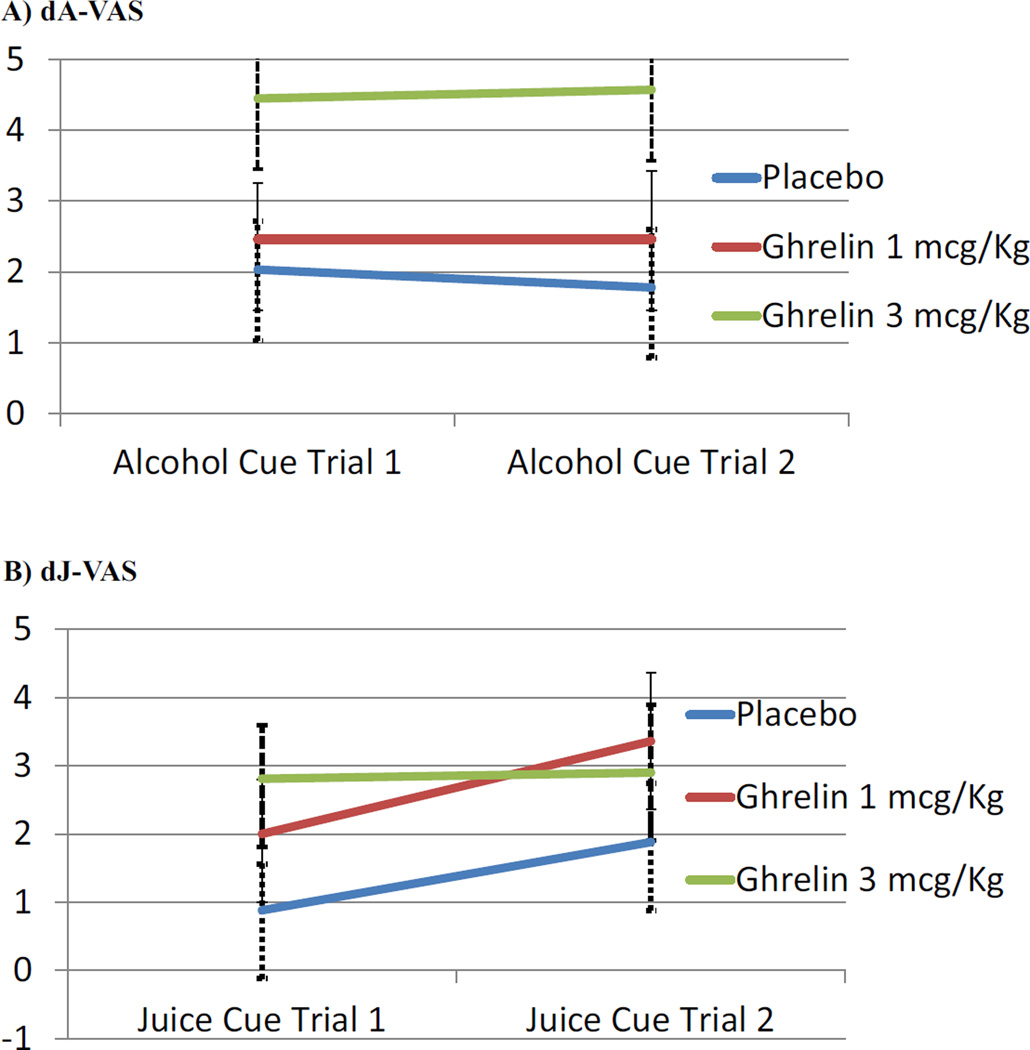
Increase in alcohol urge after intravenous ghrelin administration, versus as compared to placebo, was the primary aim. Repeated measures (two trials) ANCOVAs were conducted for the increase in alcohol urge (i.e., increase in the Alcohol-Visual Analogue Scale score) during the cue-reactivity procedure relative to the pre-drug urge level. Ghrelin dose was statistically related to urge increase [F(2,40) = 3.36, p = .045] (Figure 1A). Pairwise comparisons revealed that alcohol urge was significantly greater for ghrelin 3 mcg/kg than placebo (p = .046). The effect size for the increase in urge to drink alcohol for ghrelin 3 mcg/kg versus placebo was large (d = 0.94). No statistically significant differences were found in the ghrelin 1 mcg/kg vs. placebo conditions (p = 1.00) nor between ghrelin 1 mcg/kg and ghrelin 3 mcg/kg (p = .23).
Figure 1.
(A) Increase in alcohol urge by dose, expressed as its increase compared to the baseline (predrug) value of the Alcohol-Visual Analogue Scale (dA-VAS). Repeated measures ANCOVA indicated that ghrelin dose was statistically related to alcohol urge increase [F(2,40) = 3.36, p = .045], and Bonferroni-corrected pairwise comparisons revealed that alcohol urge was significantly greater for ghrelin 3 mcg/kg than placebo (p = .046). The effect size for the increase in alcohol urge for ghrelin 3 mcg/kg versus placebo was large (d = 0.94). (B) Increase in juice urge by dose, expressed as its increase compared to the baseline (pre-drug) value of the Juice-Visual Analogue Scale (dJ-VAS). Repeated measures ANCOVA indicated that ghrelin dose was not statistically related to juice urge increase [F(2,40) = 1.16, p = .32].
Other secondary outcomes
Urge to Drink Juice and Food Craving
Ghrelin dose was not statistically related to increased urge to drink juice [F(2,40) = 1.16, p = .32; Figure 1B], nor to food cravings questionnaire scores [F(2,39) = 0.28, p = .76]. Table S1 shows the mean and standard deviation for alcohol and juice urge increases in the three groups collapsed across the two alcohol trials and collapsed across the two juice trials.
Although the study was not powered to examine a dose by cue type interaction, this was also explored via a 3 (dose)×2 (cue)×2 (trial) ANCOVA, with SDUs the day before and baseline ghrelin as covariates, and change in urge as the DV. However, relative to Rohsenow et al.(26) which had an average cell size of 26 and only two doses conditions, this exploratory analyses were compromised by a lack of statistical power with a cell size of 15 (relative to a main effect, the power to detect an interaction is based on half the cell size). In this analysis, a cue effect was demonstrated [F(1,45) = 4.08, p = .049] and there was a trend for a dose effect [F(2,45) = 3.05, p = .057] but the cue by dose interaction was not significant [F(2,45) = 1.12, p = .33].
We also examined correlations between increases in urge for juice, food, and alcohol in trials 1 and 2, and then again in trials 3 and 4. For the whole sample, alcohol and juice urge increases were related, first and second trials: r(43) = .45, p = .002; third and fourth trials: r(43) = .48, p = .001. However, the correlations with an increase in food craving were not correlated with increases in the other urges, perhaps due to methodological reasons, given the differing format and number of items (r’s ranged from −.08 to +.14, p’s ranged from .38 to .73). Regarding the correlations between an increase alcohol and juice urge, this was most pronounced for the first and second trials for the low ghrelin dose group [r(11) = .61, p = .03] and for the third and fourth trials for the high ghrelin dose [r(12) = .83, p < .001].
Ghrelin Safety
As summarized in Table S2, ghrelin dose did not significantly predict adverse events for the three post-injection assessments [F(2,40) = 0.97, p = .39]. Due to the short half-life of ghrelin, we also ran a parallel analysis on only the first two post-injection assessments, and no significant group differences were found [F2,40) = 1.37, p = .27]. There was a significant dose-dependent effect of ghrelin in reducing MAP for both the first [F(2,34) = 8.39, p = .001] and the second [F(2,36) = 9.04, p = .001] alcohol trials; as well as for both the first [F(2,35) = 6.17, p = .005] and the second [F(2,32) = 12.13, p < .001] juice trials (Table S3).
Alcohol and Juice Attention Scales
Ghrelin dose did not predict the Alcohol Attention Scale [F(2,39) = 1.01, p = .38] nor the Juice Attention Scale [F(2,39) = 0.50, p = .61].
Heart Rate
HR was analyzed in the same manner as MAP. No significant difference were found across drug conditions in HR in all cue-reactivity trials, i.e. first alcohol trial [F(2,34) = 0.72, p = .49], second alcohol trial [F(2,36) = 2.71, p = .08], first juice trial [F(2,35) = 1.57, p = .22], second juice trial [F(2,32) = 3.20, p = .054] (Table S3).
Salivation
Ghrelin dose-dependently reduced saliva mass during both the alcohol trials [F(2,39) = 5.48, p = .008] and the juice trials [F(2,39) = 8.31, p = .001] (Table S4).
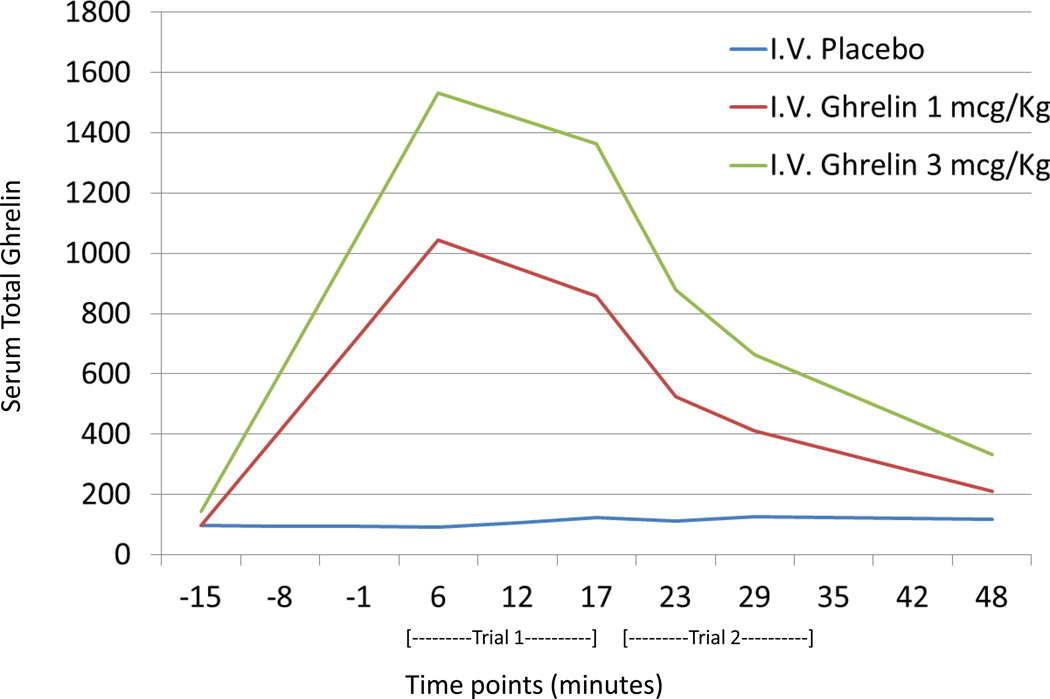
Effects of intravenous ghrelin administration to serum ghrelin levels
As expected, there was a pronounced main effect for dose administered [F(2,39) = 88.0, p < .001] as well as a dose by time interaction [F(2,39) = 82.1, p < .001] (Figure 2). Pairwise comparisons revealed that all three conditions were statistically significantly different [all three p’s < .001; ghrelin 3mcg/kg: 934.5 (174.5); ghrelin 1mcg/kg: 618.7 (172.7); and placebo: 122.5 (173.0)].
Figure 2.
Total serum ghrelin levels in the three study groups measured at baseline (−15 min), and then after the first juice trial (+6 min), first alcohol trial (+17 min), second juice trial (+23 min), second alcohol trial (+29 min) and after the experiment (+48). There was a pronounced main effect for dose administered [F(2,39) = 88.06.7, p < .001] as well as a dose by time interaction [F(2,39) = 82.179.5, p < .001]. Bonferroni-corrected pairwise comparisons also revealed that all three conditions were statistically significantly different (all three p’s < .001).
Correlations between serum ghrelin levels and urges to drink alcohol or juice
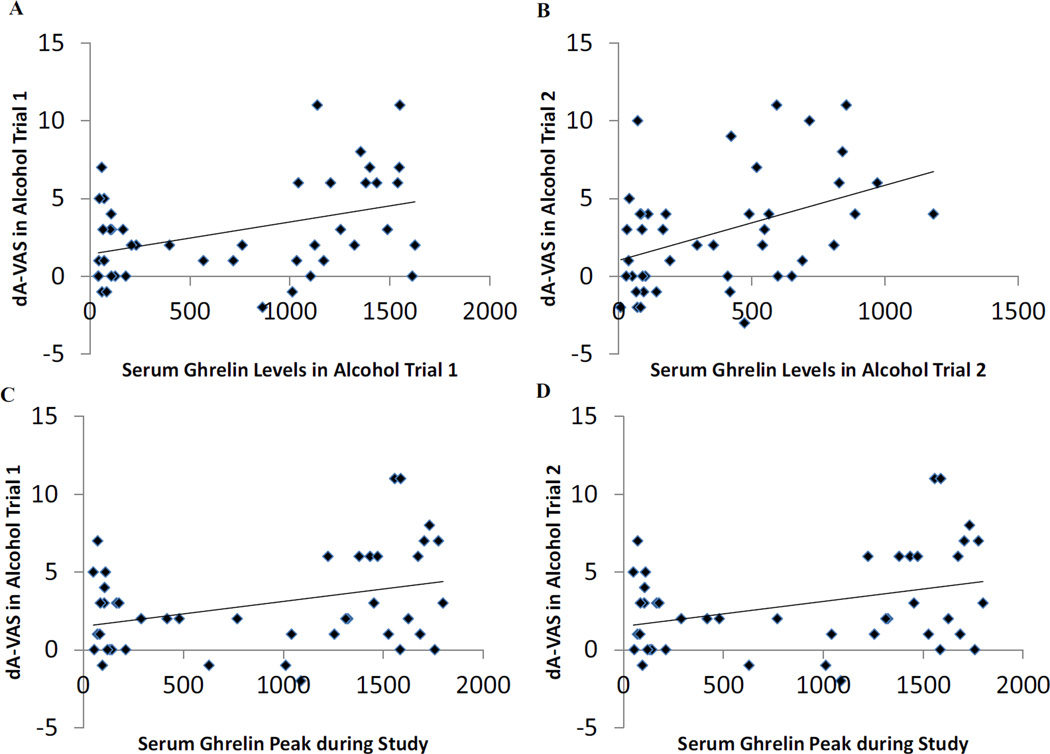
Serum total ghrelin level was correlated with the increase in alcohol urge during both the first alcohol trial (r(42) = .40, p = .008, n = 44; Figure 3A) and the second alcohol trial (r(42) = .42, p = .005, n = 44; Figure 3B). Similarly, the maximum serum ghrelin peak level across the six measurements correlated with the increase in alcohol urge during both the first alcohol trial (r(42) = .35, p = .02, n = 44; Figure 3C) and second alcohol trial (r(42) = .31, p = .04, n = 44; Figure 3D). [Likewise, when only the active cells were considered, these correlations were: r(24) = .49, p = .01; r(24) = .47, p = .02; r(24) = .44, p = .03, and r(24) = .29, p = .16, respectively.] By contrast, neither urge to drink juice craving nor food craving questionnaire were significantly correlated with serum ghrelin levels.
Figure 3.
Serum total ghrelin level was correlated with the increase in alcohol urge measured by the increase in the Alcohol-Visual Analogue Scale (dA-VAS) during both the first (p = .008; Figure 3A) and the second (p = .005; Figure 3B) alcohol cue trials. The maximum serum total ghrelin peak level across the six measurements was correlated with the increase in alcohol urge both in the first (p = .02; Figure 3C) and the second (p = .04; Figure 3D) alcohol cue trials.
Correlations between serum ghrelin levels and MAP, HR and saliva mass
Serum ghrelin levels were significantly and inversely correlated with MAP and saliva weight during all trials, while correlations between serum ghrelin levels and HR were not statistically significant.
Discussion
This is the first study in which the effects of exogenous intravenous ghrelin administration on alcohol craving were studied directly in alcohol-dependent individuals. This study provides preliminary evidence that ghrelin administration significantly increases alcohol craving. This was observed using a well-validated procedure in a well-controlled setting.
Elevated craving levels are associated with increased relapse rates, therefore craving has been proposed as a clinically relevant endophenotype able to predict alcohol use outcomes (3). Notably, cue-reactivity has demonstrated utility in eliciting urge to drink in alcoholics (24) and medications (e.g., the FDA-approved naltrexone) that reduce alcohol consumption also reduce alcohol craving in cue-reactivity human studies (37). As such, the present findings have potentially important clinical implications as they suggest ghrelin may play an important role in increased alcohol craving, which in turn may result in alcohol relapse. Manupulations of the ghrelin signaling may represent a novel pharmacological approach for treatment. This is consistent with preclinical experiments where alcohol intake and measures of alcohol reward and motivation to consume alcohol were suppressed by GHS-R1a antagonism (11, 38–40).
The results of this study may also have additional implications. Examining a treatment target that worsens a pharmacologically-induced symptom as a pathway to developing better treatments for alcoholism is innovative. Consistent with previous studies investigating different targets (41), this study shows that, with appropriate safeguards, a pharmacologically-triggered symptom provoking study can be safely executed. The ability of ghrelin administration to increase alcohol urge posits this procedure as a potential novel pharmacological challenge. As such, the laboratory model used here might be considered a novel way to test craving in the laboratory with anti-craving treatments be they pharmacological or behavioral.
Consistent with previous literature showing a positive correlation between endogenous blood ghrelin and alcohol craving (13, 18, 19), this study provides evidence of a positive significant correlation between cue-induced increase in alcohol craving and blood ghrelin levels after exogenous ghrelin administration. Measuring alcohol craving is difficult to operationalize (28), and this study suggests that ghrelin may represent a novel biomarker to indirectly quantify alcohol craving. Here we only measured total ghrelin serum levels, although it is reasonable that this did not influence the study conclusions. In fact, a recent intravenous acetylated ghrelin infusion pharmacokinetics study demonstrated a relatively constant acetylated/desacetylated ghrelin ratio, with a linear relationship between ghrelin infused dose and acetylated ghrelin levels, as well as between ghrelin infused dose and total serum ghrelin levels (42). Future studies will have to assess the acetylated/desacetylated ghrelin ratio after intravenous ghrelin administration in alcoholic individuals in order to further shed light on the potential role of ghrelin as a novel biomarker in alcoholism.
Ghrelin-related effects on appetite are known (5, 6). Notably, post-infusion serum ghrelin levels were significantly and positively correlated with alcohol urge, but not with juice urge or food craving. Reward processing plays an important role in the neural circuitry of cue-elicited alcohol craving (43). As such, our study provides evidence that ghrelin may play a key role facilitating alcohol-seeking behaviors. These findings support the concept that ghrelin’s effects extend beyond energy homeostasis and the hedonic values of substances, and involve mechanisms underlying the search for rewarding substances such as alcohol, at least in addicted individuals. This is consistent with preclinical experiments suggesting that, ghrelin-induced alcohol intake is driven by reward and independent from the caloric value of alcohol (10). However, it is important to note that, there was not a dose by cue type interaction, probably due to the small sample enrolled in this study or alternatively due to a non-specific effect of ghrelin on appetitive behaviors. As such, this study does not fully answer the question if ghrelin effects were specific for alcohol urge. Future larger studies are needed to address this question.
The highest expression of GHS-R1a’s is in the brain. In this study, intravenous (i.e. peripheral) ghrelin administration resulted in an acute increase in a brain-mediated behavior, i.e. alcohol craving. In preclinical experiments, intraperitoneal ghrelin results in the same brain effects that are observed when ghrelin is administered centrally (10). Furthermore, not only is there evidence that ghrelin is produced centrally (44, 45), but also circulating human ghrelin may pass from blood-to-brain by a saturable system (46).
We observed a significant ghrelin effect in decreasing MAP. Animal studies consistently show central ghrelin effects in suppressing sympathetic activity, and decreasing MAP and HR (47, 48). Furthermore, ghrelin has shown to increase nitric oxide bioactivity in blood vessels, thus decreasing peripheral vascular resistance (49). In normal subjects, intravenous ghrelin administration causes a significant MAP decrease without changes in HR (50). Additionally, suppression of the sympathetic activity is associated with reduced salivation, which is consistent with reports of dry mouth as a possible ghrelin-induced effect (51). While the cue-elicited craving is usually associated with increased MAP, HR and salivation, on the other hand these measures can be independent of each other in alcoholic individuals (28). Here, intravenous ghrelin, versus placebo, did increase cue-elicited craving, and it did so specifically for alcohol. On the other hand, ghrelin reduced MAP and salivation with no significant changes in HR; these effects are consistent with previous literature (47–51) and suggest a non-specific pharmacological effect of exogenous ghrelin which may have washed-out or masked cue-specific effects on MAP, HR and salivation. These observations are consistent with the centrally-mediated sympathetic effects of ghrelin, thus suggesting that in our subjects, ghrelin might have been centrally active. On the other hand, one may argue that, given the large amout of GHS-R1s in the vagal nerve (52), the effects might have been peripheral. Either way, it is interesting to note that, in addition to the significant effects of intravenous ghrelin versus placebo on MAP and salivation, we also found a negative significant correlation between post-infusion serum ghrelin levels and both MAP and saliva during all cue trials, thus further supporting the direct and non-specific pharmacological effects of ghrelin on these physiological outcomes. Additionally, a question may be what effects ghrelin had on catecholamine and acetylcholine systems and whether this may translate into any cognitive/affective effects. Ghrelin did not result in changes in adverse events possibly related to the catecholamine and acetylcholine systems (e.g. restlessness, nervousness or anxiety, irritability, depression or mood disturbances). Additionally, these symptoms were not significantly related to increase in craving (all p’s > 0.05; data not shown), thus making it unlikely that these symptoms may drive some of the alcohol craving measures.
Among the intravenous ghrelin studies conducted with other populations (53), only a few looked at possible dose-response effects. Here, the fact that only the highest ghrelin dose significantly affected alcohol craving is consistent with a study – upon which we based ours – indicated ghrelin 3 mcg/kg had a significantly different pharmacokinetic profile than 1 mcg/kg (22).
This study has a number of strengths, in fact this study: a) was the first study that used a potential pharmacologically-triggered symptom provoking study to directly demonstrate the role of ghrelin in alcohol craving; b) was the first to administer exogenous intravenous ghrelin to an addicted population; c) included a well-validated procedure that was modified ad hoc; and d) was conducted in a strict and well-controlled environment, thus allowing us to measure in real time cue-elicited craving and control carefully for several possible confounders (e.g. recent alcohol and food intake). Limitations of the study include the small sample and the short duration of intravenous ghrelin administration. Future studies should test the effects of more prolonged exposure to ghrelin on alcohol craving and larger samples should be considered. It is also important to keep in mind that subjective measures of craving are highly variable and therefore additional paradigms should also be considered. For example, future studies may investigate the effects of ghrelin on alcohol self-administration in order to expand the clinical relevance of this research. Furthermore, future research should consider genetic and neuroimaging tools to further investigate the role of ghrelin in alcoholism.
Consistent with previous studies, cues were presented more than once in order to provide a more stable assessment of craving, and were always presented in the same order. The use of a fixed order was done to allow for the most conservative assessment of alcohol cue-reactivity (24–26) as previous studies reported a general lowering of cue-reactivity to any stimulus presented second (25). Although ghrelin effects were more robust for the second and the fourth trials (i.e., the alcohol ones), the inclusion of only one fixed juice-alcohol order still represents a limitation that should be taken into account in future studies.
Strict inclusionary/exclusionary criteria were applied, therefore future studies will need to assess the generalizability of these results. In particular, obesity was an exclusion in order to enroll a sample as homogenous as possible. In fact, ghrelin plays a key role in regulating the gut-brain axis mechanisms that contribute to obesity (54), thus the role of ghrelin might differ in obese vs. non-obese alcoholic individuals. As such, we excluded obese patients in order to minimize the risk that differences in ghrelin signaling among participants might represent a confound to test our hypothesis. On the other hand, possible future studies might consider enrolling participants stratified by groups to further investigate ghrelin specificity to alcohol craving. Finally, the between-subject (as compared to a cross-over) design allowed us to avoid a possible "learning" effect on the cue-reactivity and minimize drop-outs. However, while participants were urn randomized, a crossover design is usually better in terms of matching study groups.
In conclusion, this study provides the first direct evidence in humans that intravenous ghrelin administration significantly increases alcohol craving in alcohol-dependent heavy drinking individuals. As such, this study suggests that ghrelin plays an important role in the mechanisms how alcohol-dependent individuals develop craving for alcohol and that the ghrelin signalling may represent a novel neuropharmacological target for alcohol-dependent patients. Additionally, this study suggests that intravenous ghrelin administration may represent a novel pharmacological challange to trigger alcohol craving in human laboratory studies; and that ghrelin may represent a novel biomarker of alcohol craving in alcoholic individuals. However, given the small sample and preliminary nature of our findings, future confirmatory studies are needed.
Supplementary Material
Acknowledgments
This study was funded by the National Institute on Alcohol Abuse and Alcoholism (NIAAA), grant R21-AA019709 awarded to Dr. Leggio, while he was at Brown University. Dr. Leggio’s current work is supported by the Division of Intramural Clinical and Biological Research of the NIAAA and the Intramural Research Program of the National Institute on Drug Abuse (NIDA). The authors would like to acknowledge the nursing support received by Tamara A. Sequeira, R.N. and Julia Nadeau, R.N. at the Brown University Center for Alcohol and Addiction Studies, and the technical support received by Valerie Zabala, B.S. and Ming Tong, M.D., at Rhode Island Hospital, and Chetram Deochand and Rosa Yu at Brown University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures
All authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Whiteford HA, Degenhardt L, Rehm J, Baxter AJ, Ferrari AJ, Erskine HE, et al. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet. 2013;382:1575–1586. doi: 10.1016/S0140-6736(13)61611-6. [DOI] [PubMed] [Google Scholar]
- 2.Edwards S, Kenna GA, Swift RM, Leggio L. Current and promising pharmacotherapies, and novel research target areas in the treatment of alcohol dependence: a review. Current pharmaceutical design. 2011;17:1323–1332. doi: 10.2174/138161211796150765. [DOI] [PubMed] [Google Scholar]
- 3.Leggio L. Understanding and treating alcohol craving and dependence: recent pharmacological and neuroendocrinological findings. Alcohol and alcoholism. 2009;44:341–352. doi: 10.1093/alcalc/agp026. [DOI] [PubMed] [Google Scholar]
- 4.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 5.Tschop M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–913. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- 6.Druce MR, Wren AM, Park AJ, Milton JE, Patterson M, Frost G, et al. Ghrelin increases food intake in obese as well as lean subjects. International journal of obesity. 2005;29:1130–1136. doi: 10.1038/sj.ijo.0803001. [DOI] [PubMed] [Google Scholar]
- 7.Katayama M, Nogami H, Nishiyama J, Kawase T, Kawamura K. Developmentally and regionally regulated expression of growth hormone secretagogue receptor mRNA in rat brain and pituitary gland. Neuroendocrinology. 2000;72:333–340. doi: 10.1159/000054602. [DOI] [PubMed] [Google Scholar]
- 8.Zigman JM, Jones JE, Lee CE, Saper CB, Elmquist JK. Expression of ghrelin receptor mRNA in the rat and the mouse brain. The Journal of comparative neurology. 2006;494:528–548. doi: 10.1002/cne.20823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang H, Betancourt L, Smith RG. Ghrelin amplifies dopamine signaling by cross talk involving formation of growth hormone secretagogue receptor/dopamine receptor subtype 1 heterodimers. Molecular endocrinology. 2006;20:1772–1785. doi: 10.1210/me.2005-0084. [DOI] [PubMed] [Google Scholar]
- 10.Jerlhag E. Systemic administration of ghrelin induces conditioned place preference and stimulates accumbal dopamine. Addiction biology. 2008;13:358–363. doi: 10.1111/j.1369-1600.2008.00125.x. [DOI] [PubMed] [Google Scholar]
- 11.Jerlhag E, Egecioglu E, Landgren S, Salome N, Heilig M, Moechars D, et al. Requirement of central ghrelin signaling for alcohol reward. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:11318–11323. doi: 10.1073/pnas.0812809106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jerlhag E, Egecioglu E, Dickson SL, Douhan A, Svensson L, Engel JA. Ghrelin administration into tegmental areas stimulates locomotor activity and increases extracellular concentration of dopamine in the nucleus accumbens. Addiction biology. 2007;12:6–16. doi: 10.1111/j.1369-1600.2006.00041.x. [DOI] [PubMed] [Google Scholar]
- 13.Addolorato G, Capristo E, Leggio L, Ferrulli A, Abenavoli L, Malandrino N, et al. Relationship between ghrelin levels, alcohol craving, and nutritional status in current alcoholic patients. Alcoholism, clinical and experimental research. 2006;30:1933–1937. doi: 10.1111/j.1530-0277.2006.00238.x. [DOI] [PubMed] [Google Scholar]
- 14.Badaoui A, De Saeger C, Duchemin J, Gihousse D, de Timary P, Starkel P. Alcohol dependence is associated with reduced plasma and fundic ghrelin levels. European journal of clinical investigation. 2008;38:397–403. doi: 10.1111/j.1365-2362.2008.01947.x. [DOI] [PubMed] [Google Scholar]
- 15.de Timary P, Cani PD, Duchemin J, Neyrinck AM, Gihousse D, Laterre PF, et al. The loss of metabolic control on alcohol drinking in heavy drinking alcohol-dependent subjects. PloS one. 2012;7:e38682. doi: 10.1371/journal.pone.0038682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim DJ, Yoon SJ, Choi B, Kim TS, Woo YS, Kim W, et al. Increased fasting plasma ghrelin levels during alcohol abstinence. Alcohol and alcoholism. 2005;40:76–79. doi: 10.1093/alcalc/agh108. [DOI] [PubMed] [Google Scholar]
- 17.Kraus T, Schanze A, Groschl M, Bayerlein K, Hillemacher T, Reulbach U, et al. Ghrelin levels are increased in alcoholism. Alcoholism, clinical and experimental research. 2005;29:2154–2157. doi: 10.1097/01.alc.0000191753.82554.7e. [DOI] [PubMed] [Google Scholar]
- 18.Leggio L, Ferrulli A, Cardone S, Nesci A, Miceli A, Malandrino N, et al. Ghrelin system in alcohol-dependent subjects: role of plasma ghrelin levels in alcohol drinking and craving. Addiction biology. 2012;17:452–464. doi: 10.1111/j.1369-1600.2010.00308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koopmann A, von der Goltz C, Grosshans M, Dinter C, Vitale M, Wiedemann K, et al. The association of the appetitive peptide acetylated ghrelin with alcohol craving in early abstinent alcohol dependent individuals. Psychoneuroendocrinology. 2012;37:980–986. doi: 10.1016/j.psyneuen.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 20.Leggio L. Role of the ghrelin system in alcoholism: Acting on the growth hormone secretagogue receptor to treat alcohol-related diseases. Drug news & perspectives. 2010;23:157–166. doi: 10.1358/dnp.2010.23.3.1429490. [DOI] [PubMed] [Google Scholar]
- 21.Vestergaard ET, Hansen TK, Gormsen LC, Jakobsen P, Moller N, Christiansen JS, et al. Constant intravenous ghrelin infusion in healthy young men: clinical pharmacokinetics and metabolic effects. American journal of physiology Endocrinology and metabolism. 2007;292:E1829–E1836. doi: 10.1152/ajpendo.00682.2006. [DOI] [PubMed] [Google Scholar]
- 22.Paulo RC, Brundage R, Cosma M, Mielke KL, Bowers CY, Veldhuis JD. Estrogen elevates the peak overnight production rate of acylated ghrelin. The Journal of clinical endocrinology and metabolism. 2008;93:4440–4447. doi: 10.1210/jc.2008-0783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50:1714–1719. doi: 10.2337/diabetes.50.8.1714. [DOI] [PubMed] [Google Scholar]
- 24.Monti PM, Rohsenow DJ, Hutchison KE, Swift RM, Mueller TI, Colby SM, et al. Naltrexone's effect on cue-elicited craving among alcoholics in treatment. Alcoholism, clinical and experimental research. 1999;23:1386–1394. [PubMed] [Google Scholar]
- 25.Monti PM, Binkoff JA, Abrams DB, Zwick WR, Nirenberg TD, Liepman MR. Reactivity of alcoholics and nonalcoholics to drinking cues. Journal of abnormal psychology. 1987;96:122–126. doi: 10.1037//0021-843x.96.2.122. [DOI] [PubMed] [Google Scholar]
- 26.Rohsenow DJ, Monti PM, Hutchison KE, Swift RM, Colby SM, Kaplan GB. Naltrexone's effects on reactivity to alcohol cues among alcoholic men. Journal of abnormal psychology. 2000;109:738–742. [PubMed] [Google Scholar]
- 27.Nijs IM, Franken IH, Muris P. The modified Trait and State Food-Cravings Questionnaires: development and validation of a general index of food craving. Appetite. 2007;49:38–46. doi: 10.1016/j.appet.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 28.Monti PM, Rohsenow DJ, Hutchison KE. Toward bridging the gap between biological, psychobiological and psychosocial models of alcohol craving. Addiction. 2000;95(Suppl 2):S229–S236. doi: 10.1080/09652140050111799. [DOI] [PubMed] [Google Scholar]
- 29.White KD. Salivation: a review and experimental investigation of major techniques. Psychophysiology. 1977;14:203–212. doi: 10.1111/j.1469-8986.1977.tb03379.x. [DOI] [PubMed] [Google Scholar]
- 30.Johnson BA, Ait-Daoud N, Roache JD. The COMBINE SAFTEE: a structured instrument for collecting adverse events adapted for clinical studies in the alcoholism field. Journal of studies on alcohol Supplement. 2005:157–167. doi: 10.15288/jsas.2005.s15.157. discussion 140. [DOI] [PubMed] [Google Scholar]
- 31.Levine J, Schooler NR. SAFTEE: a technique for the systematic assessment of side effects in clinical trials. Psychopharmacology bulletin. 1986;22:343–381. [PubMed] [Google Scholar]
- 32.Holder HD, Cisler RA, Longabaugh R, Stout RL, Treno AJ, Zweben A. Alcoholism treatment and medical care costs from Project MATCH. Addiction. 2000;95:999–1013. doi: 10.1046/j.1360-0443.2000.9579993.x. [DOI] [PubMed] [Google Scholar]
- 33.Drummond DC. What does cue-reactivity have to offer clinical research? Addiction. 2000;95(Suppl 2):S129–S144. doi: 10.1080/09652140050111708. [DOI] [PubMed] [Google Scholar]
- 34.Zywiak WH, Westerberg VS, Connors GJ, Maisto SA. Exploratory findings from the Reasons for Drinking Questionnaire. Journal of substance abuse treatment. 2003;25:287–292. doi: 10.1016/s0740-5472(03)00118-1. [DOI] [PubMed] [Google Scholar]
- 35.Costantini VJ, Vicentini E, Sabbatini FM, Valerio E, Lepore S, Tessari M, et al. GSK1614343, a novel ghrelin receptor antagonist, produces an unexpected increase of food intake and body weight in rodents and dogs. Neuroendocrinology. 2011;94:158–168. doi: 10.1159/000328968. [DOI] [PubMed] [Google Scholar]
- 36.Cremonini F, Camilleri M, Vazquez Roque M, McKinzie S, Burton D, Baxter K, et al. Obesity does not increase effects of synthetic ghrelin on human gastric motor functions. Gastroenterology. 2006;131:1431–1439. doi: 10.1053/j.gastro.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 37.Ray LA, Hutchison KE, Tartter M. Application of human laboratory models to pharmacotherapy development for alcohol dependence. Current pharmaceutical design. 2010;16:2149–2158. doi: 10.2174/138161210791516422. [DOI] [PubMed] [Google Scholar]
- 38.Kaur S, Ryabinin AE. Ghrelin receptor antagonism decreases alcohol consumption and activation of perioculomotor urocortin-containing neurons. Alcoholism, clinical and experimental research. 2010;34:1525–1534. doi: 10.1111/j.1530-0277.2010.01237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bahi A, Tolle V, Fehrentz JA, Brunel L, Martinez J, Tomasetto CL, et al. Ghrelin knockout mice show decreased voluntary alcohol consumption and reduced ethanol-induced conditioned place preference. Peptides. 2013;43:48–55. doi: 10.1016/j.peptides.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 40.Cruz MT, Herman MA, Cote DM, Ryabinin AE, Roberto M. Ghrelin increases GABAergic transmission and interacts with ethanol actions in the rat central nucleus of the amygdala. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2013;38:364–375. doi: 10.1038/npp.2012.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Umhau JC, Schwandt ML, Usala J, Geyer C, Singley E, George DT, et al. Pharmacologically induced alcohol craving in treatment seeking alcoholics correlates with alcoholism severity, but is insensitive to acamprosate. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2011;36:1178–1186. doi: 10.1038/npp.2010.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tong J, Dave N, Mugundu GM, Davis HW, Gaylinn BD, Thorner MO, et al. The pharmacokinetics of acyl, des-acyl, and total ghrelin in healthy human subjects. European journal of endocrinology / European Federation of Endocrine Societies. 2013;168:821–828. doi: 10.1530/EJE-13-0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heinz A, Beck A, Grusser SM, Grace AA, Wrase J. Identifying the neural circuitry of alcohol craving and relapse vulnerability. Addiction biology. 2009;14:108–118. doi: 10.1111/j.1369-1600.2008.00136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lu S, Guan JL, Wang QP, Uehara K, Yamada S, Goto N, et al. Immunocytochemical observation of ghrelin-containing neurons in the rat arcuate nucleus. Neuroscience letters. 2002;321:157–160. doi: 10.1016/s0304-3940(01)02544-7. [DOI] [PubMed] [Google Scholar]
- 45.Mondal MS, Date Y, Yamaguchi H, Toshinai K, Tsuruta T, Kangawa K, et al. Identification of ghrelin and its receptor in neurons of the rat arcuate nucleus. Regulatory peptides. 2005;126:55–59. doi: 10.1016/j.regpep.2004.08.038. [DOI] [PubMed] [Google Scholar]
- 46.Banks WA, Tschop M, Robinson SM, Heiman ML. Extent and direction of ghrelin transport across the blood-brain barrier is determined by its unique primary structure. The Journal of pharmacology and experimental therapeutics. 2002;302:822–827. doi: 10.1124/jpet.102.034827. [DOI] [PubMed] [Google Scholar]
- 47.Freeman JN, do Carmo JM, Adi AH, da Silva AA. Chronic central ghrelin infusion reduces blood pressure and heart rate despite increasing appetite and promoting weight gain in normotensive and hypertensive rats. Peptides. 2013;42:35–42. doi: 10.1016/j.peptides.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nagaya N, Uematsu M, Kojima M, Ikeda Y, Yoshihara F, Shimizu W, et al. Chronic administration of ghrelin improves left ventricular dysfunction and attenuates development of cardiac cachexia in rats with heart failure. Circulation. 2001;104:1430–1435. doi: 10.1161/hc3601.095575. [DOI] [PubMed] [Google Scholar]
- 49.Iantorno M, Chen H, Kim JA, Tesauro M, Lauro D, Cardillo C, et al. Ghrelin has novel vascular actions that mimic PI 3-kinase-dependent actions of insulin to stimulate production of NO from endothelial cells. American journal of physiology Endocrinology and metabolism. 2007;292:E756–E764. doi: 10.1152/ajpendo.00570.2006. [DOI] [PubMed] [Google Scholar]
- 50.Nagaya N, Kojima M, Uematsu M, Yamagishi M, Hosoda H, Oya H, et al. Hemodynamic and hormonal effects of human ghrelin in healthy volunteers. American journal of physiology Regulatory, integrative and comparative physiology. 2001;280:R1483–R1487. doi: 10.1152/ajpregu.2001.280.5.R1483. [DOI] [PubMed] [Google Scholar]
- 51.Strasser F, Lutz TA, Maeder MT, Thuerlimann B, Bueche D, Tschop M, et al. Safety, tolerability and pharmacokinetics of intravenous ghrelin for cancer-related anorexia/cachexia: a randomised, placebo-controlled, double-blind, double-crossover study. British journal of cancer. 2008;98:300–308. doi: 10.1038/sj.bjc.6604148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Inui A, Asakawa A, Bowers CY, Mantovani G, Laviano A, Meguid MM, et al. Ghrelin, appetite, and gastric motility: the emerging role of the stomach as an endocrine organ. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2004;18:439–456. doi: 10.1096/fj.03-0641rev. [DOI] [PubMed] [Google Scholar]
- 53.Garin MC, Burns CM, Kaul S, Cappola AR. Clinical review: The human experience with ghrelin administration. The Journal of clinical endocrinology and metabolism. 2013;98:1826–1837. doi: 10.1210/jc.2012-4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dickson SL, Egecioglu E, Landgren S, Skibicka KP, Engel JA, Jerlhag E. The role of the central ghrelin system in reward from food and chemical drugs. Molecular and cellular endocrinology. 2011;340:80–87. doi: 10.1016/j.mce.2011.02.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.