Abstract
Myeloid-derived suppressor cells are increased in the peripheral blood of advanced-stage cancer patients; however, no studies have shown a correlation of these immunosuppressive cells with clinical outcomes in melanoma patients. We characterized the frequency and suppressive function of multiple subsets of myeloid-derived suppressor cells in the peripheral blood of 34 patients with Stage IV melanoma, 20 patients with Stage I melanoma, and 15 healthy donors. The frequency of CD14+ MDSCs (Lin− CD11b+ HLA-DR− CD14+ CD33+) and CD14− MDSCs (Lin− CD11b+ HLA-DR− CD14− CD33+) was increased in the peripheral blood of Stage IV melanoma patients relative to healthy donors. The frequency of CD14+ and CD14− MDSCs correlated with each other and with the increased frequency of regulatory T cells, but not with classically defined monocytes. CD14− MDSCs isolated from the peripheral blood of Stage IV melanoma patients suppressed T cell activation more than those isolated from healthy donors, and the frequency of these cells correlated with disease progression and decreased overall survival. Our study provides the first evidence that the frequency of CD14− MDSCs negatively correlates with clinical outcomes in advanced-stage melanoma patients. These data indicate that suppressive MDSCs should be considered as targets for future immunotherapies.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-013-1475-x) contains supplementary material, which is available to authorized users.
Keywords: Myeloid-derived suppressor cells, Immunotherapy, Immunosuppression, Melanoma
Background
Due to the early identification of tumor-specific immune responses, multiple tumor-specific target antigens, and the importance of regulatory T cells as a known correlate of patient outcomes [1, 2], melanoma is at the forefront of tumor immunology and immunotherapy research. Both high-dose interleukin-2 (IL-2) and ipilimumab [anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4)] are approved by the Food and Drug Administration for treating patients with metastatic melanoma [3, 4]. There is also ongoing research using PD-1/PD-L1-blocking antibodies and adoptive cell therapies as potential treatments [5–7]. While ipilimumab is the first therapy shown to prolong survival in advanced-stage melanoma patients, it has a relatively low overall response rate of 10–20 % [4, 8]. However, subsets of ipilimumab-treated patients develop complete clinical responses lasting many years, highlighting the potential of immunotherapies to generate durable responses in melanoma patients [9]. While these currently available immunotherapies are promising, there remains an opportunity for the development of additional strategies to improve response rates, including combined therapies that aim to prevent or reduce tumor-induced immunosuppression.
Originally described in cancer models as “natural suppressor cells” that lack lymphocyte lineage markers [10, 11], myeloid-derived suppressor cells (MDSCs) are a mixed population of myeloid cells consisting of monocytic and granulocytic populations [12–14]. MDSCs in mice are characterized as Gr-1+ CD115+ CD11b+ cells with immunosuppressive capabilities, including the ability to suppress cytokine production, proliferation, and specific killing by T lymphocytes [15, 16]. Although generally characterized as lineage negative (CD3, CD14, CD19, CD56), HLA-DRneg, CD33+, and CD11b+ [17, 18], human MDSCs have also been divided into monocytic and granulocytic populations based on expression of the monocytic marker CD14 [19, 20] or the granulocytic marker CD15 [21]. MDSCs have recently been implicated as key contributors in tumor-induced immunosuppression in humans. Increased numbers of circulating MDSCs correlate with larger tumor burdens and disease stage in hepatocellular carcinoma, non-small-cell lung cancer, renal cell carcinoma, breast cancer, and glioblastoma [17, 21–26]. Furthermore, MDSCs isolated from cancer patients inhibit the function of human T cells [27, 28]. Treatments aimed at inhibiting the soluble factors that contribute to MDSC accumulation or function may help maximize the benefits of immunotherapy. For example, treatment of human MDSCs with all-trans-retinoic acid (ATRA) abrogates immune suppression in vitro [27] and reduces the frequency of MDSCs in vivo [29, 30]. These data demonstrate that therapies targeting MDSCs may improve anti-tumor immune responses, suggesting the need for further research in the accumulation and immunosuppressive function of MDSCs.
Although suppressive MDSCs are associated with disease stage in several cancers, very few studies demonstrate a correlation with prognosis or overall survival. High frequencies of Lin− HLA-DR− CD11b+ CD33+ are associated with decreased overall survival in studies of heterogeneous advanced-stage cancer patients including gastrointestinal malignancies, pancreatic cancer, and breast cancer [31–34]. In addition, breast cancer patients with low levels of circulating MDSCs are more likely to respond to an experimental treatment with NOV-002 (a chemopotentiator and chemoprotectant) in combination with chemotherapy [35]. In advanced-stage melanoma patients, several studies indicate an increased number of circulating MDSCs relative to healthy donors and that these cells are immunosuppressive ex vivo [19, 28, 36, 37]. Furthermore, one study showed reductions in circulating MDSCs in melanoma patients with a complete or partial response after treatment with a combination of CTLA-4 antibody and interferon alpha [38]. However, there are no published studies showing an association of MDSCs with overall survival or disease progression in melanoma patients. There are also few reports that provide a detailed comparison of multiple subsets of MDSCs in melanoma patients.
In this study, we compared the frequency and immunosuppressive function of MDSCs isolated from advanced-stage melanoma patients, Stage I melanoma patients, and healthy donors. In agreement with previous reports in melanoma patients, we found an increase in both Lin− (CD3, CD19, CD56) CD11b+ HLA-DR− CD33+ CD14+ MDSCs, referred to as CD14+ MDSCs, and Lin− CD11b+ HLA-DR− CD33+ CD14− MDSCs, referred to as CD14− MDSCs, in the peripheral blood of Stage IV melanoma patients. The CD14− MDSCs consistently suppressed T cell responses ex vivo, and the frequency of these cells correlated with overall survival and disease progression in our study. Furthermore, we show that a high frequency of CD14− MDSCs predicts a significantly increased risk of disease progression and may serve as a potential biomarker for patient prognosis. This study demonstrates that CD14− MDSCs may play a role in immunosuppression in melanoma patients and that immunotherapies targeting these cells should be considered for future combinatorial treatments.
Materials and methods
Study population
Patients with Stage I or Stage IV melanoma at the University of Colorado Cancer Center at the University of Colorado Hospital (Aurora, CO, USA) were enrolled from April 2011 through April 2012. Eligible Stage IV patients had ECOG performance status grades 0 or 1 and were previously untreated (n = 19) or untreated for 4 weeks prior to the blood draw (n = 15). Most of the enrolled Stage IV patients had a high metastatic disease burden (sub-staging: 9.5 % M1a, 6.5 % M1b, 84 % M1c). Of the 15 previously treated patients, 10 received chemotherapy or radiation treatment regimens, 6 received targeted inhibitors, and 8 received immunotherapy (see Table 1). Healthy donors were also enrolled during this time period (n = 15). Eligible healthy donors had no history of autoimmune disease or immunosuppression due to a known disease or medication. Age and gender distributions were approximately matched between healthy donors and melanoma patients. Informed consent was obtained for all subjects through protocols approved by the Colorado Multiple Institutional Review Board. Eleven additional Stage I and Stage IV melanoma patients and 10 additional healthy donors with similar clinical characteristics were recruited for the functional assays in Fig. 4 (see Online Resource 1).
Table 1.
Clinical characteristic of enrolled patients
| Stage of disease at blood drawa | No. of patients | Average age (range)b | Gender (M/F) |
|---|---|---|---|
| Healthy donor | 15 | 55.8 (28–77) | 8/7 |
| I | 20 | 54.0 (25–77) | 9/11 |
| IV | 34 | 55.0 (23–87) | 22/12 |
| Stage at initial diagnosisc | No. of patients | Average age (range)d | Gender (M/F) |
|---|---|---|---|
| Early (I–II) | 16 | 52.9 (19–85) | 13/3 |
| Late (III–IV) | 18 | 53.1 (30–67) | 9/9 |
| Rounds of prior treatmentsc | No. of patients | Average age (range)b | Gender (M/F) |
|---|---|---|---|
| None | 19 | 54.7 (23–87) | 13/6 |
| 1 Regimen | 6 | 61.4 (45–74) | 3/3 |
| 2–3 Regimens | 9 | 58.1 (36–78) | 6/3 |
Eligible patients were diagnosed with Stage I or Stage IV melanoma
aAll patients enrolled in the study
bAge at blood draw
cOnly patients with Stage IV disease at blood draw
dAge at initial diagnosis
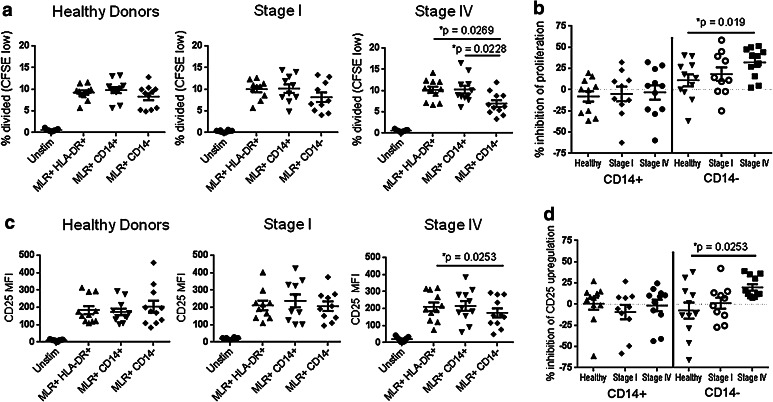
Fig. 4.
CD14− MDSCs from advanced-stage melanoma patients suppress T cell responses more than CD14− MDSCs from healthy donors. a CFSE-labeled T cells from melanoma healthy donors (left, n = 11), Stage I melanoma patients (middle, n = 10), or Stage IV melanoma patients (right, n = 11) were incubated with stimulatory dendritic cells in a mixed-lymphocyte reaction in the presence of magnetically separated control cells (HLA-DR+) or MDSCs (HLA-DR− CD14+ or HLA-DR− CD14− CD33+). After 4 days, the cells were stained with antibodies specific for CD3, CD8, and CD25, and the percentage of divided CD8+ T cells was determined by flow cytometry. The average frequency of divided cells was compared using one-way ANOVA (Stage IV, p = 0.0121), and differences between healthy donors and melanoma patients were significant (p values shown). b The percent suppression of proliferation was determined for each sample relative to HLA-DR+ cells and compared as in (a) between melanoma patients and healthy donors (CD14− MDSCs, ANOVA p = 0.001). c The MFI of CD25 staining was determined and compared as in (a) (ANOVA p = 0.0291). d The percent suppression of CD25 upregulation was determined and compared as in (b) (ANOVA p = 0.0291)
Sample collection
Peripheral blood was collected into tubes containing acid citrate dextrose anticoagulant (BD Biosciences). Twenty patient samples were collected within 2 months of Stage IV diagnosis, 7/34 patient samples were collected within 1 year of Stage IV diagnosis, and 7/34 patients were collected at greater than 1 year after Stage IV diagnosis. Peripheral blood mononuclear cells (PBMCs) from both healthy donors and melanoma patients were isolated over a density gradient using Ficoll-Paque Plus (GE Healthcare) and stored at −80 °C in normal human serum (Gemini Bioproducts) containing 10 % DMSO.
Flow cytometry
To define the MDSC subsets, frozen PBMC were thawed and washed, and 1 x 106 cells were stained with antibodies specific for human lineage markers CD3 (OKT3), CD56 (HCD56), and CD19 (HIB19), and for other markers that define MDSCs including CD33 (WM53), HLA-DR (L243), CD14 (HCD14), CD11b (ICRF44), and CD15 (HI98). Some samples were also stained with antibodies specific for CD16 (3G8). To define T cell subsets, PBMC were stained with antibodies specific for human CD3 (SK7), CD4 (OKT4), CD8 (SK1), CD25 (BC96), and FoxP3 (PCH101). For the T cell activation assays, cells were labeled with carboxyfluorescein diacetate and succinimidyl ester (Vybrant CFDA SE cell tracer kit, Invitrogen) and stained with antibodies specific for CD3 (SK7), CD8 (SK1), and CD25 (BC96). All antibodies were obtained from BioLegend except CD15 and FoxP3 (BD Biosciences). All data were collected on a CyAn ADP Analyzer (Beckman Coulter) and analyzed using FlowJo software (Tree Star, Inc). Compensation settings were established using single-color-stained PBMCs, and analysis gates were set using isotype-matched controls. Live cells were defined as those inside the gate on the forward and side scatter plot (Fig. 1 and Online Resource 2).
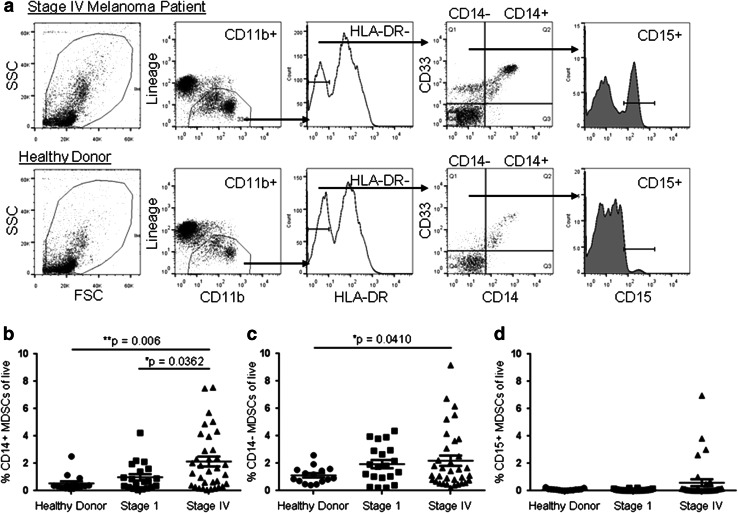
Fig. 1.
Myeloid-derived suppressor cells are increased in the peripheral blood of advanced-stage melanoma patients. a PBMCs from Stage IV melanoma patients (top) or healthy donors (bottom) were stained with antibodies specific for lineage markers (CD3, CD19, CD56), CD11b, HLA-DR, CD14, CD33, and CD15 and analyzed by flow cytometry. The gating scheme used to analyze three subsets of MDSCs are shown: CD14+ MDSCs (Lin−/low CD11b+ HLA-DR−/low CD14+ CD33+), CD14− MDSCs (Lin−/low CD11b+ HLA-DR−/low CD14− CD33+), and CD15+ MDSCs (Lin−/low CD11b+ HLA-DR−/low CD14− CD33+ CD15+). b The frequency of CD14+ MDSCs was compared between healthy donors (n = 15), Stage I melanoma patients (n = 20), and Stage IV melanoma patients (n = 34) using one-way ANOVA (p = 0.0031), and differences between healthy donor and melanoma patients were significant (p values shown are adjusted for multiple comparisons). c The frequency of CD14− MDSCs was compared as in b (p = 0.001). d The frequency of CD15+ MDSCs was compared as in b
Cells and media
All culture medium was prepared using RPMI 1640 (Mediatech), 2 mM l-glutamine (Mediatech), 100 μg/ml Streptomycin (Mediatech), 100 IU/ml Penicillin (Mediatech), and 10 % normal human serum (Gemini Bioproducts). Monocyte-derived dendritic cells for mixed-lymphocyte reactions (MLRs) were prepared from PBMC obtained from leukapheresis cassettes of healthy donors (Bonfils Blood Center, Denver, CO, USA). CD14+ cells from these healthy donors were enriched using magnetic separation according to the manufacturer’s instructions (Miltenyi Biotec), and 1 × 106 CD14+ cells were incubated with recombinant human IL-4 (100 ng/ml, Gemini Bioproducts) and GM-CSF (70 ng/ml, Gemini Bioproducts) for 4–6 days at 37 °C in 5 % CO2 in a 24-well plate [39, 40]. Lipopolysaccharide from Escherichia coli 0111:B4 (0.1 μg/ml, Sigma-Aldrich) was added 24 h prior to T cell stimulation assays.
T cell suppression assays
MDSCs were separated using a three-step sequential purification over magnetic columns according to the manufacturer’s instructions (Miltenyi Biotec). HLA-DR+ cells were removed from fresh PBMC (HLA-DR+ control cells), the negative fraction was enriched for CD14+ cells (80–90 % HLA-DR−/low CD14+ MDSC, Online Resource 3b), and the CD14 negative fraction was enriched for CD33+ cells (60–70 % HLA-DR− CD14− CD33+ MDSCs, Online Resource 3c). The remaining cells (>90 % T cells, Online Resource 3b) were labeled with CFSE, and 1 × 105 cells were stimulated in an MLR with 2 × 104 monocyte-derived dendritic cells from a healthy donor (preparation described above) in the presence of 1 × 105 HLA-DR+ control cells, CD14+ MDSCs, or CD14− MDSCs. After 4 days, the supernatants were removed, and the cells were stained for T cell activation markers and analyzed by flow cytometry. The percentage of divided cells was determined by analyzing the frequency of CD3+ CD8+ CFSElow CD25high cells (gating scheme shown in Online Resource 3d and e). Percent suppression was calculated by dividing the frequency of divided cells (CFSElow) or the MFI of CD25 staining in the presence of MDSCs by the frequency of divided cells or the MFI of CD25 staining in the presence of the control HLA-DR+ cells.
Statistical analysis
All graphical and statistical analyses were performed using GraphPad Prism Software (version 6) and SAS Software 9.3. Group means were compared using an unpaired two-tailed Student’s t test for two groups and one-way ANOVA for multiple groups with p values adjusted for multiple comparisons using Tukey’s approach. Correlations were evaluated using Pearson correlation coefficients with p values reported. Receiver operating characteristic (ROC) curves were used to define the cutoffs for high frequencies of Tregs, CD14+ MDSCs, and CD14− MDSCs for overall survival and disease progression events. Kaplan–Meir curves for progression-free survival and overall survival were plotted using GraphPad Prism software and compared between groups using a log-rank test. Cox proportional hazard regression models were used to obtain hazard ratios and 95 % confidence intervals. A stepwise selection procedure was applied to the Cox model to select variables associated with progression-free survival and overall survival, with entry p values ≤0.2 and stay p values ≤0.25. p < 0.05 was considered significant throughout this study. Error bars represent the standard error of the mean (SE).
Results
MDSCs are increased in advanced-stage melanoma patients and correlate with regulatory T cells
Although they have been well described in experimental cancer models in rodents, MDSCs are less clearly defined in human cancer patients due to the lack of specific lineage markers. In this study, we quantified the frequency of the three most commonly described MDSC populations in human cancer: the Lin−/low (CD3, CD19, CD56) CD11b+ HLA-DR−/low CD33+ CD14+ MDSCs [19], the Lin−/low CD11b+ HLA-DR−/low CD33+ CD14− MDSCs [17, 29, 30], and a subset of CD14− MDSCs that express the granulocytic marker CD15 [21, 23, 24]. PBMCs from the peripheral blood of patients with metastatic melanoma (Stage IV), Stage I melanoma, or healthy donors (Table 1) were stained with antibodies specific for the cellular markers of MDSCs and analyzed by flow cytometry. Lin−/low HLA-DR− CD11b+ cells were analyzed for expression of CD14, CD33, and CD15 (Fig. 1a). Compared to healthy donors, we found a significant increase in the frequency of both the CD14+ (0.54 % ± 0.16 to 2.14 % ± 0.36, p = 0.006) and CD14− (1.12 % ± 0.16 to 2.19 % ± 0.37, p = 0.041) MDSC populations in the peripheral blood of Stage IV melanoma patients (Fig. 1b, c). A subset of CD14− MDSCs expressing the granulocytic marker CD15 was evident in only 4 of the Stage IV melanoma patients (Fig. 1d) and may be underrepresented in our study due to its sensitivity to cryopreservation [20, 41].
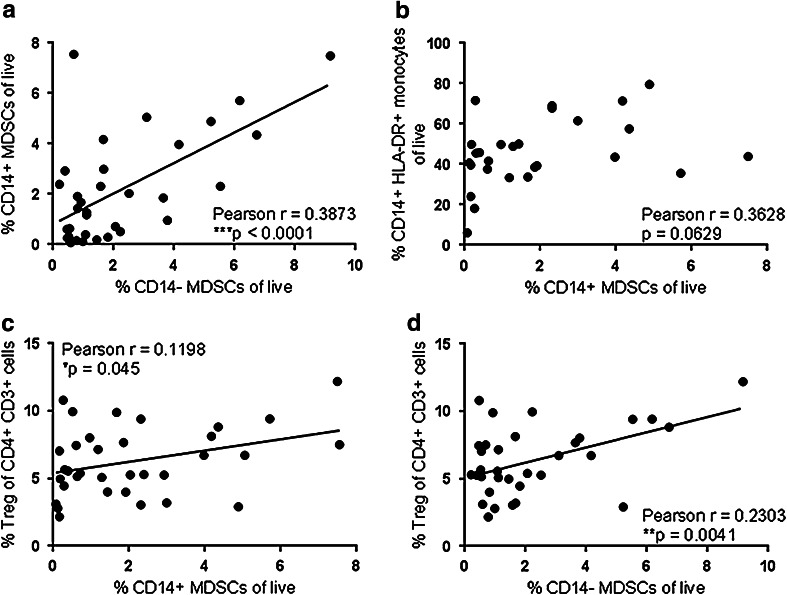
We next determined whether the increased frequency of MDSCs in melanoma patients was associated with changes in other PBMC populations. We found that the frequency of CD14+ and CD14− MDSCs correlated with each other (p < 0.0001, Fig. 2a). The frequency of classically defined monocytes (HLA-DR+ CD14+ CD16−) in advanced-stage melanoma patients was increased relative to healthy donors (data not shown); however, the frequency of these cells did not correlate with the frequency of MDSCs (p = 0.0629, Fig. 2b). The overall frequency of CD3+ T lymphocytes also did not correlate with the frequency of MDSCs (data not shown). However, similar to previous reports in melanoma patients [22, 42], we found a significant increase in the frequency of CD3+ CD4+ CD25+ FoxP3+ regulatory T cells (Tregs, 3.82 % ± 0.35 to 6.30 % ± 0.44, p = 0.0044, Online Resource 2) and a significant correlation between Tregs and both populations of MDSCs in Stage IV melanoma patients (p = 0.045 and 0.0041, respectively, Fig. 2c, d). These data suggest that immunosuppressive cells, including Tregs, CD14+ MDSCs, and CD14− MDSCs, are specifically increased in metastatic melanoma patients and are found in association with each other.
Fig. 2.
The frequency of CD14+ and CD14− MDSCs correlates with regulatory T cells and with each other, but not with HLA-DR+ monocytes. The frequency of CD14+ MDSCs was compared with the frequency of CD14− MDSCs (a) or the frequency of CD14+ HLA-DR+ CD16− monocytes (b) in Stage IV melanoma patients using a Pearson correlation test. The frequency of CD14+ (c) or CD14− (d) MDSCs was compared with the frequency of Tregs (Online Resource 2) in Stage IV melanoma patients using a Pearson correlation test
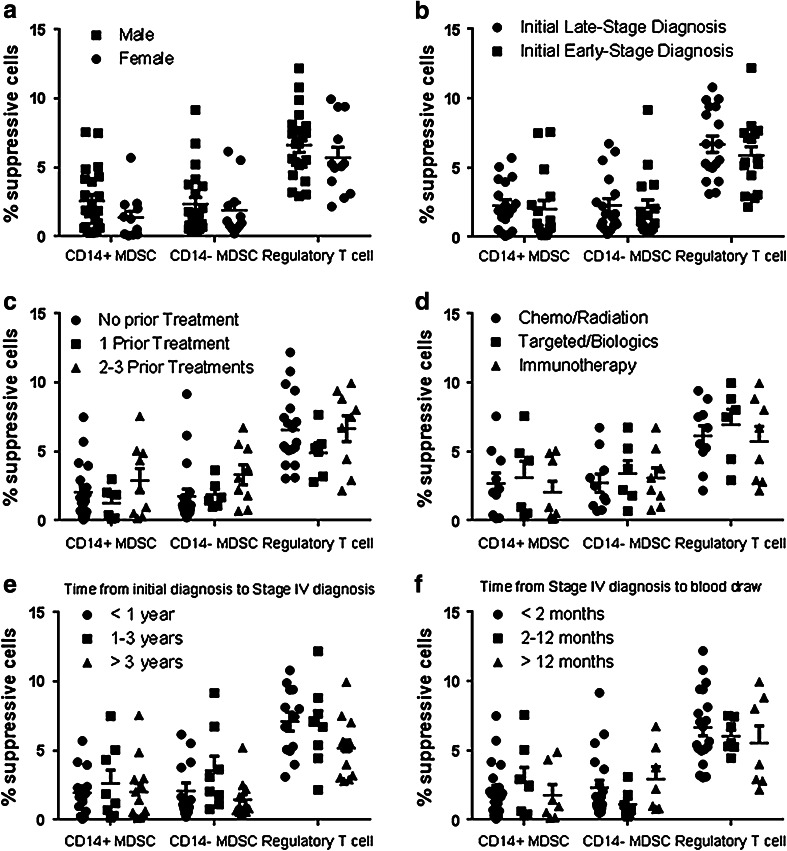
Although all of the advanced-stage melanoma patients were diagnosed with Stage IV disease at the time of the blood draw and analysis, there were differences in the clinical characteristics of these patients that may influence the frequency of immunosuppressive cells (Table 1). Therefore, we grouped the Stage IV melanoma patients according to gender, to the stage of disease at their initial diagnosis (Stage I or II disease versus Stage III or IV disease), to prior treatment regimens, and to the length of time between diagnosis and blood draw. There were no significant differences in the frequencies of Tregs or MDSCs between genders (Fig. 3a) or between patients that were initially diagnosed with early- versus late-stage disease (Fig. 3b). The frequency of immunosuppressive cells was also not associated with the number of prior treatment regimens (Fig. 3c) or the type of prior treatment received (Fig. 3d). Finally, the length of time between initial diagnosis and Stage IV diagnosis and the length of time between Stage IV diagnosis and enrollment in the study were also not associated with the frequency of MDSCs or Tregs (Fig. 3e, f).
Fig. 3.
The frequency of MDSCs is not affected by gender, stage of initial diagnosis, or prior treatments. Stage IV melanoma patients were grouped according to gender (a male = 22, female = 12 patients), the stage of disease at initial diagnosis (b late = 18, early = 16 patients), the number of prior treatment regimens (c no treatment = 19, 1 treatment = 6, 2–3 treatments = 9 patients), and the type of prior treatment regimens (d chemo/radiation = 10, targeted inhibitors = 6, immunotherapy = 8 patients). Patients that had received multiple treatments prior to enrollment in this study are represented in each treatment regimen they received. Stage IV melanoma patients were also grouped according to the length of time between initial diagnosis and Stage IV diagnosis (e <1 year = 13, 1–3 years = 8, >3 year = 13 patients) and the length of time between Stage IV diagnosis and enrollment in the study (f <2 months = 20, 2–12 months = 7, >12 months = 7 patients). The frequency of CD14+ MDSCs, CD14− MDSCs, and Tregs was compared using a Student’s t test (a and b) or a one-way ANOVA (c–f). No significant differences were observed
MDSCs from advanced-stage melanoma patients are immunosuppressive
Due to the lack of specific cellular markers, human MDSCs are often defined by their ability to suppress T cell responses ex vivo. To determine whether the MDSCs we characterized phenotypically were immunosuppressive, we added magnetically separated HLA-DR− CD14+ or HLA-DR− CD14− CD33+ MDSCs to T cells stimulated with allogeneic dendritic cells in a mixed-lymphocyte reaction (MLR). These assays were performed using fresh non-cryopreserved samples and are a heterogeneous population consisting of both CD15− and granulocytic CD15+ subsets (Online Resource 3c). We found that CD14− MDSCs isolated from the peripheral blood of Stage IV melanoma patients significantly reduced proliferation (10.2 ± 0.7 % to 6.9 ± 0.8 %, p = 0.0269, Fig. 4a and Online Resource 3d) and activation of CD8+ T cells (MFI of CD25 210.6 ± 25.6 to 174.8 ± 26.1, p = 0.0253, Fig. 4c). In contrast, CD14− MDSCs isolated from healthy donors and Stage I melanoma patients did not significantly reduce T cell responses (Fig. 4a, c). There was also a significant difference in the calculated percent suppression between CD14− MDSCs from healthy donors and Stage IV melanoma patients (inhibition of proliferation 11.1 ± 7.0 % to 32.2 ± 5.3 %, p = 0.019; inhibition of CD25 upregulation −7.5 ± 9.5 % to 19.8 ± 3.8 %, p = 0.0253, Fig. 4b, d). In contrast to previous studies using antibody-coupled beads or mitogens to stimulate T cells [19, 36, 37], the HLA-DR− CD14+ MDSCs did not suppress T cell responses in our study (Fig. 4). These data suggest that peripheral CD14− MDSCs are immunosuppressive and therefore may be relevant for targeted therapeutic approaches in this patient cohort.
The frequency of CD14− MDSCs in advanced-stage melanoma patients predicts overall survival and disease progression
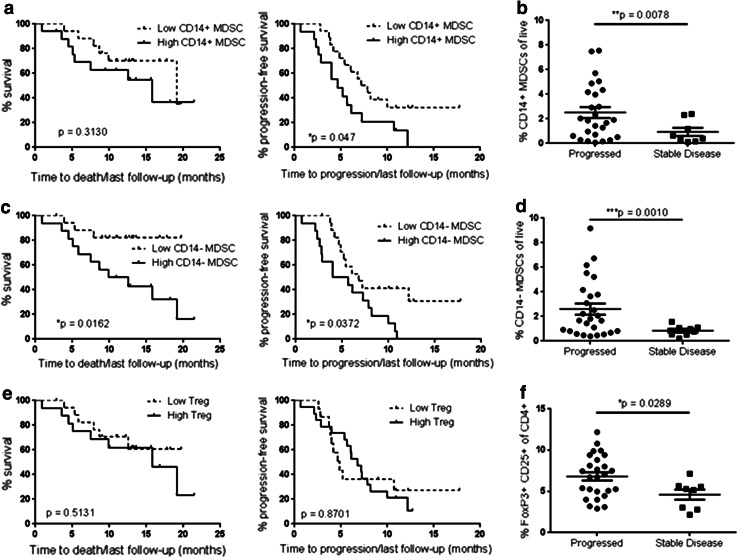
The association between MDSCs and tumor growth and progression has been well described in mouse cancer models [43, 44]. However, there are few studies that demonstrate a correlation between MDSCs and clinical outcomes in human cancer patients [31–34]. We are not aware of any currently published studies that correlate CD14− MDSCs with overall survival or disease progression in melanoma patients. ROC curves were used to assess the cutoff values for high frequencies of Tregs or MDSCs in our cohort of Stage IV melanoma patients according to desirable sensitivity and specificity for predicting survival (CD14+ MDSCs ≥ 1.6 %, CD14− MDSCs ≥ 1.6 %, Tregs ≥ 6.0 %) or disease progression (CD14+ MDSCs ≥ 1.7 %, CD14− MDSCs ≥ 1.6 %, Tregs ≥ 5.3 %). We then analyzed the time to death and time to progression for patients with either a high or low frequency of Tregs or MDSCs. In agreement with their suppressive activity, we found a significant association between a high frequency of CD14− MDSCs and poorer overall survival (p = 0.0162) as well as a significant correlation between a high frequency of CD14− MDSCs and a shorter time from enrollment in the study to disease progression (p = 0.0372, Fig. 5c). We also found a marginally significant association between CD14+ MDSCs and disease progression in our study (p = 0.047, Fig. 5a).
Fig. 5.
The frequency of CD14− MDSCs is associated with disease progression in Stage IV melanoma patients. The length of time between enrollment (blood draw) and death (left) or clinical disease progression (right) was determined for Stage IV patients from Fig. 1 (n = 34). The time between enrollment and last follow-up was determined for surviving patients and patients with no disease progression. The overall survival probability was compared between patients with a high or low/average frequency of CD14+ MDSCs (a high = 17 patients, low = 17), CD14− MDSCs (c high = 16, low = 18), or FoxP3+ CD25+ Tregs (e high = 16, low = 18) using a log-rank test. The mean follow-up time and time between Stage IV diagnosis and blood draw were not significantly different between patients with low or high frequencies of suppressor cells (Online Resource 4). The frequency of CD14+ MDSCs (b), CD14− MDSCs (d), and FoxP3+ CD25+ Tregs (f) in patients with stable disease during the study or those with clinical disease progression was compared using a Student’s t test. Mean follow-up time for patients with a progression event was not significantly different from patients with stable disease (Online Resource 4d)
We next determined whether other clinical factors contributed to the association between CD14− MDSCs and patient outcomes. We found no significant differences in age between the patients with high or low frequencies of CD14− MDSCs (Online Resource 4a). Importantly, there were no significant differences in the mean follow-up times between patients with high or low CD14− MDSC frequencies (Online Resource 4b), or the time between Stage IV diagnosis and blood draw (Online Resource 4c). Finally, we found that gender, age, prior treatment regimens, stage of initial diagnosis, and BRAF mutation status were not univariately significant in predicting overall survival or disease progression. We used a stepwise selection procedure in the Cox model to calculate the risk of death and disease progression for patients with a high frequency of CD14− MDSCs. We found that a high frequency of CD14− MDSCs was significantly associated with an increased risk of death (hazard ratio of 4.83, *p = 0.016) and an increased risk of progression (hazard ratio of 2.39, p = 0.039, Online Resource 5). Therefore, a high frequency of CD14− MDSCs independently predicted poorer survival and disease progression in our study.
To further characterize the association between suppressive cells and melanoma disease progression, we divided our Stage IV cohort into patients with progressive disease and those with stable or responsive disease during the time frame of our study. We found that patients with progressive disease had an increased frequency of all three suppressive cell types, CD14+ MDSCs (Fig. 5b), CD14− MDSCs (Fig. 5d), and Tregs (Fig. 5f) compared to patients with stable or responsive disease. Furthermore, 15 of 16 (94 %) patients with high levels of CD14− MDSCs and 11 of 18 (61 %) patients with low levels of CD14− MDSCs progressed during the study. The mean follow-up time between patients with progressive or stable/responsive disease was similar (Online Resource 4d). Therefore, CD14− MDSCs may serve as a predictor of clinical outcomes in Stage IV melanoma patients, and these immunosuppressive cells may be a mediator of disease progression.
Discussion
In this study, we showed that Stage IV melanoma patients have an increased frequency of CD14+ and CD14− MDSCs that correlate with the frequency of Tregs as well as with each other (Figs. 1, 2). We were unable to reliably quantify the CD14− CD15+ population of MDSCs because their numbers are reduced in cryopreserved samples [41]. However, CD14− CD15+ cells are equally sensitive to cryopreservation in both cancer patients and healthy donors, and the length of time in freezer storage does not affect their frequency [41]. Furthermore, the frequency of CD14+ MDSCs is consistent regardless of cryopreservation [20, 41]. Despite this limitation, we found that the increased frequency of CD14− MDSCs correlates with poorer overall survival and disease progression, the first report of its kind for melanoma (Fig. 5). Furthermore, patients with high levels of CD14− MDSCs have a nearly 5 times greater risk of death and a 2.4 times greater risk of disease progression. These data suggest that a high frequency of peripheral CD14− MDSCs may be a useful prognostic biomarker for Stage IV melanoma. Interestingly, some of the Stage I melanoma patients in our study have an increase in the frequency of CD14− MDSCs, with 8 out of 20 patients (40 %) above the average frequency in healthy donors. The survival and recurrence of melanoma in these Stage I patients will be tracked to determine if an increased level of peripheral MDSCs also correlates with disease relapse in these patients.
Our data suggest that advanced-stage melanoma patients not only have an increased frequency of peripheral MDSCs, but also an increase in the suppressive activity of these cells. Similar to studies of other cancers, we showed that the CD14− MDSCs from Stage IV melanoma patients suppress T cell responses more than MDSCs isolated from healthy donors [27, 28]. As indicated by numerous studies in rodent cancer models, our results support the hypothesis that MDSCs acquire immunosuppressive functions in tumor-bearing hosts [45–47]. In contrast, the CD14+ MDSCs in our study did not suppress T cell responses ex vivo. CD14+ MDSCs produce TGFβ, recruit, or induce regulatory T cells, and are immunosuppressive in several studies [19, 22, 36, 37]. Consistent with our study, several groups have shown that granulocytic MDSCs are more suppressive than CD14+ HLA-DRlow/− MDSCs, which require high MDSC to T cell ratios to achieve immunosuppression [20, 22]. We speculate that the lack of immunosuppression by CD14+ MDSCs in our study may be due to lower MDSC to T cell ratios, different methods of T cell stimulation, or different methods of MDSC isolation. We stimulated T cells using mixed-lymphocyte reactions, resulting in a smaller frequency of responding T cells than mitogen or antibody stimulations used in previous studies. Although MLRs more closely mimic natural immune responses, the reduced frequency of responding T cells may have reduced the sensitivity of our assays to an extent that immunosuppression by CD14+ MDSCs was not apparent. In addition, despite magnetic separation of HLA-DR− cells, the CD14+ MDSCs in our assays express a low level of HLA-DR (Online Resource 3a) and, thus, may be less enriched for HLA-DR− CD14+ cells than previous studies using fluorescence-activated cell sorting.
Despite the strong evidence for the immunosuppressive role of MDSCs in the microenvironment of mouse cancer models [48], few studies of MDSCs have been performed in human tumor tissue. One group recently showed that melanoma tumor-infiltrating CD14+ and CD14− cells, which express high levels of HLA-DR, did not suppress T cell proliferation [42]. However, MDSCs isolated from the tumor tissue of head and neck cancer and breast cancer patients suppress T cell responses ex vivo [49, 50]. In light of these results, the suppressive function of HLA-DR− MDSCs isolated from melanoma tumor tissue needs to be evaluated to fully understand their role in the immunosuppressive environment of human melanoma.
Immunotherapy has provided clinical benefit for patients with advanced melanoma. However, there remains an opportunity to improve upon these treatments through the targeting of additional or multiple immunosuppressive mechanisms, such as reducing the frequency or activity of MDSCs. Our study suggests that CD14− MDSCs are an important component of immunosuppression in melanoma patients. It remains to be clarified whether the frequency of MDSCs could serve as a predictive biomarker for the response to immunotherapy and whether these responses could be improved by the addition of therapies that target this MDSC population in melanoma patients. One recent trial using a dendritic cell vaccine in combination with ATRA demonstrated a reduced frequency of MDSCs and increased p53-specific T cells in small-cell lung cancer patients treated with the combined therapy [30], suggesting that combinatorial therapies specifically targeting MDSCs are promising. These insights may contribute to the growing knowledge of immunotherapeutic treatments for melanoma patients and help to understand the manipulation of the human immune response to cancer.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This work was supported by a University of Colorado Cancer Center Fellowship, the University of Colorado Cancer Center Support Grant (P30CA046934), the American Cancer Society 2012 Roaring Fork Valley Postdoctoral Research Award, and the Conner Family Foundation Grant. We would like to thank the laboratory members of Dr. Virginia Borges and Dr. Pepper Schedin for critical reviews of the data and Eric Spongberg for his technical support. We would also like to thank Dr. Sonali Jindal and Pat Bell for their assistance and histology expertise.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Baumgartner JM, Gonzalez R, Lewis KD, Robinson WA, Richter DA, Palmer BE, Wilson CC, McCarter MD. Increased survival from stage IV melanoma associated with fewer regulatory T Cells. J Surg Res. 2009;154:13–20. doi: 10.1016/j.jss.2008.04.043. [DOI] [PubMed] [Google Scholar]
- 2.Cote AL, Usherwood EJ, Turk MJ. Tumor-specific T-cell memory: clearing the regulatory T-cell hurdle. Cancer Res. 2008;68:1614–1617. doi: 10.1158/0008-5472.CAN-07-6012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atkins MB, Lotze MT, Dutcher JP, Fisher RI, Weiss G, Margolin K, Abrams J, Sznol M, Parkinson D, Hawkins M, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999;17:2105–2116. doi: 10.1200/JCO.1999.17.7.2105. [DOI] [PubMed] [Google Scholar]
- 4.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenberg SA, Yang JC, Sherry RM, Kammula US, Hughes MS, Phan GQ, Citrin DE, Restifo NP, Robbins PF, Wunderlich JR, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res. 2011;17:4550–4557. doi: 10.1158/1078-0432.CCR-11-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sarnaik AA, Yu B, Yu D, Morelli D, Hall M, Bogle D, Yan L, Targan S, Solomon J, Nichol G, et al. Extended dose ipilimumab with a peptide vaccine: immune correlates associated with clinical benefit in patients with resected high-risk stage IIIc/IV melanoma. Clin Cancer Res. 2011;17:896–906. doi: 10.1158/1078-0432.CCR-10-2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prieto PA, Yang JC, Sherry RM, Hughes MS, Kammula US, White DE, Levy CL, Rosenberg SA, Phan GQ. CTLA-4 blockade with ipilimumab: long-term follow-up of 177 patients with metastatic melanoma. Clin Cancer Res. 2012;18:2039–2047. doi: 10.1158/1078-0432.CCR-11-1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seung LP, Rowley DA, Dubey P, Schreiber H. Synergy between T-cell immunity and inhibition of paracrine stimulation causes tumor rejection. Proc Natl Acad Sci USA. 1995;92:6254–6258. doi: 10.1073/pnas.92.14.6254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pekarek LA, Starr BA, Toledano AY, Schreiber H. Inhibition of tumor growth by elimination of granulocytes. J Exp Med. 1995;181:435–440. doi: 10.1084/jem.181.1.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Talmadge JE. Pathways mediating the expansion and immunosuppressive activity of myeloid-derived suppressor cells and their relevance to cancer therapy. Clin Cancer Res. 2007;13:5243–5248. doi: 10.1158/1078-0432.CCR-07-0182. [DOI] [PubMed] [Google Scholar]
- 13.Movahedi K, Guilliams M, Van den Bossche J, Van den Bergh R, Gysemans C, Beschin A, De Baetselier P, Van Ginderachter JA. Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity. Blood. 2008;111:4233–4244. doi: 10.1182/blood-2007-07-099226. [DOI] [PubMed] [Google Scholar]
- 14.Youn JI, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol. 2008;181:5791–5802. doi: 10.4049/jimmunol.181.8.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol. 2009;182:4499–4506. doi: 10.4049/jimmunol.0802740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kusmartsev S, Nagaraj S, Gabrilovich DI. Tumor-associated CD8+ T cell tolerance induced by bone marrow-derived immature myeloid cells. J Immunol. 2005;175:4583–4592. doi: 10.4049/jimmunol.175.7.4583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diaz-Montero CM, Salem ML, Nishimura MI, Garrett-Mayer E, Cole DJ, Montero AJ. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol Immunother. 2009;58:49–59. doi: 10.1007/s00262-008-0523-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Almand B, Clark JI, Nikitina E, van Beynen J, English NR, Knight SC, Carbone DP, Gabrilovich DI. Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J Immunol. 2001;166:678–689. doi: 10.4049/jimmunol.166.1.678. [DOI] [PubMed] [Google Scholar]
- 19.Poschke I, Mougiakakos D, Hansson J, Masucci GV, Kiessling R. Immature immunosuppressive CD14+ HLA-DR-/low cells in melanoma patients are Stat3hi and overexpress CD80, CD83, and DC-sign. Cancer Res. 2010;70:4335–4345. doi: 10.1158/0008-5472.CAN-09-3767. [DOI] [PubMed] [Google Scholar]
- 20.Duffy A, Zhao F, Haile L, Gamrekelashvili J, Fioravanti S, Ma C, Kapanadze T, Compton K, Figg WD, Greten TF. Comparative analysis of monocytic and granulocytic myeloid-derived suppressor cell subsets in patients with gastrointestinal malignancies. Cancer Immunol Immunother. 2013;62:299–307. doi: 10.1007/s00262-012-1332-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zea AH, Rodriguez PC, Atkins MB, Hernandez C, Signoretti S, Zabaleta J, McDermott D, Quiceno D, Youmans A, O’Neill A, et al. Arginase-producing myeloid suppressor cells in renal cell carcinoma patients: a mechanism of tumor evasion. Cancer Res. 2005;65:3044–3048. doi: 10.1158/0008-5472.CAN-04-4505. [DOI] [PubMed] [Google Scholar]
- 22.Hoechst B, Ormandy LA, Ballmaier M, Lehner F, Kruger C, Manns MP, Greten TF, Korangy F. A new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4(+)CD25(+)Foxp3(+) T cells. Gastroenterology. 2008;135:234–243. doi: 10.1053/j.gastro.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 23.Liu CY, Wang YM, Wang CL, Feng PH, Ko HW, Liu YH, Wu YC, Chu Y, Chung FT, Kuo CH, et al. Population alterations of L-arginase- and inducible nitric oxide synthase-expressed CD11b+/CD14(−)/CD15+/CD33+ myeloid-derived suppressor cells and CD8+ T lymphocytes in patients with advanced-stage non-small cell lung cancer. J Cancer Res Clin Oncol. 2010;136:35–45. doi: 10.1007/s00432-009-0634-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodriguez PC, Ernstoff MS, Hernandez C, Atkins M, Zabaleta J, Sierra R, Ochoa AC. Arginase I-producing myeloid-derived suppressor cells in renal cell carcinoma are a subpopulation of activated granulocytes. Cancer Res. 2009;69:1553–1560. doi: 10.1158/0008-5472.CAN-08-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodrigues JC, Gonzalez GC, Zhang L, Ibrahim G, Kelly JJ, Gustafson MP, Lin Y, Dietz AB, Forsyth PA, Yong VW, et al. Normal human monocytes exposed to glioma cells acquire myeloid-derived suppressor cell-like properties. Neuro Oncol. 2010;12:351–365. doi: 10.1093/neuonc/nop023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Porembka MR, Mitchem JB, Belt BA, Hsieh CS, Lee HM, Herndon J, Gillanders WE, Linehan DC, Goedegebuure P. Pancreatic adenocarcinoma induces bone marrow mobilization of myeloid-derived suppressor cells which promote primary tumor growth. Cancer Immunol Immunother. 2012;61:1373–1385. doi: 10.1007/s00262-011-1178-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kusmartsev S, Su Z, Heiser A, Dannull J, Eruslanov E, Kubler H, Yancey D, Dahm P, Vieweg J. Reversal of myeloid cell-mediated immunosuppression in patients with metastatic renal cell carcinoma. Clin Cancer Res. 2008;14:8270–8278. doi: 10.1158/1078-0432.CCR-08-0165. [DOI] [PubMed] [Google Scholar]
- 28.Mandruzzato S, Solito S, Falisi E, Francescato S, Chiarion-Sileni V, Mocellin S, Zanon A, Rossi CR, Nitti D, Bronte V, et al. IL4Ralpha+ myeloid-derived suppressor cell expansion in cancer patients. J Immunol. 2009;182:6562–6568. doi: 10.4049/jimmunol.0803831. [DOI] [PubMed] [Google Scholar]
- 29.Mirza N, Fishman M, Fricke I, Dunn M, Neuger AM, Frost TJ, Lush RM, Antonia S, Gabrilovich DI. All-trans-retinoic acid improves differentiation of myeloid cells and immune response in cancer patients. Cancer Res. 2006;66:9299–9307. doi: 10.1158/0008-5472.CAN-06-1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iclozan C, Antonia S, Chiappori A, Chen DT, Gabrilovich D. Therapeutic regulation of myeloid-derived suppressor cells and immune response to cancer vaccine in patients with extensive stage small cell lung cancer. Cancer Immunol Immunother. 2013;62:909–918. doi: 10.1007/s00262-013-1396-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choi J, Suh B, Ahn YO, Kim TM, Lee JO, Lee SH, Heo DS. CD15+/CD16low human granulocytes from terminal cancer patients: granulocytic myeloid-derived suppressor cells that have suppressive function. Tumour Biol. 2012;33:121–129. doi: 10.1007/s13277-011-0254-6. [DOI] [PubMed] [Google Scholar]
- 32.Gabitass RF, Annels NE, Stocken DD, Pandha HA, Middleton GW. Elevated myeloid-derived suppressor cells in pancreatic, esophageal and gastric cancer are an independent prognostic factor and are associated with significant elevation of the Th2 cytokine interleukin-13. Cancer Immunol Immunother. 2011;60:1419–1430. doi: 10.1007/s00262-011-1028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Solito S, Falisi E, Diaz-Montero CM, Doni A, Pinton L, Rosato A, Francescato S, Basso G, Zanovello P, Onicescu G, et al. A human promyelocytic-like population is responsible for the immune suppression mediated by myeloid-derived suppressor cells. Blood. 2011;118:2254–2265. doi: 10.1182/blood-2010-12-325753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mundy-Bosse BL, Young GS, Bauer T, Binkley E, Bloomston M, Bill MA, Bekaii-Saab T, Carson WE, 3rd, Lesinski GB. Distinct myeloid suppressor cell subsets correlate with plasma IL-6 and IL-10 and reduced interferon-alpha signaling in CD4(+) T cells from patients with GI malignancy. Cancer Immunol Immunother. 2011;60:1269–1279. doi: 10.1007/s00262-011-1029-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Montero AJ, Diaz-Montero CM, Deutsch YE, Hurley J, Koniaris LG, Rumboldt T, Yasir S, Jorda M, Garret-Mayer E, Avisar E, et al. Phase 2 study of neoadjuvant treatment with NOV-002 in combination with doxorubicin and cyclophosphamide followed by docetaxel in patients with HER-2 negative clinical stage II-IIIc breast cancer. Breast Cancer Res Treat. 2012;132:215–223. doi: 10.1007/s10549-011-1889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Valenti R, Huber V, Filipazzi P, Pilla L, Sovena G, Villa A, Corbelli A, Fais S, Parmiani G, Rivoltini L. Human tumor-released microvesicles promote the differentiation of myeloid cells with transforming growth factor-beta-mediated suppressive activity on T lymphocytes. Cancer Res. 2006;66:9290–9298. doi: 10.1158/0008-5472.CAN-06-1819. [DOI] [PubMed] [Google Scholar]
- 37.Filipazzi P, Valenti R, Huber V, Pilla L, Canese P, Iero M, Castelli C, Mariani L, Parmiani G, Rivoltini L. Identification of a new subset of myeloid suppressor cells in peripheral blood of melanoma patients with modulation by a granulocyte-macrophage colony-stimulation factor-based antitumor vaccine. J Clin Oncol. 2007;25:2546–2553. doi: 10.1200/JCO.2006.08.5829. [DOI] [PubMed] [Google Scholar]
- 38.Tarhini AA, Butterfield LH, Shuai Y, Gooding WE, Kalinski P, Kirkwood JM. Differing patterns of circulating regulatory T cells and myeloid-derived suppressor cells in metastatic melanoma patients receiving anti-CTLA4 antibody and interferon-alpha or TLR-9 agonist and GM-CSF with peptide vaccination. J Immunother. 2012;35:702–710. doi: 10.1097/CJI.0b013e31825481fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ganguly D, Paul K, Bagchi J, Rakshit S, Mandal L, Bandyopadhyay G, Bandyopadhyay S. Granulocyte-macrophage colony-stimulating factor drives monocytes to CD14low CD83+ DCSIGN- interleukin-10-producing myeloid cells with differential effects on T-cell subsets. Immunology. 2007;121:499–507. doi: 10.1111/j.1365-2567.2007.02596.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tedder TF, Jansen PJ (2001) Isolation and generation of human dendritic cells. Curr Protoc Immunol Chapter 7: Unit 7 32 [DOI] [PubMed]
- 41.Kotsakis A, Harasymczuk M, Schilling B, Georgoulias V, Argiris A, Whiteside TL. Myeloid-derived suppressor cell measurements in fresh and cryopreserved blood samples. J Immunol Methods. 2012;381:14–22. doi: 10.1016/j.jim.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gros A, Turcotte S, Wunderlich JR, Ahmadzadeh M, Dudley ME, Rosenberg SA. Myeloid cells obtained from the blood but not from the tumor can suppress T-cell proliferation in patients with melanoma. Clin Cancer Res. 2012;18:5212–5223. doi: 10.1158/1078-0432.CCR-12-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kodumudi KN, Weber A, Sarnaik AA, Pilon-Thomas S. Blockade of myeloid-derived suppressor cells after induction of lymphopenia improves adoptive T cell therapy in a murine model of melanoma. J Immunol. 2012;189:5147–5154. doi: 10.4049/jimmunol.1200274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bunt SK, Yang L, Sinha P, Clements VK, Leips J, Ostrand-Rosenberg S. Reduced inflammation in the tumor microenvironment delays the accumulation of myeloid-derived suppressor cells and limits tumor progression. Cancer Res. 2007;67:10019–10026. doi: 10.1158/0008-5472.CAN-07-2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sinha P, Clements VK, Fulton AM, Ostrand-Rosenberg S. Prostaglandin E2 promotes tumor progression by inducing myeloid-derived suppressor cells. Cancer Res. 2007;67:4507–4513. doi: 10.1158/0008-5472.CAN-06-4174. [DOI] [PubMed] [Google Scholar]
- 46.Bronte V, Chappell DB, Apolloni E, Cabrelle A, Wang M, Hwu P, Restifo NP. Unopposed production of granulocyte-macrophage colony-stimulating factor by tumors inhibits CD8+ T cell responses by dysregulating antigen-presenting cell maturation. J Immunol. 1999;162:5728–5737. [PMC free article] [PubMed] [Google Scholar]
- 47.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ostrand-Rosenberg S. Myeloid-derived suppressor cells: more mechanisms for inhibiting antitumor immunity. Cancer Immunol Immunother. 2010;59:1593–1600. doi: 10.1007/s00262-010-0855-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Corzo CA, Condamine T, Lu L, Cotter MJ, Youn JI, Cheng P, Cho HI, Celis E, Quiceno DG, Padhya T, et al. HIF-1alpha regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J Exp Med. 2010;207:2439–2453. doi: 10.1084/jem.20100587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yu J, Du W, Yan F, Wang Y, Li H, Cao S, Yu W, Shen C, Liu J, Ren X. Myeloid-derived suppressor cells suppress antitumor immune responses through IDO expression and correlate with lymph node metastasis in patients with breast cancer. J Immunol. 2013;190:3783–3797. doi: 10.4049/jimmunol.1201449. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.