Abstract
Objectives
Tenofovir disoproxil fumarate (TDF) has been associated with renal insufficiency. Co-administration with boosted protease inhibitors (PIs), which increases its exposure, may further increase the risk of renal insufficiency.
Methods
We compared the incidence of renal events among women taking TDF co-administered with lopinavir/ritonavir (LPV/r) versus those co-administering TDF with nevirapine (NVP). Renal events were defined as a confirmed drop in creatinine clearance associated with a serum creatinine grade 2 or higher, or that leading to treatment modification.
Results
Overall, 741 HIV-infected women were enrolled into the study. Of these, 24 (3.2%) had reportable renal events (18 in LPV/r arm, 6 in NVP arm). In multivariate analysis, renal events were significantly associated with the LPV/r arm (odds ratio [OR]=3.12, 95% confidence interval [CI] 1.21, 8.05]; p=0.019), baseline HIV-1 RNA (OR=2.65, 95% CI: 1.23, 5.69 per 1 log10 copies/mL higher; p=0.013) and baseline creatinine clearance (OR=0.83, 95% CI 0.70–0.98 per 10 mL/min higher; p=0.030). In multivariate analysis evaluating renal events requiring treatment modification, only baseline HIV-1 RNA and creatinine clearance were significantly associated (OR=4.41, 95% CI 1.65, 11.78 per 1 log10 copies/mL higher; p= 0.003 and OR=0.80, 95% CI 0.64, 0.99 per 10 mL/min higher; p= 0.040, respectively).
Conclusion
The rates of renal events were relatively low in the two treatment arms. However, patients taking TDF co-administered with LPV/r had significantly more renal events compared to those co-administered with NVP. Furthermore, higher baseline HIV RNA and lower creatinine clearance were associated with the development of renal insufficiency requiring treatment modification.
Keywords: renal insufficiency, tenofovir, lopinavir/ritonavir, nevirapine
INTRODUCTION
HIV/AIDS remains an important medical challenge globally, especially in sub-Saharan Africa. The roll-out of combination antiretroviral therapy (cART) across the region has led to significant reduction in AIDS mortality [1]. While a cART treatment gap exists with respect to those in need of therapy, the long term safety issues of those on treatment are moving to the forefront. The most recent WHO guidelines [2] recommend that ART programs shift away from stavudine (d4T) based therapy toward tenofovir (TDF) or zidovudine (AZT) based first line cART due to such safety concerns.
As TDF use becomes more widespread, one safety concern is the potential for renal toxicity. Depending on program guidelines, TDF may be a component of either first line non-nucleoside reverse transcriptase inhibitor (NNRTI)-based therapy, or second line protease inhibitor (PI)-based therapy. The renal safety profile in each scenario may differ; this issue has particular importance for programs with limited safety monitoring. Although the clinical significance of this renal toxicity remains controversial [3–5], the need for continued vigilance cannot be overemphasized. When TDF is co-administered with ritonavir(r) boosted PIs, TDF exposure rises by about 20–30% [6–7], although it is unclear if this change in TDF exposure increases the risk of nephrotoxicity. Estimated glomerular filtration rate declined significantly more among patients who took TDF with boosted PIs compared with those taking TDF with NNRTI agents [8–9]. However, other studies have failed to demonstrate such incident renal insufficiency in association with the use of TDF co-administered with boosted PIs [3, 10–11].
The ACTG A5208 clinical trial randomized HIV-infected women to receive tenofovir/emtricitabine (TDF/FTC) plus nevirapine (NVP) or TDF/FTC plus lopinavir/ritonavir (LPV/r) with a goal of determining the impact of previous single dose NVP exposure on virologic response [12–13]. Using data from this randomized trial, we evaluated the incidence of renal events and examined if co-administration of LPV/r with TDF as a risk factor for TDF treatment discontinuation.
METHODOLOGY
STUDY POPULATION
This analysis is derived from the A5208 study, “Optimal Combination Therapy After Nevirapine Exposure (OCTANE)”. Participants were cART-naive HIV-infected women aged ≥13 years with a CD4 count of < 200 cells/mm3 enrolled at sites in eastern and southern Africa. For entry, all had creatinine clearance (CrCl) of at least ≥60 mL/min, calculated using the Cockcroft-Gault formula [14, 15], and a Karnofsky score of ≥70.
STUDY PROTOCOL
The OCTANE trial has previously been described [12–13]. Briefly, OCTANE study was a phase III study comprising two randomized controlled trials running concurrently. One trial enrolled women who reported prior exposure to single dose nevirapine (sdNVP) for prevention of mother-to-trial transmission of HIV, and the other trial enrolled women who reported no prior sdNVP exposure. Both trials compared the virological response to NNRTI-based versus PI-based ART. Participants were randomized to start cART with either NVP and FTC/TDF or LPV/r and FTC/TDF. Participants who discontinued NVP or LPV/r, either because of virologic failure, toxicity, or intolerability, could be switched to LPV/r or NVP, respectively.
Following randomization, study participants were seen at weeks 2, 4, 8, 12, 16, 24, and every 12 weeks thereafter until the last woman randomized reached 48 weeks. Chemistry tests, including serum creatinine, were performed at weeks 4, 12, 24, and then every 12 weeks. Chemistry laboratory results that were Grade 2 or higher per the Division of AIDS (DAIDS) toxicity table [16], or those resulting in treatment change were reported to the database. Per protocol, any calculated CrCl less than 50mL/min required repeat testing within 1 week. For confirmed levels less than 50mL/min, all cART was held or TDF was replaced with an appropriately renal-dose-adjusted NRTI alternative while investigations for alternative etiologies for renal insufficiency were explored. TDF was permanently discontinued if no other potential cause was determined. Those with alternative etiologies of their renal insufficiency could be rechallenged with TDF once CrCl was greater than or equal to 60 mL/min, but if CrCl decreased after rechallenge, TDF was permanently replaced with another NRTI agent.
IDENTIFICATION OF RENAL EVENTS
The primary outcome for this analysis was the development of a renal event, defined as a drop in creatinine clearance associated with a serum creatinine value of Grade 2 or greater (>1.4 times the upper limit of normal), as well as any renal events, including confirmed calculated CrCl less than 50 mL/min, which led to interruption or discontinuation of TDF. The secondary outcome for this analysis included only those renal events leading to temporary or permanent discontinuation of TDF.
To identify potential renal events for inclusion in the analysis, the adverse event database for the OCTANE study was screened for adverse events related to serum creatinine levels, CrCl, or renal diagnoses. This included a search of adverse event records in which the term “RENAL” was used in the MedDRA [17] preferred or lower level terms for the events. In addition, we reviewed causes of death for potential additional events and the cART records for reasons for changes in cART regimens. Adverse events that were identified by this search had no associated serum creatinine or CrCl results, or had serum creatinine of Grade 1 or lower and CrCl greater than 50mL/min, and which did not lead to any change in cART were excluded from the analysis. Renal events that were pre-existing prior to the start of study treatment were also excluded from the analysis.
DATA ANALYSIS
The log rank test was used to compare randomized arms with respect to time to a renal event. Exact logistic regression was used to identify factors associated with increased risk of a renal event. In multivariate modeling, stepwise variable selection was used initially to identify factors predictive of increased odds of a renal event with a p-value of less than 0.05 when added to a model which included variables for randomized treatment and prior exposure to sdNVP. Age was also included as a potential confounder of creatinine clearance.
RESULTS
Overall, 741 HIV infected women were enrolled in the OCTANE study, including 241 women (33%) with prior sdNVP exposure. Baseline characteristics were comparable between the two randomized treatment arms with a mean creatinine and CrCl of 0.71 mg/dL and 112 mL/min, respectively (Table 1). The median duration of follow-up was 2.3 years (10th, 90th percentiles: 1.2, 3.1 years).
Table 1.
Baseline characteristics of participants by randomized treatment arm
| LPV/r | NVP | Total | |
|---|---|---|---|
| Characteristic | (N=371) | (N=370) | (N=741) |
| Age (years) | |||
| Mean (SD) | 33(7) | 34(7) | 34(7) |
| Height (cm) | |||
| Mean (SD) | 160(6) | 159(6) | 160(6) |
| Weight (kg) | |||
| Mean (SD) | 60(12) | 60(13) | 60(13) |
| BMI (kg/m2) | |||
| Mean (SD) | 24(5) | 24(5) | 24(5) |
| HIV-1 RNA (log10 copies/mL) | |||
| Mean (SD) | 5.0(0.7) | 5.0(0.7) | 5.0(0.7) |
| CD4 count (cells/mm3) | |||
| Mean (SD) | 124(73) | 125(53) | 124(64) |
| Hemoglobin (g/dL) | |||
| Mean (SD) | 11.6(1.8) | 11.6(1.6) | 11.6(1.7) |
| Serum creatinine (mg/dL) | |||
| Mean (SD) | 0.71(0.21) | 0.70(0.15) | 0.71(0.18) |
| Serum creatinine grade, number (%)± | |||
| Normal | 368(99.2) | 368(99.5) | 736(99.3) |
| Mild (Grade 1) | 1(0.3) | 1(0.3) | 2(0.3) |
| Moderate (Grade 2) | 0 | 0 | 0 |
| Severe (Grade 3) | 2(0.5) | 0 | 2(0.3) |
| Potentially Life-threatening(Grade 4) | 0 | 1(0.3) | 1(0.1) |
| Creatinine Clearance (mL/min) | |||
| Mean (SD) | 112(34) | 113(37) | 112(35) |
| Single Dose NVP Exposure, number (%) | |||
| Yes | 121(32.6) | 121(32.7) | 242(32.7) |
| No | 250(67.3) | 249(67.3) | 499(67.3) |
SD: standard deviation; BMI: body mass index; NVP: nevirapine; ULN: Upper Limit of Normal
Subjects with baseline grade 3 and 4 events were excluded from renal analysis. Grade 1 (1.1–1.3 X ULN), Grade 2(1.4–1.8 x ULN), Grade 3 (1.9–3.4 x ULN), Grade 4 (>=3.5 x ULN)
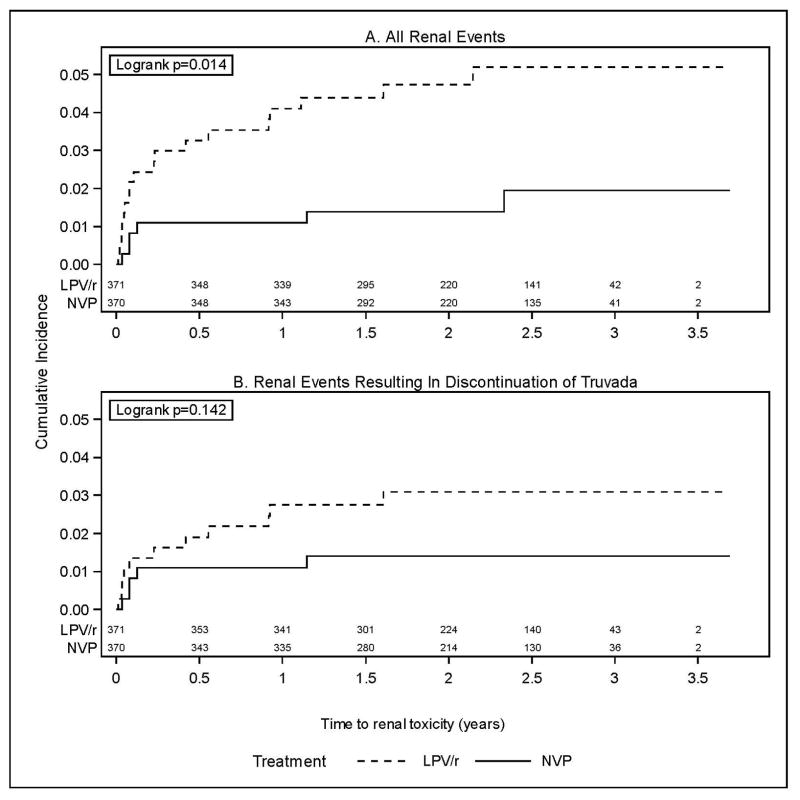
Twenty-four women experienced renal events, including 18 (4.9%) in the LPV/r arm and 6 (1.6%) in the NVP arm. All 24 women were receiving TDF and all except one were receiving their randomized regimen at the time of the event (i.e. LPV/r or NVP; one woman randomized to LPV/r was receiving efavirenz because of concomitant tuberculosis treatment). Eighteen of the 24 women had moderate (Grade 2) or higher elevated serum creatinine values (14 [3.8%] among women randomized to LPV/r and 4 [1.1%] among women randomized to NVP), including 12 with severe (Grade 3) or higher values (9 [2.4%] for the LPV/r arm and 3 [0.8%] for the NVP arm). Figure 1A shows the cumulative incidence over time of renal events. Events primarily occurred early after starting treatment, with a decreasing rate over time. The rate of renal events was significantly higher in the LPV/r arm compared to the NVP arm (p=0.014).
Figure 1.
Twenty-two of the 24 women had CrCl <50 mL/min; both women with higher CrCl had moderately elevated serum creatinine values (both were receiving LPV/r). Sixteen of the 22 also met criteria for temporary (2 women) or permanent discontinuation (14 women) of TDF, including 11 [3.0%] among women randomized to LPV/r and 5 [1.4%] among women randomized to NVP. The 2 women who discontinued temporarily were receiving LPV/r; they subsequently restarted and continued receiving TDF for the duration of the study. Figure 1B shows the cumulative incidence over time of renal events resulting in discontinuation of TDF. The difference between arms was not statistically significant (p=0.14).
Among all 741 women enrolled, there were 20 deaths, 5 of which were among patients with renal events (5/24, 21%); 2 in the LPV/r arm and 3 in the NVP arm. For the 2 women receiving LPV/r, the deaths occurred approximately 1 and 2 months (respectively) after the onset of the renal event, and acute renal failure was the reported primary or contributing cause of death in both patients. Among the 3 patients on the NVP arm with renal events who died, 1 died approximately one week after the onset of the renal event with acute renal failure as the reported primary cause; 1 died approximately 3 weeks after the onset of the renal event with pulmonary embolus as the reported primary cause and the renal event was ongoing; and 1 died approximately 6 months after onset and 5 months after the resolution of the renal event from unknown causes (but had continued on study-provided antiretroviral therapy after discontinuing TDF).
Logistic regression analysis was used to evaluate possible baseline (pre-treatment) factors that might be associated with risk of development of a renal event (Table 2). Reflecting the difference between randomized arms described above, there was a significantly higher odds of a renal event in the LPV/r arm compared with the NVP arm (OR = 3.09, 95% confidence interval [CI]: 1.21, 7.88; p=0.018). Additionally, in univariate analysis, several baseline factors were associated with increased odds of developing a renal event: older age, higher HIV-1 RNA, lower CrCl and lower CD4 count. However, when considering renal events resulting in treatment change as the outcome, while randomized treatment was not statistically significant (OR = 2.23 for LPV/r versus NVP, 95% CI: 0.77, 6.48; p=0.14), older age, higher HIV-1 RNA, lower CrCl, lower CD4 count as well as lower hemoglobin were significant (Table 2).
Table 2.
Univariate exact logistic regression results for association of odds of a renal event and selected baseline characteristics
| Variable | All Renal Events | All Renal Events leading to Treatment change | ||||||
|---|---|---|---|---|---|---|---|---|
| Odds Ratio (OR) | 95% Confidence Interval for OR | p-value | OR | 95% Confidence Interval for OR | p-value | |||
| Randomized Treatment – LPV/r versus NVP | 3.09 | 1.21 | 7.88 | 0.018 | 2.23 | 0.77 | 6.48 | 0.14 |
| Prior Single Dose NVP Exposure– No versus Yes | 1.88 | 0.69 | 5.09 | 0.22 | 2.13 | 0.60 | 7.55 | 0.24 |
| Age, per 10 years higher | 1.97 | 1.18 | 3.32 | 0.010 | 2.57 | 1.39 | 4.75 | 0.003 |
| Weight, per 10 kg higher | 0.72 | 0.48 | 1.08 | 0.11 | 0.68 | 0.41 | 1.13 | 0.13 |
| Height, per 10 cm higher | 1.42 | 0.73 | 2.77 | 0.30 | 0.71 | 0.31 | 1.61 | 0.41 |
| BMI, per kg/m2 higher | 0.91 | 0.81 | 1.01 | 0.078 | 0.94 | 0.83 | 1.06 | 0.31 |
| HIV-1 RNA, per 1 log10 copies/mL higher | 2.68 | 1.28 | 5.64 | 0.009 | 4.14 | 1.64 | 10.47 | 0.003 |
| Hemoglobin, per 1 g/dL higher | 0.79 | 0.62 | 1.00 | 0.054 | 0.73 | 0.54 | 0.98 | 0.033 |
| Creatinine Clearance, per 10 mL/min higher | 0.80 | 0.68 | 0.94 | 0.008 | 0.75 | 0.61 | 0.93 | 0.008 |
| CD4 count, per 100 cells/mm3 higher | 0.39 | 0.19 | 0.83 | 0.014 | 0.33 | 0.13 | 0.83 | 0.018 |
LPV/r: lopinavir/ritonavir; NVP: nevirapine; BMI: body mass index
In multivariate analysis, we fitted a model which included randomized treatment, prior exposure to single dose NVP, CrCl, HIV-1 RNA and age as covariates (Table 3; see Methods for rationale for this model); other covariates considered in univariate analysis were not significant when added to this model. In this model, LPV/r treatment arm was significantly associated with having a renal event (OR = 3.12, 95% CI [1.21, 8.05], p = 0.019) as were higher baseline HIV-1 RNA and lower baseline CrCl (Table 3). However, when considering renal events resulting in treatment modification as the outcome, higher baseline HIV-1 RNA and lower baseline creatinine clearance were significantly associated with increased odds of an event though there was a non-significant association with treatment arm (p=0.16).
Table 3.
Multivariate exact logistic regression results for association of odds of a renal event and selected baseline characteristics.
| Variable | All Renal Events | Events leading to change of tenofovir in Treatment | ||||||
|---|---|---|---|---|---|---|---|---|
| Odds Ratio (OR) | 95% Confidence Interval for OR | p-value | OR | 95% Confidence Interval for OR | p-value | |||
| Randomized Treatment – LPV/r versus NVP | 3.12 | 1.21 | 8.05 | 0.019 | 2.20 | 0.73 | 6.59 | 0.160 |
| Prior Single Dose NVP Exposure – No versus Yes | 1.44 | 0.50 | 4.17 | 0.501 | 1.40 | 0.36 | 5.44 | 0.629 |
| Creatinine Clearance, per 10mL/min higher | 0.83 | 0.70 | 0.98 | 0.030 | 0.80 | 0.64 | 0.99 | 0.040 |
| HIV-1 RNA, per 1 log10 copies/mL higher | 2.65 | 1.23 | 5.69 | 0.013 | 4.41 | 1.65 | 11.78 | 0.003 |
| Age, per 10 years higher | 1.55 | 0.89 | 2.69 | 0.121 | 1.94 | 0.99 | 3.79 | 0.053 |
LPV/r: lopinavir/ritonavir; NVP: nevirapine;
Discussion
In this large randomized clinical trial of HIV-infected women from resource limited settings starting TDF-based cART regimens, 3% of women experienced renal events and 2% required permanent treatment modification. The LPV/r treatment arm was associated with increased overall renal events compared to the NVP treatment arm (4.9% vs. 1.6%) and higher, though not statistically significant, rates of renal events leading to treatment modification (3.0% vs. 1.4%). Additionally, higher baseline HIV RNA and lower baseline CrCl were associated with both increased renal events and renal events leading to treatment modification.
The increased risk of renal events with LPV/r therapy and TDF co-administration is plausible and consistent with previous research [7, 9, 10, 18]. TDF is eliminated by renal clearance, with 20–30% of the filtered drug actively transported into renal proximal tubules. From these proximal tubular cells, the drug is excreted by the multidrug resistance proteins (MRPs). Ritonavir is a potent inhibitor of MRP-2[19–20]. Consequently, co-administration of TDF with ritonavir could lead to accumulation of TDF within the proximal tubular cells and possibly the development of TDF-associated renal proximal tubulopathy. Unfortunately, we did not perform pharmacokinetic analysis of TDF levels in the study so we cannot confirm if levels were increased in the LPV/r arm.
In one non-randomized study, greater declines in renal function have been observed among patients treated with TDF and PI/r compared to TDF+NNRTI regimens [18]. Although the degree of renal impairment found was relatively low, probably in part to a short period of follow up (48 weeks), potential serious clinical implications for patients taking TDF co-administered with LPV/r for longer durations were postulated. In our randomized study that included a median 2.3 years of follow-up, while the LPV/r arm had more renal events over time compared to the NVP arm, the events tended to occur earlier following treatment initiation and the overall risk of serious renal toxicity due to TDF with long term use remains low. However, as we did not measure glomerular filtration rate (GFR) for all patients, subtle decreases in GFR may have been missed. Continued exposure to TDF even in patients with subtle renal impairment may lead to progressive and significant renal insufficiency [21]. Irreversible TDF-associated renal toxicity may be more likely among those with a gradual decline in GFR as opposed to more acute renal damage [22]. In our study, among those surviving their renal event there were no cases of chronic kidney disease; this was likely due to close monitoring and cART modification, as mandated per the protocol.
We found high baseline HIV-1 RNA and lower baseline CrCl to be associated with renal insufficiency in multivariate analysis. Direct effects of HIV play an important role in the development of HIV-associated nephropathy [23–24]. HIV-associated nephropathy has been shown to be the most common cause of end stage renal disease in HIV patients, typically occurring among patients with a relatively high viral burden and a CD4 count of < 200 cells/mm3 [25–26]. Indirectly, HIV proteins may mediate endothelial damage causing platelet deposition within renal microvasculature through various mechanisms, leading to HIV-induced thrombotic microangiopathy. This could explain the association in increasing viral burden to the development of renal insufficiency we found in our analysis. In addition, the low baseline CrCl is probably indicative of underlying renal disease even before exposure to TDF. Others have also shown that baseline serum creatinine, age, CD4 count, low hemoglobin, BMI, and CrCl are associated with decline in renal function among TDF-treated patients [27–31].
Our study had several methodological limitations. The primary events, any renal event, and, in particular, renal events leading to treatment change, consistent with previous studies involving the use of TDF in resource limited settings [32–33], were uncommon (3%), thereby limiting power to evaluate possible factors associated with risk. Also, we were not able to evaluate factors associated with more subtle decreases in CrCl that remained above 60mL/min but could represent significant declines compared to baseline. Urinalysis and urine dipstick evaluation, as measure of tubular function, were not routinely collected, thereby limiting interpretation of the renal events. In addition, our patient population was exclusively female and so our results may not apply to males who generally have higher body weights and muscle mass. In other settings, however, gender has not been associated with development of renal insufficiency. We also did not explore the effect of other concomitant medications or concurrent diseases that could potentially have caused of renal insufficiency. However, despite these limitations, our study results are strengthened by the fact that patients were randomized to the treatment groups and therefore had comparable baseline characteristics and were managed by standardized toxicity protocols.
In most sub-saharan African settings, programs rely on public health approaches and renal monitoring and determination of alternative etiologies of renal events are not readily available or would require referral for specialist evaluation. Hence, the frequency of laboratory monitoring of ART patients remains a relevant topic. The DART trial suggested that toxicity monitoring did not improve overall outcomes among individuals receiving either AZT/3TC/TDF or AZT/3TC/NVP [32] and renal events have been low in other clinical trials using TDF in resource limited settings [31]. Based on these findings, many countries using TDF-based therapy for first line therapy are not conducting routine renal toxicity monitoring. However, the higher rates of renal events seen in our study among those randomized to TDF combined with LPV/r may suggest monitoring in this population may be beneficial. The timing of events in our study, occurring somewhat early in the treatment course, may suggest a simplified toxicity algorithm could be applied to detect most cases. Alternatively, policy makers may favor initial treatment of TDF with an NNRTI as first line rather than using it as a component of second line when a boosted PI would be used. Additionally, with expansion of use of LPV/r or TDF for prevention of mother-to-child-transmission, further evaluation of renal function in this population is necessary.
In summary, the rate of renal events in the A5208 study was low, although women receiving TDF co-administered with LPV/r had significantly more renal events both in magnitude and over time, compared to those co-administering TDF with NVP. However, renal events did not always require treatment modification, given our safety monitoring strategy. Finally, high baseline HIV RNA and low baseline CrCl were factors associated with increased risk of renal events, including those requiring treatment modifications.
Acknowledgments
We thank all patients for participating in the study. The main study A5208, (OCTANE) was supported in part by grants (U01AI068636, AI38838, and SDMC AI68634) from the National Institute of Allergy and Infectious Diseases to the AIDS Clinical Trials Group, by grants from the National Center for Research to the General Clinical Research Center Units, and by grants (K24 AI56933, to Dr. Currier, and 5401A1068636-04, to the Virology Support Laboratory for Adult AIDS Clinical Trials Group) from the National Institutes of Health. Study drugs were provided by AbbVie Inc, Boehringer Ingelheim Pharmaceuticals, Gilead Sciences, Bristol-Myers Squibb, and GlaxoSmithKline.
Footnotes
Disclaimers: None
References
- 1.Floyd S, Molesworth A, Dube A, Banda E, Jahn A, Mwafulirwa C, et al. Population-Level Reduction in Adult Mortality after Extension of Free Anti-Retroviral Therapy Provision into Rural Areas in Northern Malawi. [10.1371/journal.pone.0013499];PLoS ONE. 5(10):e13499. doi: 10.1371/journal.pone.0013499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO. WHO Antiretroviral therapy for HIV infection in adults and adolescents: Recommendations for a public health approach. 2010 revision, http://whqlibdoc.who.int/publications/2010/9789241599764_eng.pdf. [PubMed]
- 3.Fux C, Christen A, Zgraggen S, Mohaupt M, Furrer H. Effect of tenofovir on renal glomerular and tubular function. AIDS. 2007;21 (11):1483–85. doi: 10.1097/QAD.0b013e328216f15b. [DOI] [PubMed] [Google Scholar]
- 4.Antoniuo T, Raboud J, Chirhin S, Yoong D, Govan K. Incidence of and risk factors for tenofovir-induced nephrotoxicity: a retrospective cohort study. HIV Medicine. 2005;6:284–290. doi: 10.1111/j.1468-1293.2005.00308.x. [DOI] [PubMed] [Google Scholar]
- 5.Cooper R, Wiebe N, Smith N, Kieser P, Naicker S, Tonelli M. Systematic Review and Meta-analysis: Renal Safety of Tenofovir Disoproxil Fumarate in HIV-Infected Patients. Clinical Infectious Diseases. 2010;51(5):496–505. doi: 10.1086/655681. [DOI] [PubMed] [Google Scholar]
- 6.Gilead Sciences. Viread Medication insert. http://www.gilead.com/pdf/viread_pi.pdf.
- 7.Kearney B, Mathias A, Miltan A, Sayre J, Ebrahim R, Cheng A. Pharmacokinetics and safety of tenofovir Disoproxil Fumarate on co administration with Lopinavir/ritonavir. JAIDS. 2006;43 (3):278–83. doi: 10.1097/01.qai.0000243103.03265.2b. [DOI] [PubMed] [Google Scholar]
- 8.Gallant J, Moore R. Renal function with use of tenofovir-containing initial antiretroviral regimen. AIDS. 2009;23:1971–1975. doi: 10.1097/QAD.0b013e32832c96e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buchacz K, Young B, Baker R, Moorman A, Chmiel J, Wood K. Renal function in patients receiving tenofovir with Ritonavir/lopinavir or Ritonavir/Atazanavir in the HIV Outpatient Study (HOPS) cohort. JAIDS. 2006;43(5):626–28. doi: 10.1097/01.qai.0000242461.35768.45. [DOI] [PubMed] [Google Scholar]
- 10.Kiser J, Carten M, Aquilante C, Anderson P, Wolfe, King T, et al. The effect of Lopinavir/ritonavir on renal clearance of tenofovir in HIV-infected patients. Clinical Pharmacology and Therapeutics. 2008;83:265–72. doi: 10.1038/sj.clpt.6100269. [DOI] [PubMed] [Google Scholar]
- 11.Zimmermann A, Pizzoferrato T, Bedford J, Morris A, Hoffman R, Braden G. Tenofovir-associated acute and chronic kidney disease: A case of Multiple drug interactions. Clinical Infectious Diseases. 2006;42:283–90. doi: 10.1086/499048. [DOI] [PubMed] [Google Scholar]
- 12.Lockman S, Shapiro R, Smeaton L, Wester C, Thior I, Stevens L, et al. Response to Antiretroviral Therapy after a Single, Peripartum Dose of Nevirapine. N Eng J Med. 2007;356:135–47. doi: 10.1056/NEJMoa062876. [DOI] [PubMed] [Google Scholar]
- 13.Lockman S, Hughes M, Sawe F, Zhegh Y, McIntyres J, Chipato T, et al. Nevirapine-Versus Lopinavir/Ritonavir-Based Initial Therapy for HIV-1 Infection among Women in Africa: A Randomized Trial. PLoS Med. 9(6):e1001236. doi: 10.1371/journal.pmed.1001236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levey A, Bosch J, Lewis J. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Ann Intern Med. 1999;130:461–70. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 15.Sanusi A, Akinsola A, Ajayi A. Creatinine clearance estimation from serum creatinine values ; evaluation and comparison of five prediction formulae in Nigerian patients. Afr J Med Sci. 2000;29:7–11. [PubMed] [Google Scholar]
- 16.National institute of allergy and Infectious diseases. Division of AIDS table of grading severity of adult and pediatric adverse events. http://rsc.tech-res.com/Document/safetyandpharmacovigilance/Table_for_Grading_Severity_of_Adult_Pediatric_Adverse_Events.pdf.
- 17.Medical dictionary for regulatory activities. MedDRA, Error! Hyperlink reference not valid. http://www.meddramsso.com.
- 18.Goicoechea M, Liu S, Best B, Sun S, Jain S, Kemper C, et al. Greater tenofovir associated renal function decline with PI-based versus Non-nucleoside reverse –transcriptase inhibitor–based therapy. JAIDS. 2008;197:102–08. doi: 10.1086/524061. [DOI] [PubMed] [Google Scholar]
- 19.Ray A, Cihlar T, Robinson K, Tong L, Vela J, Fuller M, et al. Mechanism of active renal tubular Efflux of tenofovir. Antimicrobial agents and chemotherapy. 2006;50(10):3297–3304. doi: 10.1128/AAC.00251-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Izzedine H, Launay-Vacher V, Deray G. Renal tubular transporters and antiviral drugs: an update. JAIDS. 2006;43 (5):455–62. doi: 10.1097/01.aids.0000162333.35686.4c. [DOI] [PubMed] [Google Scholar]
- 21.Horberg M, Tang B, Towner W, Silverberg M, Bersoff-Matcha S, Hurley L, et al. Impact of Tenofovir on renal function in HIV-infected, antiretroviral-naive patients. J Acquir Immune Defic Syndr. 2010;53 (1):62–69. doi: 10.1097/QAI.0b013e3181be6be2. [DOI] [PubMed] [Google Scholar]
- 22.Wever K, Agtmael M, Carr A. Incomplete reversibility of Tenofovir-related renal toxicity in HIV-infected men. J Acquir Immune Defic Syndr. 2010;(55):78–81. doi: 10.1097/QAI.0b013e3181d05579. [DOI] [PubMed]
- 23.Rao T, Freidman E, Nicastri A. The types of renal disease on acquired immunodeficiency syndrome. The New England Journal of Medicine. 1987;316(17):1062–1068. doi: 10.1056/NEJM198704233161705. [DOI] [PubMed] [Google Scholar]
- 24.R_ling J, Schmid H, Fischereder M, Draenert R, Goebel D. HIV-associated renal disease and Highly Active Antiretroviral Therapy- induced nephropathy. Clinical infectious disease. 2006;42:1488–95. doi: 10.1086/503566. [DOI] [PubMed] [Google Scholar]
- 25.Krawczyka S, Holmbergb S, Moormanb A, Gardnerb L. Factors associated with chronic renal failure in HIV-infected ambulatory patients. AIDS. 2004;18:2171–2178. doi: 10.1097/00002030-200411050-00009. [DOI] [PubMed] [Google Scholar]
- 26.Szczech l, Gange S, Van Der Horst C, Bartlett J, et al. Predictors of proteinuria and renal failure among women with HIV infection. Kidney International. 2002;61:195–202. doi: 10.1046/j.1523-1755.2002.00094.x. [DOI] [PubMed] [Google Scholar]
- 27.Hall A, Hendry B, Nitsch D, Connolly Tenofovir-associated kidney toxicity in HIV-infected patients: a review of the evidence. Am J Kidney Disease. 2011;57 (5):p773–80. doi: 10.1053/j.ajkd.2011.01.022. [DOI] [PubMed] [Google Scholar]
- 28.Winston J, Deray G, Hawkins T, Szcech L, Wyatt C, Young B. Kidney Disease in Patients with HIV Infection and AIDS. HIV/AIDS. 2008;47:p1449– 57. doi: 10.1086/593099. [DOI] [PubMed] [Google Scholar]
- 29.Nelson M, Katlama, Montaner J, Cooper, Gazzard B, Clotet B, et al. The safety of tenofovir for the treatment of HIV infection in adults: the first four years. AIDS. 2007;21:1273–1281. doi: 10.1097/QAD.0b013e3280b07b33. [DOI] [PubMed] [Google Scholar]
- 30.Barrios A, García-Benayas T, Lahoz J, Soriano V. Tenofovir-related nephrotoxicity in HIV-infected patients. AIDS. 2004;18 (6):960–962. doi: 10.1097/00002030-200404090-00019. [DOI] [PubMed] [Google Scholar]
- 31.Sahly H, Teeter L, Zerai T, Roberto A, Andrade A, Munoz A, et al. Serum creatinine changes in HIV-seropositive patients receiving tenofovir. AIDS. 2006;20(5):786–787. doi: 10.1097/01.aids.0000216386.60481.47. [DOI] [PubMed] [Google Scholar]
- 32.Campbell T, Smeaton L, Kumarasamy N, Flanigan T, Klingman K, Firnhaber C, et al. Efficacy and safety of three Antiretroviral regimens for initial treatment of HIV-1: A Randomized Clinical Trial in Diverse Multinational Settings. PLoS Med. 2012;9(8):e1001290. doi: 10.1371/journal.pmed.1001290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reid A, Stohr W, Walker AS, Hakim J, Ssali F, Munderi P, et al. Glomerular dysfunction and associated risk factors through four years following initiation of ART in adults with HIV Infection in Africa in the DART trial; 5th International AIDS Society. [Google Scholar]